Preparation of Hydrophobic Modified Silica with Si69 and Its Reinforcing Mechanical Properties in Natural Rubber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
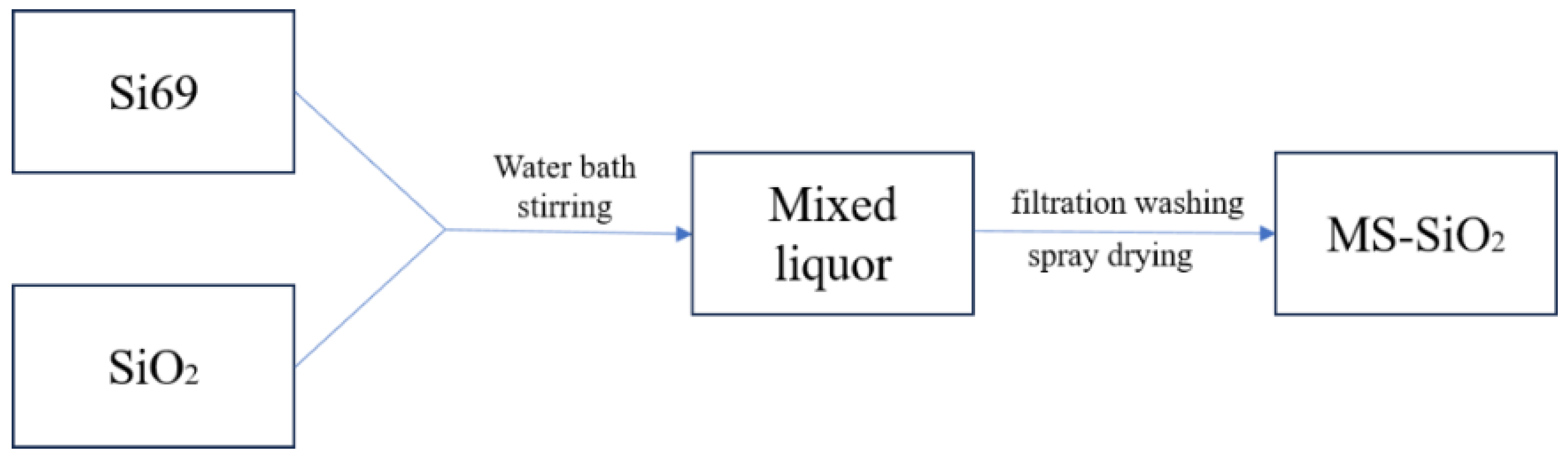
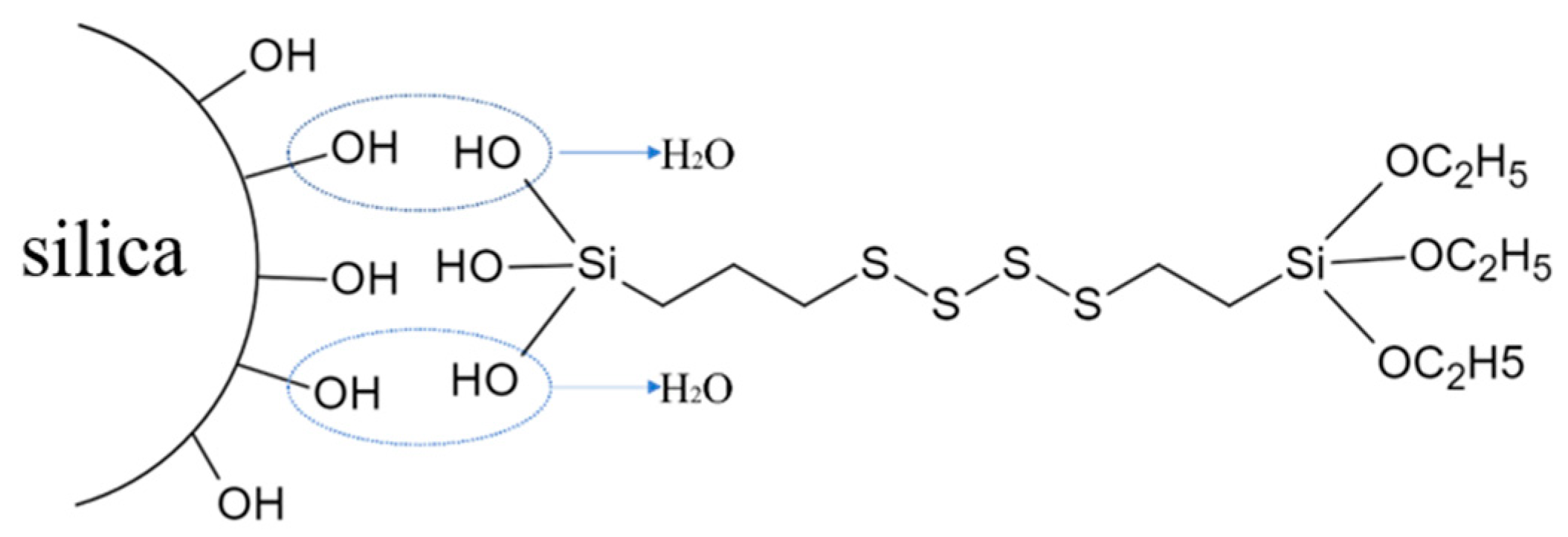
2.2. Preparation of Modified Silica (MS-SiO2)
2.3. Preparation of SiO2/NR and MS-SiO2/NR Composites
2.4. Characterization
3. Results and Discussion
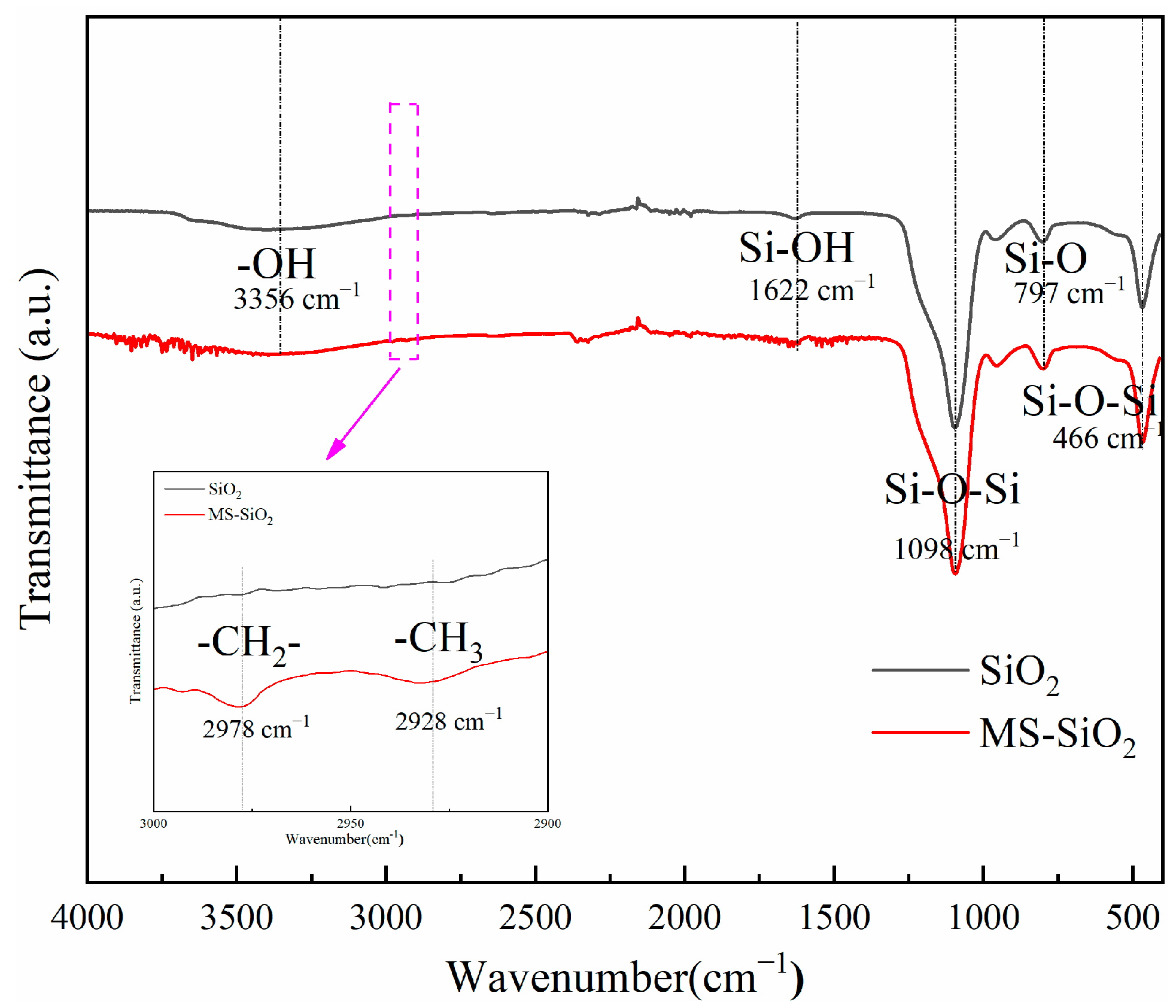
3.1. FTIR Analysis
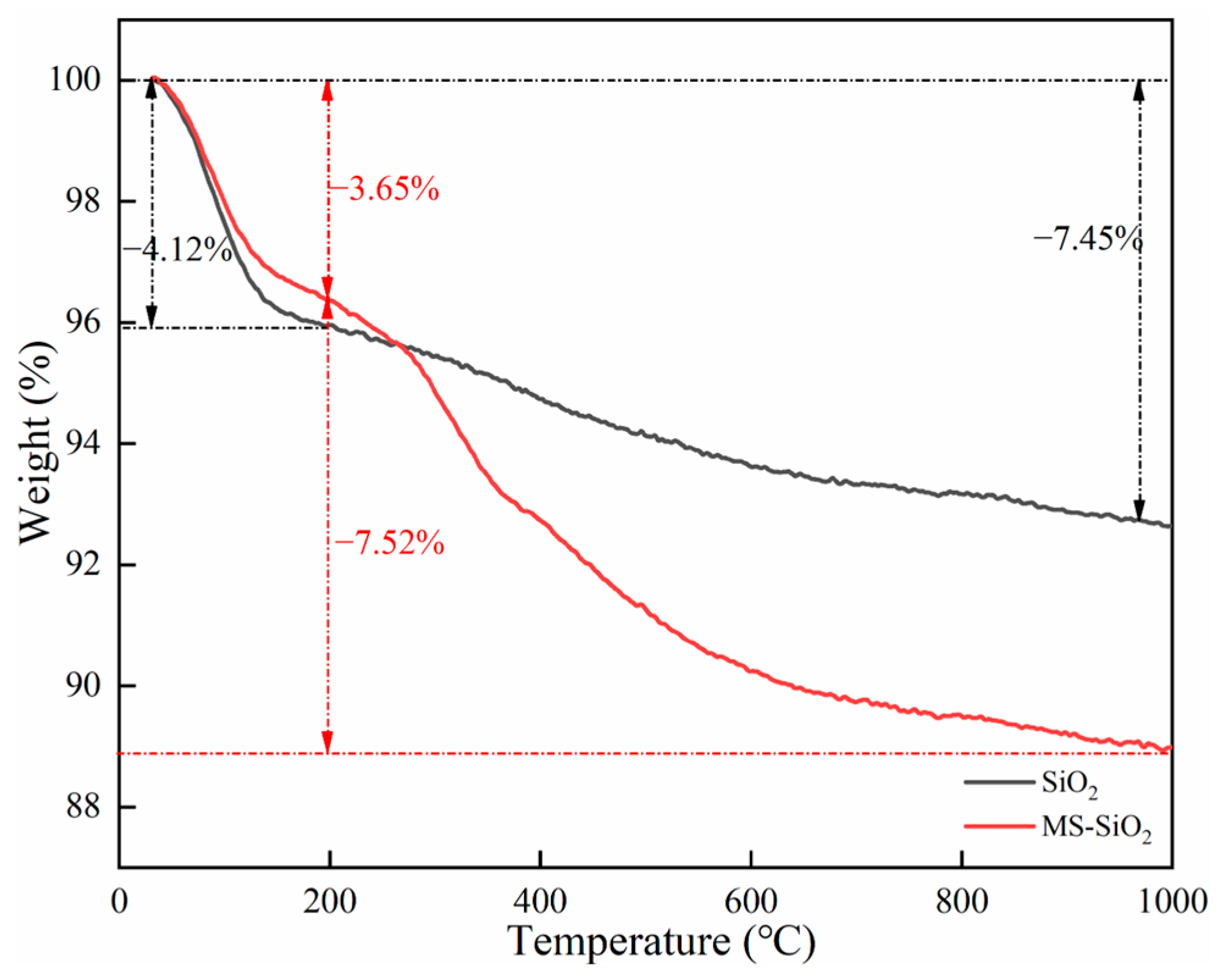
3.2. Thermal Decomposition Analysis
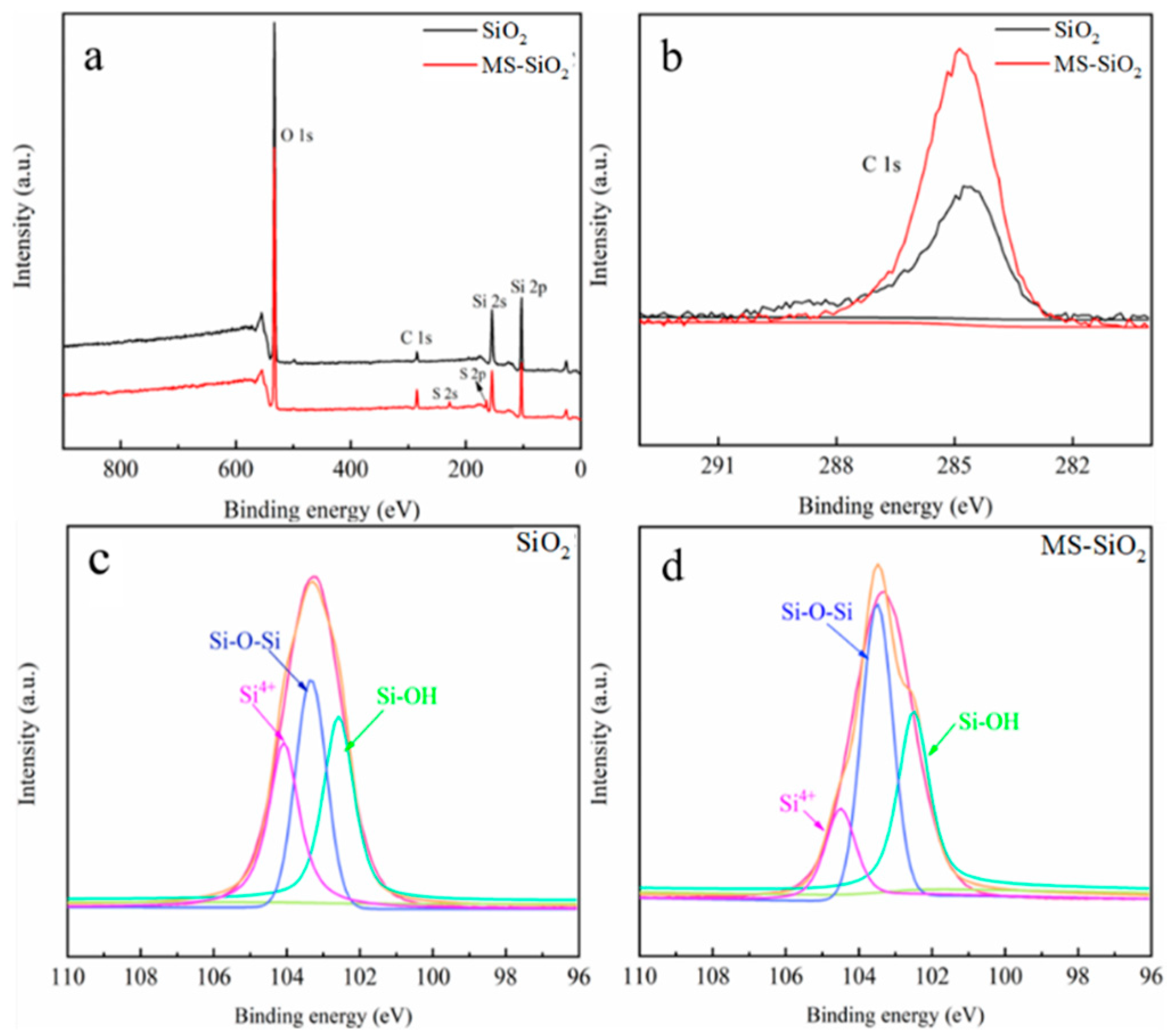
3.3. XPS Analysis
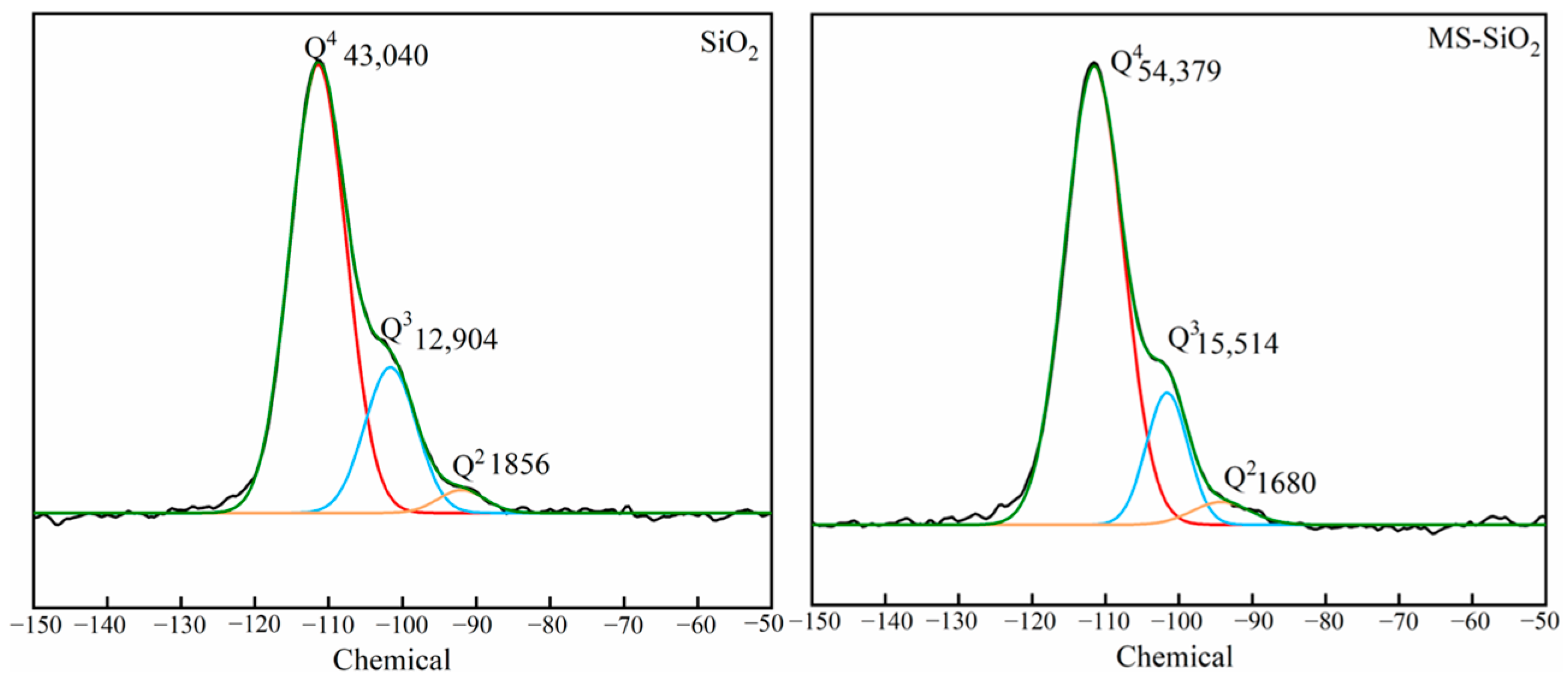
3.4. 29Si NMR Spectrum Analysis
3.5. Surface Wettability of SiO2 and MS-SiO2
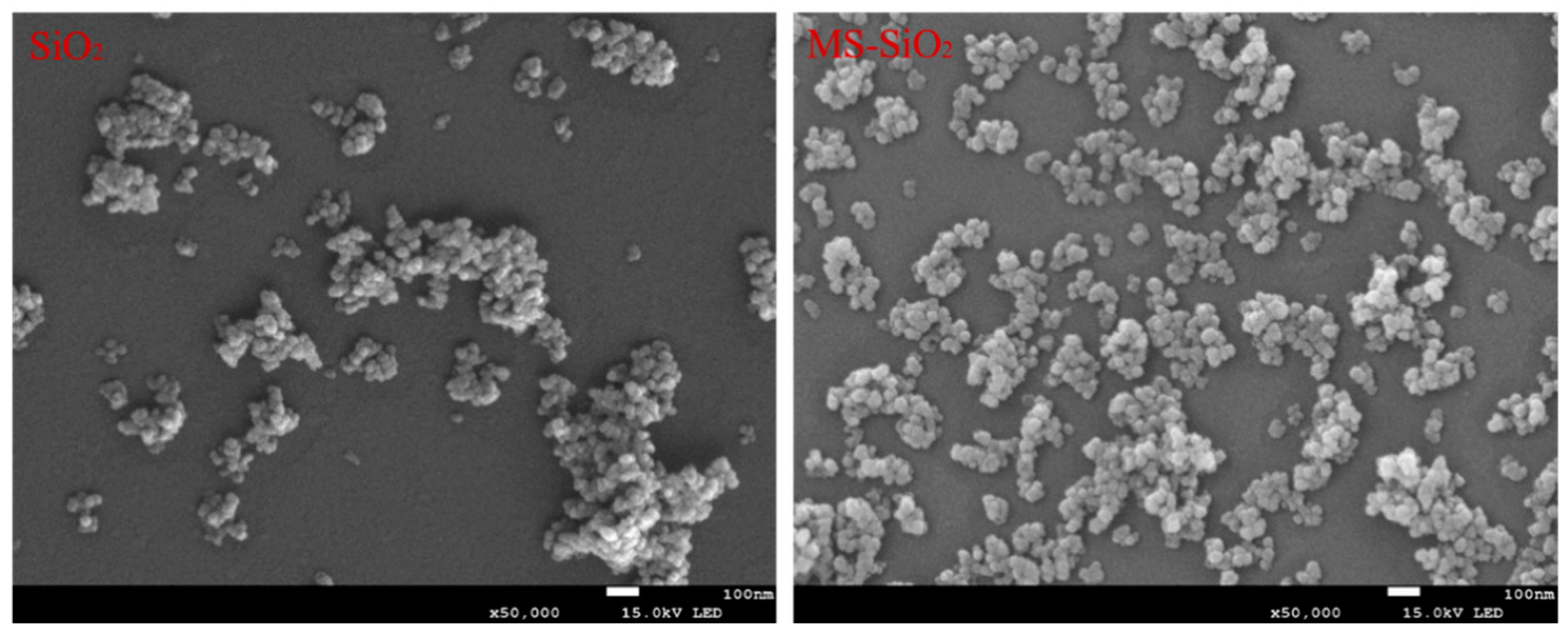
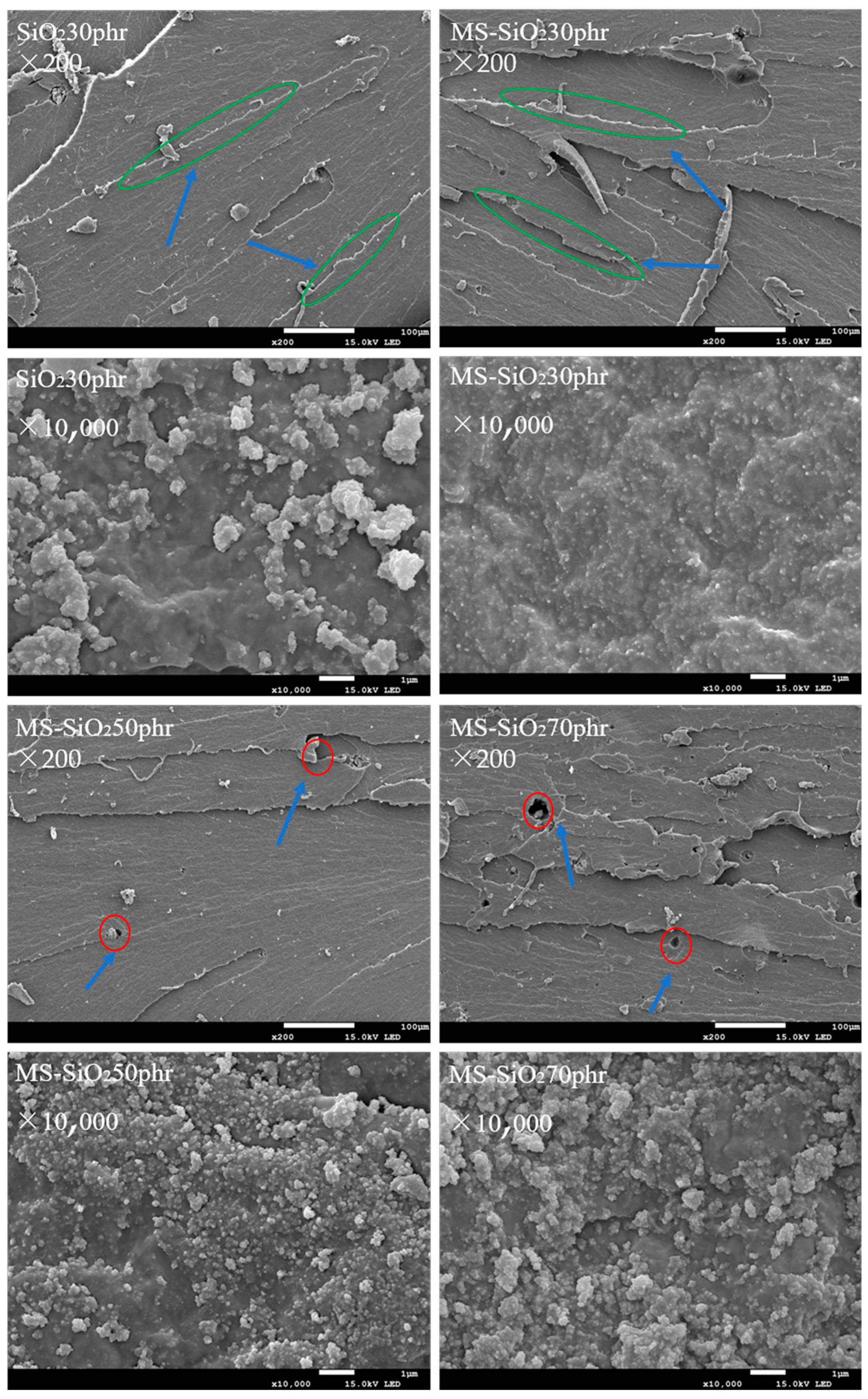
3.6. The Micromorphology of SiO2 and MS-SiO2
3.7. Mechanical Properties of SiO2/NR and MS-SiO2/NR
3.8. Dynamic Thermomechanical Analysis of SiO2/NR and MS-SiO2/NR
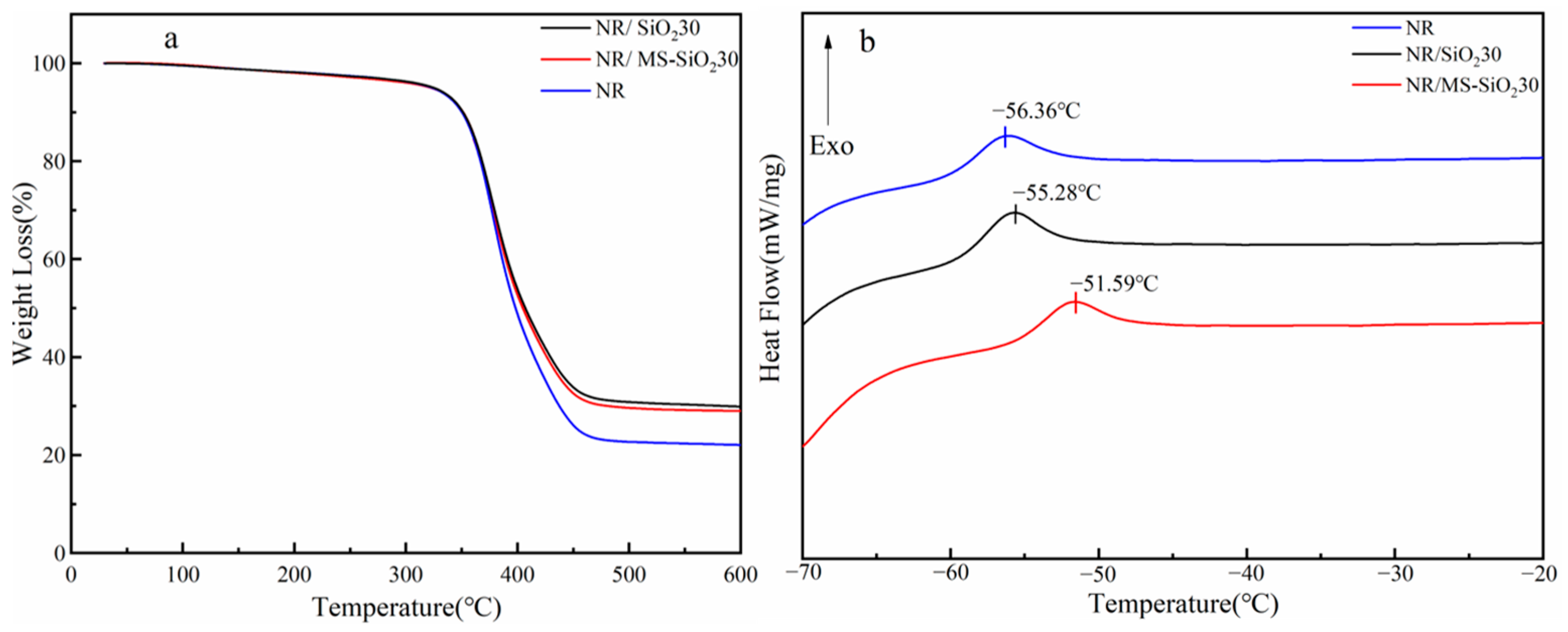
3.9. TG and DSC Analysis of NR, NR/SiO230 and NR/MS-SiO230
3.10. Vulcanization Properties of SiO2/NR and MS-SiO2/NR
3.11. Binding Adhesive Content of NR, NR/SiO230 and NR/MS-SiO230 Composites
4. Conclusions
- The wet modification process successfully grafted Si69 onto the surface of silica powder. FTIR spectrum analysis revealed the presence of distinct peaks corresponding to the -CH3 and -CH2 groups, indicating successful modification. This transformation converted the hydrophilic silica slurry into hydrophobic silica powder, resulting in a substantial increase in the contact angle of MS-SiO2 from 23.71° to 61.31°, representing a 158.6% augmentation. Furthermore, SEM analysis revealed that the Si69-modified silica powder exhibited superior dispersion characteristics.
