Glass-Forming Ability, Mechanical Properties, and Energetic Characteristics of ZrCuNiAlNbHfY Bulk Metallic Glasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Zr59.62Cu18.4-xNi12Al6+xNb3Hf0.78Y0.2 Alloy
2.2. Microstructure Characterization
2.3. Performance Test
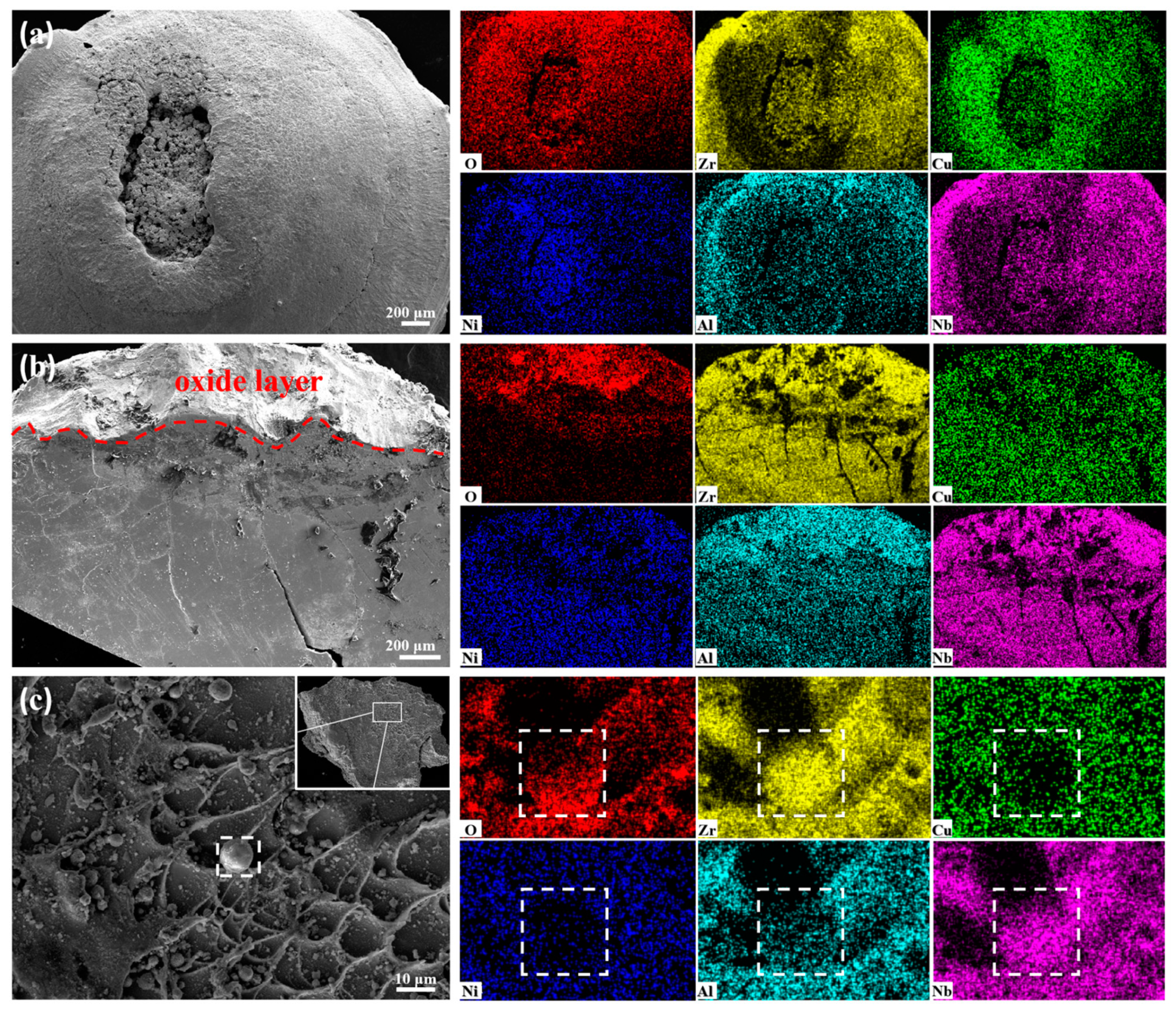
3. Results and Discussion
3.1. GFA and Thermal Ability
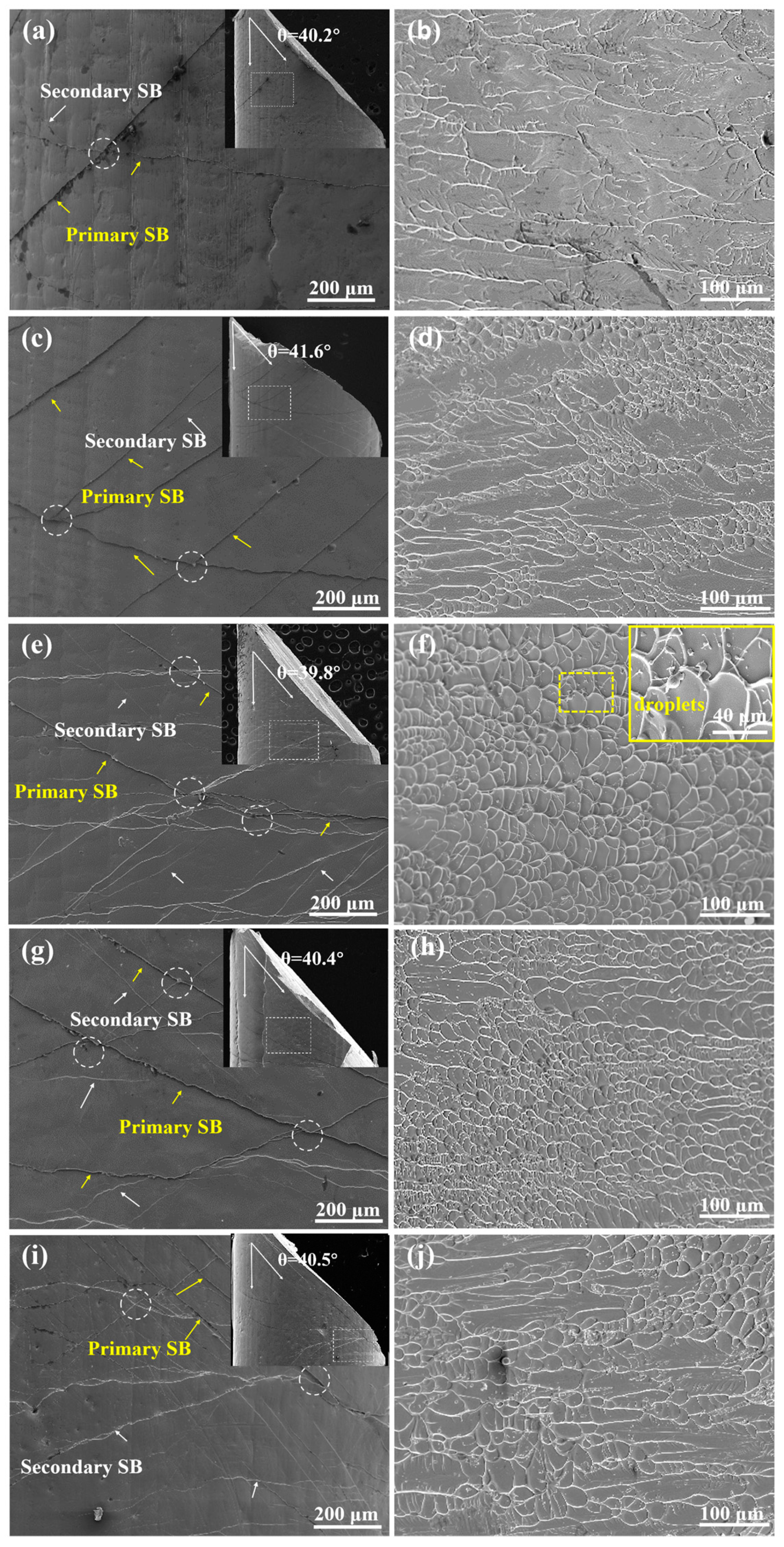
3.2. Quasi-Static and Dynamic Mechanical Properties of BMGs
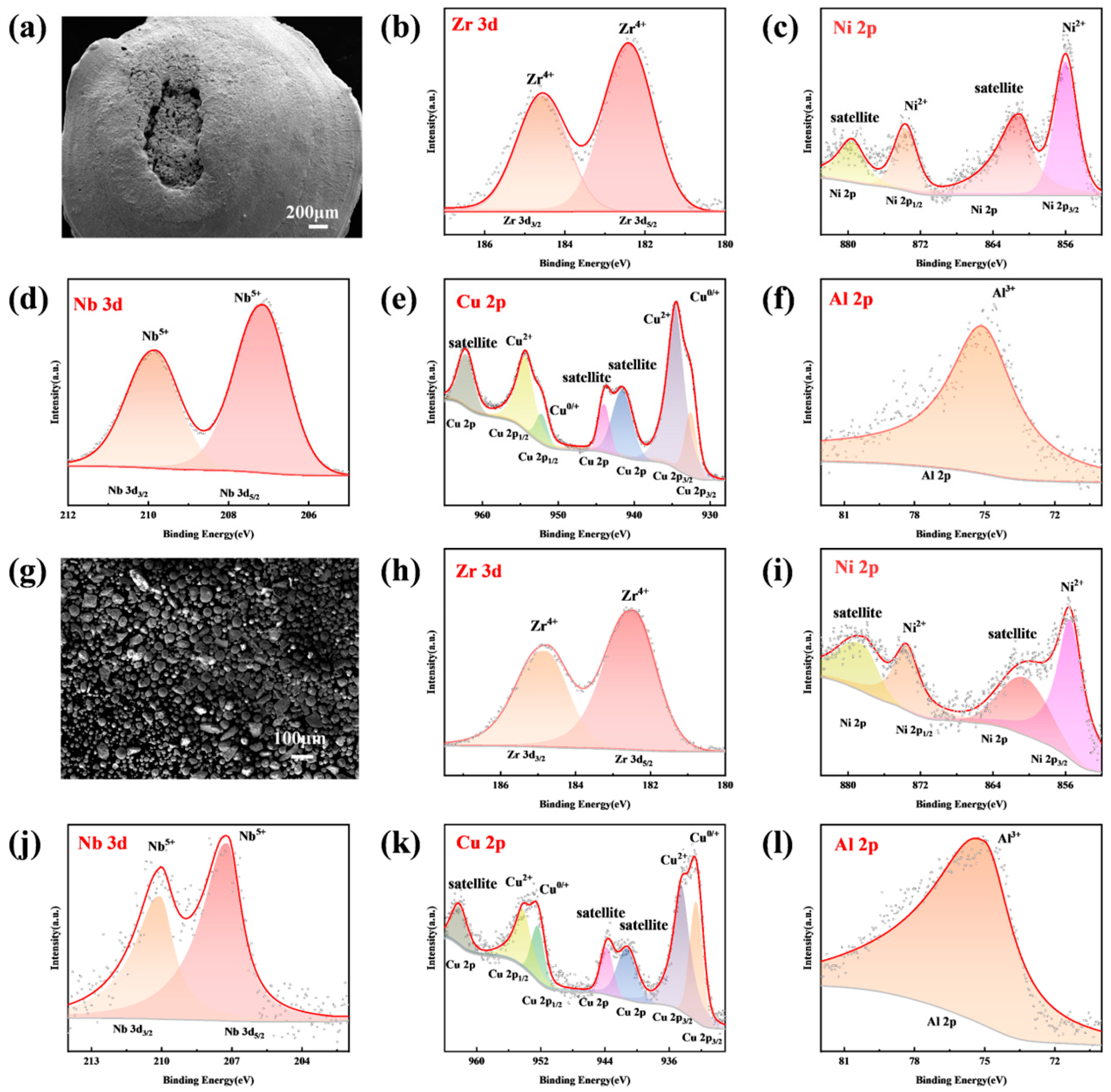
3.3. Energetic Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.Q.; Li, M.C.; Tong, X.; Zhao, Y.; Xie, J.Y.; Guo, S.W.; Huang, P.; Wang, F.; Ke, H.B.; Sun, B.A.; et al. Hardening and Toughening Effects of Intermediate Nanosized Structures in a Confined Amorphous Alloy Film. J. Mater. Sci. Technol. 2022, 118, 44–53. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of Metallic Supercooled Liquid and Bulk Amorphous Alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Akbarpour, A.; Milkova, D.A.; Zanaeva, E.N.; Parkhomenko, M.S.; Cheverikin, V.V.; Lubenchenko, A.; Bazlov, A.I. Influence of Cold Rolling Process and Chemical Composition on the Mechanical Properties and Corrosion Behavior of Zr-Based Metallic Glasses. Metals 2021, 11, 1514. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Wu, Y.; Xie, G.; Shao, L.; Li, Y.; Shan, K.; Jiang, S.; Liu, X.; Huang, J.; et al. Combustion Behavior and Mechanism of Cu46Zr46Al8 Bulk Metallic Glass in Oxygen-Enriched Environments. Corros. Sci. 2022, 204, 110415. [Google Scholar] [CrossRef]
- Luo, P.; Wang, Z.; Jiang, C.; Mao, L.; Li, Q. Experimental Study on Impact-Initiated Characters of W/Zr Energetic Fragments. Mater. Des. 2015, 84, 72–78. [Google Scholar] [CrossRef]
- Shi, J.; Huang, Z.; Zu, X.; Xiao, Q. Experimental Investigation of Zr-Based Amorphous Alloy as a Shaped Charge Liner. Propellants Explos. Pyrotech. 2022, 47, e202200063. [Google Scholar] [CrossRef]
