Designing Dual-Responsive Drug Delivery Systems: The Role of Phase Change Materials and Metal–Organic Frameworks
Abstract
:1. Phase Change Materials (PCMs)
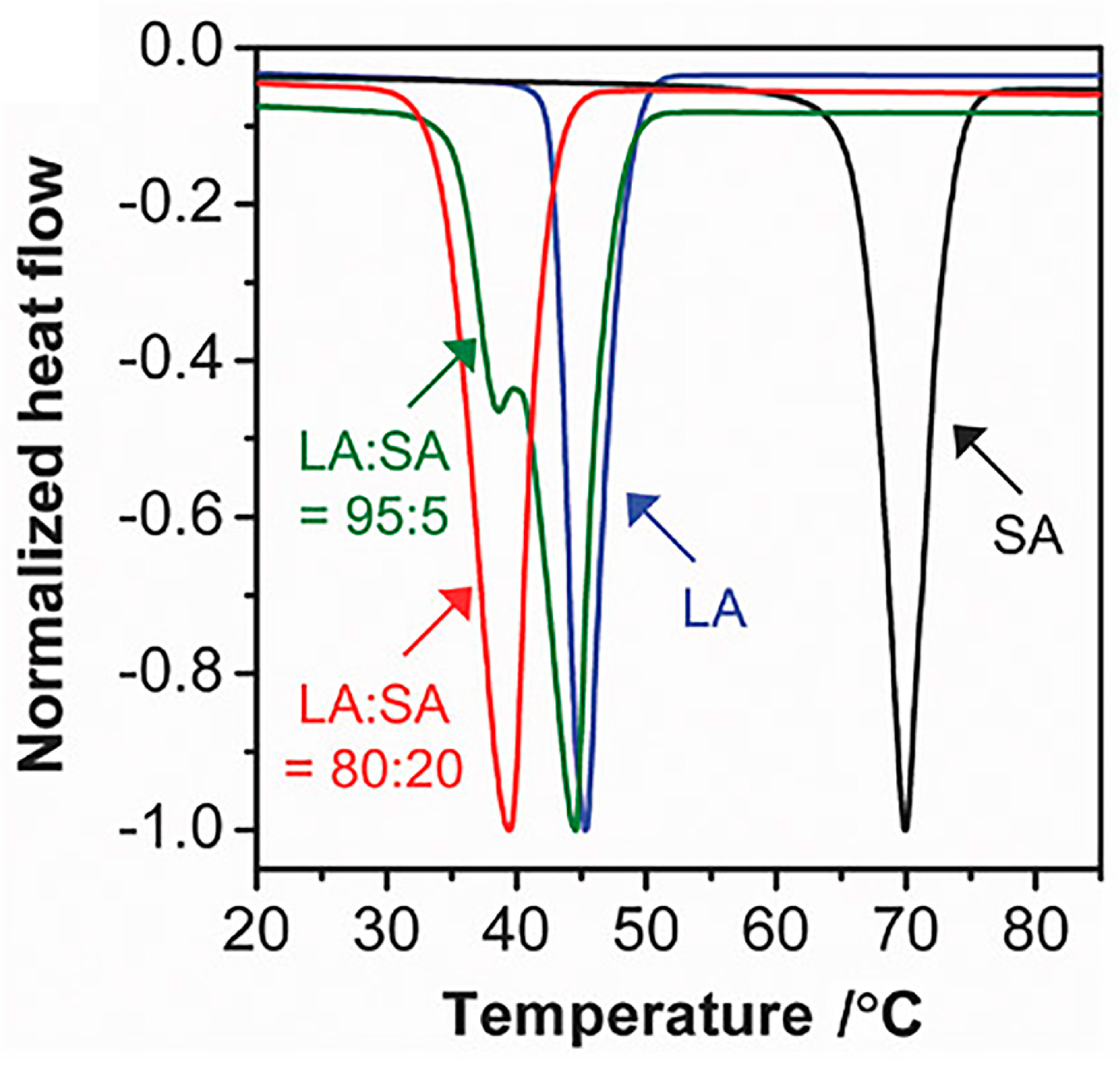
1.1. Fatty Acid PCMs
1.2. PCMs for Drug Delivery Systems
2. Metal–Organic Frameworks
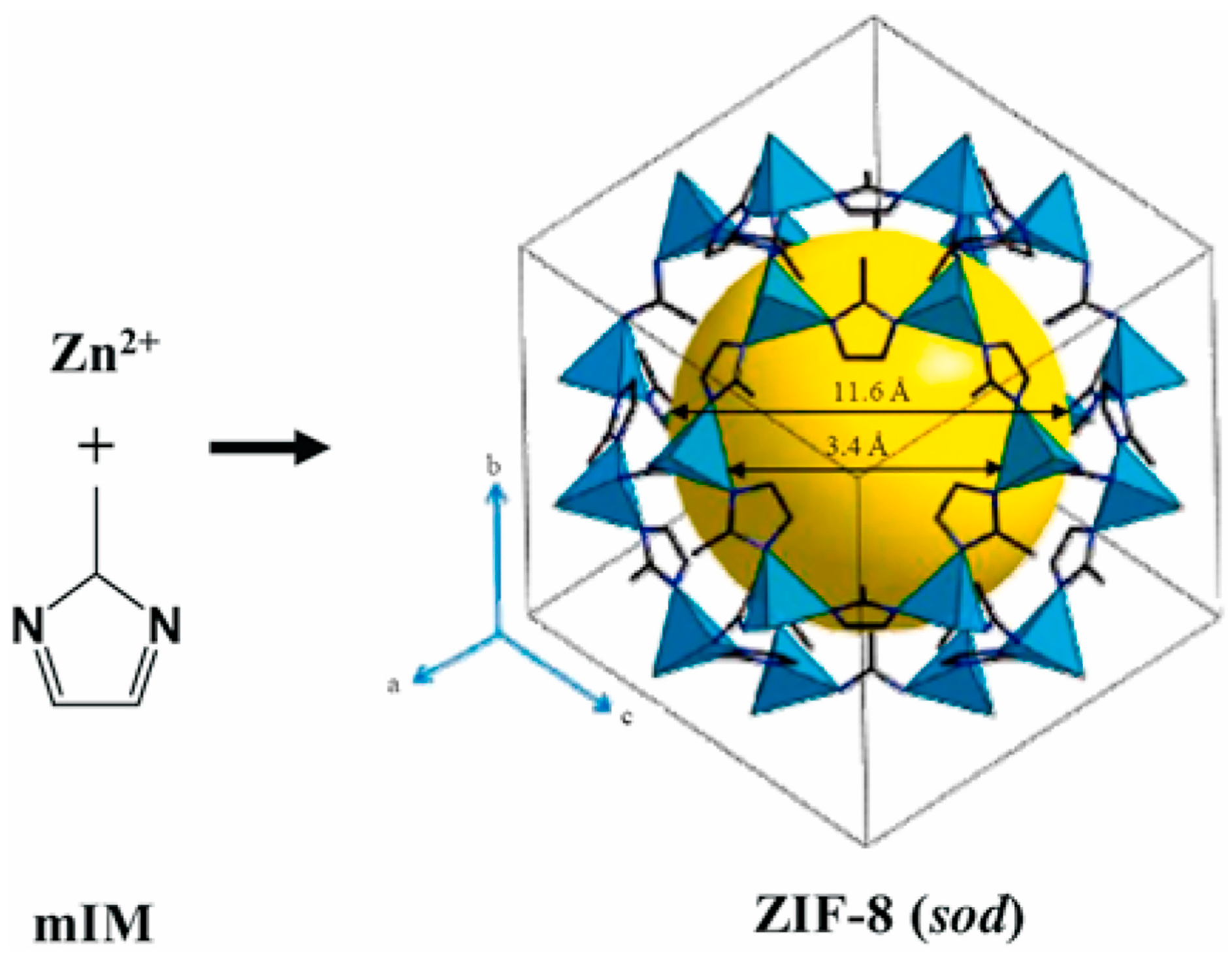
2.1. Zeolitic Imidazolate Framework-8
2.2. ZIF-8 for Drug Delivery System

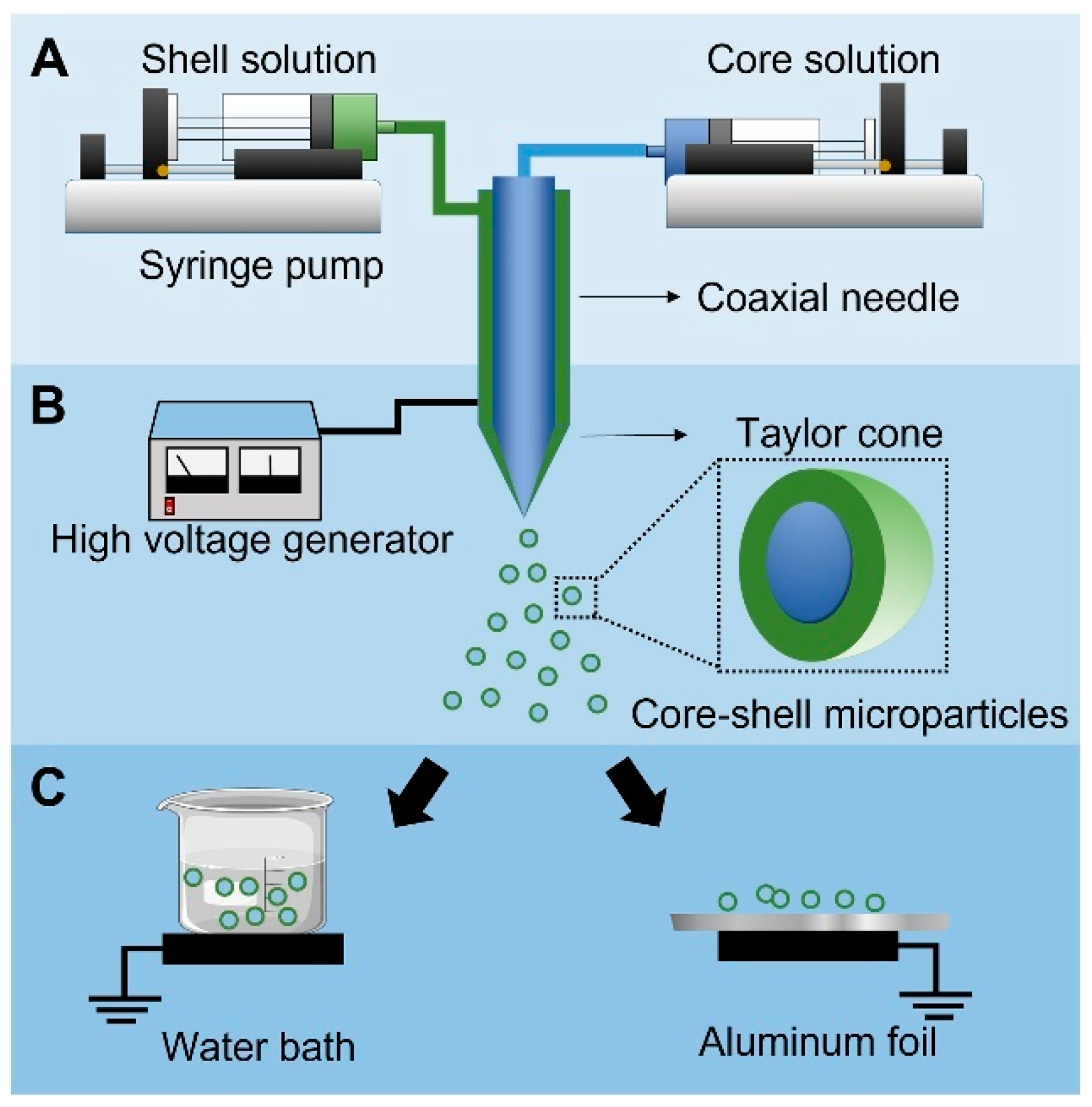
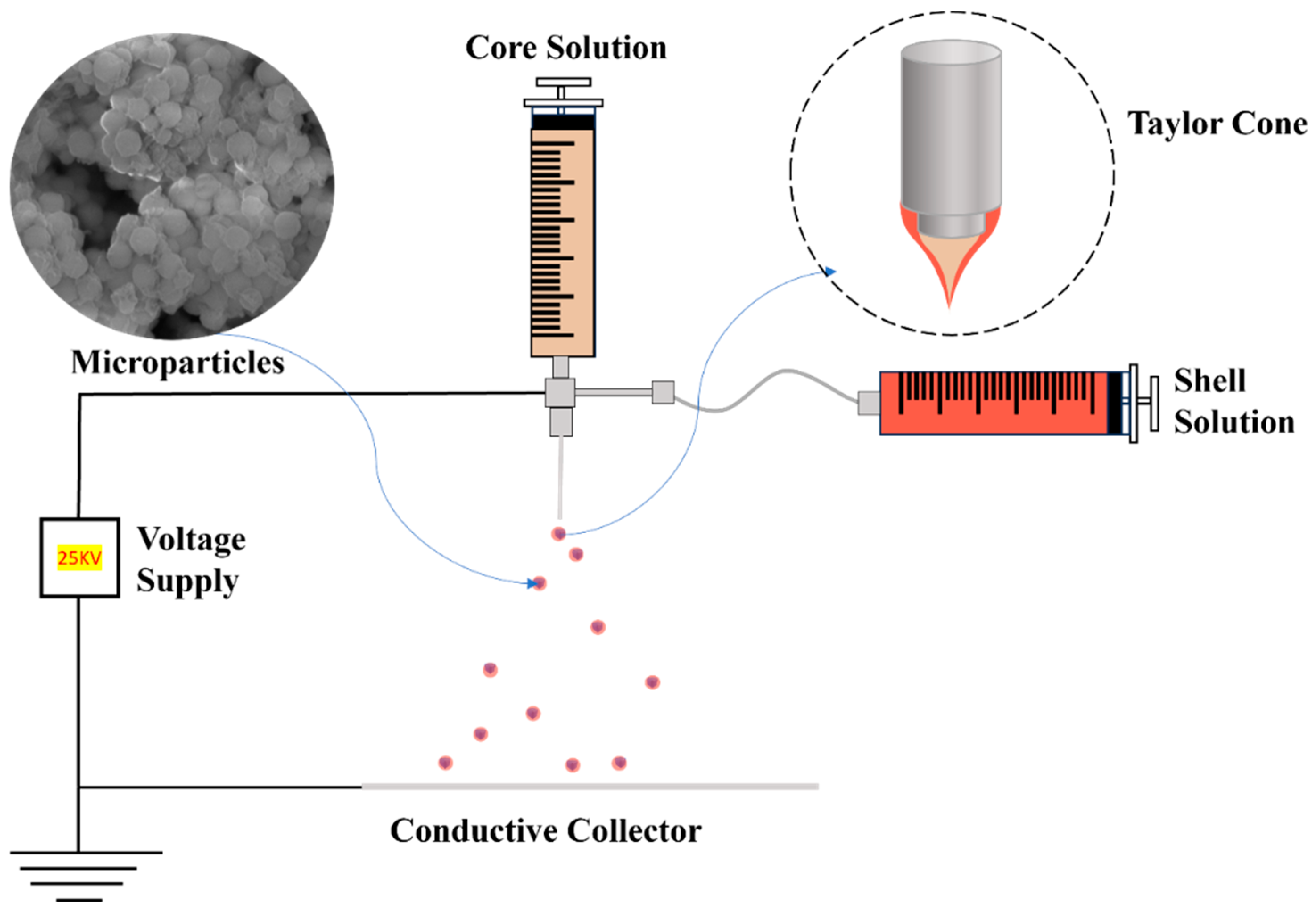
3. Coaxial Electrospray
3.1. Co-ES Setup
3.2. Nano-/Microparticles for Drug Delivery Systems
4. Stimuli-Responsive Drug Delivery Systems
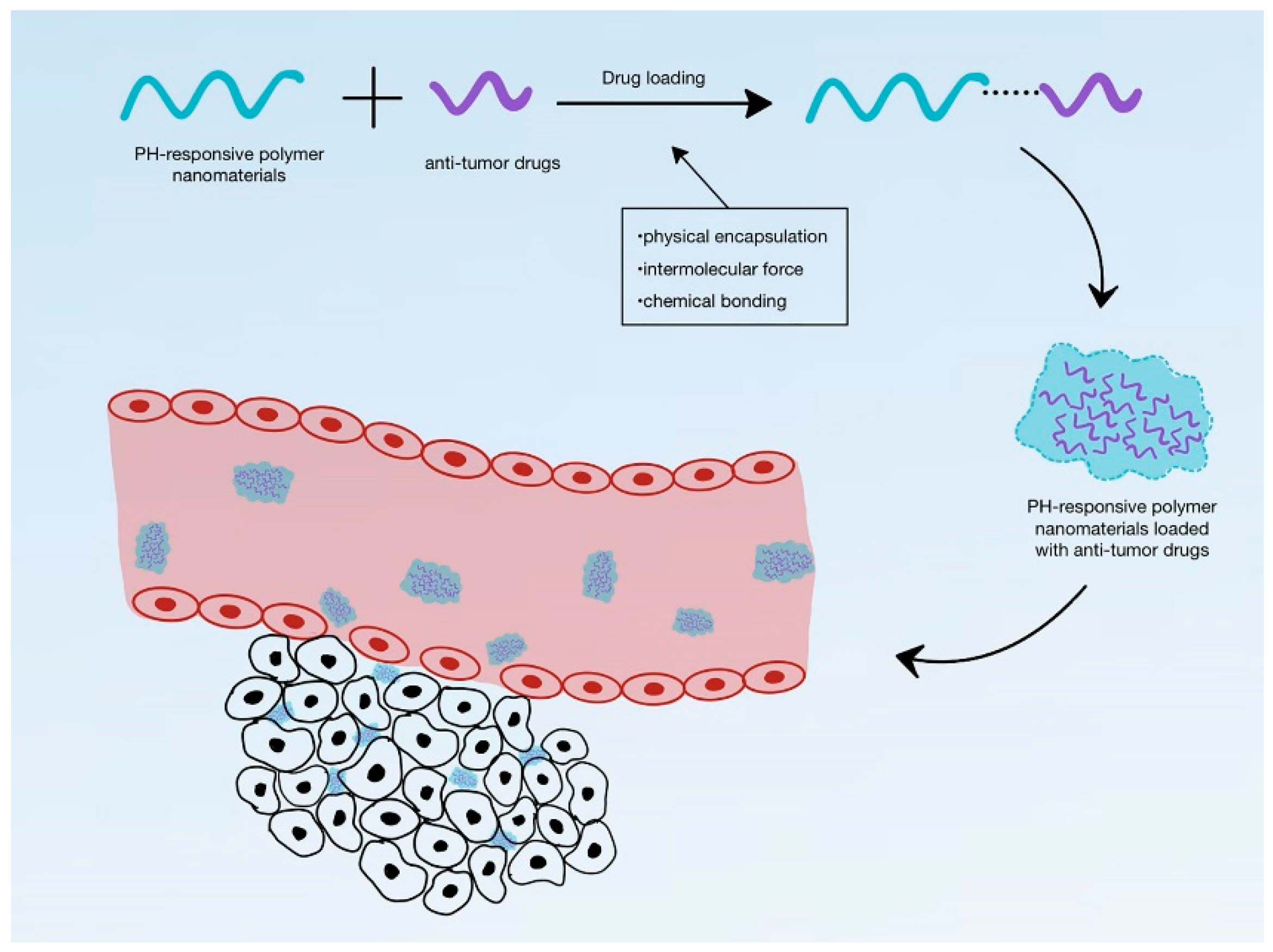
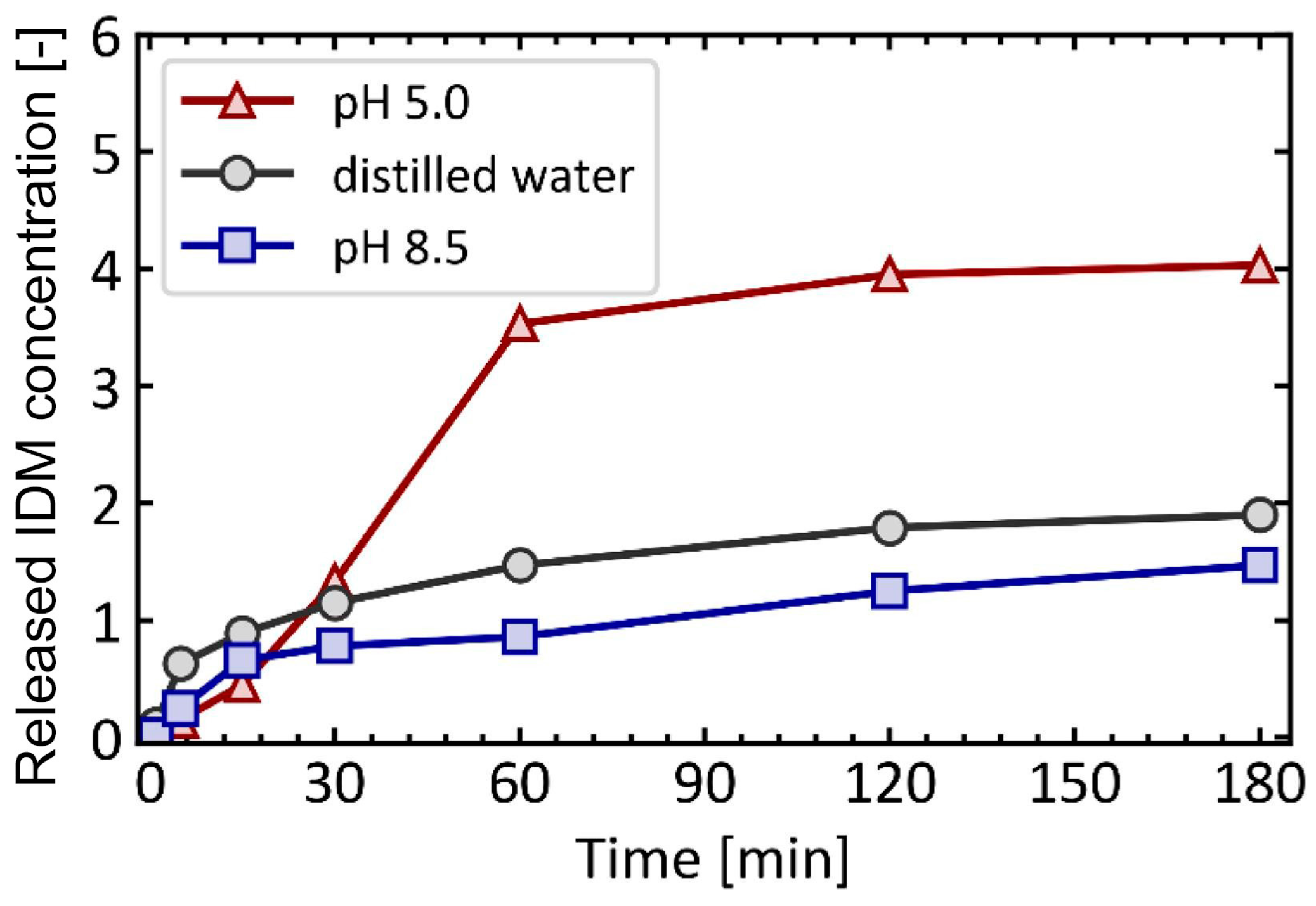
4.1. pH-Responsive DDS
4.2. Temperature-Responsive DDSs
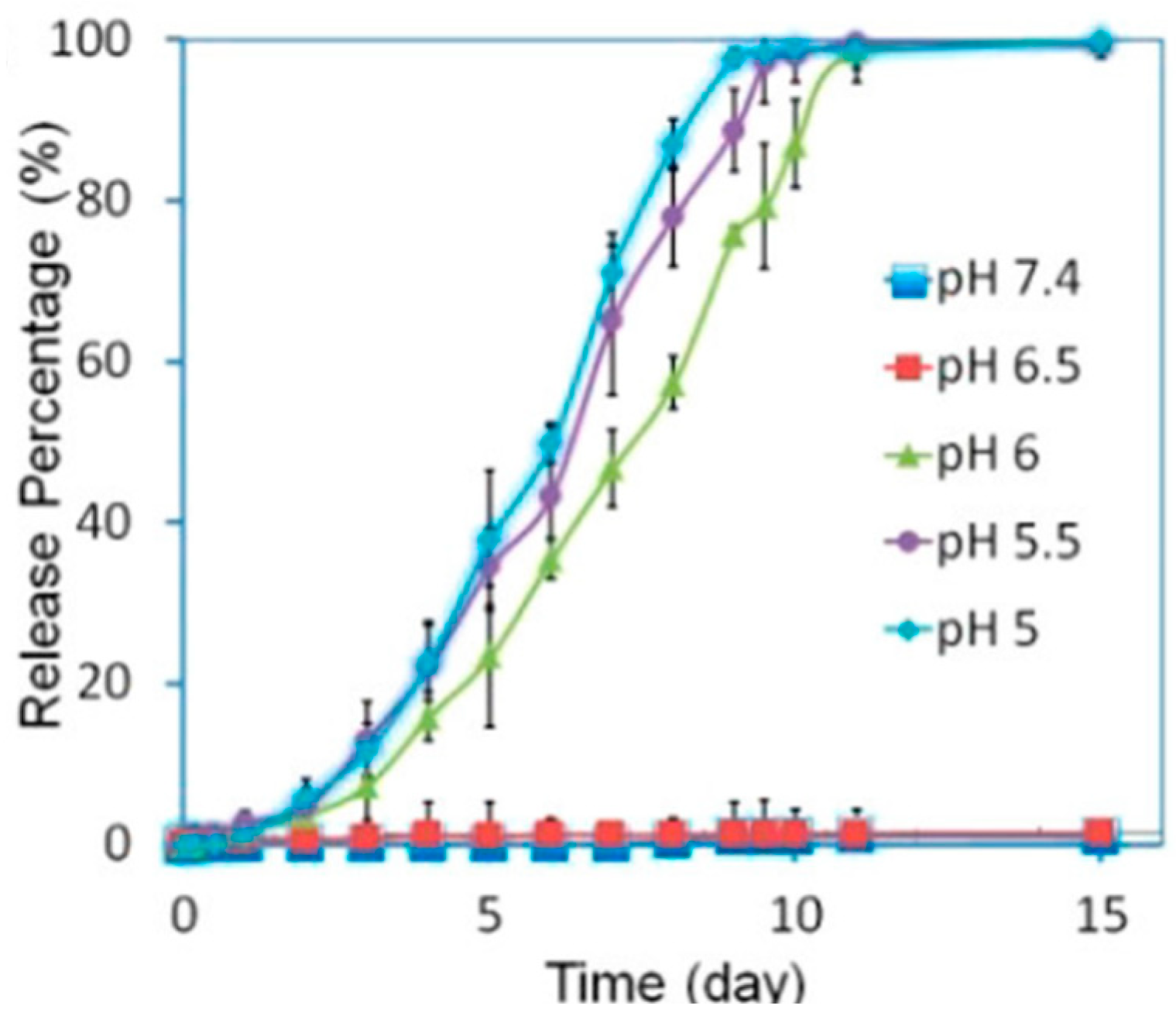
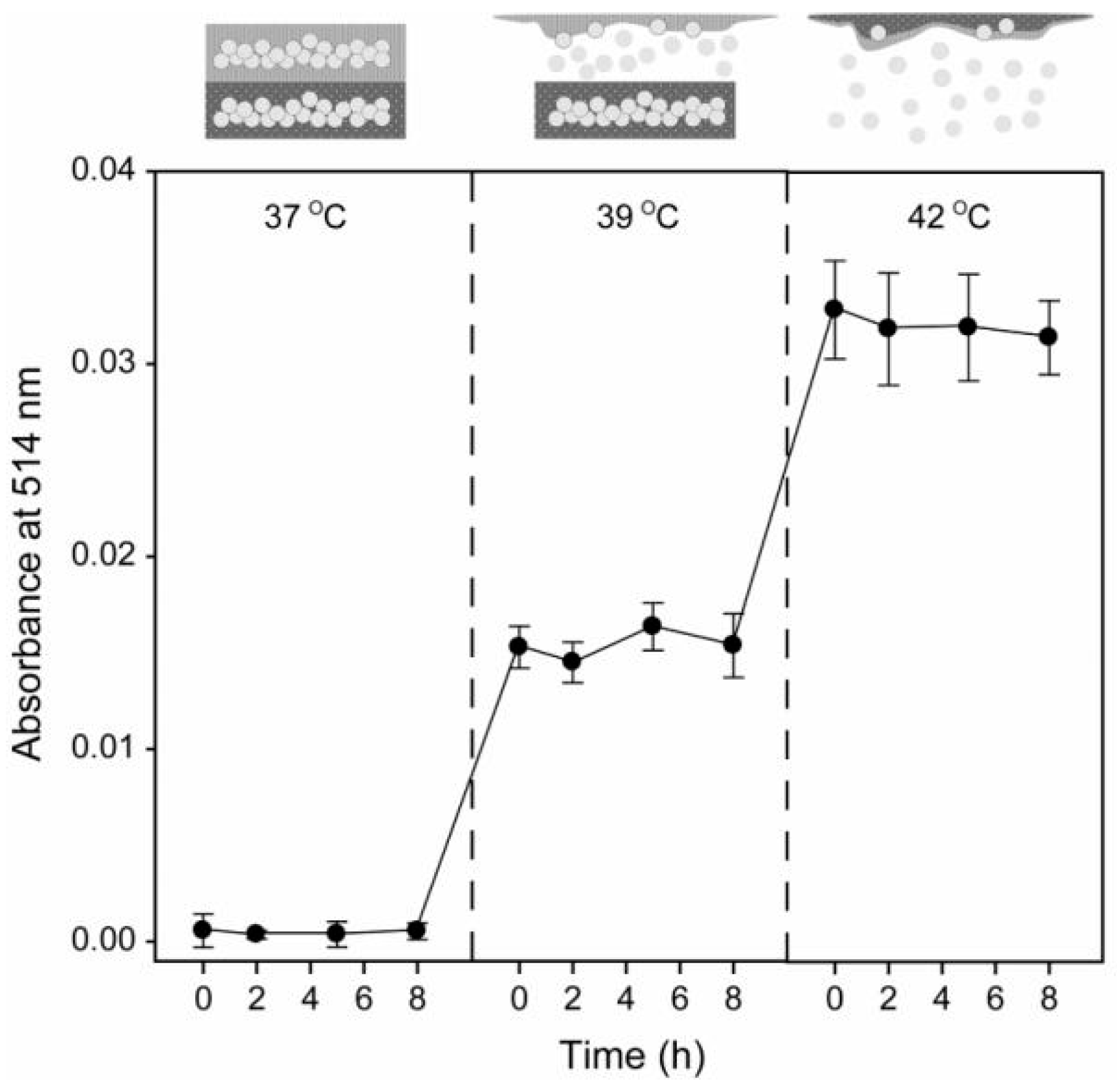
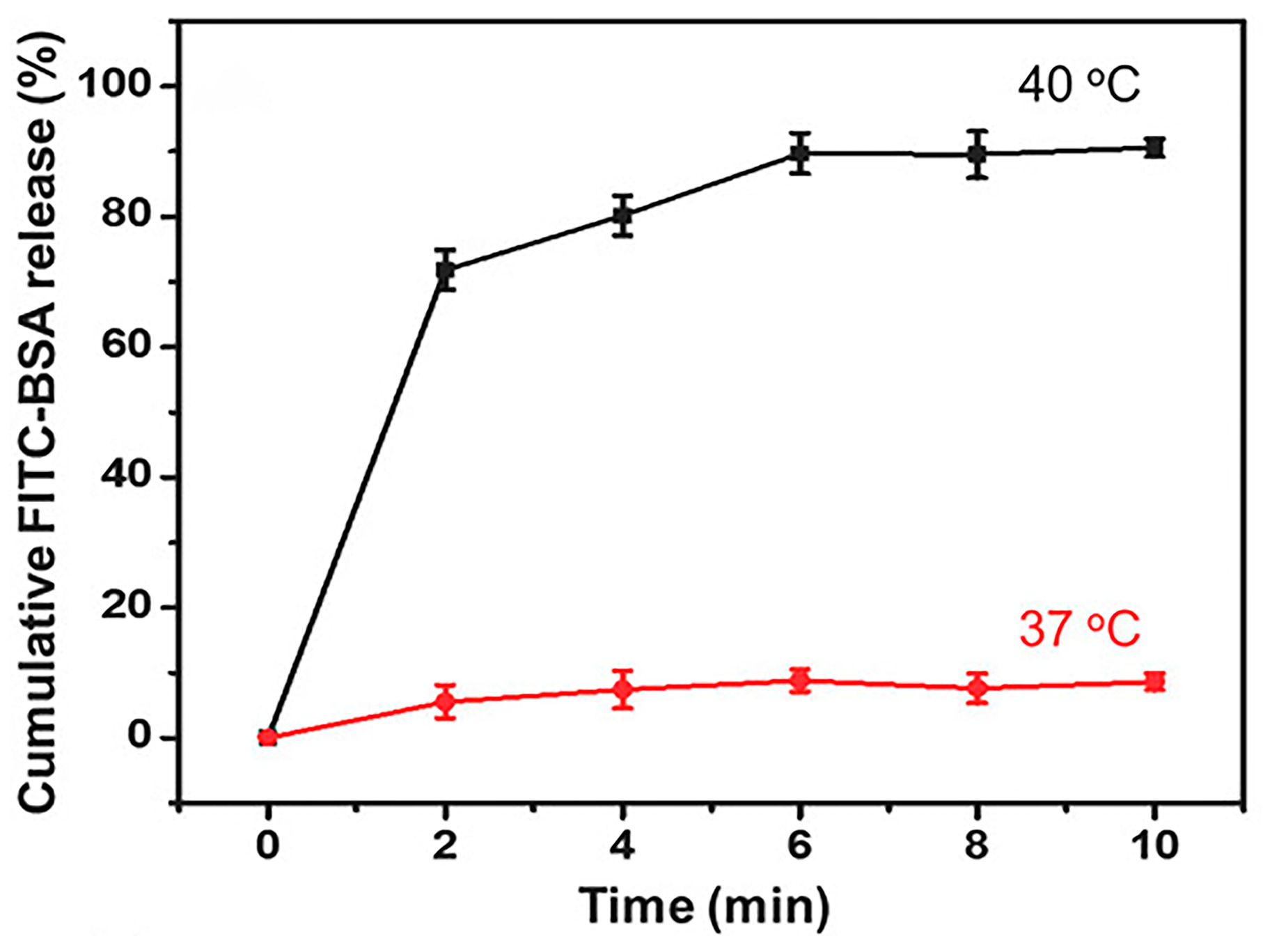
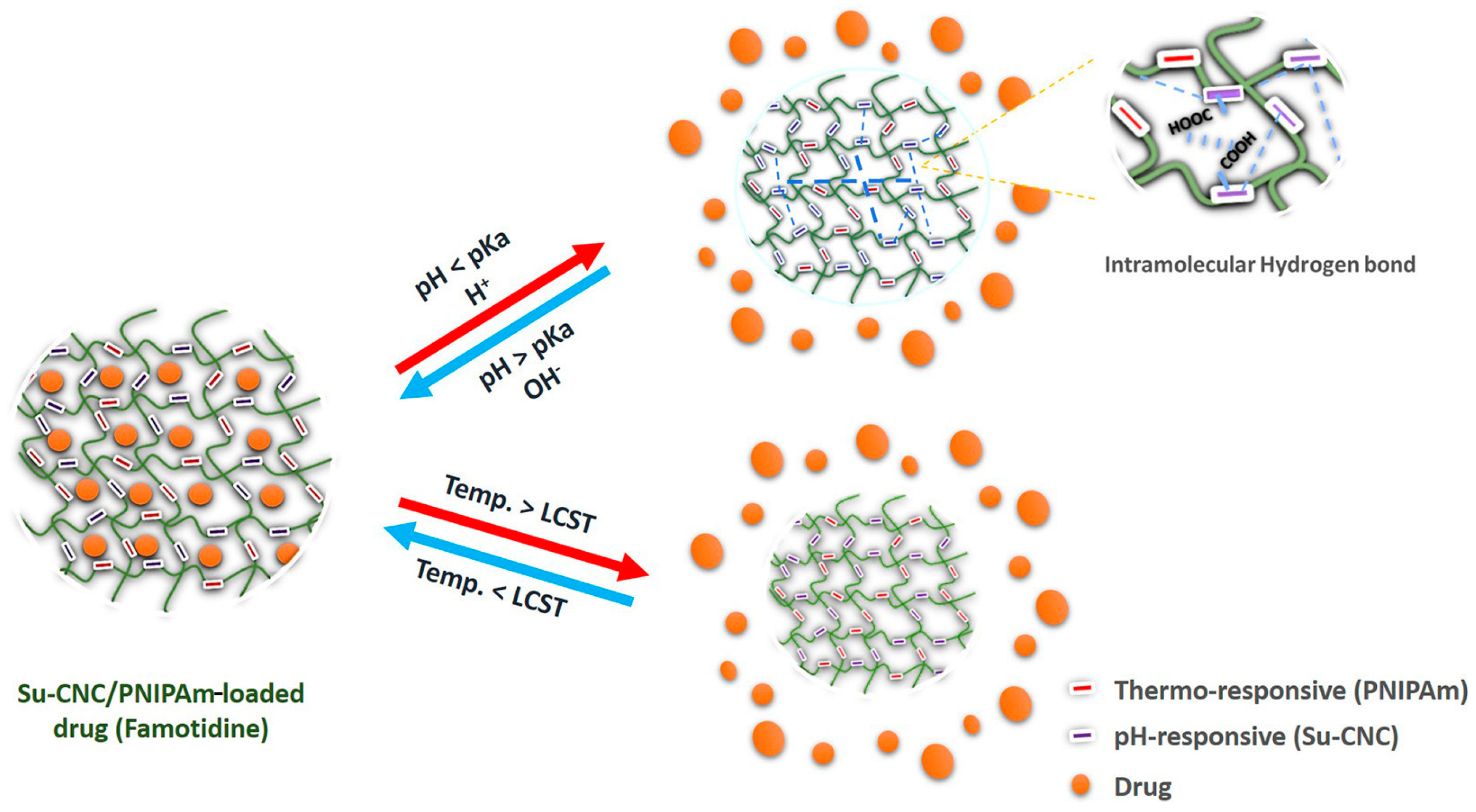
4.3. pH/Temperature Dual-Responsive DDSs
5. Summary and Outlook
Funding
Conflicts of Interest
References
- Lu, X.; Qian, R.; Xu, X.; Liu, M.; Liu, Y.; Zou, D. Modifications of microencapsulated phase change materials: Supercooling suppression, thermal conductivity enhancement and stability improvement. Nano Energy 2024, 124, 109520. [Google Scholar] [CrossRef]
- Yazdani McCord, M.R.; Baniasadi, H. Advancements in form-stabilized phase change materials: Stabilization mechanisms, multifunctionalities, and applications—A comprehensive review. Mater. Today Energy 2024, 41, 101532. [Google Scholar] [CrossRef]
- Weng, K.; Xu, X.; Chen, Y.; Li, X.; Qing, C.; Zou, D. Development and applications of multifunctional microencapsulated PCMs: A comprehensive review. Nano Energy 2024, 122, 109308. [Google Scholar] [CrossRef]
- Pause, B. 9—Phase change materials and their application in coatings and laminates for textiles. In Smart Textile Coatings and Laminates; Smith, W.C., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 236–250. [Google Scholar]
- Zhang, Q.; Ma, F.; Liu, L.; Tan, W.; Jing, M.; Wang, L.; Cai, M.; Wang, H. Recent advances in nano-enhanced phase change materials. J. Mater. Sci. 2024, 59, 5247–5267. [Google Scholar] [CrossRef]
- Tebaldi, M.L.; Belardi, R.M.; Montoro, S.R. Chapter 8—Polymers with Nano-Encapsulated Functional Polymers: Encapsulated Phase Change Materials. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Thomas, S., Shanks, R., Chandrasekharakurup, S., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 155–169. [Google Scholar]
- Junaid, M.F.; Rehman, Z.U.; Čekon, M.; Čurpek, J.; Farooq, R.; Cui, H.; Khan, I. Inorganic phase change materials in thermal energy storage: A review on perspectives and technological advances in building applications. Energy Build. 2021, 252, 111443. [Google Scholar] [CrossRef]
- Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic Salt Hydrate for Thermal Energy Storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef]
- Shah, K.W.; Huseien, G.F. Chapter 17—Nanostructures encapsulated phase-change materials for sustained thermal energy storage in concrete. In Green Nanomaterials for Industrial Applications; Shanker, U., Hussain, C.M., Rani, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 477–507. [Google Scholar]
- Casini, M. 5—Phase-change materials. In Smart Buildings; Casini, M., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 179–218. [Google Scholar]
- Sevault, A.; Vullum-Bruer, F.; Tranås, O.L. Active PCM-Based Thermal Energy Storage in Buildings. In Encyclopedia of Energy Storage; Cabeza, L.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 453–469. [Google Scholar]
- Parameshwaran, R.; Kalaiselvam, S. 15—Nanomaterial-embedded phase-change materials (PCMs) for reducing building cooling needs. In Eco-Efficient Materials for Mitigating Building Cooling Needs; Pacheco-Torgal, F., Labrincha, J.A., Cabeza, L.F., Granqvist, C.G., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 401–439. [Google Scholar]
- Oró, E.; de Gracia, A.; Castell, A.; Farid, M.M.; Cabeza, L.F. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 2012, 99, 513–533. [Google Scholar] [CrossRef]
- Bruno, F.; Belusko, M.; Liu, M.; Tay, N.H.S. 9—Solid-liquid phase change materials for thermal energy storage. In Advances in Thermal Energy Storage Systems, 2nd ed.; Cabeza, L.F., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 221–268. [Google Scholar]
- Zhang, J.; Wang, S.S.; Zhang, S.D.; Tao, Q.H.; Pan, L.; Wang, Z.Y.; Zhang, Z.P.; Lei, Y.; Yang, S.K.; Zhao, H.P. In Situ Synthesis and Phase Change Properties of Na2SO4·10H2O@SiO2 Solid Nanobowls toward Smart Heat Storage. J. Phys. Chem. C 2011, 115, 20061–20066. [Google Scholar] [CrossRef]
