Optimized Adsorption–Catalytic Conversion for Lithium Polysulfides by Constructing Bimetallic Compounds for Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Bimetallic Selenides
2.2. Preparation of Modified Separators and Sulfur Cathodes
2.3. Characterization of Bimetallic Selenide Materials
2.4. Electrochemical Performance Test of Li-S Batteries
2.5. Analysis of Adsorption and Catalytic Properties of Bimetallic Selenides
2.5.1. Adsorption Effect Evaluation
2.5.2. CV test of Li2S6 Symmetric Batteries
2.5.3. Li2S Deposition and Decomposition Test
3. Results and Discussion
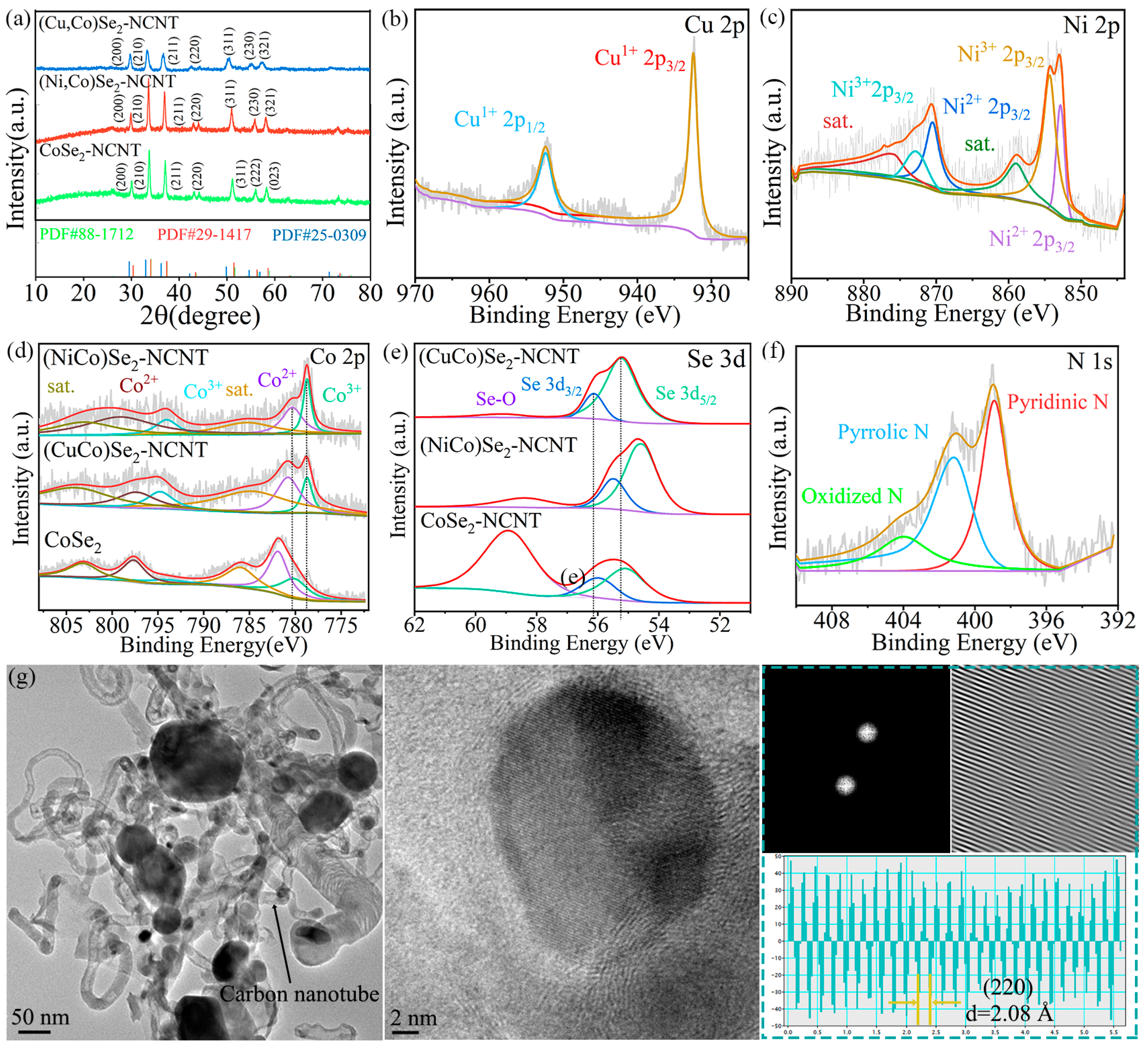
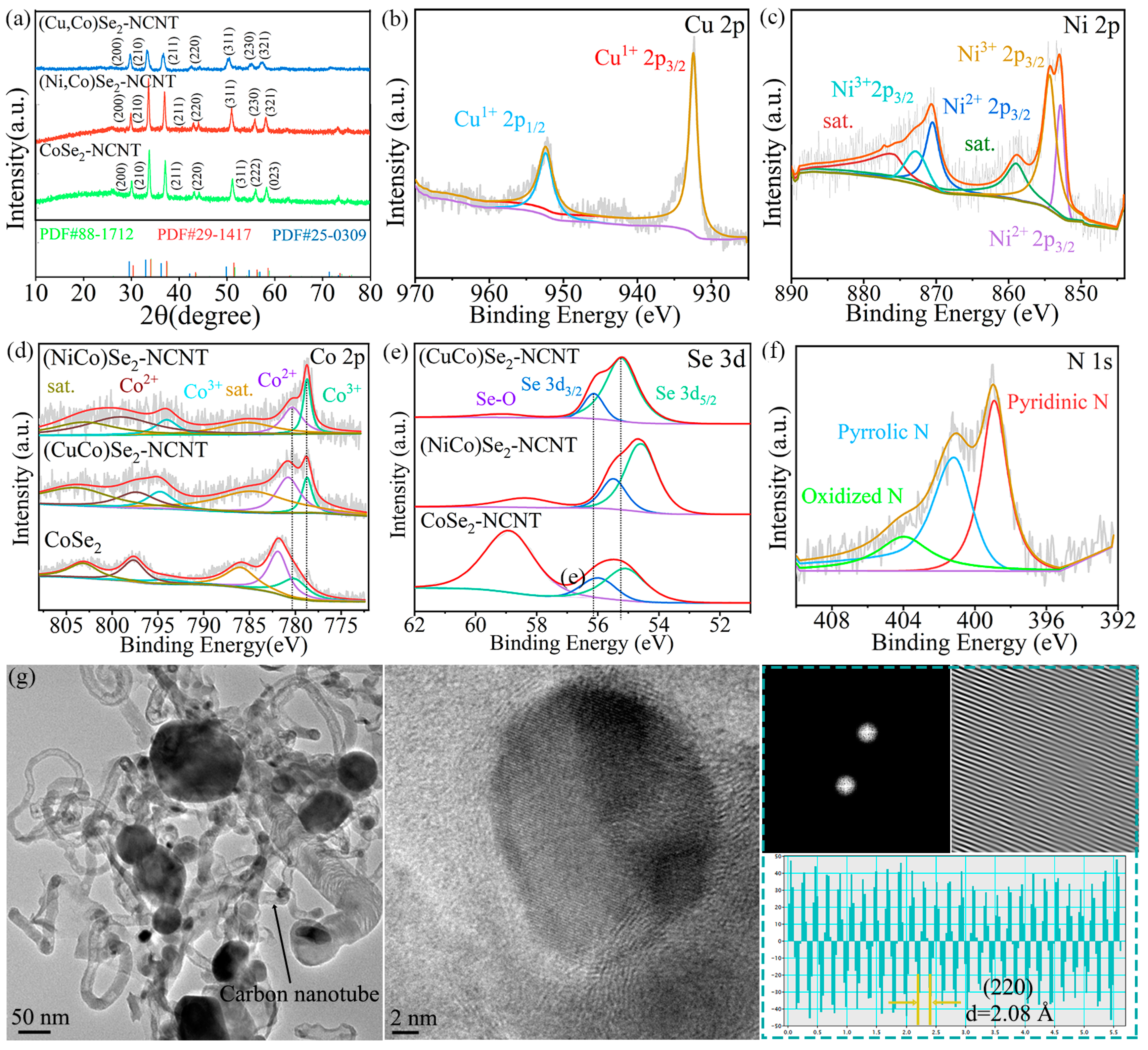
3.1. Materials Characterization
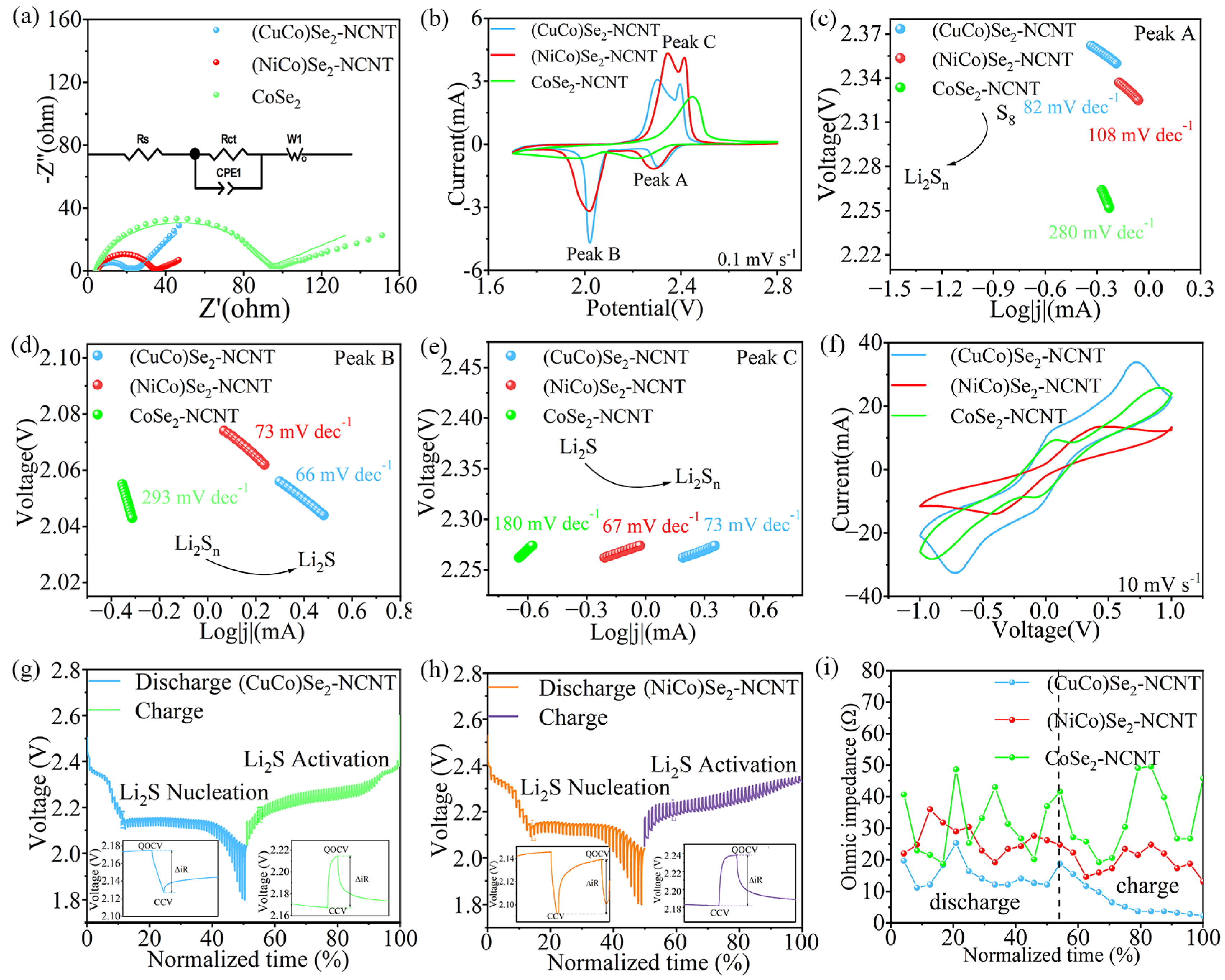
3.2. Electrochemical Performance of Li-S Batteries
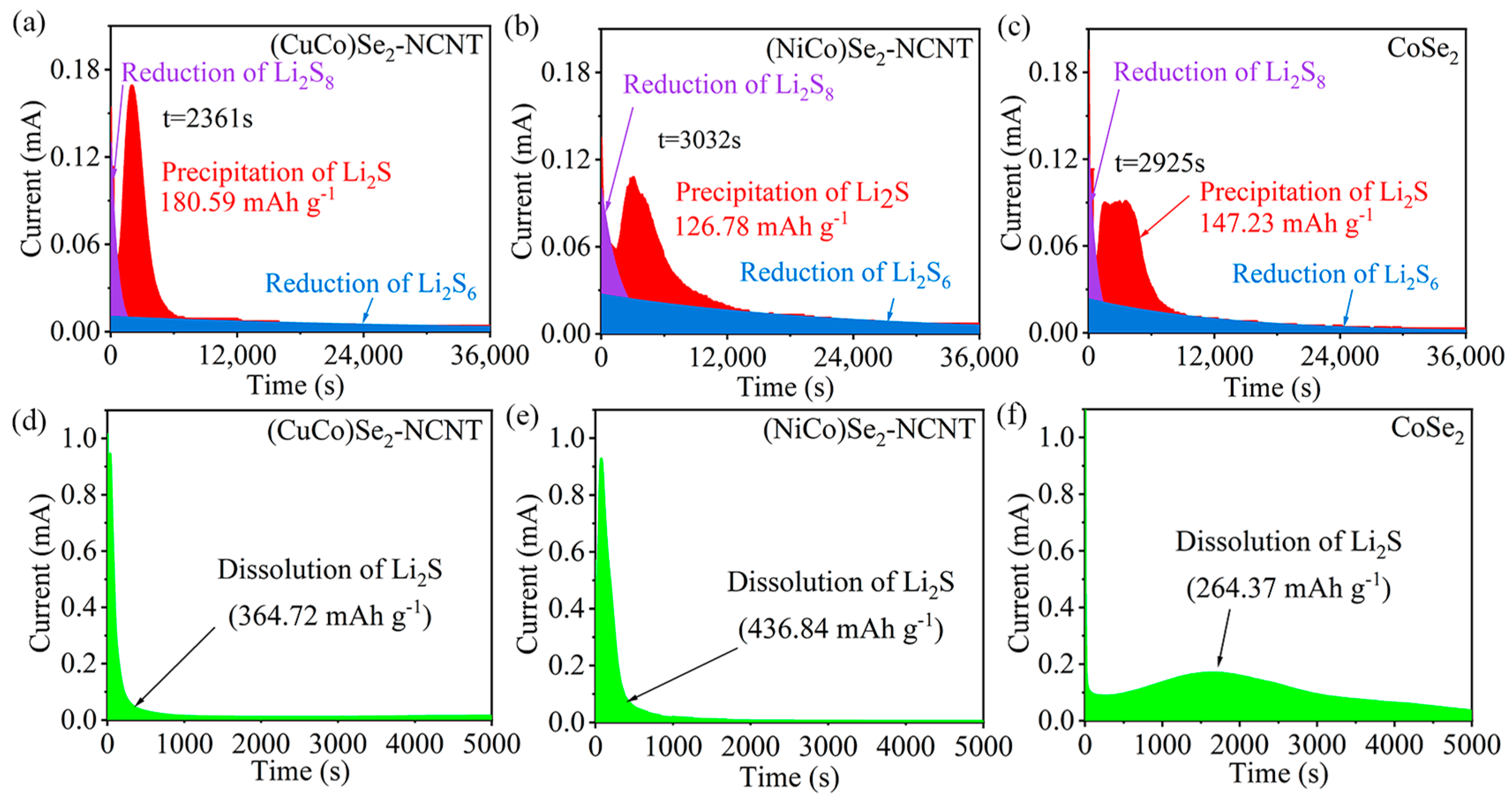
3.3. Comparison of Adsorption–Catalytic Properties of Bimetallic Selenides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Z.; Shen, J.; Xu, X.; Li, F.; Liu, J.; Yuan, B.; Yu, Y.; Zhu, M. Advances in the development of single-atom catalysts for high-energy-density lithium-sulfur batteries. Adv. Mater. 2022, 34, 2200102. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, H.; Ding, X.; Yuan, H.; Jin, C.; Nai, J.; Liu, Y.; Wang, Y.; Wan, Y.; Tao, X. 12 years roadmap of the sulfur cathode for lithium sulfur batteries. Energy Storage Mater. 2020, 30, 346–366. [Google Scholar] [CrossRef]
- Long, J.J.; Yu, H.; Liu, W.B. Structure engineering of cathode host materials for Li-S batteries. Rare Metals 2024, 43, 1370–1389. [Google Scholar] [CrossRef]
- Lv, Z.C.; Wang, P.F.; Wang, J.C.; Tian, S.H.; Yi, T.F. Key challenges, recent advances and future perspectives of rechargeable lithium-sulfur batteries. J. Ind. Eng. Chem. 2023, 124, 68–88. [Google Scholar] [CrossRef]
- Cao, G.; Duan, R.; Li, X. Controllable catalysis behavior for high performance lithium sulfur batteries: From kinetics to strategies. EnergyChem 2023, 5, 100096. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. Host materials anchoring polysulfides in Li-S batteries reviewed. Adv. Energy Mater. 2021, 11, 2001304. [Google Scholar] [CrossRef]