- The integration of MS-SiO2 with natural rubber to form a composite material significantly enhanced the mechanical properties of MS-SiO2/NR. Specifically, the elongation at break was augmented by 99%, while the Mooney viscosity was reduced by 66.97%. Notably, when MS-SiO2 at 30 phr was added, the tanθ value of the composite reached its peak of 0.21 at 0 °C, indicating optimal skid resistance properties. However, further incrementing the MS-SiO2 content for rubber reinforcement led to uneven dispersion of MS-SiO2, thereby adversely affecting the overall performance of the composite material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, C.; Wang, P.; Qian, W.; Zhang, Y.; Chen, Q. Cardanol grafted onto liquid isoprene rubber like groud brothers hanging on the vine: A green plasticizer and compatibilizer. Mater. Today Commun. 2022, 32, 104116. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Yang, C.; Huang, J.; Kuang, F.; Wang, H. Study on the effect of particle size and dispersion of SiO2 on tribological properties of nitrile rubber. Wear 2020, 460–461, 203428. [Google Scholar] [CrossRef]
- Fu, W.; Wang, L. Research on Payne effect of natural rubber reinforced by graft-modified silica. J. Appl. Polym. Sci. 2016, 133, 43891. [Google Scholar] [CrossRef]
- Li, S.; Luo, Y.; Yongjun, C.; Xu, T.; Zhong, B.; Jia, Z.; Jia, D. Enhanced Mechanical and Processing Property of Styrene-butadiene Rubber Composites with Novel Silica-supported Reactive Processing Additive. Fibers Polym. 2019, 20, 1696–1704. [Google Scholar] [CrossRef]