- Wang, C.T.; He, Y.; Ji, C.; He, Y.; Han, W.; Pan, X. Investigation on Shock-Induced Reaction Characteristics of a Zr-Based Metallic Glass. Intermetallics 2018, 93, 383–388. [Google Scholar] [CrossRef]
- Kumar, G.; Rector, D.; Conner, R.D.; Schroers, J. Embrittlement of Zr-Based Bulk Metallic Glasses. Acta Mater. 2009, 57, 3572–3583. [Google Scholar] [CrossRef]
- Cheng, Y.; Pang, S.; Chen, C.; Zhang, T. Size-Dependent Enhancement of Plasticity by Laser Surface Melting in Zr55Al10Ni5Cu30 Bulk Metallic Glass. J. Alloys Compd. 2016, 658, 49–54. [Google Scholar] [CrossRef]
- Huang, C.; Li, S.; Bai, S. Quasi-Static and Impact-Initiated Response of Zr55Ni5Al10Cu30 Alloy. J. Non-Cryst. Solids 2018, 481, 59–64. [Google Scholar] [CrossRef]
- Wang, S.P.; Li, C.Y.; Li, J.L.; Wang, H.B.; Li, Y.; Kou, S.Z. Quasi-Static and Dynamic Deformation Behavior of Zr-Based Bulk Amorphous Alloy at Cryogenic Temperature. J. Alloys Compd. 2022, 892, 162039. [Google Scholar] [CrossRef]
- Wang, C.T.; He, Y.; Ji, C.; He, Y.; Guo, L.; Meng, Y. Dynamic Fragmentation of a Zr-Based Metallic Glass under Various Impact Velocities. J. Mater. Sci. 2021, 56, 2900–2911. [Google Scholar] [CrossRef]
- Ji, C.; He, Y.; Wang, C.T.; He, Y.; Guo, Z.; Guo, L. Effect of Dynamic Fragmentation on the Reaction Characteristics of a Zr-Based Metallic Glass. J. Non-Cryst. Solids 2019, 515, 149–156. [Google Scholar] [CrossRef]
- Long, Z.; Tao, P.; Wang, G.; Zhu, K.; Chen, Y.; Zhang, W.; Zhao, Z.; Yang, Y.; Huang, Z. Effect of Nb and Ta Addition on Mechanical Properties of Zr-Based Bulk Metallic Glasses and Composites. J. Alloys Compd. 2022, 912, 165071. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, C.; Jiang, J.-Z. Bulk Metallic Glass Composites Containing B2 Phase. Prog. Mater. Sci. 2021, 121, 100799. [Google Scholar] [CrossRef]