- Han, J.; Wang, P.; Zhang, H.; Song, N.; An, X.; Xi, B.; Xiong, S. Performance optimization of chalcogenide catalytic materials in lithium-sulfur batteries: Structural and electronic engineering. Chin. Chem. Lett. 2024, 35, 109543. [Google Scholar] [CrossRef]
- Chen, L.; Cao, G.; Li, Y.; Zu, G.; Duan, R.; Bai, Y.; Xue, K.; Fu, Y.; Xu, Y.; Wang, J.; et al. review on engineering transition metal compound catalysts to accelerate the redox kinetics of sulfur cathodes for lithium-sulfur batteries. Nano-Micro Lett. 2024, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, R.; Yu, C.; Xu, C.; Shi, Z. Application of transition metal compounds in cathode materials for lithium-sulfur battery. Ionics 2022, 28, 5275–5288. [Google Scholar] [CrossRef]
- Shi, F.; Yu, J.; Chen, C.; Lau, S.P.; Lv, W.; Xu, Z.-L. Advances in understanding and regulation of sulfur conversion processes in metal-sulfur batteries. J. Mater. Chem. A 2022, 10, 19412–19443. [Google Scholar] [CrossRef]
- Du, S.; Yu, Y.; Liu, X.; Lu, D.; Yue, X.; Liu, T.; Yin, Y.; Wu, Z. Catalytic engineering for polysulfide conversion in high-performance lithium-sulfur batteries. J. Mater. Sci. Technol. 2024, 186, 110–131. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, J.; Niu, R.; Zhao, Z.; Wang, X. High-entropy materials: Features for lithium-sulfur battery applications. Metals 2023, 13, 833. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Arshad, H.M.U.; Li, H.J.; Liu, S.; Li, G.R.; Gao, X.P. Crystalline multi-metallic compounds as host materials in cathode for lithium-sulfur batteries. Small 2021, 17, 2005332. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, Z.; Von Lim, Y.; Wang, Y.; Li, Y.; Zhang, D.; Yang, H.Y. Recent advances in heterostructure engineering for lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2003689. [Google Scholar] [CrossRef]