- Neena, G.; Amrutha, S.R.; Rani, J.; Jose, P.M.; Mathiazhagan, A. Nano-silica as reinforcing filler in NR latex: Role of processing method on filler morphology inside the rubber and properties of the nanocomposite. Express Polym. Lett. 2021, 15, 1101–1112. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, C.; Tang, Y.; Liu, Y.; Niu, L.; Ding, T.; Li, X.; Zhang, Z. Preparation of hexamethyl disilazane-surface functionalized nano-silica by controlling surface chemistry and its “agglomeration-collapse” behavior in solution polymerized styrene butadiene rubber/butadiene rubber composites. Compos. Sci. Technol. 2021, 201, 108482. [Google Scholar] [CrossRef]
- Jayabalakrishnan, D.; Saravanan, K.; Ravi, S.; Prabhu, P.; Maridurai, T.; Prakash, V.R.A. Fabrication and Characterization of Acrylonitrile Butadiene Rubber and Stitched E-Glass Fibre Tailored Nano-Silica Epoxy Resin Composite. Silicon 2020, 13, 2509–2517. [Google Scholar] [CrossRef]
- Pöschl, M.; Vašina, M.; Zádrapa, P.; Měřínská, D.; Žaludek, M. Study of Carbon Black Types in SBR Rubber: Mechanical and Vibration Damping Properties. Materials 2020, 13, 2394. [Google Scholar] [CrossRef]
- Chen, J.; Hu, M.; Li, Y.; Li, R.; Qing, L. Significant Influence of Bound Rubber Thickness on the Rubber Reinforcement Effect. Polymers 2023, 15, 2051. [Google Scholar] [CrossRef]
- Pang, S.; Yu, Y.; Zhang, L.; Wu, Y. Adjusting silica/rubber interfacial interactions and properties via the click reactions between liquid polybutadiene and silane. Compos. Sci. Technol. 2021, 213, 108903. [Google Scholar] [CrossRef]
