Nanocones: A Compressive Review of Their Electrochemical Synthesis and Applications
Abstract
:1. Introduction
2. Cones or Pyramids?
3. Methods of Synthesis
3.1. One-Step Method
3.2. Deposition in Prepared Substrates
3.3. Other Methods
4. Applications
5. Concerns and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armarego, W.L.F. Nanomaterials. In Purification of Laboratory Chemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 586–630. [Google Scholar]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Bratovcic, A. Different Applications of Nanomaterials and Their Impact on the Environment. Int. J. Mater. Sci. Eng. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Gajanan, K.; Tijare, S.N. Applications of nanomaterials. Mater. Today Proc. 2018, 5, 1093–1096. [Google Scholar] [CrossRef]
- Bashir, S.; Liu, J. Nanomaterials and Their Application. In Advanced Nanomaterials and Their Applications in Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–50. [Google Scholar]
- Hossain, U.H.; Jantsen, G.; Muench, F.; Kunz, U.; Ensinger, W. Increasing the structural and compositional diversity of ion-track templated 1D nanostructures through multistep etching, plastic deformation, and deposition. Nanotechnology 2022, 33, 245603. [Google Scholar] [CrossRef] [PubMed]
- Kac, M.; Mis, A.; Dubiel, B.; Kowalski, K.; Zarzycki, A.; Dobosz, I. Template-Assisted Iron Nanowire Formation at Different Electrolyte Temperatures. Materials 2021, 14, 4080. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, I.; Kutyła, D.; Kac, M.; Włoch, G.; Żabiński, P. The influence of homogenous external magnetic field on morphology and magnetic properties of CoRu nanowire arrays. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 262, 114795. [Google Scholar] [CrossRef]
- Zaraska, L.; Gawlak, K.; Kurowska-Tabor, E.; Jaskuła, M.; Sulka, G.D. Template-assisted synthesis of rough Ag nanorods and their application for amperometric sensing of H2O2. Comptes Rendus Chim. 2017, 20, 693–696. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Das, K. Fabrication of Sn nanostructures by template assisted pulse electrodeposition. Surf. Eng. 2016, 32, 378–384. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhang, J.; Liu, Y.; Ma, X.; Chen, H.Y. Electrochemical synthesis of selenium nanotubes by using CTAB soft-template. Electrochim. Acta 2005, 50, 4365–4370. [Google Scholar] [CrossRef]
- Machín, A.; Fontánez, K.; Arango, J.C.; Ortiz, D.; De León, J.; Pinilla, S.; Nicolosi, V.; Petrescu, F.I.; Morant, C.; Márquez, F. One-Dimensional (1D) Nanostructured Materials for Energy Applications. Materials 2021, 14, 2609. [Google Scholar] [CrossRef]
- Li, P.; Chen, W. Recent advances in one-dimensional nanostructures for energy electrocatalysis. Cuihua Xuebao/Chin. J. Catal. 2019, 40, 4–22. [Google Scholar] [CrossRef]
- Daulbayev, C.; Sultanov, F.; Bakbolat, B.; Daulbayev, O. 0D, 1D and 2D nanomaterials for visible photoelectrochemical water splitting. A Review. Int. J. Hydrogen Energy 2020, 45, 33325–33342. [Google Scholar] [CrossRef]
- Saldan, I.; Dobrovetska, O.; Sus, L.; Makota, O.; Pereviznyk, O.; Kuntyi, O.; Reshetnyak, O. Electrochemical synthesis and properties of gold nanomaterials. J. Solid State Electrochem. 2018, 22, 637–656. [Google Scholar] [CrossRef]
- Stine, K.J. Biosensor Applications of Electrodeposited Nanostructures. Appl. Sci. 2019, 9, 797. [Google Scholar] [CrossRef]
- Xiao, F.; Hangarter, C.; Yoo, B.; Rheem, Y.; Lee, K.H.; Myung, N.V. Recent progress in electrodeposition of thermoelectric thin films and nanostructures. Electrochim. Acta 2008, 53, 8103–8117. [Google Scholar] [CrossRef]
- Li, X.; Keshavarz, M.; Kassanos, P.; Kidy, Z.; Roddan, A.; Yeatman, E.; Thompson, A.J. SERS Detection of Breast Cancer-Derived Exosomes Using a Nanostructured Pt-Black Template. Adv. Sens. Res. 2023, 2, 2200039. [Google Scholar] [CrossRef]