- Wang, J.; Han, W.-Q. A review of heteroatom doped materials for advanced lithium-sulfur batteries. Adv. Funct. Mater. 2022, 32, 2107166. [Google Scholar] [CrossRef]
- Li, S.; Xu, P.; Aslam, M.K.; Chen, C.; Rashid, A.; Wang, G.; Zhang, L.; Mao, B. Propelling polysulfide conversion for high-loading lithium-sulfur batteries through highly sulfiphilic NiCo2S4 nanotubes. Energy Storage Mater. 2020, 27, 51–60. [Google Scholar] [CrossRef]
- Duan, J.; Zou, Y.; Li, Z.; Long, B.; Du, Y. Hollow quasi-polyhedron structure of NiCoP with strong constraint sulfur effect for lithium sulfur battery. Electroanal. Chem. 2019, 847, 113187. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Zhang, F.; Guo, J. Li4Ti5O12 nanowire array as a sulfur host for high performance lithium sulfur battery. J. Alloys Compd. 2019, 805, 873–879. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.; Cheng, C.; Yan, T.; Gao, W.; Mao, J.; Dai, K.; Zhang, L. Boosting polysulfide redox conversion of Li-S batteries by one-step-synthesized Co-Mo bimetallic nitride. J. Energy Chem. 2021, 61, 336–346. [Google Scholar] [CrossRef]
- Wang, X.; Han, J.; Luo, C.; Zhang, B.; Ma, J.; Li, Z.; He, Y.-B.; Yang, Q.-H.; Kang, F.; Lv, W. Coordinated adsorption and catalytic conversion of polysulfides enabled by perovskite bimetallic hydroxide nanocages for lithium-sulfur batteries. Small 2021, 17, 2101538. [Google Scholar] [CrossRef]
- Shen, Z.; Cao, M.; Zhang, Z.; Pu, J.; Zhong, C.; Li, J.; Ma, H.; Li, F.; Zhu, J.; Pan, F.; et al. Efficient Ni2Co4P3 nanowires catalysts enhance ultrahigh-loading lithium-sulfur conversion in a microreactor-like battery. Adv. Funct. Mater. 2020, 30, 1906661. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of heterostructure materials in energy storage: A review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, L.; Ding, D.; Zhai, Y.; Wang, F.; Jing, Z.; Yang, X.; Kong, Y.; Qian, Y.; Xu, L. Zinc-assisted cobalt ditelluride polyhedra inducing lattice strain to endow efficient adsorption-catalysis for high-energy lithium-sulfur batteries. Adv. Mater. 2022, 34, 2204403. [Google Scholar] [CrossRef]
