Pb(II) Adsorption Properties of a Three-Dimensional Porous Bacterial Cellulose/Graphene Oxide Composite Hydrogel Subjected to Ultrasonic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BC/GO Composite Hydrogel
2.3. Characterization
2.4. Adsorption Experiment
3. Results and Discussion
3.1. Characterization of BC/GO
3.2. TEM and SEM Studies
3.3. FT-IR Studies
3.4. NMR Studies
3.5. Zeta Potential Studies
3.6. Adsorption of Pb(II)
3.6.1. Effect of GO Concentration on Adsorption
3.6.2. Effect of Contact Time on Adsorption
3.6.3. Effect of Initial Concentration on Adsorption
3.7. Adsorption Kinetics
3.8. Adsorption Isotherm
3.9. FT-IR Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Liu, M.; Zhao, W.; Wang, S.; Gui, M.; Li, H.; Yu, R. DNAzyme-based cascade signal amplification strategy for highly sensitive detection of lead ions in the environment. J. Hazard. Mater. 2022, 429, 128347. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Liu, Y.; Wang, F.; Luo, D. Highly sensitive and selective optical sensor for lead ion detection based on liquid crystal decorated with DNAzyme. Opt. Express 2019, 27, 30421. [Google Scholar] [CrossRef] [PubMed]
- Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Daňo, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Qiu, H.; Wang, C.; Yuan, B.; Huang, H.; Li, B. Thermodynamic and Kinetic Studies on Adsorption of Vanadium with Glutamic Acid. ACS Omega 2021, 6, 21563–21570. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Guo, J.; Li, B.; Huang, H. Vanadium properties, toxicity, mineral sources and extraction methods: A review. Environ. Chem. Lett. 2022, 20, 1249–1263. [Google Scholar] [CrossRef]
- Souza, L.R.R.; Cicolani, R.S.; De Freitas, B.E.S.; Floriano, G.L.; De Oliveira, M.L.; De Oliveira Filho, A.G.S.; Da Veiga, M.A.M.S.; Demets, G.J.-F. Polyurethane sponges bearing cucurbituril adsorb Cr(III) and Pb(II) ions from contaminated water samples. Environ. Sci. Pollut. Res. 2024, 31, 29749–29762. [Google Scholar] [CrossRef] [PubMed]
- El-Wakeel, S.T.; Fathy, N.A.; Tawfik, M.E. Porous carbons prepared from a novel hard wood composite waste for effective adsorption of Pb(II) and Cd(II) ions. RSC Adv. 2023, 13, 34935–34946. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-R.; Kim, S.W.; Lee, C.-H.; Choi, E.-K.; Oh, M.H.; Seo, S.N.; Park, H.-J.; Hamm, S.-Y. Performance of composite mineral adsorbents for removing Cu, Cd, and Pb ions from polluted water. Sci. Rep. 2019, 9, 13598. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, J.; Doumane, G.; Iraqi, O.; Elhenawy, A.A.; Ouaddari, H.; Okla, M.K.; Nafidi, H.-A.; Younous, Y.A.; Bourhia, M.; Habsaoui, A. Optimization of an experimental study of cationic Pb metal adsorption by resin polymer. Sci. Rep. 2023, 13, 20060. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Joshi, S.K.; Bhandari, N.S. Estimation of sorption–desorption characteristics of biosorbent of Lantana camara leaves for removal of Pb (II) ions from wastewater. Environ. Monit. Assess. 2022, 195, 42. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Sadeghalvad, B.; Ghasemi, I.; Ebrahimi, H.; Rezaeian, I. Fabrication of Polyethersulfone/Functionalized MWCNTs Nanocomposite and Investigation its Efficiency as an Adsorbent of Pb(II) Ions. Arab. J. Sci. Eng. 2021, 46, 6259–6273. [Google Scholar] [CrossRef]
