Impact of Chemical Corrosion on Mechanical Properties of Boroaluminosilicate Pharmaceutical Glasses
Abstract
:1. Introduction
2. Materials and Method
3. Results and Discussion
3.1. Weight-Loss Ratio
3.2. Surface Morphology and Roughness
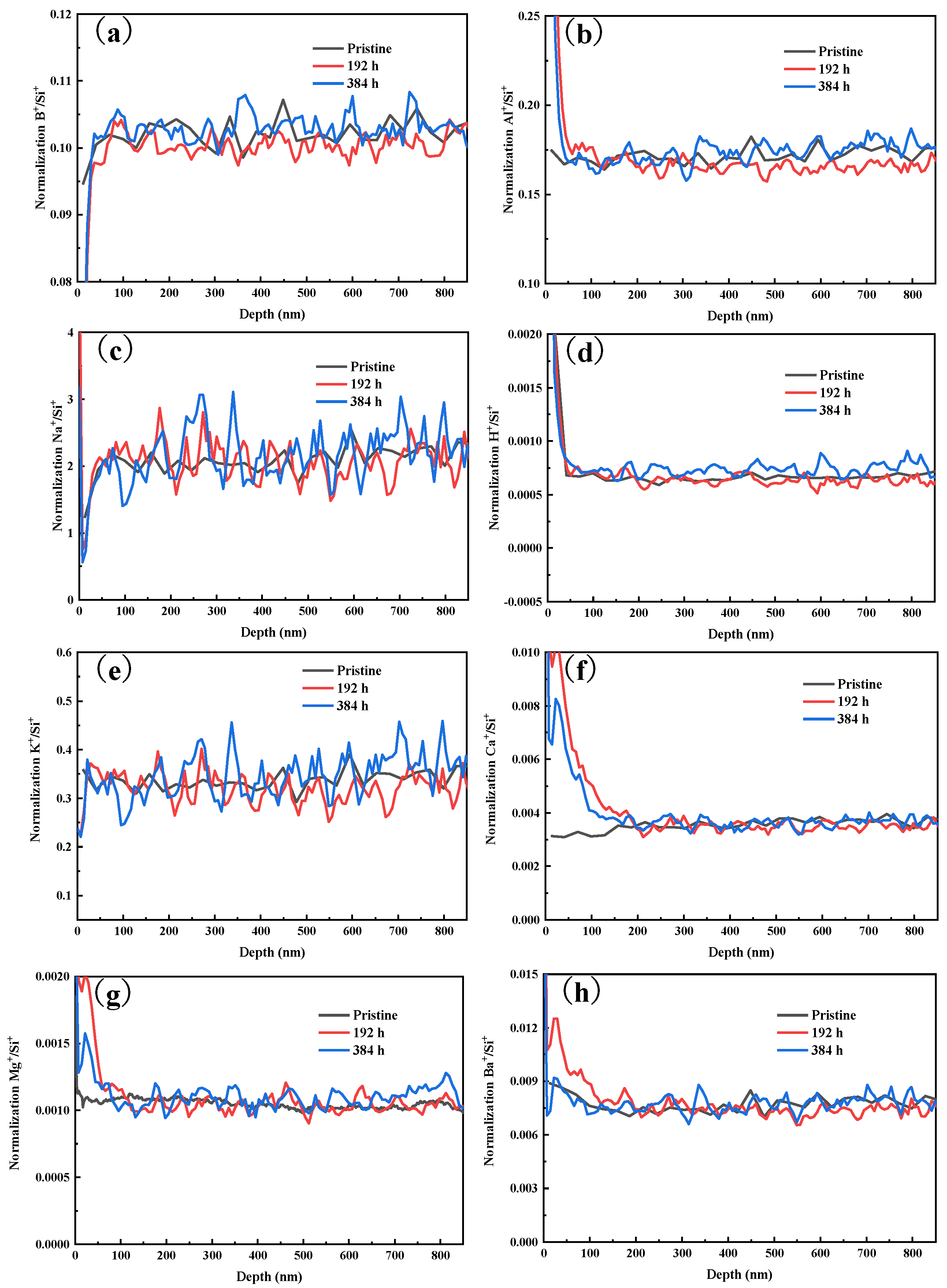
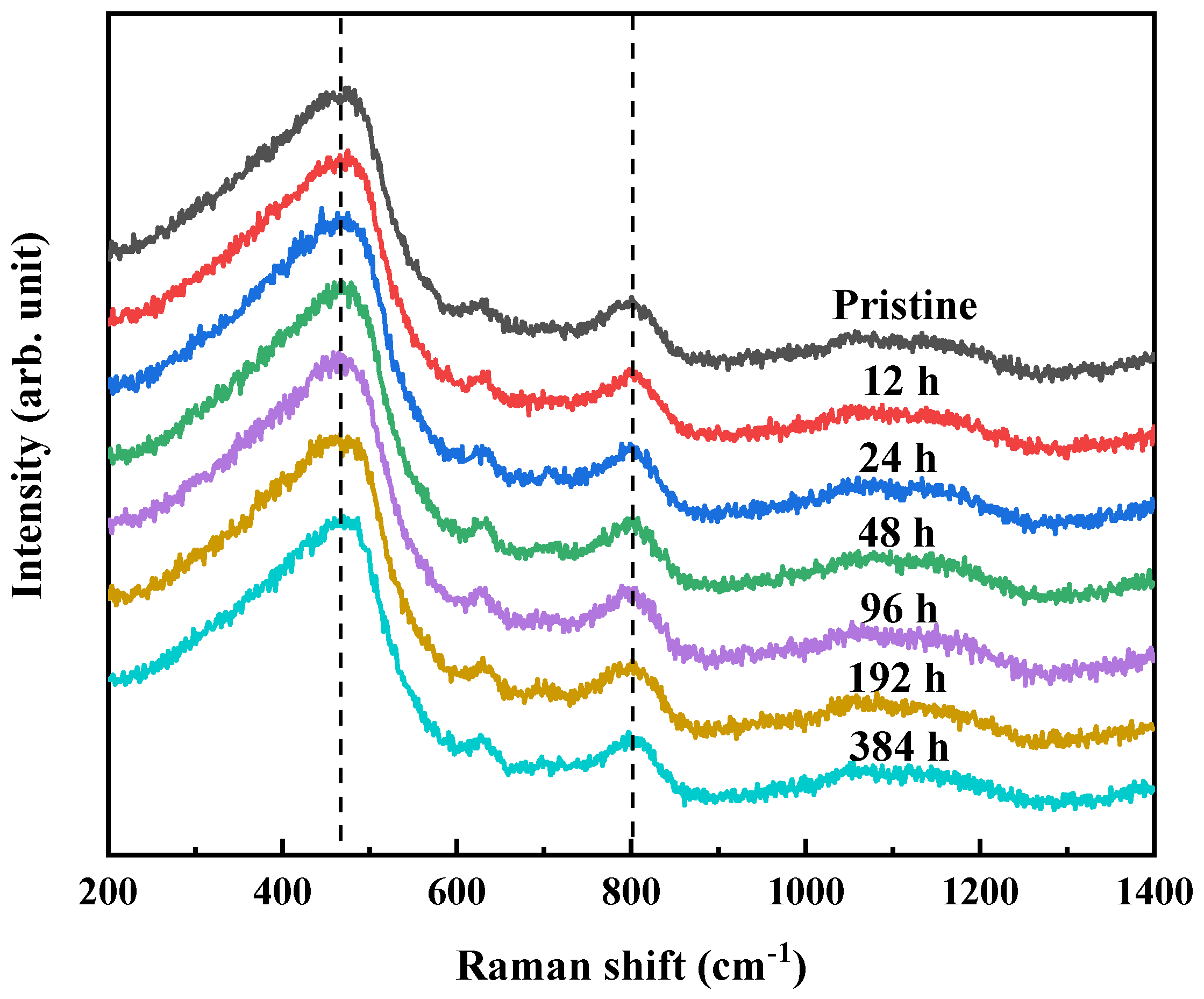
3.3. Chemical Structure Change in Glass Surface
3.4. Mechanical Properties of Glass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EscolàVergé, L.; BorràsBermejo, B.; LosArcos, I.; Esperalba, J.; Ferrer, C.; FernándezHidalgo, N. Nosocomial COVID-19. Prospective study in a referral hospital. Med. Clin. 2022, 159, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Solís, A.; Reding-Bernal, A.; Cantú-Torres, V.P. Clinical behaviour of SARS-CoV-2 infection (COVID-19) in patients with type-2 diabetes mellitus in a respiratory care unit. Med. Clin. 2023, 161, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Shivji, R.; Conocchia, R.; Korakianiti, E.; Jekerle, V. Considerations for the chemistry, manufacturing and Controls (CMC)—Quality package for COVID-19 vaccines- interim lessons learnt by the European medicines Agency (EMA). Vaccine 2022, 40, 5539–5541. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ashraf, M.; Srinivasan, C. Microscopic Evaluation of Pharmaceutical Glass Container-Formulation Interactions under Stressed Conditions. Int. J. Pharm. 2021, 596, 120248. [Google Scholar] [CrossRef] [PubMed]
- Oni, Y.; Franck, J.; Evans, C.; Paniagua, D.; Kulshrestha, A.; Mantri, R.V. Container Closure Integrity of Vial Primary Packaging Systems under Frozen Storage Conditions: A Case Study. PDA J. Pharm. Sci. Technol. 2023, 77, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, K.; Gin, S.; Naranayasamy, S.; Delaye, J.M. On the effect of Al on alumino-borosilicate glass chemical durability. Npj Mater. Degrad. 2023, 7, 46. [Google Scholar] [CrossRef]
- Sahu, P.; Ali, S.K.M.; Shenoy, K.T.; Arvind, A.; Banerjee, D.; Kumar, S.; Manohar, S.; Bhatt, K. Understanding the correlation of microscopic structure and macroscopic properties of multi-component glass through atomistic simulations. J. Chem. Sci. 2023, 135, 31. [Google Scholar] [CrossRef]