- Shang, C.; Li, G.; Wei, B.; Wang, J.; Gao, R.; Tian, Y.; Chen, Q.; Zhang, Y.; Shui, L.; Zhou, G.; et al. Dissolving vanadium into titanium nitride lattice framework for rational polysulfide regulation in Li-S batteries. Adv. Energy Mater. 2021, 11, 2003020. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Z.; Wan, X.; Hua, W.; Li, H.; Yang, Q.H.; Wang, W. Li2S4 anchoring governs the catalytic sulfur reduction on defective SmMn2O5 in lithium-sulfur battery. Adv. Energy Mater. 2022, 12, 2200340. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, Y.; Zhang, W.; Xu, M. Boosting polysulfide conversion in lithium-sulfur batteries by cobalt-doped vanadium nitride microflowers. ACS Appl. Energy Mater. 2020, 3, 4523–4530. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, X.; Tian, J.; Li, M.; Yuan, Y.; Zhang, S.; Fang, S.; Fan, X.; Xu, W.; Lu, H.; et al. Cation-doped ZnS catalysts for polysulfide conversion in lithium-sulfur batteries. Nat. Catal. 2022, 5, 555–563. [Google Scholar] [CrossRef]
- Wang, M.; Fan, L.; Sun, X.; Guan, B.; Jiang, B.; Wu, X.; Tian, D.; Sun, K.; Qiu, Y.; Yin, X.; et al. Nitrogen-doped CoSe2 as a bifunctional catalyst for high areal capacity and lean electrolyte of Li-S battery. ACS Energy Lett. 2020, 5, 3041–3050. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, J.; Rao, D.; Zhu, L.; Niu, S.; Cai, J.; Fang, Y.; Liu, Y.; Liu, X.; Zang, Y.; et al. Regulating the electron filling state of d orbitals in Ta-based compounds for tunable lithium-sulfur chemistry. Sustain. Mater. Techno. 2021, 28, e00271. [Google Scholar] [CrossRef]
- Al-Tahan, M.A.; Dong, Y.; Shrshr, A.E.; Liu, X.; Zhang, R.; Guan, H.; Zhang, J. Enormous-sulfur-content cathode and excellent electrochemical performance of Li-S battery accouched by surface engineering of Ni-doped WS2@rGO nanohybrid as a modified separator. J. Colloid Interf. Sci. 2022, 609, 235–248. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, Y.; Al-Tahan, M.A.; Zhang, Y.; Wei, R.; Ma, Y.; Yang, C.; Zhang, J. Insights into the sandwich-like ultrathin Ni-doped MoS2/rGO hybrid as effective sulfur hosts with excellent adsorption and electrocatalysis effects for lithium-sulfur batteries. J. Energy Chem. 2021, 60, 85–94. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, R.; Zhu, S.; Zhou, L.; Xu, Q.; Min, Y. Bimetallic nitride modified separator constructs internal electric field for high-performance lithium-sulfur battery. Chem. Eng. J. 2022, 429, 132454. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, Y.; Tan, L.; Li, M.; Peng, K. Self-reconstruction of (CoNiFeCuCr)Se high-entropy selenide for efficient oxygen evolution reaction. Appl. Surf. Sci. 2023, 627, 157282. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Z.; Liu, S.; Li, G.; Gao, X. High-entropy spinel oxide nanofibers as catalytic sulfur hosts promise the high gravimetric and volumetric capacities for lithium-sulfur batteries. Energy Environ. Mater. 2022, 5, 645–654. [Google Scholar] [CrossRef]
- Yao, W.; Tian, C.; Yang, C.; Xu, J.; Meng, Y.; Manke, I.; Chen, N.; Wu, Z.; Zhan, L.; Wang, Y.; et al. P-doped NiTe2 with Te-vacancies in lithium-sulfur batteries prevents shuttling and promotes polysulfide conversion. Adv. Mater. 2022, 34, 2106370. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Xie, Y.; Lai, Y.; Yang, M.; Xu, J.; Wu, F.; Cheng, S.; Wang, X. CoSe2 nanoparticles-decorated carbon nanofibers as a hierarchical self-supported sulfur host for high-energy lithium-sulfur batteries. Sci. China Mater. 2023, 66, 3075–3083. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wei, X.; Wei, C.; Song, Y. A review of size engineering-enabled electrocatalysts for Li-S chemistry. Nanoscale Adv. 2021, 3, 5777–5784. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Cheng, C.; Yan, T.; Liu, G.; Jiang, J.; Mao, J.; Dai, K.; Li, J.; Wu, J.; Zhang, L. Synergistic effect of Co3Fe7 alloy and N-doped hollow carbon spheres with high activity and stability for high-performance lithium-sulfur batteries. Nano Energy 2021, 86, 106111. [Google Scholar] [CrossRef]