- Bessa, R.A.; França, A.M.M.; Pereira, A.L.S.; Alexandre, N.P.; Pérez-Page, M.; Holmes, S.M.; Nascimento, R.F.; Rosa, M.F.; Anderson, M.W.; Loiola, A.R. Hierarchical zeolite based on multiporous zeolite A and bacterial cellulose: An efficient adsorbent of Pb2+. Microporous Mesoporous Mater. 2021, 312, 110752. [Google Scholar] [CrossRef]
- Oktaviani, O.; Puspitasari, T.; Pangerteni, D.S.; Nuryanthi, N.; Syahputra, A.R. Synthesis and Application of Bacterial Cellulose-co-(poly)acrylamide as an Adsorbent for Cu and Pb Metal Ions. Macromol. Symp. 2020, 391, 1900154. [Google Scholar] [CrossRef]
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium oxide-bacterial cellulose bioadsorbent for the removal of lead ions from aqueous solution. Int. J. Biol. Macromol. 2019, 129, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Njeh, F.; Hamza, M.; Bouaziz, I.; Abdelhedi, R.; Abdelmouleh, M. Isolation, characterization and methylene blue adsorption: Application of cellulose from olive sawdust. Korean J. Chem. Eng. 2022, 39, 760–774. [Google Scholar] [CrossRef]
- Sakirler, F.; Wong, H.-W. Cellulose Fast Pyrolysis Activated by Intramolecular Hydrogen Bonds. J. Phys. Chem. A 2022, 126, 7806–7819. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhan, X.; Wen, C.; Xu, F.; Luo, L. Amino-functionalized magnetic bacterial cellulose/activated carbon composite for Pb2+ and methyl orange sorption from aqueous solution. J. Mater. Sci. Technol. 2018, 34, 855–863. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Huang, X.; Qian, X. Surface Functionalization of Graphene Oxide with Hyperbranched Polyamide-Amine and Microcrystalline Cellulose for Efficient Adsorption of Heavy Metal Ions. ACS Omega 2022, 7, 10944–10954. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.C.; Rawat, B.S.; Kumar, P.; Kumar, N.; Upadhyay, S.; Chetana, S.; Gururani, P.; Kimothi, S. Sustainable synthetic approach and applications of ZnO/r-GO in the adsorption of toxic Pb2+ and Cr6+ ions. Inorg. Chem. Commun. 2022, 145, 110040. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Lan, G.; Qiu, H.; Xu, B.; Xu, Q.; Sun, N.; Zhang, L. Pb(II) adsorption characteristics of magnetic GO-hydroxyapatite and the contribution of GO to enhance its acid resistance. J. Environ. Chem. Eng. 2021, 9, 105310. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, X.; Liang, C.; Zhu, W.; Meng, X. Characteristics and mechanism of Pb(II) adsorption/desorption on GO/r-GO under sulfide-reducing conditions. J. Ind. Eng. Chem. 2019, 73, 233–240. [Google Scholar] [CrossRef]
- Mensah, A.; Lv, P.; Narh, C.; Huang, J.; Wang, D.; Wei, Q. Sequestration of Pb(II) Ions from Aqueous Systems with Novel Green Bacterial Cellulose Graphene Oxide Composite. Materials 2019, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Walling, B.; Bharali, P.; Ramachandran, D.; Viswanathan, K.; Hazarika, S.; Dutta, N.; Mudoi, P.; Manivannan, J.; Manjunath Kamath, S.; Kumari, S.; et al. In-situ biofabrication of bacterial nanocellulose (BNC)/graphene oxide (GO) nano-biocomposite and study of its cationic dyes adsorption properties. Int. J. Biol. Macromol. 2023, 251, 126309. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Zhang, J.; Jiao, C.; Ding, L.; Yang, S. Synthesis and Adsorption Properties of Novel Bacterial Cellulose/Graphene Oxide/Attapulgite Materials for Cu and Pb Ions in Aqueous Solutions. Materials 2020, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- GB/T 1725-1979; Methods of Test for Non-Volatile Matter of Varnishes, Paints and Their Vehicles. China Standard Press: Beijing, China, 1979.