- Shoul, B.; Marfavi, Y.; Sadeghi, B.; Kowsari, E.; Sadeghi, P.; Ramakrishna, S. Investigating the potential of sustainable use of green silica in the green tire industry: A review. Environ. Sci. Pollut. Res. 2022, 29, 51298–51317. [Google Scholar] [CrossRef]
- Lolage, M.; Parida, P.; Chaskar, M.; Gupta, A.; Rautaray, D. Green Silica: Industrially scalable & sustainable approach towards achieving improved “nano filler–Elastomer” interaction and reinforcement in tire tread compounds. Sustain. Mater. Technol. 2020, 26, e00232. [Google Scholar] [CrossRef]
- Zheng, X.; Song, S.K.; Zhou, Z.; Jiang, X.; Sui, Y.; Che, M.; Xu, Q.; Wang, Y.; Zhao, S.; Li, L. Effect of silica dispersed by special dispersing agents with green strategy on tire rolling resistance and energy consumption. J. Appl. Polym. Sci. 2022, 139, e52933. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, L.; Zhang, G.; Shang, C.; Fang, H. Numerical modeling of sintering and dehydroxylation of porous silica preform for low-hydroxyl silica glass fabrication. Ceram. Int. 2020, 46, 23067–23083. [Google Scholar] [CrossRef]
- Wang, B.; Hu, J.; Liu, K.; Zhang, L.; Jiang, H.; Li, C. Reinforcement mechanism of silica surface hydroxyl: The opposite effect. Appl. Surf. Sci. 2023, 623, 157000. [Google Scholar] [CrossRef]
- Linec, M.; Mušič, B. The Effects of Silica-Based Fillers on the Properties of Epoxy Molding Compounds. Materials 2019, 12, 1811. [Google Scholar] [CrossRef]
- Jong, L. Synergistic Effect of Calcium Carbonate and Biobased Particles for Rubber Reinforcement and Comparison to Silica Reinforced Rubber. J. Compos. Sci. 2020, 4, 113. [Google Scholar] [CrossRef]