- Jiang, B.; Qiu, Y.; Tian, D.; Zhang, Y.; Song, X.; Zhao, C.; Wang, M.; Sun, X.; Huang, H.; Zhao, C.; et al. Crystal facet engineering induced active tin dioxide nanocatalysts for highly stable lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2102995. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, W.; Xu, J.; Tian, C.; Han, K.; Sun, W.; Xiao, S. ZnS-SnS@NC heterostructure as robust lithiophilicity and sulfiphilicity mediator toward high-rate and long-life lithium-sulfur batteries. ACS Nano. 2021, 15, 7114–7130. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, Y.; Fan, L.; Sun, X.; Jiang, B.; Wang, M.; Wu, X.; Tian, D.; Song, X.; Yin, X.; et al. Dual-atoms iron sites boost the kinetics of reversible conversion of polysulfide for high-performance lithium-sulfur batteries. Energy Storage Mater. 2023, 63, 103026. [Google Scholar] [CrossRef]
- Song, X.; Tian, D.; Qiu, Y.; Sun, X.; Jiang, B.; Zhao, C.; Zhang, Y.; Fan, L.; Zhang, N. Accelerating sulfur redox reactions by topological insulator Bi2Te3 for high-performance Li-S batteries. Adv. Funct. Mater. 2022, 32, 2109413. [Google Scholar] [CrossRef]
- Cai, D.; Liu, B.; Zhu, D.; Chen, D.; Lu, M.; Cao, J.; Han, W. Ultrafine Co3Se4 nanoparticles in nitrogen-doped 3D carbon matrix for high-stable and long-cycle-life lithium sulfur batteries. Adv. Energy Mater. 2020, 10, 1904273. [Google Scholar] [CrossRef]
- Wen, K.; Huang, L.; Qu, L.; Deng, T.; Men, X.; Chen, L.; Wang, J. g-C3N4/MoO3 composite with optimized crystal face: A synergistic adsorption-catalysis for boosting cathode performance of lithium-sulfur batteries. J. Colloid Interf. Sci. 2023, 649, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wang, J.; Chen, L.; Men, X.; Deng, T.; Wen, K.; Huang, L. Accelerated polysulfide conversion using nitrogen-doped carbon nanofibers embedded with V2O3 as interlayers for lithium-sulfur batteries. Ionics 2023, 29, 2299–2310. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Yuan, G.; Guo, J.; Zheng, Y.; Huang, Y.; Shao, H. Elucidating the volcanic-type catalytic behavior in lithium-sulfur batteries via defect engineering. ACS Nano. 2023, 17, 18253–18265. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qu, Y.; Zhao, L.; Zhao, M. Monolayer Fe3GeX2 (X = S, Se, and Te) as highly efficient electrocatalysts for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2021, 13, 11845–11851. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fang, H.; Yang, X.; Xue, J.; Li, C.; Hou, S.; Hu, C. Fe-cation doping in NiSe2 as an effective method of electronic structure modulation towards high-performance lithium-sulfur batteries. ChemSusChem 2021, 14, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, R.; Zhang, Y.; Yan, H.; Zhu, Q.; Shang, J.; Yang, S.; Li, B. Nano high-entropy alloy with strong affinity driving fast polysulfide conversion towards stable lithium sulfur batteries. Energy Storage Mater. 2021, 43, 212–220. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, H.; Han, N.; Feng, S.; Zhang, X.; Guo, W.; Tan, P.; Xie, S.; Zhou, Z.; Ma, Q.; et al. Balancing adsorption, catalysis, and desorption in cathode catalyst for Li-S batteries. Adv. Energy Mater. 2023, 13, 2301551. [Google Scholar] [CrossRef]
- Seo, H.Y.; Choi, J.H.; Kim, Y.B.; Cho, J.S.; Kang, Y.C.; Park, G.D. Tailoring the shell thickness of yolk-shell structured carbon microspheres: Applications in metal selenide and carbon composite microspheres for enhanced sodium ion storage properties. J. Mater. Chem. A 2023, 11, 24738–24753. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Chen, S.; Gao, S.; Wang, L.; Liu, X.; Li, M.; Shangguan, E. Facile preparation of bimetallic cobalt-copper selenide nanosheets as stable cathode materials for rechargeable magnesium batteries. J. Alloys Compd. 2024, 976, 173223. [Google Scholar] [CrossRef]
- Meshesha, M.M.; Chanda, D.; Jang, S.G.; Yang, B.L. Enhancing cobalt-based bimetallic selenide performance for urea and water electrolysis through interface engineering. Chem. Eng. J. 2023, 474, 145708. [Google Scholar] [CrossRef]