- Majó, M.; Sánchez, R.; Barcelona, P.; García, J.; Fernández, A.; Barreneche, C. Degradation of Fatty Acid Phase-Change Materials (PCM): New Approach for Its Characterization. Molecules 2021, 26, 982. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmed, A.; Sarı, A.; Mazumder, M.A.J.; Salhi, B.; Hekimoğlu, G.; Al-Sulaiman, F.A.; Inamuddin. Thermal energy storage and thermal conductivity properties of fatty acid/fatty acid-grafted-CNTs and fatty acid/CNTs as novel composite phase change materials. Sci. Rep. 2020, 10, 15388. [Google Scholar] [CrossRef]
- Dimaano, M.N.R.; Watanabe, T. The capric–lauric acid and pentadecane combination as phase change material for cooling applications. Appl. Therm. Eng. 2002, 22, 365–377. [Google Scholar] [CrossRef]
- Barlina, R.; Dewandari, K.T.; Mulyawanti, I.; Herawan, T. Chapter 30—Chemistry and composition of coconut oil and its biological activities. In Multiple Biological Activities of Unconventional Seed Oils; Mariod, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 383–395. [Google Scholar]
- Salsinha, A.S.; Machado, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Chapter 1—Bioactive lipids: Chemistry, biochemistry, and biological properties. In Bioactive Lipids; Pintado, M., Machado, M., Gomes, A.M., Salsinha, A.S., Rodríguez-Alcalá, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–35. [Google Scholar]
- Dayrit, F.M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Garti, N.; Wellner, E.; Sarig, S. Stearic acid polymorphs in correlation with crystallization conditions and solvents. Krist. Und Tech. 1980, 15, 1303–1310. [Google Scholar] [CrossRef]
- Jubie, S.; Ramesh, P.N.; Dhanabal, P.; Kalirajan, R.; Muruganantham, N.; Shanish Antony, A. Synthesis, antidepressant and antimicrobial activities of some novel stearic acid analogues. Eur. J. Med. Chem. 2012, 54, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, H.; Aliofkhazraei, M. 3.19 Effect of Surface Roughness on Wetting Properties. In Comprehensive Materials Finishing; Hashmi, M.S.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 276–305. [Google Scholar]
- Zhen, Z.; Xi, T.F.; Zheng, Y.F. 11—Surface modification by natural biopolymer coatings on magnesium alloys for biomedical applications. In Surface Modification of Magnesium and its Alloys for Biomedical Applications; Narayanan, T.S.N.S., Park, I.-S., Lee, M.-H., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 301–333. [Google Scholar]
- Sari, A.; Kaygusuz, K. Thermal performance of palmitic acid as a phase change energy storage material. Energy Convers. Manag. 2002, 43, 863–876. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and characterization of myristic acid/expanded graphite composite phase change materials for thermal energy storage. Sci. Rep. 2020, 10, 10889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yue, Q.; He, H.; Gao, B.; Wang, Y.; Li, Q. Study on phase diagram of fatty acids mixtures to determine eutectic temperatures and the corresponding mixing proportions. Appl. Energy 2014, 115, 483–490. [Google Scholar] [CrossRef]
- Prudnikov, E.; Polishchuk, I.; Sand, A.; Hamad, H.A.; Massad-Ivanir, N.; Segal, E.; Pokroy, B. Self-assembled fatty acid crystalline coatings display superhydrophobic antimicrobial properties. Mater. Today Bio 2022, 18, 100516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huo, D.; Chen, Q.; Xue, J.; Shen, S.; Xia, Y. A Eutectic Mixture of Natural Fatty Acids Can Serve as the Gating Material for Near-Infrared-Triggered Drug Release. Adv. Mater. 2017, 29, 1703702. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Randhawa, J.K. Solid lipid nanoparticles of stearic acid for the drug delivery of paliperidone. RSC Adv. 2015, 5, 68743–68750. [Google Scholar] [CrossRef]
- Choi, S.-W.; Zhang, Y.; Xia, Y. A Temperature-Sensitive Drug Release System Based on Phase-Change Materials. Angew. Chem. Int. Ed. 2010, 49, 7904–7908. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; McFearin, C.; Sermsakdi, M.; Edigin, O.; Almutairi, A. UV and Near-IR Triggered Release from Polymeric Nanoparticles. J. Am. Chem. Soc. 2010, 132, 9540–9542. [Google Scholar] [CrossRef]
- Bao, J.; Tu, H.; Li, J.; Li, Y.; Yu, S.; Gao, J.; Lei, K.; Zhang, F.; Li, J. Applications of phase change materials in smart drug delivery for cancer treatment. Front. Bioeng. Biotechnol. 2022, 10, 991005. [Google Scholar] [CrossRef]
- Xue, J.; Zhu, C.; Li, J.; Li, H.; Xia, Y. Integration of Phase-Change Materials with Electrospun Fibers for Promoting Neurite Outgrowth under Controlled Release. Adv. Funct. Mater. 2018, 28, 1705563. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhu, C.; Huo, D.; Yang, M.; Xue, J.; Xia, Y. A Hybrid Nanomaterial for the Controlled Generation of Free Radicals and Oxidative Destruction of Hypoxic Cancer Cells. Angew. Chem. Int. Ed. 2017, 56, 8801–8804. [Google Scholar] [CrossRef]
- Bergaoui, M.; Khalfaoui, M.; Awadallah-F, A.; Al-Muhtaseb, S. A review of the features and applications of ZIF-8 and its derivatives for separating CO2 and isomers of C3− and C4− hydrocarbons. J. Nat. Gas Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Sharma, D.; Rasaily, S.; Pradhan, S.; Baruah, K.; Tamang, S.; Pariyar, A. HKUST-1 Metal Organic Framework as an Efficient Dual-Function Catalyst: Aziridination and One-Pot Ring-Opening Transformation for Formation of β-Aryl Sulfonamides with C–C, C–N, C–S, and C–O Bonds. Inorg. Chem. 2021, 60, 7794–7802. [Google Scholar] [CrossRef] [PubMed]