- Torati, S.R.; Kasturi, K.C.S.B.; Lim, B.; Kim, C.G. Hierarchical gold nanostructures modified electrode for electrochemical detection of cancer antigen CA125. Sens. Actuators B Chem. 2017, 243, 64–71. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shi, C.; Guo, X.; He, Z.; Pan, L.; Huang, Z.; Zhang, X.; Zou, J. Rational Design of Better Hydrogen Evolution Electrocatalysts for Water Splitting: A Review. Adv. Sci. 2022, 9, 2200307. [Google Scholar] [CrossRef]
- Ferriday, T.B.; Middleton, P.H.; Kolhe, M.L. Review of the Hydrogen Evolution Reaction—A Basic Approach. Energies 2021, 14, 8535. [Google Scholar] [CrossRef]
- Yusuf, B.A.; Yaseen, W.; Xie, M.; Zayyan, R.S.; Muhammad, A.I.; Nankya, R.; Xie, J.; Xu, Y. Recent advances in understanding and design of efficient hydrogen evolution electrocatalysts for water splitting: A comprehensive review. Adv. Colloid Interface Sci. 2023, 311, 102811. [Google Scholar] [CrossRef]
- Brinkmann, G.; Van Cleemput, N. Classification and generation of nanocones. Discret. Appl. Math. 2011, 159, 1528–1539. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, Q.; Jiang, W.; Zhu, H.; Xu, K.; Ren, Y.; Xu, C. Achieving of bionic super-hydrophobicity by electrodepositing nano-Ni-pyramids on the picosecond laser-ablated micro-Cu-cone surface. Surf. Coat. Technol. 2019, 363, 170–178. [Google Scholar] [CrossRef]
- Skibińska, K.; Kołczyk-Siedlecka, K.; Kutyła, D.; Gajewska, M.; Żabiński, P. Synthesis of Co–Fe 1D Nanocone Array Electrodes Using Aluminum Oxide Template. Materials 2021, 14, 1717. [Google Scholar] [CrossRef] [PubMed]
- Skibinska, K.; Kolczyk-Siedlecka, K.; Kutyla, D.; Jedraczka, A.; Leszczyńska-Madej, B.; Marzec, M.M.; Zabinski, P. Electrocatalytic Properties of Co Nanoconical Structured Electrodes Produced by a One-Step or Two-Step Method. Catalysts 2021, 11, 544. [Google Scholar] [CrossRef]
- Skibińska, K.; Elsharkawy, S.; Kula, A.; Kutyła, D.; Żabiński, P. Influence of Substrate Preparation on the Catalytic Activity of Conical Ni Catalysts. Coatings 2023, 13, 2067. [Google Scholar] [CrossRef]
- Worstell, J. Fundamentals of Fixed-Bed Reactors. In Adiabatic Fixed-Bed Reactors; Elsevier: Amsterdam, The Netherlands, 2014; pp. 13–33. [Google Scholar]
- Hang, T.; Hu, A.; Ling, H.; Li, M.; Mao, D. Super-hydrophobic nickel films with micro-nano hierarchical structure prepared by electrodeposition. Appl. Surf. Sci. 2010, 256, 2400–2404. [Google Scholar] [CrossRef]
- Salehikahrizsangi, P.; Raeissi, K.; Karimzadeh, F.; Calabrese, L.; Patane, S.; Proverbio, E. Erosion-corrosion behavior of highly hydrophobic hierarchical nickel coatings. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 558, 446–454. [Google Scholar] [CrossRef]
- Skibińska, K.; Kutyła, D.; Kula, A.; Gajewska, M.; Marzec, M.M.; Żabiński, P. Hydrogen evolution reaction (HER) activity of conical Co–Fe alloy structures and their application as a sensitive and rapid sensor for H2O2 detection. Arch. Civ. Mech. Eng. 2022, 22, 76. [Google Scholar] [CrossRef]
- Zou, R.; Zhou, Y.; Wang, J.; Li, Y.; Gu, L.; Wang, Y. Electrochemical approach towards the controllable synthesis of nickel nanocones based on the screw dislocation. Appl. Nanosci. 2020, 10, 1625–1638. [Google Scholar] [CrossRef]
- Meng, F.; Morin, S.A.; Forticaux, A.; Jin, S. Screw Dislocation Driven Growth of Nanomaterials. Acc. Chem. Res. 2013, 46, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Jung, K.K.; Ko, J.S. Effect of NaCl in a nickel electrodeposition on the formation of nickel nanostructure. J. Mater. Sci. 2016, 51, 3036–3044. [Google Scholar] [CrossRef]
- Hang, T.; Ling, H.; Hu, A.; Li, M. Growth Mechanism and Field Emission Properties of Nickel Nanocones Array Fabricated by One-Step Electrodeposition. J. Electrochem. Soc. 2010, 157, D624. [Google Scholar] [CrossRef]