- Chae, S.H.; Muthurasu, A.; Kim, T.; Kim, J.S.; Khil, M.S.; Lee, M.; Kim, H.; Lee, J.Y.; Kim, H.Y. Templated fabrication of perfectly aligned metal-organic framework-supported iron-doped copper-cobalt selenide nanostructure on hollow carbon nanofibers for an efficient trifunctional electrode material. Appl. Catal. B Environ. 2021, 293, 120209. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.H.; Ha, J.S.; Kim, I.T.; Park, S.H.; Park, H.K.; Park, H.J.; Kang, B.K.; Park, Y.S. Highly Active cobalt-copper-selenide electrocatalysts for solar-driven oxygen evolution reaction: An electrochemical activation energy aspect. ACS Sustain. Chem. Eng. 2024, 12, 275–282. [Google Scholar] [CrossRef]
- Hui, X.; Zhao, J.; Mao, J.; Zhao, H. Reduced graphene oxide-wrapped copper cobalt selenide composites as anode materials for high-performance lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 130979. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo)Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, R.; Li, N.; Bai, Y.; Zhou, Y.; Wang, J. Optimized Adsorption–Catalytic Conversion for Lithium Polysulfides by Constructing Bimetallic Compounds for Lithium–Sulfur Batteries. Materials 2024, 17, 3075. https://doi.org/10.3390/ma17133075
Chen L, Wang R, Li N, Bai Y, Zhou Y, Wang J. Optimized Adsorption–Catalytic Conversion for Lithium Polysulfides by Constructing Bimetallic Compounds for Lithium–Sulfur Batteries. Materials. 2024; 17(13):3075. https://doi.org/10.3390/ma17133075
Chicago/Turabian StyleChen, Liping, Runhua Wang, Nan Li, Yang Bai, Yimo Zhou, and Juan Wang. 2024. "Optimized Adsorption–Catalytic Conversion for Lithium Polysulfides by Constructing Bimetallic Compounds for Lithium–Sulfur Batteries" Materials 17, no. 13: 3075. https://doi.org/10.3390/ma17133075
APA StyleChen, L., Wang, R., Li, N., Bai, Y., Zhou, Y., & Wang, J. (2024). Optimized Adsorption–Catalytic Conversion for Lithium Polysulfides by Constructing Bimetallic Compounds for Lithium–Sulfur Batteries. Materials, 17(13), 3075. https://doi.org/10.3390/ma17133075