- Chi, C.; Li, X.; Zhang, Y.; Miao, S.; Chen, L.; Li, L.; Liang, Y. Understanding the effect of freeze-drying on microstructures of starch hydrogels. Food Hydrocoll. 2020, 101, 105509. [Google Scholar] [CrossRef]
- Hoo, D.Y.; Low, Z.L.; Low, D.Y.S.; Tang, S.Y.; Manickam, S.; Tan, K.W.; Ban, Z.H. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason. Sonochem. 2022, 90, 106176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Zhang, Y.; Xu, M.; Shi, S.Q. The three-dimensional heterostructure synthesis of ZnO/cellulosic fibers and its application for rubber composites. Compos. Sci. Technol. 2019, 177, 10–17. [Google Scholar] [CrossRef]
- Pignon, F.; Semeraro, E.F.; Chèvremont, W.; Bodiguel, H.; Hengl, N.; Karrouch, M.; Sztucki, M. Orientation of Cellulose Nanocrystals Controlled in Perpendicular Directions by Combined Shear Flow and Ultrasound Waves Studied by Small-Angle X-ray Scattering. J. Phys. Chem. C 2021, 125, 18409–18419. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Wei, J.; Long, Y.; Li, T.; Gao, H.; Nie, Y. Exploring hydrogen-bond structures in cellulose during regeneration with anti-solvent through two-dimensional correlation infrared spectroscopy. Int. J. Biol. Macromol. 2024, 267, 131204. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, E.; Babaeipour, V.; Chegeni, A.; Khodamoradi, N.; Omidi, M. Synthesis and characterization of bacterial cellulose/graphene oxide nano-biocomposites. Polym. Compos. 2021, 42, 4698–4706. [Google Scholar] [CrossRef]
- Sereshti, H.; Rezvani, F.; Soltani, S.; Karami, F.; Nodeh, H.R. Designing a bacterial cellulose-based hydrogel incorporated with manganese sulfide and graphene oxide for green extraction of acrylamide in bread samples. Sep. Sci. Plus 2024, 7, 2300169. [Google Scholar] [CrossRef]
- El-Khouly, A.S.; Takahashi, Y. Synthesis, Characterization, and Evaluation of the Adsorption Behavior of Cellulose-Graft-Poly(Acrylonitrile-co-Acrylic Acid) and Cellulose-Graft-Poly(Acrylonitrile-co-Styrene) towards Ni(II) and Cu(II) Heavy Metals. Polymers 2024, 16, 445. [Google Scholar] [CrossRef]
- Brouwer, D.H.; Mikolajewski, J.G. Resolving the discrepancies in reported 13C solid state NMR chemical shifts for native celluloses. Cellulose 2023, 30, 4827–4839. [Google Scholar] [CrossRef]