- Ashling, C.W.; Johnstone, D.N.; Widmer, R.N.; Hou, J.; Collins, S.M.; Sapnik, A.F.; Bumstead, A.M.; Midgley, P.A.; Chater, P.A.; Keen, D.A.; et al. Synthesis and Properties of a Compositional Series of MIL-53(Al) Metal–Organic Framework Crystal-Glass Composites. J. Am. Chem. Soc. 2019, 141, 15641–15648. [Google Scholar] [CrossRef]
- Yan, S.; Li, W.; He, D.; He, G.; Chen, H. Recent research progress of metal-organic frameworks (MOFs) based catalysts for CO2 cycloaddition reaction. Mol. Catal. 2023, 550, 113608. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Alinia Asli, M.; Vossoughi, M. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Strauss, I.; Mundstock, A.; Treger, M.; Lange, K.; Hwang, S.; Chmelik, C.; Rusch, P.; Bigall, N.C.; Pichler, T.; Shiozawa, H.; et al. Metal–Organic Framework Co-MOF-74-Based Host–Guest Composites for Resistive Gas Sensing. ACS Appl. Mater. Interfaces 2019, 11, 14175–14181. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Das, M.; Pal, K.; Jana, S.; Dutta, B.; Ray, P.P.; Jana, K.; Sinha, C. Three-Dimensional-Coordination Polymer of Zn(II)-Carboxylate: Structural Elucidation, Photoelectrical Conductivity, and Biological Activity. ACS Omega 2019, 4, 17649–17661. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Xu, W.; Hanikel, N.; Lomachenko, K.A.; Atzori, C.; Lund, A.; Lyu, H.; Zhou, Z.; Angell, C.A.; Yaghi, O.M. High-Porosity Metal-Organic Framework Glasses. Angew. Chem. Int. Ed. 2023, 62, e202300003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, S.; Han, N. Metal organic frameworks derived functional materials for energy and environment related sustainable applications. Chemosphere 2023, 313, 137330. [Google Scholar] [CrossRef] [PubMed]
- Pascanu, V.; González Miera, G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef]
- Kacem, M.; Dib, M. An overview of the progress of MOFs-based hybrid materials as efficient catalysts for Knoevenagel condensation. Inorg. Chem. Commun. 2023, 158, 111561. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.C.; Lai, S.O.; Mah, S.K.; Thiam, H.S.; Chong, W.C.; Shuit, S.H.; Lee, S.S.; Chong, W.E. A Review of HKUST-1 Metal-Organic Frameworks in Gas Adsorption. IOP Conf. Ser. Earth Environ. Sci. 2023, 1135, 012030. [Google Scholar] [CrossRef]
- Mao, Y.; Li, J.; Cao, W.; Ying, Y.; Sun, L.; Peng, X. Pressure-Assisted Synthesis of HKUST-1 Thin Film on Polymer Hollow Fiber at Room Temperature toward Gas Separation. ACS Appl. Mater. Interfaces 2014, 6, 4473–4479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Babucci, M.; Zhang, Y.; Wen, Y.; Peng, L.; Yang, B.; Gates, B.C.; Yang, D. Dialing in Catalytic Sites on Metal Organic Framework Nodes: MIL-53(Al) and MIL-68(Al) Probed with Methanol Dehydration Catalysis. ACS Appl. Mater. Interfaces 2020, 12, 53537–53546. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-M.T.; Chen, J.-W.; Pham, M.-T.; Bui, H.M.; Hu, C.-C.; You, S.-J.; Wang, Y.-F. A high-performance ZIF-8 membrane for gas separation applications: Synthesis and characterization. Environ. Technol. Innov. 2023, 31, 103169. [Google Scholar] [CrossRef]
- Suresh, K.; Aulakh, D.; Purewal, J.; Siegel, D.J.; Veenstra, M.; Matzger, A.J. Optimizing Hydrogen Storage in MOFs through Engineering of Crystal Morphology and Control of Crystal Size. J. Am. Chem. Soc. 2021, 143, 10727–10734. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Liu, D.; Zhang, J. From Zeolitic Imidazolate Framework-8 to Metal-Organic Frameworks (MOFs): Representative Substance for the General Study of Pioneering MOF Applications. Energy Environ. Mater. 2018, 1, 209–220. [Google Scholar] [CrossRef]
- Deacon, A.; Briquet, L.; Malankowska, M.; Massingberd-Mundy, F.; Rudić, S.; Hyde, T.L.; Cavaye, H.; Coronas, J.; Poulston, S.; Johnson, T. Understanding the ZIF-L to ZIF-8 transformation from fundamentals to fully costed kilogram-scale production. Commun. Chem. 2022, 5, 18. [Google Scholar] [CrossRef]
- Linder-Patton, O.M.; de Prinse, T.J.; Furukawa, S.; Bell, S.G.; Sumida, K.; Doonan, C.J.; Sumby, C.J. Influence of nanoscale structuralisation on the catalytic performance of ZIF-8: A cautionary surface catalysis study. CrystEngComm 2018, 20, 4926–4934. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Grzelczak, M. Growing anisotropic crystals at the nanoscale. Science 2017, 356, 1120–1121. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Gil, D.; Figueiredo, F.M.L. High Surface Proton Conduction in Nanostructured ZIF-8. Nanomaterials 2019, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Heryadi, D.; Zhou, F.; Zhao, L.; Lestari, G.; Su, H.; Lai, Z. Tuning the crystal morphology and size of zeolitic imidazolate framework-8 in aqueous solution by surfactants. CrystEngComm 2011, 13, 6937–6940. [Google Scholar] [CrossRef]