- Chen, S.S.; Todd, I. Enhanced Plasticity in the Zr–Cu–Ni–Al–Nb Alloy System by in-Situ Formation of Two Glassy Phases. J. Alloys Compd. 2015, 646, 973–977. [Google Scholar] [CrossRef]
- Kozieł, T.; Pajor, K.; Cios, G.; Bała, P. Effect of Strain Rate and Crystalline Inclusions on Mechanical Properties of Bulk Glassy and Partially Crystallized Zr52.5Cu17.9Ni14.6Al10Ti5 Alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 1036–1045. [Google Scholar] [CrossRef]
- Huang, C.; Bai, S.; Li, S.; Tang, Y.; Liu, X.; Ye, Y.; Zhu, L. Effect of In-Situ Crystalline Phases on the Mechanical Properties and Energy Release Behaviors of Zr55Ni5Al10Cu30 Bulk Metallic Glasses. Intermetallics 2020, 119, 106720. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Ma, E. Atomic-Level Structure and Structure–Property Relationship in Metallic Glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Song, Y.; Zhang, S.; Li, H.; Wang, A.; Zhang, H.; Zhu, Z. Atomic-Scale Nb Heterogeneity Induced Icosahedral Short-Range Ordering in Metallic Glasses. J. Mater. Sci. Technol. 2022, 108, 73–81. [Google Scholar] [CrossRef]
- Geng, G.; Yan, Z.; Hu, Y.; Wang, Z.; Ketov, S.V.; Eckert, J. Correlation between the Atomic Configurations and the Amorphous-to-Icosahedral Phase Transition in Metallic Glasses. J. Mater. Res. 2018, 33, 2775–2783. [Google Scholar] [CrossRef]
- Li, B.; Sun, W.C.; Qi, H.N.; Lv, J.W.; Wang, F.L.; Ma, M.Z.; Zhang, X.Y. Effects of Ag Substitution for Fe on Glass-Forming Ability, Crystallization Kinetics, and Mechanical Properties of Ni-Free Zr–Cu–Al–Fe Bulk Metallic Glasses. J. Alloys Compd. 2020, 827, 154385. [Google Scholar] [CrossRef]