- Chen, S.; Zou, Y.; Yan, Z.; Shen, W.; Shi, S.; Zhang, X.; Wang, H. Carboxymethylated-bacterial cellulose for copper and lead ion removal. J. Hazard. Mater. 2008, 161, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, S.; Wan, T.; Jia, Y.; Yang, H.; Li, J.; Yan, L.; Zhong, C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr. Polym. 2011, 86, 1558–1564. [Google Scholar] [CrossRef]
- Chen, S.; Shen, W.; Yu, F.; Hu, W.; Wang, H. Preparation of amidoximated bacterial cellulose and its adsorption mechanism for Cu2+ and Pb2+. J. Appl. Polym. Sci. 2010, 117, 8–15. [Google Scholar] [CrossRef]
- Shen, W.; Chen, S.; Shi, S.; Li, X.; Zhang, X.; Hu, W.; Wang, H. Adsorption of Cu(II) and Pb(II) onto diethylenetriamine-bacterial cellulose. Carbohydr. Polym. 2008, 75, 110–114. [Google Scholar] [CrossRef]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Luo, P. Preparation, Kinetics, and Adsorption Mechanism Study of Microcrystalline Cellulose-Modified Bone Char as an Efficient Pb (II) Adsorbent. Water. Air. Soil Pollut. 2020, 231, 328. [Google Scholar] [CrossRef]
- Hashem, A.; Fletcher, A.J.; Younis, H.; Mauof, H.; Abou-Okeil, A. Adsorption of Pb(II) ions from contaminated water by 1,2,3,4-butanetetracarboxylic acid-modified microcrystalline cellulose: Isotherms, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2020, 164, 3193–3203. [Google Scholar] [CrossRef] [PubMed]
- Neeli, S.T.; Ramsurn, H.; Ng, C.Y.; Wang, Y.; Lu, J. Removal of Cr (VI), As (V), Cu (II), and Pb (II) using cellulose biochar supported iron nanoparticles: A kinetic and mechanistic study. J. Environ. Chem. Eng. 2020, 8, 103886. [Google Scholar] [CrossRef]
- Wu, D.; Hu, L.; Wang, Y.; Wei, Q.; Yan, L.; Yan, T.; Li, Y.; Du, B. EDTA modified β-cyclodextrin/chitosan for rapid removal of Pb(II) and acid red from aqueous solution. J. Colloid Interface Sci. 2018, 523, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yuvaraja, G.; Liu, C.; Kong, L.; Guo, K.; Reddy, G.M.; Zyryanov, G.V. Removal of Pb(II) ions from aqueous media using epichlorohydrin crosslinked chitosan Schiff’s base@Fe3O4 (ECCSB@Fe3O4). Int. J. Biol. Macromol. 2018, 117, 1305–1313. [Google Scholar] [CrossRef] [PubMed]