- Gashti, M.P.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.-E. Various nano-silica particles affecting dyeability of poly(ethylene terephthalate)/silica nanocomposite films. Fibers Polym. 2013, 14, 743–751. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Wu, Q.; Zhu, H.; Liu, Y. Thermal properties of metakaolin-based geopolymer modified by the silane coupling agent. Mater. Chem. Phys. 2021, 267, 124655. [Google Scholar] [CrossRef]
- Rong, Z.; Zhao, M.; Wang, Y. Effects of Modified Nano-SiO2 Particles on Properties of High-Performance Cement-Based Composites. Materials 2020, 13, 646. [Google Scholar] [CrossRef]
- Kumkrong, N.; Dittanet, P.; Saeoui, P.; Loykulnant, S.; Prapainainar, P. Properties of silica/natural rubber composite film and foam: Effects of silica content and sulfur vulcanization system. J. Polym. Res. 2022, 29, 302. [Google Scholar] [CrossRef]
- Nuinu, P.; Sirisinha, C.; Suchiva, K.; Daniel, P.; Phinyocheep, P. Improvement of mechanical and dynamic properties of high silica filled epoxide functionalized natural rubber. J. Mater. Res. Technol. 2023, 24, 2155–2168. [Google Scholar] [CrossRef]
- Olejnik, O.; Masek, A. Natural Phenolic Compounds as Modifiers for Epoxidized Natural Rubber/Silica Hybrids. Molecules 2022, 27, 2214. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, K.; Xiong, Y. Multiple Intermolecular Interaction to Improve the Abrasion Resistance and Wet Skid Resistance of Eucommia Ulmoides Gum/Styrene Butadiene Rubber Composite. Materials 2021, 14, 5246. [Google Scholar] [CrossRef] [PubMed]
- Saengdee, L.; Pasetto, P.; Sukhawipat, N. Functionalization of mesoporous silica with oligoisoprene brushes: Application as charge for elastomers reinforcement. Prog. Org. Coat. 2024, 186, 107988. [Google Scholar] [CrossRef]