- Hashemzadeh, M.; Raeissi, K.; Ashrafizadeh, F.; Khorsand, S. Effect of ammonium chloride on microstructure, super-hydrophobicity and corrosion resistance of nickel coatings. Surf. Coat. Technol. 2015, 283, 318–328. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, K.K.; Lee, S.H.; Ko, J.S. One-step fabrication of nickel nanocones by electrodeposition using CaCl2·2H2O as capping reagent. Appl. Surf. Sci. 2016, 369, 163–169. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, K.K.; Lee, S.H.; Ko, J.S. Formation of nickel microcones by using an electrodeposition solution containing H3BO3. Curr. Appl. Phys. 2016, 16, 261–266. [Google Scholar] [CrossRef]
- Rahimi, E.; Davoodi, A.; Kiani Rashid, A.R. Characterization of screw dislocation-driven growth in nickel micro-nanostructure electrodeposition process by AFM. Mater. Lett. 2018, 210, 341–344. [Google Scholar] [CrossRef]
- Šupicová, M.; Rozik, R.; Trnková, L.; Oriňáková, R.; Gálová, M. Influence of boric acid on the electrochemical deposition of Ni. J. Solid State Electrochem. 2006, 10, 61–68. [Google Scholar] [CrossRef]
- Skibińska, K.; Huang, M.; Mutschke, G.; Eckert, K.; Włoch, G.; Wojnicki, M.; Żabiński, P. On the electrodeposition of conically nano-structured nickel layers assisted by a capping agent. J. Electroanal. Chem. 2022, 904, 115935. [Google Scholar] [CrossRef]
- Deng, Y.; Ling, H.; Feng, X.; Hang, T.; Li, M. Electrodeposition and characterization of copper nanocone structures. CrystEngComm 2015, 17, 868–876. [Google Scholar] [CrossRef]
- Tang, L.; Peng, Y.; Han, S.; Hang, T.; Ling, H.; Li, M.; Wu, Y. Fabrication of Pyramid-Like Structured Cu Coatings by Pulse-Reverse Current Electrodeposition. J. Electrochem. Soc. 2022, 169, 092513. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C.; Cai, M.; Yi, X.; Li, J. Growth and morphology tuning of ordered nickel nanocones routed by one-step pulse electrodeposition. Appl. Surf. Sci. 2020, 508, 145291. [Google Scholar] [CrossRef]
- Geng, W.; Hu, A.; Li, M. Super-hydrophilicity to super-hydrophobicity transition of a surface with Ni micro-nano cones array. Appl. Surf. Sci. 2012, 263, 821–824. [Google Scholar] [CrossRef]
- Ren, Q.; Feng, L.; Ye, C.; Xue, X.; Lin, D.; Eisenberg, S.; Kou, T.; Duoss, E.B.; Zhu, C.; Li, Y. Nanocone-Modified Surface Facilitates Gas Bubble Detachment for High-Rate Alkaline Water Splitting. Adv. Energy Mater. 2023, 13, 2302073. [Google Scholar] [CrossRef]
- Skibińska, K.; Semeniuk, S.; Kutyła, D.; Jędraczka, A.; Żabiński, P. Study on Synthesis and Modification of Conical Ni Structures by One-Step Method. Arch. Metall. Mater. 2021, 66, 861–869. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; He, Z.; Chen, J.; Gao, W.; Cao, P. Superhydrophobic Ni nanocone surface prepared by electrodeposition and its overall performance. Surf. Coat. Technol. 2023, 464, 129548. [Google Scholar] [CrossRef]
- Hang, T.; Li, M.; Fei, Q.; Mao, D. Characterization of nickel nanocones routed by electrodeposition without any template. Nanotechnology 2008, 19, 035201. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Li, Q.; Wang, Z.; Li, L.; Chen, F.; Zhou, J. Researching the fabrication of anticorrosion superhydrophobic surface on magnesium alloy and its mechanical stability and durability. Chem. Eng. J. 2013, 228, 415–424. [Google Scholar] [CrossRef]
- Dong, M.; Chen, P.; Hang, T.; Li, M. Nanotwinned copper micro-cone array fabricated by pulse electrodeposition for low-temperature bonding. Mater. Lett. 2021, 290, 129470. [Google Scholar] [CrossRef]
- Lei, H.; Hu, H.; Xie, J.; Hu, A.; Li, M. Co micro-cone arrays with stable and uniform morphology for low-temperature solid-state bonding. Mater. Today Commun. 2023, 37, 107365. [Google Scholar] [CrossRef]
- Hang, T.; Hu, A.; Li, M.; Mao, D. Structural control of a cobalt nanocone array grown by directional electrodeposition. CrystEngComm 2010, 12, 2799–2802. [Google Scholar] [CrossRef]