- Václavek, L.; Tomáštík, J.; Čtvrtlík, R. Effect of Machining on Mechanical Properties of Borosilicate Glasses. Powder Metall. Prog. 2023, 22, 62–74. [Google Scholar] [CrossRef]
- Demirel, B.; Erol, T.M. Antibacterial Borosilicate Glass and Glass Ceramic Materials Doped with ZnO for Usage in the Pharmaceutical Industry. ACS Omega 2023, 8, 18735–18742. [Google Scholar] [CrossRef]
- Guadagnino, E.; Guglielmi, M.; Nicoletti, F. Glass: The best material for pharmaceutical packaging International. J. Appl. Glass Sci. 2022, 13, 281–291. [Google Scholar] [CrossRef]
- Watkins, M.A.; Iacocca, R.G.; Shelbourn, T.L.; Dong, X.; Stobba-Wiley, C. Impact of Glass Corrosion on Drug Substance Stability. J. Pharm. Sci. 2014, 103, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Bødker, M.S.; Sørensen, S.S.; Mauro, J.C.; Smedskjaer, M.M. Predicting Composition-Structure Relations in Alkali Borosilicate Glasses Using Statistical Mechanics. Front. Mater. 2019, 6, 175. [Google Scholar] [CrossRef]
- Kerisit, S.; Du, J. Monte Carlo simulation of borosilicate glass dissolution using molecular dynamics-generated glass structures. J. Non-Cryst. Solids 2019, 522, 119601. [Google Scholar] [CrossRef]
- He, H.; Li, B.; Yu, J.; Ma, X.; Ma, Y.; Yue, Y.; Zheng, Q. Mixed alkaline earth effect on nanomechanical properties of glass. J. Non-Cryst. Solids 2024, 626, 122808. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhang, S.; Shi, Z.L.; Wang, F.L.; Wei, C.; Ma, M.Z.; Liu, R.P. Corrosion behavior and mechanical properties of Vit1 metallic glasses prepared at different cooling rates. J. Alloys Compd. 2023, 934, 167848. [Google Scholar] [CrossRef]
- Zhen-Wu, B.Y.; Prentice, D.P.; Simonetti, D.; Ryan, J.V.; Sant, G.; Bauchy, M. Predicting zeolites’ stability during the corrosion of nuclear waste immobilization glasses: Comparison with glass corrosion experiments. J. Nucl. Mater. 2021, 547, 152813. [Google Scholar] [CrossRef]
- Perea, D.E.; Schreiber, D.K.; Ryan, J.V.; Wirth, M.G.; Deng, L.; Lu, X.; Du, J.; Vienna, J.D. Tomographic mapping of the nanoscale water-filled pore structure in corroded borosilicate glass. Npj Mater. Degrad. 2020, 4, 2952–2962. [Google Scholar] [CrossRef]