- Yang, F.; Mu, H.; Wang, C.; Xiang, L.; Yao, K.X.; Liu, L.; Yang, Y.; Han, Y.; Li, Y.; Pan, Y. Morphological Map of ZIF-8 Crystals with Five Distinctive Shapes: Feature of Filler in Mixed-Matrix Membranes on C3H6/C3H8 Separation. Chem. Mater. 2018, 30, 3467–3473. [Google Scholar] [CrossRef]
- Fan, X.; Wang, W.; Li, W.; Zhou, J.; Wang, B.; Zheng, J.; Li, X. Highly Porous ZIF-8 Nanocrystals Prepared by a Surfactant Mediated Method in Aqueous Solution with Enhanced Adsorption Kinetics. ACS Appl. Mater. Interfaces 2014, 6, 14994–14999. [Google Scholar] [CrossRef]
- Qiu, J.; Xu, X.; Liu, B.; Guo, Y.; Wang, H.; Yu, L.; Jiang, Y.; Huang, C.; Fan, B.; Zeng, Z.; et al. Size-Controllable Synthesis of ZIF-8 and Derived Nitrogen-Rich Porous Carbon for CO2 and VOCs Adsorption. ChemistrySelect 2022, 7, e202203273. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Li, S.; Zhang, P.; Yao, Q. Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy. RSC Adv. 2020, 10, 37600–37620. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, A.; Garg, N.; Randhawa, J.K. Curcumin encapsulated zeolitic imidazolate frameworks as stimuli responsive drug delivery system and their interaction with biomimetic environment. Sci. Rep. 2017, 7, 12598. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Liu, R.; Qu, J.; Wang, H.; Zhang, X. Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. RSC Adv. 2015, 5, 48433–48441. [Google Scholar] [CrossRef]
- Gao, X.; Hai, X.; Baigude, H.; Guan, W.; Liu, Z. Fabrication of functional hollow microspheres constructed from MOF shells: Promising drug delivery systems with high loading capacity and targeted transport. Sci. Rep. 2016, 6, 37705. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Yang, L.; Li, Y.; Wang, L. Continuous and scalable fabrication of stable and biocompatible MOF@SiO2 nanoparticles for drug loading. J. Mater. Chem. B 2018, 6, 7936–7942. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, X.; Zhang, X.; Wu, X.; Li, C.; Sun, Z.; Chu, H. Facile synthesis of degradable DOX/ICG co-loaded metal–organic frameworks for targeted drug release and thermoablation. Cancer Nanotechnol. 2022, 13, 18. [Google Scholar] [CrossRef]
- Molina, M.A.; Rodríguez-Campa, J.; Flores-Borrell, R.; Blanco, R.M.; Sánchez-Sánchez, M. Sustainable Synthesis of Zeolitic Imidazolate Frameworks at Room Temperature in Water with Exact Zn/Linker Stoichiometry. Nanomaterials 2024, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.A.H.M.; Ismail, A.F.; Mustafa, A.; Murali, R.S.; Matsuura, T. The impact of ZIF-8 particle size and heat treatment on CO2/CH4 separation using asymmetric mixed matrix membrane. RSC Adv. 2014, 4, 52530–52541. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Zhang, C.; Gee, J.A.; Sholl, D.S.; Lively, R.P. Crystal-Size-Dependent Structural Transitions in Nanoporous Crystals: Adsorption-Induced Transitions in ZIF-8. J. Phys. Chem. C 2014, 118, 20727–20733. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, H.C. Surface and Bulk Integrations of Single-Layered Au or Ag Nanoparticles onto Designated Crystal Planes {110} or {100} of ZIF-8. Chem. Mater. 2013, 25, 1761–1768. [Google Scholar] [CrossRef]
- Avci, C.; Ariñez-Soriano, J.; Carné-Sánchez, A.; Guillerm, V.; Carbonell, C.; Imaz, I.; Maspoch, D. Post-Synthetic Anisotropic Wet-Chemical Etching of Colloidal Sodalite ZIF Crystals. Angew. Chem. Int. Ed. 2015, 54, 14417–14421. [Google Scholar] [CrossRef]
- Soltani, B.; Nabipour, H.; Nasab, N.A. Efficient Storage of Gentamicin in Nanoscale Zeolitic Imidazolate Framework-8 Nanocarrier for pH-Responsive Drug Release. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1090–1097. [Google Scholar] [CrossRef]
- Little, S.R.; Lynn, D.M.; Ge, Q.; Anderson, D.G.; Puram, S.V.; Chen, J.; Eisen, H.N.; Langer, R. Poly-β amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc. Natl. Acad. Sci. USA 2004, 101, 9534–9539. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, I.-H.; Lee, E.; Park, J.; Jon, S. pH-Sensitive Polymer Nanospheres for Use as a Potential Drug Delivery Vehicle. Biomacromolecules 2007, 8, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- Engin, K.; Leeper, D.B.; Cater, J.R.; Thistlethwaite, A.J.; Tupchong, L.; McFarlane, J.D. Extracellular pH distribution in human tumours. Int. J. Hyperth. 1995, 11, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.; McSheehy, P.M.J.; Griffiths, J.R.; Bashford, C.L. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 2000, 6, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Huang, X.; Ma, Y.; Huang, Y.; Yang, R.; Duan, H.; Chen, Y. Multi-functionalized graphene oxide based anticancer drug-carrier with dual-targeting function and pH-sensitivity. J. Mater. Chem. 2011, 21, 3448–3454. [Google Scholar] [CrossRef]