- Skibińska, K.; Wojtaszek, K.; Krause, L.; Kula, A.; Yang, X.; Marzec, M.M.; Wojnicki, M.; Żabiński, P. Tuning up catalytical properties of electrochemically prepared nanoconical Co-Ni deposit for HER and OER. Appl. Surf. Sci. 2023, 607, 155004. [Google Scholar] [CrossRef]
- Skibińska, K.; Kutyła, D.; Kołczyk-Siedlecka, K.; Marzec, M.M.; Żabiński, P.; Kowalik, R. Synthesis of conical Co-Fe alloys structures obtained with crystal modifier in superimposed magnetic. Arch. Civ. Mech. Eng. 2021, 21, 165. [Google Scholar] [CrossRef]
- Skibińska, K.; Elsharkawy, S.; Kutyła, D.; Boryczko, B.; Marzec, M.M.; Żabiński, P. Electrocatalytic properties of Ni–Cu structures fabricated by electrodeposition of Cu on Ni cones. Arch. Civ. Mech. Eng. 2024, 24, 138. [Google Scholar] [CrossRef]
- Wu, L.K.; Zhu, Y.X.; Liu, M.; Hou, G.Y.; Tang, Y.P.; Cao, H.Z.; Zhang, H.B.; Zheng, G.Q. Ultrafast fabrication of amorphous bimetallic hydroxide layer on nickel nanocones array for oxygen evolution electrocatalyst. Int. J. Hydrogen Energy 2019, 44, 5899–5911. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion resistance and long-term durability of super-hydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014, 305, 498–505. [Google Scholar] [CrossRef]
- Rasaili, P.; Sharma, N.K.; Bhattarai, A. Comparison of Ferromagnetic Materials: Past Work, Recent Trends, and Applications. Condens. Matter 2022, 7, 12. [Google Scholar] [CrossRef]
- Huang, M.; Eckert, K.; Mutschke, G. Magnetic-field-assisted electrodeposition of metal to obtain conically structured ferromagnetic layers. Electrochim. Acta 2021, 365, 137374. [Google Scholar] [CrossRef]
- Huang, M.; Skibinska, K.; Zabinski, P.; Mutschke, G.; Eckert, K. Enhancing magnetic field support for the electrodeposition of nano-structured metal layers by reducing global cell flow. Magnetohydrodynamics 2022, 58, 465–474. [Google Scholar] [CrossRef]
- Pérez-Page, M.; Yu, E.; Li, J.; Rahman, M.; Dryden, D.M.; Vidu, R.; Stroeve, P. Template-based syntheses for shape controlled nanostructures. Adv. Colloid Interface Sci. 2016, 234, 51–79. [Google Scholar] [CrossRef]
- Lee, W. The anodization of aluminum for nanotechnology applications. JOM 2010, 62, 57–63. [Google Scholar] [CrossRef]
- Syamdri, F.D.; Wibowo, R. The effect of sulphuric acid and oxalic acid on the formation of anodic aluminium oxide templates using two-step anodization method. J. Phys. Conf. Ser. 2020, 1442, 012063. [Google Scholar] [CrossRef]
- Asoh, H.; Sano, T. Forming Hard Anodic Films on Aluminum by Anodization in Oxalic Acid and Alcohol. J. Electrochem. Soc. 2021, 168, 103506. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Low-Cost Nanostructured Coating of Anodic Aluminium Oxide Synthesized in Sulphuric Acid as Electrolyte. Coatings 2021, 11, 309. [Google Scholar] [CrossRef]
- Sriram Sudhan, A.L.; Brusly Solomon, A. Effect of Temperature on the Surface Characteristics of Anodized Aluminium Tubes. In Trends in Manufacturing and Engineering Management; Springer: Singapore, 2021; pp. 591–600. [Google Scholar]
- Białek, E.; Włodarski, M.; Norek, M. Influence of Anodization Temperature on Geometrical and Optical Properties of Porous Anodic Alumina(PAA)-Based Photonic Structures. Materials 2020, 13, 3185. [Google Scholar] [CrossRef] [PubMed]
- Chernyakova, K.; Tzaneva, B.; Vrublevsky, I.; Videkov, V. Effect of Aluminum Anode Temperature on Growth Rate and Structure of Nanoporous Anodic Alumina. J. Electrochem. Soc. 2020, 167, 103506. [Google Scholar] [CrossRef]
- Dobosz, I. Influence of the anodization conditions and chemical treatment on the formation of alumina membranes with defined pore diameters. J. Porous Mater. 2021, 28, 1011–1022. [Google Scholar] [CrossRef]
- Yang, J.-J.; Yin, S.-Y.; Fan, C.-H.; Ou, L.; Wang, J.-H.; Peng, H.; Wang, B.-W.; Luo, D.; Dong, S.-Y.; Zhang, Z.-Y. Effect of voltage on structure and properties of 2024 aluminum alloy surface anodized aluminum oxide films. Surf. Coat. Technol. 2024, 479, 130508. [Google Scholar] [CrossRef]