- Chen, S.; Qi, K.; Yin, J.; Zou, J.; Song, P.; Peng, G. Significant Plasticity and Atomic-Scale Deformation Mechanism of High Glass-Forming Zr-Cu-Ni-Ti-Al Bulk Glassy Alloys. J. Non-Cryst. Solids 2021, 566, 120897. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Y.W.; Cheng, X.W.; Fu, Q.; Xin, Z.H.; Xu, Z.Q.; Cheng, H.W. Effects of Al Replacement on Glass Forming Ability and Mechanical Properties of Zr-Based Bulk Metallic Glasses. J. Non-Cryst. Solids 2021, 568, 120962. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, W.; Wei, X.; Ding, Y.; Shen, X.; Liu, W. Effect of Ti Addition on Mechanical Properties and Corrosion Resistance of Ni-Free Zr-Based Bulk Metallic Glasses for Potential Biomedical Applications. J. Alloys Compd. 2020, 815, 152636. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Gu, L.; Xie, G.Q.; Zhang, W.; Inoue, A.; Zhang, H.F.; Hu, Z.Q. Relation between Icosahedral Short-Range Ordering and Plastic Deformation in Zr–Nb–Cu–Ni–Al Bulk Metallic Glasses. Acta Mater. 2011, 59, 2814–2822. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhu, Z.W.; Chen, S.; Fu, H.M.; Zhang, H.W.; Li, H.; Wang, A.M.; Zhang, H.F. Simultaneously Enhancing Strength and Toughness of Zr-Based Bulk Metallic Glasses via Minor Hf Addition. Intermetallics 2020, 118, 106685. [Google Scholar] [CrossRef]
- Krimer, Y.; Aronhime, N.; Ohodnicki, P.; McHenry, M.E. Prediction of Good Glass Forming Ability in Amorphous Soft Magnetic Alloys by Thermocalc Simulation with Experimental Validation. J. Alloys Compd. 2020, 814, 152294. [Google Scholar] [CrossRef]
- Pan, J.; Liu, L.; Chan, K.C. Enhanced Plasticity by Phase Separation in CuZrAl Bulk Metallic Glass with Micro-Addition of Fe. Scr. Mater. 2009, 60, 822–825. [Google Scholar] [CrossRef]
- Xing, L.Q.; Ochin, P. Investigation of the Effects of Al and Ti on the Glass Forming Ability of Zr-Cu-Al and Zr-Ti-Al-Cu-Ni Alloys through Their Solidification Characteristics. Acta Mater. 1997, 45, 3765–3774. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.J.; Cheng, J.L.; Li, F.; Zhao, W.; Chen, G. Investigation of the Effects of Al on the Glass Forming Ability of Zr-Cu-Ni-Al Alloys through Their Solidification Characteristics. Intermetallics 2019, 109, 105–109. [Google Scholar] [CrossRef]