- Li, A.; Luo, X.; Jia, Q.; Jiang, Q.; Liu, X.; Yang, Y.; Zhang, L.; Zeng, H. Anti-acid corrosion mechanism of yttrium oxide doped barium borosilicate glass. Ceram. Int. 2023, 49, 27030–27039. [Google Scholar] [CrossRef]
- Yang, R.; Liu, H.; Ding, Z.; Zheng, J.; Mauro, J.C.; Kim, S.H.; Zheng, Q. Chemical durability of borosilicate pharmaceutical glasses: Mixed alkaline earth effect with varying [MgO]/[CaO] ratio. J. Am. Ceram. Soc. 2021, 104, 3973–3981. [Google Scholar] [CrossRef]
- Stone-Weiss, N.; Youngman, R.E.; Thorpe, R.; Smith, N.J.; Pierce, E.M.; Goel, A. An insight into the corrosion of alkali aluminoborosilicate glasses in acidic environments. Phys. Chem. Chem. Phys. 2020, 22, 1881–1896. [Google Scholar] [CrossRef]
- Strachan, D.; Neeway, J.J.; Pederson, L.; Schreiber, D.K.; Mitroshkov, A.; Zhu, Z.; Ryan, J.V. On the dissolution of a borosilicate glass with the use of isotopic tracing—Insights into the mechanism for the long-term dissolution rate. Geochim. Cosmochim. Acta 2022, 318, 213–229. [Google Scholar] [CrossRef]
- Kadikova, I.F.; Morozova, E.A.; Yuryeva, T.V.; Grigorieva, I.A.; Yuryev, V.A. Glass depolymerization in the process of long-term corrosion: A study of deteriorating semiopaque turquoise glass beads using micro-FTIR spectroscopy. Mater. Res. Express 2020, 7, 025203. [Google Scholar] [CrossRef]
- Dabbas, A.A.; Kopecskó, K. Investigation of silicic acid saturation and gel formation during ISG leaching: 180 days duration reaction at 90 °C. J. Phys. Conf. Ser. 2022, 2315, 012007. [Google Scholar] [CrossRef]
- Schalm, O.; Anaf, W. Laminated altered layers in historical glass: Density variations of silica nanoparticle random packings as explanation for the observed lamellae. J. Non-Cryst. Solids 2016, 442, 1–16. [Google Scholar] [CrossRef]
- Ma, T.; Jivkov, A.P.; Li, W.; Liang, W.; Wang, Y.; Xu, H.; Han, X. A mechanistic model for long-term nuclear waste glass dissolution integrating chemical affinity and interfacial diffusion barrier. J. Nucl. Mater. 2017, 486, 70–85. [Google Scholar] [CrossRef]