- Ilango, M.S.; Mutalikdesai, A.; Ramasesha, S.K. Anodization of Aluminium using a fast two-step process. J. Chem. Sci. 2016, 128, 153–158. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, J.; Yao, T.; Li, L.; Zhao, J.; Guo, Y. Influence of anodization parameters on film thickness and volume expansion of thick- and large-sized anodic aluminum oxide film. J. Mater. Sci. Mater. Electron. 2021, 32, 13708–13718. [Google Scholar] [CrossRef]
- Raffin, F.; Echouard, J.; Volovitch, P. Influence of the Anodizing Time on the Microstructure and Immersion Stability of Tartaric-Sulfuric Acid Anodized Aluminum Alloys. Metals 2023, 13, 993. [Google Scholar] [CrossRef]
- Brzózka, A.; Szeliga, D.; Kurowska-Tabor, E.; Sulka, G.D. Synthesis of copper nanocone array electrodes and its electrocatalytic properties toward hydrogen peroxide reduction. Mater. Lett. 2016, 174, 66–70. [Google Scholar] [CrossRef]
- Laocharoensuk, R.; Sattayasamitsathit, S.; Burdick, J.; Kanatharana, P.; Thavarungkul, P.; Wang, J. Shape-tailored porous gold nanowires: From nano barbells to nano step- Cones. ACS Nano 2007, 1, 403–408. [Google Scholar] [CrossRef]
- Duan, J.L.; Lei, D.Y.; Chen, F.; Lau, S.P.; Milne, W.I.; Toimil-Molares, M.E.; Trautmann, C.; Liu, J. Vertically-Aligned Single-Crystal Nanocone Arrays: Controlled Fabrication and Enhanced Field Emission. ACS Appl. Mater. Interfaces 2016, 8, 472–479. [Google Scholar] [CrossRef]
- Toimil Molares, M.E.; Brötz, J.; Buschmann, V.; Dobrev, D.; Neumann, R.; Scholz, R.; Schuchert, I.U.; Trautmann, C.; Vetter, J. Etched heavy ion tracks in polycarbonate as template for copper nanowires. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 185, 192–197. [Google Scholar] [CrossRef]
- Acharya, N.K. Microscopic Observation of Nuclear Track Pores in Polymeric Membranes. Engineering 2011, 3, 639–642. [Google Scholar] [CrossRef]
- Batista, E.; dos Santos, D.; Andrade, G.S.; Sant’Ana, A.; Brolo, A.; Temperini, M.A. Using Polycarbonate Membranes as Templates for the Preparation of Au Nanostructures for Surface-Enhanced Raman Scattering. J. Nanosci. Nanotechnol. 2009, 9, 3233–3238. [Google Scholar] [CrossRef]
- Naderi, N.; Hashim, M.R.; Rouhi, J. Synthesis and characterization of pt nanowires electrodeposited into the cylindrical pores of polycarbonate membranes. Int. J. Electrochem. Sci. 2012, 7, 8481–8486. [Google Scholar] [CrossRef]
- Schönenberger, C.; van der Zande, B.M.I.; Fokkink, L.G.J.; Henny, M.; Schmid, C.; Krüger, M.; Bachtold, A.; Huber, R.; Birk, H.; Staufer, U. Template Synthesis of Nanowires in Porous Polycarbonate Membranes: Electrochemistry and Morphology. J. Phys. Chem. B 1997, 101, 5497–5505. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Gu, Z. A comprehensive review of template-synthesized multi-component nanowires: From interfacial design to sensing and actuation applications. Sens. Actuators Rep. 2021, 3, 100029. [Google Scholar] [CrossRef]
- da Câmara Santa Clara Gomes, T.; Marchal, N.; Abreu Araujo, F.; Velázquez Galván, Y.; de la Torre Medina, J.; Piraux, L. Magneto-Transport in Flexible 3D Networks Made of Interconnected Magnetic Nanowires and Nanotubes. Nanomaterials 2021, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Nagaura, T.; Takeuchi, F.; Yamauchi, Y.; Wada, K.; Inoue, S. Fabrication of ordered Ni nanocones using a porous anodic alumina template. Electrochem. Commun. 2008, 10, 681–685. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Nagaura, T.; Takai, K.; Suzuki, N.; Sato, K.; Fukata, N.; Inoue, S.; Kishimoto, S. Generation of electron moiré fringes on designed nanoporous anodic alumina films and their replicated ni cone arrays: Exploration of domain sizes and nanopore arrangements. J. Phys. Chem. C 2009, 113, 9632–9637. [Google Scholar] [CrossRef]
- Nagaura, T.; Wada, K.; Inoue, S. Hexagonally Ordered Ni Nanocone Array; Controlling the Aspect Ratio. Mater. Trans. 2010, 51, 1237–1241. [Google Scholar] [CrossRef]