- de Moura Ferraz, L.R.; Tabosa, A.É.G.A.; da Silva Nascimento, D.D.S.; Ferreira, A.S.; de Albuquerque Wanderley Sales, V.; Silva, J.Y.R.; Júnior, S.A.; Rolim, L.A.; de Souza Pereira, J.J.; Rolim-Neto, P.J. ZIF-8 as a promising drug delivery system for benznidazole: Development, characterization, in vitro dialysis release and cytotoxicity. Sci. Rep. 2020, 10, 16815. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal–Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Morad, M.R.; Rajabi, A.; Razavi, M.; Sereshkeh, S.R.P. A Very Stable High Throughput Taylor Cone-jet in Electrohydrodynamics. Sci. Rep. 2016, 6, 38509. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ho, C.S.; Lam, C.W.K.; Chan, M.H.; Cheung, R.C.K.; Law, L.K.; Lit, L.C.W.; Ng, K.F.; Suen, M.W.M.; Tai, H. Electrospray ionisation mass spectrometry: Principles and clinical applications. Clin. Biochem. Rev. 2003, 24, 3. [Google Scholar]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization–principles and practice. Mass Spectrom. Rev. 1990, 9, 37–70. [Google Scholar] [CrossRef]
- He, X.-X.; Zheng, J.; Yu, G.-F.; You, M.-H.; Yu, M.; Ning, X.; Long, Y.-Z. Near-Field Electrospinning: Progress and Applications. J. Phys. Chem. C 2017, 121, 8663–8678. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Si, T.; Xu, R.X. Coaxial electrospray of microparticles and nanoparticles for biomedical applications. Expert Rev. Med. Devices 2012, 9, 595–612. [Google Scholar] [CrossRef]
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Gañán-Calvo, A.M. Micro/Nano Encapsulation via Electrified Coaxial Liquid Jets. Science 2002, 295, 1695–1698. [Google Scholar] [CrossRef]
- Xie, J.; Ng, W.J.; Lee, L.Y.; Wang, C.H. Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray. J. Colloid Interface Sci. 2008, 317, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Farook, U.; Stride, E.; Edirisinghe, M.J. Stability of microbubbles prepared by co-axial electrohydrodynamic atomisation. Eur. Biophys. J. 2009, 38, 713–718. [Google Scholar] [CrossRef]
- Figueroa-Enriquez, C.E.; Rodríguez-Félix, F.; Plascencia-Jatomea, M.; Sánchez-Escalante, A.; Vargas-López, J.M.; Tapia-Hernández, J.; Canizales-Rodríguez, D.F.; Castro-Enriquez, D.D.; Ruiz-Cruz, S.; Santos-Sauceda, I.; et al. Nanoparticles of Betalain-Gelatin with Antioxidant Properties by Coaxial Electrospraying: Preparation and Characterization. ACS Omega 2023, 8, 41156–41168. [Google Scholar] [CrossRef]
- Yuan, S.; Lei, F.; Liu, Z.; Tong, Q.; Si, T.; Xu, R.X. Coaxial Electrospray of Curcumin-Loaded Microparticles for Sustained Drug Release. PLoS ONE 2015, 10, e0132609. [Google Scholar] [CrossRef]
- Ibili, H.; Dasdemir, M.; Çankaya, İ.İ.T.; Orhan, M.; Güneşoğlu, C.; Arabacı Anul, S. Investigation of poly(lactic acid) nanocapsules containing the plant extract via coaxial electrospraying method for functional nonwoven applications. J. Ind. Text. 2021, 51 (Suppl. S3), 5304S–5327S. [Google Scholar] [CrossRef]
- Si, T.; Zhang, L.; Li, G.; Roberts, C.J.; Yin, X.; Xu, R. Experimental design and instability analysis of coaxial electrospray process for microencapsulation of drugs and imaging agents. J. Biomed. Opt. 2013, 18, 075003. [Google Scholar] [CrossRef]
- Chen, X.; Jia, L.; Yin, X.; Cheng, J.; Lu, J. Spraying modes in coaxial jet electrospray with outer driving liquid. Phys. Fluids 2005, 17, 032101. [Google Scholar] [CrossRef]
- Chang, M.W.; Stride, E.; Edirisinghe, M. Controlling the thickness of hollow polymeric microspheres prepared by electrohydrodynamic atomization. J. R. Soc. Interface 2010, 7, S451–S460. [Google Scholar] [CrossRef] [PubMed]
- Farook, U.; Stride, E.; Edirisinghe, M.J.; Moaleji, R. Microbubbling by co-axial electrohydrodynamic atomization. Med. Biol. Eng. Comput. 2007, 45, 781–789. [Google Scholar] [CrossRef]
- Tang, J.; Schutzman, R.; Rodríguez, C.A.; Lahann, J.; Rodríguez-Hornedo, N.; Prausnitz, M.R.; Schwendeman, S.P. Coaxial electrospray of uniform polylactide core-shell microparticles for long-acting contraceptive. J. Control. Release 2022, 341, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Mei, F.; Bai, M.Y.; Zhao, S.; Chen, D.R. Release profile characteristics of biodegradable-polymer-coated drug particles fabricated by dual-capillary electrospray. J. Control. Release 2010, 145, 58–65. [Google Scholar] [CrossRef] [PubMed]
- López-Herrera, J.M.; Barrero, A.; López, A.; Loscertales, I.G.; Márquez, M. Coaxial jets generated from electrified Taylor cones. Scaling laws. J. Aerosol Sci. 2003, 34, 535–552. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, F.; Chen, Y.; Yu, T.; Lou, D.; Guo, Y.; Li, P.; Wang, Z.; Ran, H. Drug release from core-shell PVA/silk fibroin nanoparticles fabricated by one-step electrospraying. Sci. Rep. 2017, 7, 11913. [Google Scholar] [CrossRef] [PubMed]
- Farook, U.; Stride, E.; Edirisinghe, M.J. Controlling size and size distribution of electrohydrodynamically prepared microbubbles. Bubble Sci. Eng. Technol. 2009, 1, 53–57. [Google Scholar] [CrossRef]