- Hellmann, R.; Cotte, S.; Cadel, E.; Malladi, S.; Karlsson, L.S.; Lozano-Perez, S.; Cabié, M.; Seyeux, A. Nanometre-scale evidence for interfacial dissolution-reprecipitation control of silicate glass corrosion. Nat. Mater. 2015, 14, 307–311. [Google Scholar] [CrossRef]
- Elbatal, H.A.; Azooz, M.A.; Saad, E.A.; EzzELDin, F.M.; Amin, M.S. Corrosion Behavior Mechanism of Borosilicate Glasses Towards Different Leaching Solutions Evaluated by the Grain Method and FTIR Spectral Analysis before and after Gamma Irradiation. Silicon 2018, 10, 1139–1149. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, C.; Xu, W.; Zhu, Q.; Ge, C.; Hou, B. Evaluation of long-term corrosion durability and self-healing ability of scratched coating systems on carbon steel in a marine environment. Chin. J. Oceanol. Limnol. 2017, 35, 1094–1107. [Google Scholar] [CrossRef]
- Mikhchian, M.; Grosvenor, A.P. An investigation of the long-term aqueous corrosion behaviour of glass-zirconolite composite materials (Fe-Al-BG-CaZrTi2O7) as a potential nuclear wasteform. Corros. Sci. 2024, 228, 111831. [Google Scholar] [CrossRef]
- Wang, F.; Balasubramanya, N.; Qin, Q.; Youngman, R.E.; Mukherjee, P.; Stone-Weiss, N.; Goel, A. Multiscale Investigation of the Mechanisms Controlling the Corrosion of Borosilicate Glasses in Hyper-Alkaline Media. J. Phys. Chem. C 2020, 124, 27542–27557. [Google Scholar] [CrossRef]
- Maraghechi, H.; Rajabipour, F.; Pantano, C.G.; Burgos, W.D. Effect of calcium on dissolution and precipitation reactions of amorphous silica at high alkalinity. Cem. Concr. Res. 2016, 87, 1–13. [Google Scholar] [CrossRef]
- Jégou, C.; Gin, S.; Larché, F. Alteration kinetics of a simplified nuclear glass in an aqueous medium: Effects of solution chemistry and of protective gel properties on diminishing the alteration rate. J. Nucl. Mater. 2000, 280, 216–229. [Google Scholar] [CrossRef]
- Gin, S.; Jollivet, P.; Fournier, M.; Angeli, F.; Frugier, P.; Charpentier, T. Origin and consequences of silicate glass passivation by surface layers. Nat. Commun. 2015, 6, 6360. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, T.S.; Du, J. Surface reaction, water diffusion and mechanical properties of hydrated nanoporous calcium aluminosilicate gel structures. J. Non-Cryst. Solids 2023, 621, 122604. [Google Scholar] [CrossRef]