- Thi, T.N.; Van, H.D.; Hong, H.C.; Ha, H.N.T.; Hayati, Y.N.; Kawahara, S. Preparation and properties of colloidal silica-filled natural rubber grafted with poly(methyl methacrylate). Polym. Bull. 2021, 79, 6011–6027. [Google Scholar] [CrossRef]
- Tian, Q.; Tang, Y.; Ding, T.; Li, X.; Zhang, Z. Effect of nano-silica surface-capped by bis[3-(triethoxysilyl)propyl] tetrasulfide on the mechanical properties of styrene-butadiene rubber/butadiene rubber nanocomposites. Compos. Commun. 2018, 10, 190–193. [Google Scholar] [CrossRef]
- Losev, V.N.; Borodina, E.V.; Buyko, O.V.; Samoilo, A.S.; Elsuf‘Ev, E.; Li, M. Highly selective adsorbents based on silica gel chemically modified with sulfur-containing groups of arched structure for preconcentration and determination of palladium(II) in products of processing of sulfide copper-nickel ore. Microchem. J. 2023, 195, 109452. [Google Scholar] [CrossRef]
- Jin, X.; Wu, X.; Cui, S.; Wang, W.; Zhang, Y.; Sun, S.; Sun, D. A robust adhesion between extruded polystyrene foam and mortar through different chemical linkages under ultraviolet-ozone irradiation. Constr. Build. Mater. 2022, 345, 128414. [Google Scholar] [CrossRef]
- Jalkanen, T. Isoconversional kinetic analysis for determining the rate of cross-linking for Pt and peroxide cure silicone rubbers. Thermochim. Acta 2021, 703, 178982. [Google Scholar] [CrossRef]
- Weißer, D.F.; Mayer, D.; Schmid, J.; Deckert, M.H. Novel approach to characterize the cross-linking effect of liquid silicone rubber via cavity pressure analysis during injection molding. J. Appl. Polym. Sci. 2022, 140, e53381. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, P.; Teng, Y.; Wang, S.; Zhang, L. In-situ surface modification of precipitated silica nanoparticles with 3-methacryloxypropyltrimethoxysilane in carbonation process. Res. Chem. Intermed. 2021, 47, 3037–3050. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, Y.; Tang, G.; Liu, Y.; Yang, R. Effect of alkylated surface modified silica nanoparticles on degradation products of PP during photooxidation aging: A Py-GC-MS analysis. J. Anal. Appl. Pyrolysis 2023, 169, 105862. [Google Scholar] [CrossRef]
- Alam, N.; Kumar, V.; Debnath, S.C.; Jeong, T.; Park, S.-S. Naturally abundant silica-kaolinite mixed minerals as an outstanding reinforcing filler for the advancement of natural rubber composites. J. Polym. Res. 2023, 30, 59. [Google Scholar] [CrossRef]
- Meng, Z.; Li, J.; Zou, Y.; Li, N.; Fu, X.; Zhang, R.; Hu, S.; Liu, Q. Advanced montmorillonite modification by using corrosive microorganisms as an alternative filler to reinforce natural rubber. Appl. Clay Sci. 2022, 225, 106534. [Google Scholar] [CrossRef]
- Adibi, A.; Simon, L.; Lenges, C.; Mekonnen, T.H. Sustainable natural rubber composites: Masterbatch development of epoxidized natural rubber grafted to designed enzymatic polysaccharides. Mater. Chem. Front. 2023, 7, 2208–2224. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Vu, C.M.; Vu, H.T. Effects of Co-Silanized Silica on the Mechanical Properties and Thermal Characteristics of Natural Rubber/Styrene-Butadiene Rubber Blend. Silicon 2019, 12, 1799–1809. [Google Scholar] [CrossRef]