- Barrero, A.; Gañán-Calvo, A.M.; Dávila, J.; Palacio, A.; Gómez-González, E. Low and high Reynolds number flows inside Taylor cones. Phys. Rev. E 1998, 58, 7309–7314. [Google Scholar] [CrossRef]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Nanofibres in Drug Delivery; UCL Press: London, UK, 2018. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Rietveld, I.B.; Suganuma, N.; Kobayashi, K.; Yamada, H.; Matsushige, K. Electrospray Deposition of Photoresist: A Low Impact Method for the Fabrication of Multilayered Films. Macromol. Mater. Eng. 2008, 293, 387–399. [Google Scholar] [CrossRef]
- Schiffman, J.; Schauer, C. A Review: Electrospinning of Biopolymer Nanofibers and their Applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- Boda, S.K.; Li, X.; Xie, J. Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review. J. Aerosol Sci. 2018, 125, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Almería, B.; Gomez, A. Electrospray synthesis of monodisperse polymer particles in a broad (60 nm–2 μm) diameter range: Guiding principles and formulation recipes. J. Colloid Interface Sci. 2014, 417, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Hardas, N.; Danviriyakul, S.; Foley, J.L.; Nawar, W.W.; Chinachoti, P. Accelerated Stability Studies of Microencapsulated Anhydrous Milk Fat. LWT Food Sci. Technol. 2000, 33, 506–513. [Google Scholar] [CrossRef]
- Lee, V.H.L. Encyclopedia of Controlled Drug Delivery, Volume 1 and 2: Edith Mathiowitz, editor. John Wiley & Sons, Inc.; New York, 1999, 1057pp. J. Control. Release 2001, 71, 353–354. [Google Scholar] [CrossRef]
- Lim, Q.F.; Yap, R.C.C.; Teng, C.P.; Yeo, J.C.C.; Tan, M.Y.; Toh, J.P.W.; Zhu, Q.; Thitsartarn, W.; He, C.; Liu, S.; et al. Electrospray-on-Electrospun Breathable, Biodegradable, and Robust Nanofibrous Membranes with Photocatalytic Bactericidal Activity. ACS Appl. Nano Mater. 2023, 6, 1828–1838. [Google Scholar] [CrossRef]
- Bock, N.; Dargaville, T.R.; Woodruff, M.A. Electrospraying of polymers with therapeutic molecules: State of the art. Prog. Polym. Sci. 2012, 37, 1510–1551. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Builders, P.F.; Arhewoh, M.I. Pharmaceutical applications of native starch in conventional drug delivery. Starch-Stärke 2016, 68, 864–873. [Google Scholar] [CrossRef]
- Lai, H.; Liu, S.; Yan, J.; Xing, F.; Xiao, P. Facile Fabrication of Biobased Hydrogel from Natural Resources: L-Cysteine, Itaconic Anhydride, and Chitosan. ACS Sustain. Chem. Eng. 2020, 8, 4941–4947. [Google Scholar] [CrossRef]
- Marco-Dufort, B.; Willi, J.; Vielba-Gomez, F.; Gatti, F.; Tibbitt, M. Environment Controls Biomolecule Release from Dynamic Covalent Hydrogels. Biomacromolecules 2021, 22, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef]
- Yamamoto, E. In Vitro Release Method for Liposome Drug Products. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2019, 139, 249–254. [Google Scholar] [CrossRef]
- Gul, A.; Tzirtzilakis, E.E.; Makhanov, S.S. Simulation of targeted magnetic drug delivery: Two-way coupled biomagnetic fluid dynamics approach. Phys. Fluids 2022, 34, 021911. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Ruan, G.; Winter, J.; Schmidt, C.E. Chapter I.2.19—Microparticles and Nanoparticles. In Biomaterials Science, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 360–388. [Google Scholar]
- Holder, J.E.; Ferguson, C.; Oliveira, E.; Lodeiro, C.; Trim, C.M.; Byrne, L.J.; Bertolo, E.; Wilson, C.M. The use of nanoparticles for targeted drug delivery in non-small cell lung cancer. Front. Oncol. 2023, 13, 1154318. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Matoba, T.; Koga, J.-i.; Nakano, K.; Egashira, K.; Tsutsui, H. Nanoparticle-mediated drug delivery system for atherosclerotic cardiovascular disease. J. Cardiol. 2017, 70, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yu, Z.; Xu, T.; Wang, L.; Meng, N.; Jin, H.; Xu, B. Novel Nano-Drug Delivery System for Brain Tumor Treatment. Cells 2022, 11, 3761. [Google Scholar] [CrossRef] [PubMed]
- Tanbour, R.; Martins, M.A.; Pitt, G.W.; Husseini, A.G. Drug Delivery Systems Based on Polymeric Micelles and Ultrasound: A Review. Curr. Pharm. Des. 2016, 22, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011, 10, 417–427. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Acharya, S.; Sahoo, S.K. Recent trends of nanomedicinal approaches in clinics. Int. J. Pharm. 2018, 538, 263–278. [Google Scholar] [CrossRef]
- Shi, J.; Su, Y.; Liu, W.; Chang, J.; Zhang, Z. A nanoliposome-based photoactivable drug delivery system for enhanced cancer therapy and overcoming treatment resistance. Int. J. Nanomed. 2017, 12, 8257–8275. [Google Scholar] [CrossRef]
- Hami, Z. A Brief Review on Advantages of Nano-based Drug Delivery Systems. Ann. Mil. Health Sci. Res. 2021, 19, e112274. [Google Scholar] [CrossRef]