- Collin, M.; Fournier, M.; Charpentier, T.; Moskura, M.; Gin, S. Impact of alkali on the passivation of silicate glass. Npj Mater. Degrad. 2018, 2, 16. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Zhang, H.; Luo, L.; Ding, L.; Shi, W.; Jiang, W.; Wang, H. Recycling of arsenic residue and waste soda-lime silicate glass via vitrification. J. Non-Cryst. Solids 2023, 609, 122300. [Google Scholar] [CrossRef]
- Jolivet, V.; Jossé, L.; Rivoal, M.; Paris, M.; Morizet, Y.; Carole, L.; Suzuki-Muresan, T. Quantification of boron in aluminoborosilicate glasses using Raman and 11 B NMR. J. Non-Cryst. Solids 2019, 511, 50–61. [Google Scholar] [CrossRef]
- Neuville, D.R.; de Ligny, D.; Henderson, G.S. Advances in Raman Spectroscopy Applied to Earth and Material Sciences. Rev. Miner. Geochem. 2014, 78, 509–541. [Google Scholar] [CrossRef]
- He, H.; Chen, Z.; Lin, Y.; Hahn, S.H.; Yu, J.; van Duin, A.C.T.; Gokus, T.D.; Rotkin, S.V.; Kim, S.H. Subsurface structural change of silica upon nanoscale physical contact: Chemical plasticity beyond topographic elasticity. Acta Mater. 2021, 208, 116694. [Google Scholar] [CrossRef]
- Niu, Y.; Han, K.; Guin, J. Locally enhanced dissolution rate as a probe for nanocontact-induced densification in oxide glasses. Langmuir 2012, 28, 10733–10740. [Google Scholar] [CrossRef]
- Kasimuthumaniyan, S.; Sahoo, S.; Smedskjaer, M.M.; Krishnan, N.M.A.; Gosvami, N.N. Quantifying the densification and shear flow under indentation deformation in borosilicate glasses. Int. J. Appl. Glass Sci. 2022, 13, 526–538. [Google Scholar] [CrossRef]
- Ding, L.; Xu, Y.; Yang, R.; Yang, Y.; Lu, R.; Liu, H.; He, H.; Zheng, Q.; Mauro, J.C. Lateral-pushing induced surface lift-up during nanoindentation of silicate glass. Am. Ceram. Soc. 2021, 105, 2625–2633. [Google Scholar] [CrossRef]
- Sakata, Y.; Terasaki, N. Selective detection of microcracks under the surface of glass substrates by non-contact stress-induced light-scattering method with temperature variations. Jpn. J. Appl. Phys. 2022, 61, SE1007. [Google Scholar] [CrossRef]