- Yu, P.; Bai, H.Y.; Tang, M.B.; Wang, W.L. Excellent Glass-Forming Ability in Simple Cu50Zr50-Based Alloys. J. Non-Cryst. Solids 2005, 351, 1328–1332. [Google Scholar] [CrossRef]
- Cai, A.H.; Tan, J.; Ding, D.W.; Wang, H.; Liu, Y.; Wu, H.; An, Q.; Ning, H.; Zhou, G.J. Effect of Al Addition on Properties of Cu45Zr45.5Ti9.5 Bulk Metallic Glass. Mater. Chem. Phys. 2020, 251, 123072. [Google Scholar] [CrossRef]
- Li, M.M.; Inoue, A.; Han, Y.; Kong, F.L.; Zhu, S.L.; Shalaan, E.; Al-Marzouki, F. Influence of Ag Replacement on Supercooled Liquid Region and Icosahedral Phase Precipitation of Zr65Al7.5Ni10Cu17.5-xAgx (x=0–17.5 at%) Glassy Alloys. J. Alloys Compd. 2018, 735, 1712–1721. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, D.; Chen, Z. Predicting the Thermal Stability of RE-Based Bulk Metallic Glasses. Intermetallics 2010, 18, 74–76. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T.; Dong, Y.D. Effects of Atomic Bonding Nature and Size Mismatch on Thermal Stability and Glass-Forming Ability of Bulk Metallic Glasses. J. Non-Cryst. Solids 2004, 341, 93–100. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, Y.J.; Wang, G.Y.; Liaw, P.K.; Shen, J. Influence of Strain Rate on Compressive Deformation Behavior of a Zr-Cu-Ni-Al Bulk Metallic Glass at Room Temperature. Metall. Mater. Trans. A 2011, 42, 1491–1498. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhang, H.; Shen, B.L.; Inoue, A.; Eckert, J. Shear Fracture and Fragmentation Mechanisms of Bulk Metallic Glasses. Philos. Mag. Lett. 2006, 86, 643–650. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, C.; Shi, Z.; Zhang, H.; Ma, M. Effect of Fe Addition on the Glass-Forming Ability, Stability, and Mechanical Properties of Zr50Cu34-xFexAl8Ag8 Metallic Glasses. J. Alloys Compd. 2022, 929, 167334. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, W.; Cheng, S.; Liu, X.; Yang, X.; Tian, J.; Ma, J.; Shen, J. Improving the Glass Forming Ability and Plasticity of ZrCuNiAlTi Metallic Glass by Substituting Zr with Sc. J. Alloys Compd. 2022, 909, 164679. [Google Scholar] [CrossRef]
- Fan, J.; Yang, L. Damage Mechanisms of Bulk Metallic Glasses under High-Rate Compression. Int. J. Impact Eng. 2017, 106, 217–222. [Google Scholar] [CrossRef]
- Ma, Y.-B.; Wang, B.-Z.; Zhang, Q.-D.; Jiang, Y.; Hou, D.-W.; Cui, X.; Zu, F.-Q. Change Dynamic Behaviors by Heightening Its Stored Energy of Monolithic Bulk Metallic Glass. Mater. Des. 2019, 181, 107971. [Google Scholar] [CrossRef]
- Qiao, J.W.; Feng, P.; Zhang, Y.; Zhang, Q.M.; Chen, G.L. Quasi-Static and Dynamic Deformation Behaviors of Zr-Based Bulk Metallic Glass Composites Fabricated by the Bridgman Solidification. J. Alloys Compd. 2009, 486, 527–531. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Li, B.; Wang, X.; Li, Y.; Yang, K. Quasi-Static and Dynamic Deformation Behavior of Hf28Be18Ti17Zr17Cu7.5Ni12.5 High-Entropy Bulk Metallic Glass. J. Mater. Res. Technol. 2022, 21, 1331–1343. [Google Scholar] [CrossRef]
- Hu, A.; Cai, S. Preparation, Quasi-Static, and Dynamic Compressive Mechanical Properties of BMG-W Energetic Structural Materials. J. Mater. Res. Technol. 2023, 24, 9657–9676. [Google Scholar] [CrossRef]
- Idrici, D.; Goroshin, S.; Soo, M.J.; Frost, D.L. Light Emission Signatures from Ballistic Impact of Reactive Metal Projectiles. Int. J. Impact Eng. 2021, 150, 103814. [Google Scholar] [CrossRef]
- Allison, T.C. NIST-JANAF Thermochemical Tables. 1998. Available online: https://janaf.nist.gov/ (accessed on 18 May 2024).