- Li, J.; Hu, L.; Li, C.; Gao, X. Tailoring hexagonally packed metal hollow-nanocones and taper-nanotubes by template-induced preferential electrodeposition. ACS Appl. Mater. Interfaces 2013, 5, 10376–10380. [Google Scholar] [CrossRef] [PubMed]
- Mukaibo, H.; Horne, L.P.; Park, D.; Martin, C.R. Controlling the Length of Conical Pores Etched in Ion-Tracked Poly(ethylene terephthalate) Membranes. Small 2009, 5, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Koshikawa, H.; Yamamoto, S.; Sugimoto, M.; Sawada, S.I.; Yamaki, T. Fabrication of size-and shape-controlled platinum cones by ion-track etching and electrodeposition techniques for electrocatalytic applications. Quantum Beam Sci. 2021, 5, 21. [Google Scholar] [CrossRef]
- Roustaie, F.; Bieker, J.; Cicek, R.; Schlaak, H.F. Novel fabrication method for integration of template grown metallic nanocones with controllable tip diameter and apex angle. Microelectron. Eng. 2017, 180, 81–85. [Google Scholar] [CrossRef]
- Zang, J.; Bao, S.J.; Li, C.M.; Bian, H.; Cui, X.; Bao, Q.; Sun, C.Q.; Guo, J.; Lian, K. Well-aligned cone-shaped nanostructure of polypyrrole/RuO2 and its electrochemical supercapacitor. J. Phys. Chem. C 2008, 112, 14843–14847. [Google Scholar] [CrossRef]
- Maharana, H.S.; Basu, A.; Mondal, K. Effect of CTAB on the architecture and hydrophobicity of electrodeposited Cu–ZrO2 nano-cone arrays. Surf. Coat. Technol. 2019, 375, 323–333. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, R.; Chen, Z.; Yan, H.; Cui, J.; Liu, W.; Pan, J.H.; Hong, M. Tunable hierarchical nanostructures onmicro-conical arrays of laser textured TC4 substrate by hydrothermal treatment for enhanced anti-icing property. Coatings 2020, 10, 450. [Google Scholar] [CrossRef]
- Hussain, S.; Liu, T.; Kashif, M.; Cao, S.; Zeng, W.; Xu, S.; Naseer, K.; Hashim, U. A simple preparation of ZnO nanocones and exposure to formaldehyde. Mater. Lett. 2014, 128, 35–38. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Cole, M.T.; Wang, A.; Guo, X.; Liu, X.; Lyu, W.; Teng, H.; Qv, Y.; Liu, G.; et al. Nanocone-shaped carbon nanotubes field-emitter array fabricated by laser ablation. Nanomaterials 2021, 11, 3244. [Google Scholar] [CrossRef] [PubMed]
- Murthy, N.S.; Prabhu, R.D.; Martin, J.J.; Zhou, L.; Headrick, R.L. Self-assembled and etched cones on laser ablated polymer surfaces. J. Appl. Phys. 2006, 100, 023538. [Google Scholar] [CrossRef]
- Ashfold, M.N.R.; Claeyssens, F.; Fuge, G.M.; Henley, S.J. Pulsed laser ablation and deposition of thin films. Chem. Soc. Rev. 2004, 33, 23. [Google Scholar] [CrossRef] [PubMed]
- Tanvir Ahmmed, K.M.; Grambow, C.; Kietzig, A.M. Fabrication of micro/nano structures on metals by femtosecond laser micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Pérez-Díaz, O.; Quiroga-González, E. Silicon Conical Structures by Metal Assisted Chemical Etching. Micromachines 2020, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Zuhlke, C.A.; Anderson, T.P.; Alexander, D.R. Fundamentals of layered nanoparticle covered pyramidal structures formed on nickel during femtosecond laser surface interactions. Appl. Surf. Sci. 2013, 283, 648–653. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Sabour Rouhaghdam, A. Nickel nanocones as efficient and stable catalyst for electrochemical hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 14560–14565. [Google Scholar] [CrossRef]
- Krause, L.; Skibińska, K.; Rox, H.; Baumann, R.; Marzec, M.M.; Yang, X.; Mutschke, G.; Żabiński, P.; Lasagni, A.F.; Eckert, K. Hydrogen Bubble Size Distribution on Nanostructured Ni Surfaces: Electrochemically Active Surface Area Versus Wettability. ACS Appl. Mater. Interfaces 2023, 15, 18290–18299. [Google Scholar] [CrossRef]
- Liang, Y.; Han, Y.; Li, J.S.; Wang, J.; Liu, D.; Fan, Q. Wettability control in electrocatalyst: A mini review. J. Energy Chem. 2022, 70, 643–655. [Google Scholar] [CrossRef]