- Khasanshin, R.H.; Novikov, L.S.; Primenko, D.A. Formation of Microcracks on the Surface of a Glass Irradiated with 30-Kev Protons. J. Surf. Investig.-X-ray Synchrotron Neutron Tech. 2020, 14, 906–912. [Google Scholar] [CrossRef]
- Huang, H.; Qian, Y.; Wang, C.; Yan, J. Laser induced micro-cracking of Zr-based metallic glass using 1011 W/m2 nano-pulses. Mater. Today Commun. 2020, 25, 101554. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, H.; Fu, G.; Chen, Z. Surface roughness and morphology evolution of optical glass with micro-cracks during chemical etching. Appl. Opt. 2017, 56, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Pisano, G.; Carfagni, G.R. A micromechanical derivation of the macroscopic strength statistics for pristine or corroded/abraded float glass. J. Exp. Biol. 2017, 37, 4197–4206. [Google Scholar] [CrossRef]
- Waetzig, K.; Rost, A.; Langklotz, U.; Matthey, B.; Schilm, J. An explanation of the microcrack formation in Li1.3 Al0.3 Ti1.7 (PO4)3 ceramics. J. Eur. Ceram. Soc. 2016, 36, 1995–2001. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, T.; Ding, L. Effect of Seawater Environment on the Structure and Performance of Basalt Continuous Fiber. Materials 2021, 14, 1862. [Google Scholar] [CrossRef]




| Composition | SiO2 | B2O3 | Al2O3 | Na2O + K2O | MgO + CaO + BaO + SrO |
|---|---|---|---|---|---|
| Content (wt%) | 75 | ≥8 | 2–7 | 4–8 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Liu, J.; Zhang, J.; Su, Y.; Yi, K.; Zhang, Y.; Ding, L.; Zheng, Q. Impact of Chemical Corrosion on Mechanical Properties of Boroaluminosilicate Pharmaceutical Glasses. Materials 2024, 17, 3120. https://doi.org/10.3390/ma17133120
Ma X, Liu J, Zhang J, Su Y, Yi K, Zhang Y, Ding L, Zheng Q. Impact of Chemical Corrosion on Mechanical Properties of Boroaluminosilicate Pharmaceutical Glasses. Materials. 2024; 17(13):3120. https://doi.org/10.3390/ma17133120
Chicago/Turabian StyleMa, Xinlin, Jin Liu, Jun Zhang, Yucai Su, Kangfeng Yi, Yanfei Zhang, Linfeng Ding, and Qiuju Zheng. 2024. "Impact of Chemical Corrosion on Mechanical Properties of Boroaluminosilicate Pharmaceutical Glasses" Materials 17, no. 13: 3120. https://doi.org/10.3390/ma17133120