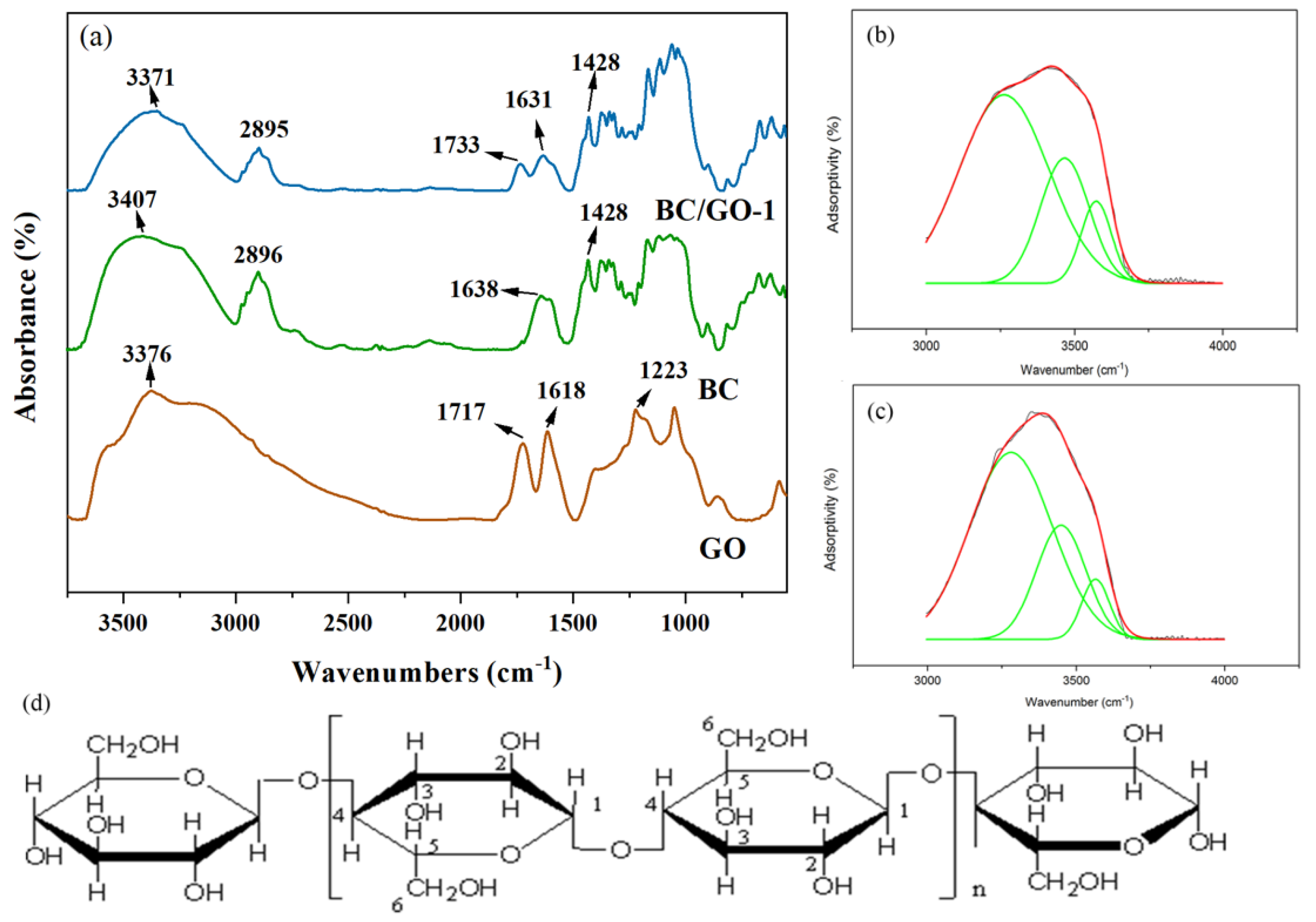







| Sample | Wavenumber/cm−1 (Area IntgP %) | ||
|---|---|---|---|
| O(2)H···O(6) | O(3)H···O(5) | O(6)H···O(3) | |
| BC | 3566 (13.7) | 3436 (46.42) | 3228 (39.88) |
| BC/GO-1 | 3563 (9.55) | 3432 (44.01) | 3251 (46.44) |
| Adsorbent | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| Carboxymethylated bacterial cellulose | 60.4 | [36] |
| Fe3O4/BC spheres | 65.0 | [37] |
| Amidoximated bacterial cellulose | 67.0 | [38] |
| Diethylenetriamine-bacterial cellulose | 87.4 | [39] |
| Polyethyleneimine-bacterial cellulose | 148.0 | [40] |
| Amino-functionalized magnetic bacterial cellulose/activated carbon | 161.8 | [17] |
| BC/GO-1 | 224.5 | This work |
| Sample | Pseudo-First-Order Model | Pseudo-Second-Order Model | Experimental qm (mg/g) | ||||
|---|---|---|---|---|---|---|---|
| K1 | qe (mg/g) | R12 | K2 | qe (mg/g) | R22 | ||
| BC | 0.03982 | 208.8 | 0.9474 | 7.460 × 10−5 | 207.5 | 0.9757 | 121.0 |
| BC/GO-1 | 0.04764 | 459.5 | 0.9505 | 3.529 × 10−5 | 398.4 | 0.9817 | 224.5 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax (mg/g) | kL | R2 | kF | n | R2 | |
| BC | 526.3 | 0.0029 | 0.6464 | 1.825 | 1.092 | 0.9983 |
| BC/GO-1 | 1272.5 | 0.0024 | 0.6448 | 3.593 | 1.081 | 0.9944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, J.; Zhang, Z.; Li, P.; He, C.; Zhong, M. Pb(II) Adsorption Properties of a Three-Dimensional Porous Bacterial Cellulose/Graphene Oxide Composite Hydrogel Subjected to Ultrasonic Treatment. Materials 2024, 17, 3053. https://doi.org/10.3390/ma17133053
Zhang X, Xu J, Zhang Z, Li P, He C, Zhong M. Pb(II) Adsorption Properties of a Three-Dimensional Porous Bacterial Cellulose/Graphene Oxide Composite Hydrogel Subjected to Ultrasonic Treatment. Materials. 2024; 17(13):3053. https://doi.org/10.3390/ma17133053
Chicago/Turabian StyleZhang, Xinxing, Jing Xu, Zhijie Zhang, Pengping Li, Chang He, and Mingfeng Zhong. 2024. "Pb(II) Adsorption Properties of a Three-Dimensional Porous Bacterial Cellulose/Graphene Oxide Composite Hydrogel Subjected to Ultrasonic Treatment" Materials 17, no. 13: 3053. https://doi.org/10.3390/ma17133053