- Enayati, M.; Ahmad, Z.; Stride, E.; Edirisinghe, M. One-step electrohydrodynamic production of drug-loaded micro- and nanoparticles. J. R. Soc. Interface 2010, 7, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Fu, Y.; Wang, C.H. Paclitaxel and suramin-loaded core/shell microspheres in the treatment of brain tumors. Biomaterials 2010, 31, 8732–8740. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Patel, M.; Patel, R. PLGA Core-Shell Nano/Microparticle Delivery System for Biomedical Application. Polymers 2021, 13, 3471. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Lawson, D.; Onyekuru, L.; Dziemidowicz, K.; Angkawinitwong, U.; Costa, P.F.; Radacsi, N.; Williams, G.R. Protein encapsulation by electrospinning and electrospraying. J. Control. Release 2021, 329, 1172–1197. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, C.; Yue, X.; Li, X.; Zhou, P.; Wu, A.; Chen, C.; Qu, Y.; Zhang, C. Application advance of electrosprayed micro/nanoparticles based on natural or synthetic polymers for drug delivery system. Mater. Des. 2022, 220, 110850. [Google Scholar] [CrossRef]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef]
- Aceves-Serrano, L.G.; Ordaz-Martinez, K.A.; Vazquez-Piñon, M.; Hwang, H. Chapter 3—Microfluidics for drug delivery systems. In Nanoarchitectonics in Biomedicine; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 55–83. [Google Scholar]
- Lopes, J.R.; Santos, G.; Barata, P.; Oliveira, R.; Lopes, C.M. Physical and chemical stimuli-responsive drug delivery systems: Targeted delivery and main routes of administration. Curr. Pharm. Des. 2013, 19, 7169–7184. [Google Scholar] [CrossRef]
- Lou, J.; Duan, H.; Qin, Q.; Teng, Z.; Gan, F.; Zhou, X.; Zhou, X. Advances in Oral Drug Delivery Systems: Challenges and Opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Saito, K.; Lee, H.R.; Lee, M.J.; Shibasaki, Y.; Oishi, Y.; Kim, B.S. Hyperbranched double hydrophilic block copolymer micelles of poly(ethylene oxide) and polyglycerol for pH-responsive drug delivery. Biomacromolecules 2012, 13, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Khoee, S.; Karimi, M.R. Dual-drug loaded Janus graphene oxide-based thermoresponsive nanoparticles for targeted therapy. Polymer 2018, 142, 80–98. [Google Scholar] [CrossRef]
- Hossion, A.M.; Bio, M.; Nkepang, G.; Awuah, S.G.; You, Y. Visible Light Controlled Release of Anticancer Drug through Double Activation of Prodrug. ACS Med. Chem. Lett. 2012, 4, 124–127. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Xu, F.; Han, Z.; Wei, D.; Jia, D.; Zhou, Y. Tough Magnetic Chitosan Hydrogel Nanocomposites for Remotely Stimulated Drug Release. Biomacromolecules 2018, 19, 3351–3360. [Google Scholar] [CrossRef]
- Neumann, S.E.; Chamberlayne, C.F.; Zare, R.N. Electrically controlled drug release using pH-sensitive polymer films. Nanoscale 2018, 10, 10087–10093. [Google Scholar] [CrossRef]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Berkmann, J.C.; Giasafaki, D.; Lozano, D.; Spyrou, K.; Manzano, M.; Steriotis, T.; Duda, G.N.; Schmidt-Bleek, K.; Charalambopoulou, G.; et al. Engineered pH-Responsive Mesoporous Carbon Nanoparticles for Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 14946–14957. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. Redox-responsive nano-carriers as tumor-targeted drug delivery systems. Eur. J. Med. Chem. 2018, 157, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Keshavarz, T. Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hu, X.; Tian, J.; Liu, T.; Zhang, G.; Liu, S. Photo-triggered release of caged camptothecin prodrugs from dually responsive shell cross-linked micelles. Macromolecules 2013, 46, 6243–6256. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.J. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm. Res. 2010, 27, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C 2017, 71, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Hashimoto, C.; Ozaki, Y.; Jung, Y.M. Understanding the phase transition of linear poly(N-isopropylacrylamide) gel under the heating and cooling processes. J. Mol. Struct. 2016, 1124, 144–150. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Wei, Y.; Cheng, G.; Ma, G. Thermo-triggered drug delivery from polymeric micelles of poly(N-isopropylacrylamide-co-acrylamide)-b-poly(n-butyl methacrylate) for tumor targeting. J. Bioact. Compat. Polym. 2014, 29, 301–317. [Google Scholar] [CrossRef]
- Akagi, K.; Ohshima, K.; Ohsaki, S.; Nakamura, H.; Watano, S. pH dependence of drug release behavior from metal-organic framework particle with different acid-base resistances. Inorganica Chim. Acta 2022, 542, 121143. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. pH-responsive drug-delivery systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef]
- Ghaffar, A.; Yameen, B.; Latif, M.; Malik, M.I. Chapter 14—pH-sensitive drug delivery systems. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Shah, M.R., Imran, M., Ullah, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 259–278. [Google Scholar]