- Chonkaew, W.; Minghvanish, W.; Kungliean, U.; Rochanawipart, N.; Brostow, W. Vulcanization Characteristics and Dynamic Mechanical Behavior of Natural Rubber Reinforced with Silane Modified Silica. J. Nanosci. Nanotechnol. 2011, 11, 2018–2024. [Google Scholar] [CrossRef]
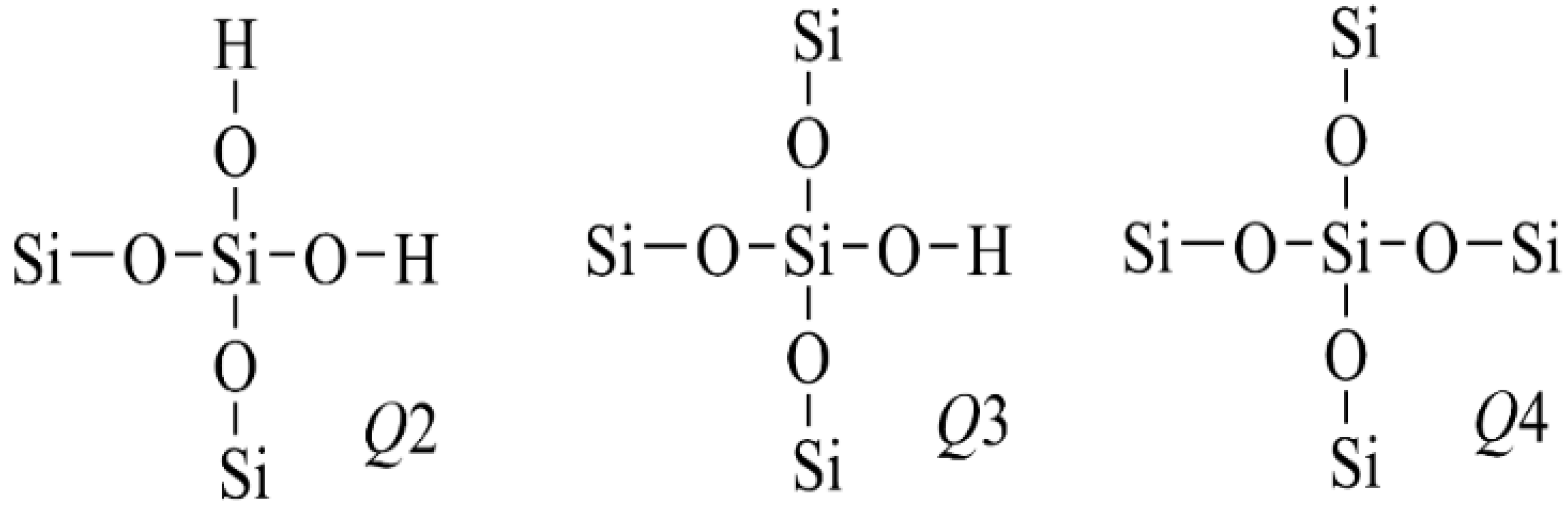




| Sample | NR | MS-SiO2 (30 phr) | MS-SiO2 (50 phr) | MS-SiO2 (70 phr) | SiO2 (30 phr) |
|---|---|---|---|---|---|
| Mooney viscosity | 20.2 | 15.1 | 24.3 | 43.3 | 45.8 |
| Tensile stress (MPa) | 24.1 | 13.7 | 12.0 | 10.8 | 10.4 |
| Elongation at break (%) | 725.3 | 1119.3 | 797.7 | 616.7 | 562.5 |
| 100% constant tensile stress (MPa) | 0.79 | 0.9 | 1.2 | 1.8 | 1.5 |
| 300% constant tensile strength (MPa) | 1.82 | 2.5 | 3.3 | 4.5 | 3.4 |
| 500% constant tensile strength (MPa) | 4.18 | 4.9 | 6.9 | 8.8 | 8.5 |
| Tear stress (MPa) | 27.51 | 19.2 | 23.1 | 26.5 | 26.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, B.; Jin, S. Preparation of Hydrophobic Modified Silica with Si69 and Its Reinforcing Mechanical Properties in Natural Rubber. Materials 2024, 17, 3131. https://doi.org/10.3390/ma17133131
You B, Jin S. Preparation of Hydrophobic Modified Silica with Si69 and Its Reinforcing Mechanical Properties in Natural Rubber. Materials. 2024; 17(13):3131. https://doi.org/10.3390/ma17133131
Chicago/Turabian StyleYou, Bo, and Shengming Jin. 2024. "Preparation of Hydrophobic Modified Silica with Si69 and Its Reinforcing Mechanical Properties in Natural Rubber" Materials 17, no. 13: 3131. https://doi.org/10.3390/ma17133131
APA StyleYou, B., & Jin, S. (2024). Preparation of Hydrophobic Modified Silica with Si69 and Its Reinforcing Mechanical Properties in Natural Rubber. Materials, 17(13), 3131. https://doi.org/10.3390/ma17133131