- Liu, J.; Fang, X.; Zhu, C.; Xing, X.; Cui, G.; Li, Z. Fabrication of superhydrophobic coatings for corrosion protection by electrodeposition: A comprehensive review. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 607, 125498. [Google Scholar] [CrossRef]
- Skibińska, K.; Kutyła, D.; Yang, X.; Krause, L.; Marzec, M.M.; Żabiński, P. Rhodium-decorated nanoconical nickel electrode synthesis and characterization as an electrochemical active cathodic material for hydrogen production. Appl. Surf. Sci. 2022, 592, 153326. [Google Scholar] [CrossRef]
- Skibińska, K.; Kornaus, K.; Yang, X.; Kutyła, D.; Wojnicki, M.; Żabiński, P. One-step synthesis of the hydrophobic conical Co-Fe structures—The comparison of their active areas and electrocatalytic properties. Electrochim. Acta 2022, 415, 140127. [Google Scholar] [CrossRef]
- Jaroniec, M.; Kruk, M.; Sayari, A. Adsorption methods for characterization of surface and structural properties of mesoporous molecular sieves. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1998; pp. 325–332. [Google Scholar]
- Lončar, A.; Jovanovič, P.; Hodnik, N.; Gaberšček, M. Determination of the Electroactive Surface Area of Supported Ir-Based Oxygen Evolution Catalysts by Impedance Spectroscopy: Observed Anomalies with Respect to Catalyst Loading. J. Electrochem. Soc. 2023, 170, 044504. [Google Scholar] [CrossRef]
- Martínez-Hincapié, R.; Wegner, J.; Anwar, M.U.; Raza-Khan, A.; Franzka, S.; Kleszczynski, S.; Čolić, V. The determination of the electrochemically active surface area and its effects on the electrocatalytic properties of structured nickel electrodes produced by additive manufacturing. Electrochim. Acta 2024, 476, 143663. [Google Scholar] [CrossRef]
- Ksibi, M. Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Eng. J. 2006, 119, 161–165. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, X.; Feng, Z.; Lu, Z.; Zhang, Z.; Huang, W.; Li, Y.; Vuckovic, D.; Li, Y.; Dai, S.; et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 2020, 4, 233–241. [Google Scholar] [CrossRef]
- Zhu, R.; Zhou, Y.-B.; Zhou, H.-Q.; Liu, F.-F. The Application of Peroxide for Organic Synthesis in Continuous Flow Chemistry. Pharm. Front. 2023, 05, e243–e253. [Google Scholar] [CrossRef]
- Watt, B.E.; Proudfoot, A.T.; Vale, J.A. Hydrogen Peroxide Poisoning. Toxicol. Rev. 2004, 23, 51–57. [Google Scholar] [CrossRef]
- Su, S.-C.; Chou, S.-S.; Chang, P.-C.; Hwang, D.-F. Identification of hydrogen peroxide as a causative agent in noodles implicated in food poisoning. J. Food Drug Anal. 2020, 9, 1. [Google Scholar] [CrossRef]
- Lin, Q.; Leung, S.F.; Tsui, K.H.; Hua, B.; Fan, Z. Programmable nanoengineering templates for fabrication of three-dimensional nanophotonic structures. Nanoscale Res. Lett. 2013, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ai, Y.; Kang, L.; Liu, W.; Ma, Z.; Song, P.; Zhao, Y.; Yang, F.; Wang, X. A Novel Nanocone Cluster Microstructure with Anti-reflection and Superhydrophobic Properties for Photovoltaic Devices. Nanoscale Res. Lett. 2018, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Souri, M. Ni-modified boron nitride nanocones as nonlinear optical active drug carriers, a DFT study. Opt. Quantum Electron. 2024, 56, 1119. [Google Scholar] [CrossRef]
- Baei, M.T.; Peyghan, A.A.; Bagheri, Z. Carbon nanocone as an ammonia sensor: DFT studies. Struct. Chem. 2013, 24, 1099–1103. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, G.; Li, B. Carbon nanocone: A promising thermal rectifier. Appl. Phys. Lett. 2008, 93, 243111. [Google Scholar] [CrossRef]
- Lu, Y.; Zhan, C.; Yu, L.; Yu, Y.; Jia, H.; Chen, X.; Zhang, D.; Gao, R. Multifunctional nanocone array as solid immunoassay plate and SERS substrate for the early diagnosis of prostate cancer on microfluidic chip. Sens. Actuators B Chem. 2023, 376, 133046. [Google Scholar] [CrossRef]