- Zeng, J.; Shirihai, O.S.; Grinstaff, M.W. Modulating lysosomal pH: A molecular and nanoscale materials design perspective. J. Life Sci. 2020, 2, 25–37. [Google Scholar] [CrossRef]
- Hopkins, E.; Sanvictores, T.; Sharma, S. Physiology, Acid Base Balance; BTI-StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yang, Q.; Wang, S.; Fan, P.; Wang, L.; Di, Y.; Lin, K.; Xiao, F.-S. pH-Responsive Carrier System Based on Carboxylic Acid Modified Mesoporous Silica and Polyelectrolyte for Drug Delivery. Chem. Mater. 2005, 17, 5999–6003. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Garbern, J.C.; Minami, E.; Stayton, P.S.; Murry, C.E. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 2011, 32, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.N.; Zhang, C.Q.; Wang, W.; Wang, P.C.; Zhou, J.P.; Liang, X.J. pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol. Med. 2014, 11, 34. [Google Scholar]
- Zhu, Y.J.; Guo, X.X.; Sham, T.K. Calcium silicate-based drug delivery systems. Expert Opin. Drug Deliv. 2017, 14, 215–228. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Ma, Y.; Jiang, Y. Calcium Phosphate-Based Nanomaterials: Preparation, Multifunction, and Application for Bone Tissue Engineering. Molecules 2023, 28, 4790. [Google Scholar] [CrossRef]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Martwong, E.; Tran, Y. Lower Critical Solution Temperature Phase Transition of Poly(PEGMA) Hydrogel Thin Films. Langmuir 2021, 37, 8585–8593. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Xing, H.; Hwang, K.; Lu, Y. Recent Developments of Liposomes as Nanocarriers for Theranostic Applications. Theranostics 2016, 6, 1336–1352. [Google Scholar] [CrossRef]
- Zhou, J.; Pishko, M.V.; Lutkenhaus, J.L. Thermoresponsive layer-by-layer assemblies for nanoparticle-based drug delivery. Langmuir 2014, 30, 5903–5910. [Google Scholar] [CrossRef] [PubMed]
- Beija, M.; Marty, J.D.; Destarac, M. Thermoresponsive poly(N-vinyl caprolactam)-coated gold nanoparticles: Sharp reversible response and easy tunability. Chem. Commun. 2011, 47, 2826–2828. [Google Scholar] [CrossRef] [PubMed]
- Jauregui, R.; Srinivasan, S.; Vojtech, L.N.; Gammill, H.S.; Chiu, D.T.; Hladik, F.; Stayton, P.S.; Lai, J.J. Temperature-Responsive Magnetic Nanoparticles for Enabling Affinity Separation of Extracellular Vesicles. ACS Appl. Mater. Interfaces 2018, 10, 33847–33856. [Google Scholar] [CrossRef] [PubMed]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Prawatborisut, M.; Oberländer, J.; Jiang, S.; Graf, R.; Avlasevich, Y.; Morsbach, S.; Crespy, D.; Mailänder, V.; Landfester, K. Temperature-Responsive Nanoparticles Enable Specific Binding of Apolipoproteins from Human Plasma. Small 2022, 18, 2103138. [Google Scholar] [CrossRef]
- Men, K.; Liu, W.; Li, L.; Duan, X.; Wang, P.; Gou, M.; Wei, X.; Gao, X.; Wang, B.; Du, Y.; et al. Delivering instilled hydrophobic drug to the bladder by a cationic nanoparticle and thermo-sensitive hydrogel composite system. Nanoscale 2012, 4, 6425–6433. [Google Scholar] [CrossRef]
- Luo, Y.L.; Yang, X.L.; Xu, F.; Chen, Y.S.; Zhang, B. Thermosensitive PNIPAM-b-HTPB block copolymer micelles: Molecular architectures and camptothecin drug release. Colloids Surfaces B Biointerfaces 2014, 114, 150–157. [Google Scholar] [CrossRef]
- Nakayama, M.; Chung, J.E.; Miyazaki, T.; Yokoyama, M.; Sakai, K.; Okano, T. Thermal modulation of intracellular drug distribution using thermoresponsive polymeric micelles. React. Funct. Polym. 2007, 67, 1398–1407. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Kan, C.W. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Emam, H.E.; Shaheen, T.I. Design of a dual pH and temperature responsive hydrogel based on esterified cellulose nanocrystals for potential drug release. Carbohydr. Polym. 2022, 278, 118925. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Thermal responsive hydrogels based on semi interpenetrating network of poly(NIPAm) and cellulose nanowhiskers. Carbohydr. Polym. 2014, 102, 159–166. [Google Scholar] [CrossRef]
- Hussain, K.; Aslam, Z.; Ullah, S.; Shah, M.R. Synthesis of pH responsive, photocrosslinked gelatin-based hydrogel system for control release of ceftriaxone. Chem. Phys. Lipids 2021, 238, 105101. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Lu, P. Designing Dual-Responsive Drug Delivery Systems: The Role of Phase Change Materials and Metal–Organic Frameworks. Materials 2024, 17, 3070. https://doi.org/10.3390/ma17133070
Wei W, Lu P. Designing Dual-Responsive Drug Delivery Systems: The Role of Phase Change Materials and Metal–Organic Frameworks. Materials. 2024; 17(13):3070. https://doi.org/10.3390/ma17133070
Chicago/Turabian StyleWei, Wanying, and Ping Lu. 2024. "Designing Dual-Responsive Drug Delivery Systems: The Role of Phase Change Materials and Metal–Organic Frameworks" Materials 17, no. 13: 3070. https://doi.org/10.3390/ma17133070