- Sun, T.; Shi, H.; Cao, L.; Liu, Y.; Tu, J.; Lu, M.; Li, H.; Zhao, W.; Li, Q.; Fu, T.; et al. Double grating high efficiency nanostructured silicon-based ultra-thin solar cells. Results Phys. 2020, 19, 103442. [Google Scholar] [CrossRef]
- Yang, X.; Shui, F.; Yu, Y.; Yi, Z.; Li, H.; Xu, Z.; Zhang, F.; Xiong, J.; Liu, X.; Wangyang, P.; et al. All layers patterned conical nanostructured thin-film silicon solar cells for light-trapping efficiency improvement. Opt. Express 2023, 31, 42111. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, P.; Liu, X.; Yi, Z.; Liu, G.; Wang, Y.; Liu, M. Truncated titanium/semiconductor cones for wide-band solar absorbers. Nanotechnology 2019, 30, 305203. [Google Scholar] [CrossRef]
- Korany, F.M.H.; Hameed, M.F.O.; Hussein, M.; Mubarak, R.; Eladawy, M.I.; Obayya, S.S.A. Conical structures for highly efficient solar cell applications. J. Nanophotonics 2018, 12, 1. [Google Scholar] [CrossRef]
- Sobhani, F.; Heidarzadeh, H.; Bahador, H. Efficiency enhancement of an ultra-thin film silicon solar cell using conical-shaped nanoparticles: Similar to superposition (top, middle, and bottom). Opt. Quantum Electron. 2020, 52, 387. [Google Scholar] [CrossRef]
- Mishra, S.; Nath, M. Growth of vertically aligned CdTe nanorod arrays through patterned electrodeposition. Nano Energy 2013, 2, 1207–1213. [Google Scholar] [CrossRef]
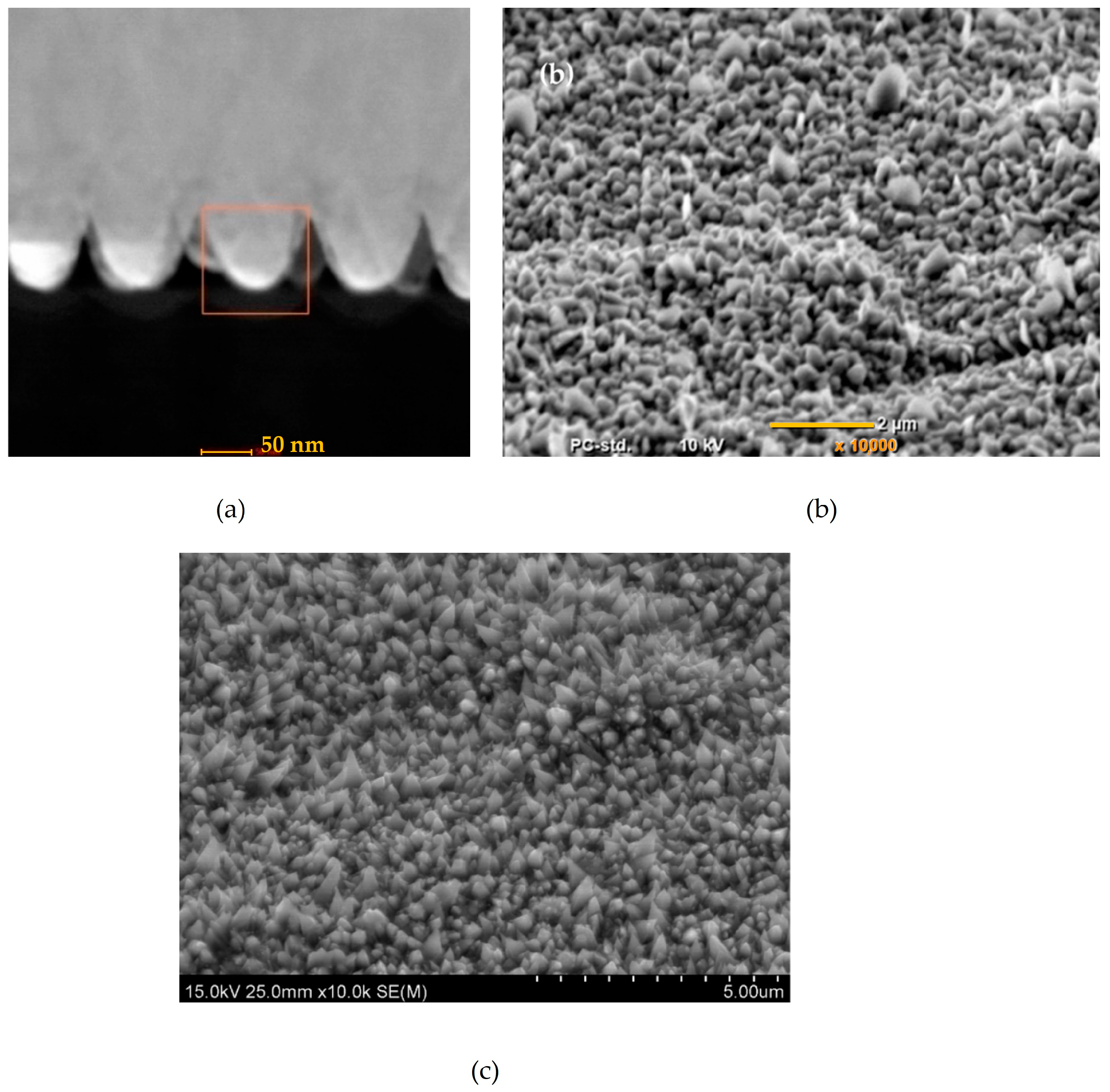
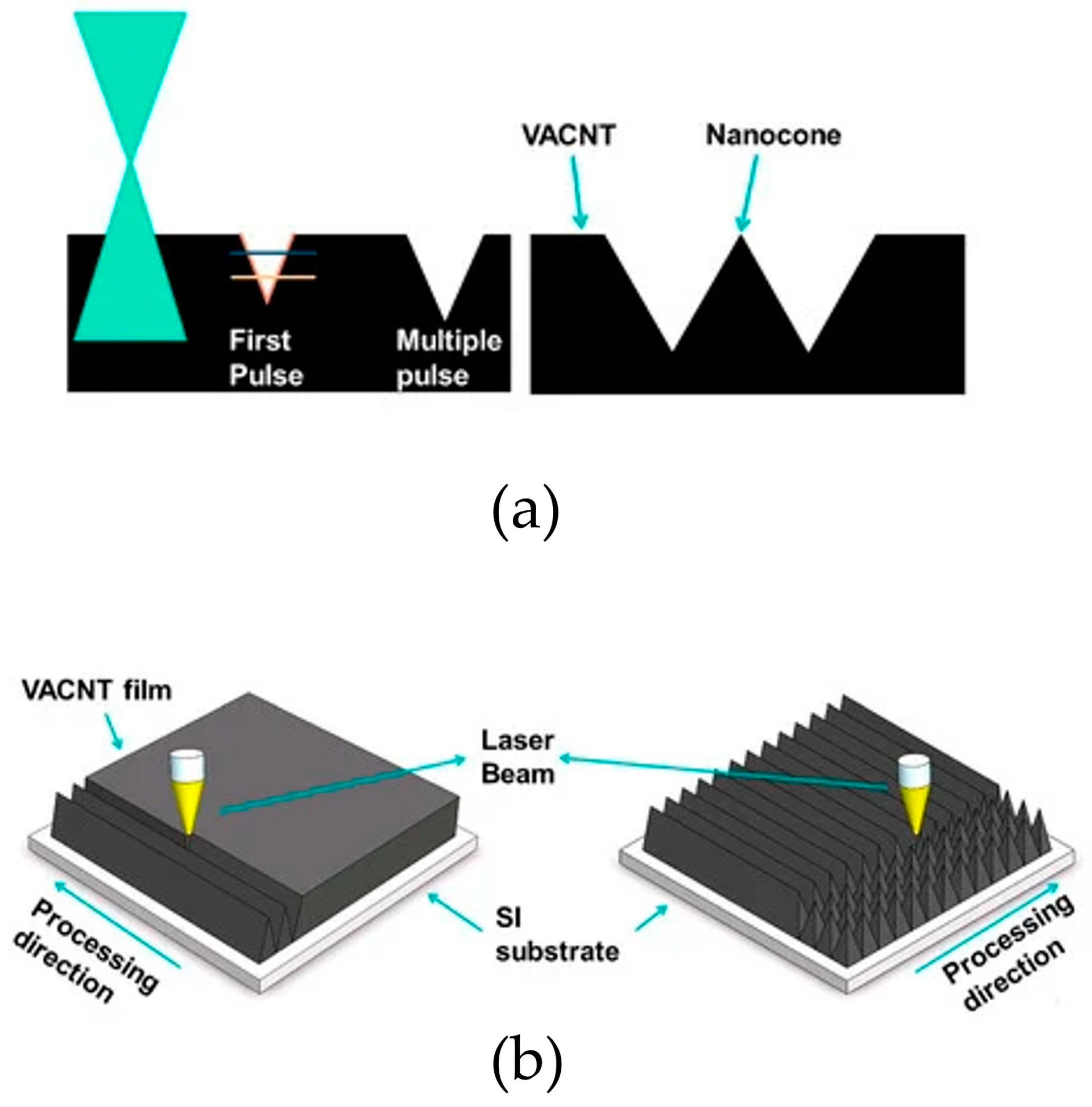

| Material | Crystal Modifier | Addition of Crystal Modifier | Duration [s] | Current Density [mA/cm2] | Reference | |
|---|---|---|---|---|---|---|
| [g/L] | [M] | |||||
| Ni | NH4Cl | 0–40 | - | 60–1500 | 10–40 | [42] |
| 40 | - | - | - | [46] | ||
| - | 1.4 | 480 | 20 | [47] | ||
| 10–40 | - | 600 | 10–40 | [48] | ||
| (NH₄)₂SO₄ | 35 | - | 300–2100 | 15 | [49] | |
| EDA·2HCl | 200 | - | 112–900 | 20 | [36] | |
| EDA·2HCl | - | 0–2.115 | 240 and 480 | 20 | [50] | |
| EDA·2HCl | - | 1.5 | 660 | 15 | [51] | |
| CaCl2·2H2O | - | 0–2.4 | 120–900 | 10–30 | [38] | |
| NaCl | - | 0–4 | 120–1200 | 10–40 | [35] | |
| H3BO3 | - | 0–2 | 1800–18,000 | 1–30 | [39] | |
| Cu | NaCl | 3·10−5 | - | 60–1800 | 100 * | [44] |
| NaCl | 0–9·10−5 | - | - | 4 ** | [52] | |
| Janus Green B (JGB) | 0–0.2 | - | 30–300 | 12 | [43] | |
| Co | NH4Cl | 100 | - | 10–1200 | 20–350 | [27] |
| NH4+ or -NH2 | - | - | 40–80 | 100 | [53] | |
| NH4+ | 0–200 | - | 40 | 100 | [54] | |
| Co-Ni | NH4Cl | 100 | - | 40 | 350 | [55] |
| Co-Fe | NH4Cl | 40 and 100 | - | 300–1200 | 20 | [56] |
| Material | Template | Diameter Base/Height [nm] | Reference |
|---|---|---|---|
| Cu | AAO | 62.3–104.9/133.5–151.8 | [76] |
| Ni | AAO | 100/100 | [86] |
| 100/100 | [87] | ||
| 100/100–500 | [88] | ||
| Ni, Cu, Fe | AAO | -/576 | [89] |
| Co-Fe | AAO | 110.4/73.5 | [26] |
| Cu | PC | 1470/28,000 | [78] |
| Au | PC | 1000–8000/1000–11,000 | [90] |
| Pt | PC | 70–1500/700–1100 | [91] |
| Ni | PC | 7000/- | [92] |
| First Step | Second Step | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Solution | Voltage [V] | Duration [min] | Temperature [°C] | Solution | Voltage [V] | Duration [s] | Temperature [°C] | |
| 0.3 M H2C2O4 | 45 | 60 | 9 | 0.3 M H2C2O4 | 45 | 25 * and 20 ** | 9 | [76] |
| 40 | 600 | 16 | 40 | [86] | ||||
| 0.3 M C6H8O7 | 40 | - | 16 | 0.3 M C6H8O7 | 40 | [87] | ||
| 0.3 M H2C2O4 | 45 | 60 | 2 | 0.3 M H2C2O4 | 45 | [26] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skibińska, K.; Żabiński, P. Nanocones: A Compressive Review of Their Electrochemical Synthesis and Applications. Materials 2024, 17, 3089. https://doi.org/10.3390/ma17133089
Skibińska K, Żabiński P. Nanocones: A Compressive Review of Their Electrochemical Synthesis and Applications. Materials. 2024; 17(13):3089. https://doi.org/10.3390/ma17133089
Chicago/Turabian StyleSkibińska, Katarzyna, and Piotr Żabiński. 2024. "Nanocones: A Compressive Review of Their Electrochemical Synthesis and Applications" Materials 17, no. 13: 3089. https://doi.org/10.3390/ma17133089