- Si, S.; He, C.; Liu, S.; Fan, B.; Xie, R.; Xue, X.; Liu, J. Influence of Impact Velocity on Impact-Initiated Reaction Behavior of Zr-Ti-Nb Alloy. Mater. Des. 2022, 220, 110846. [Google Scholar] [CrossRef]

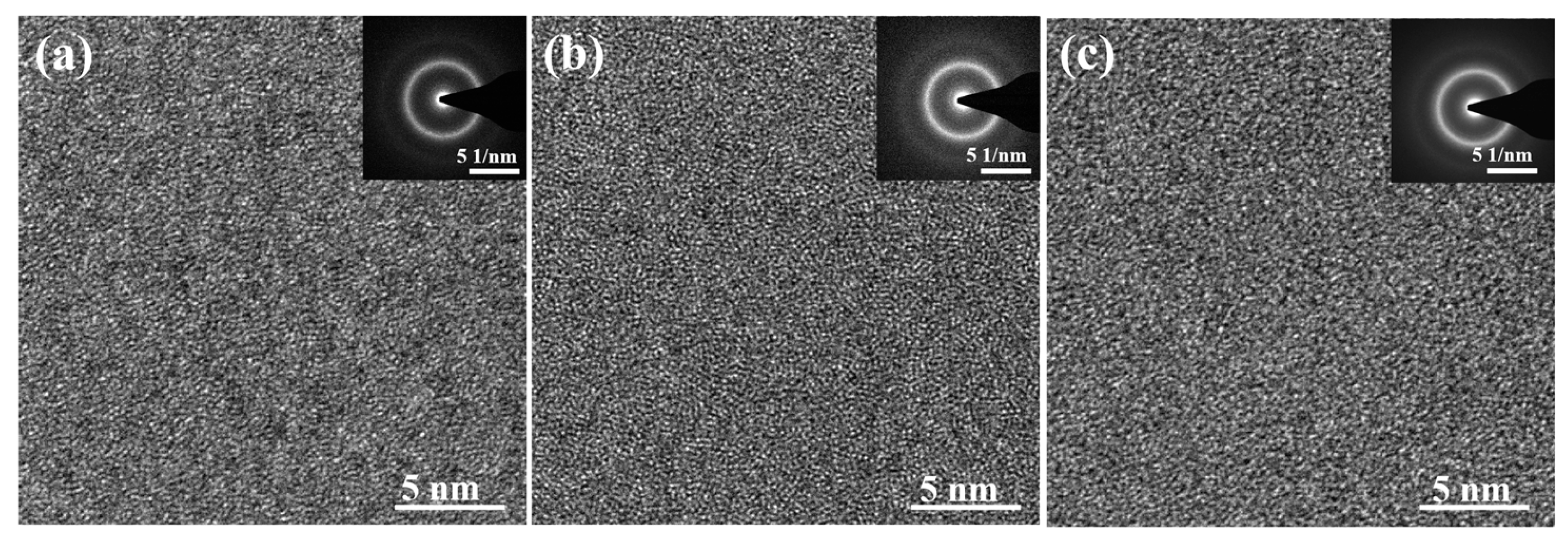
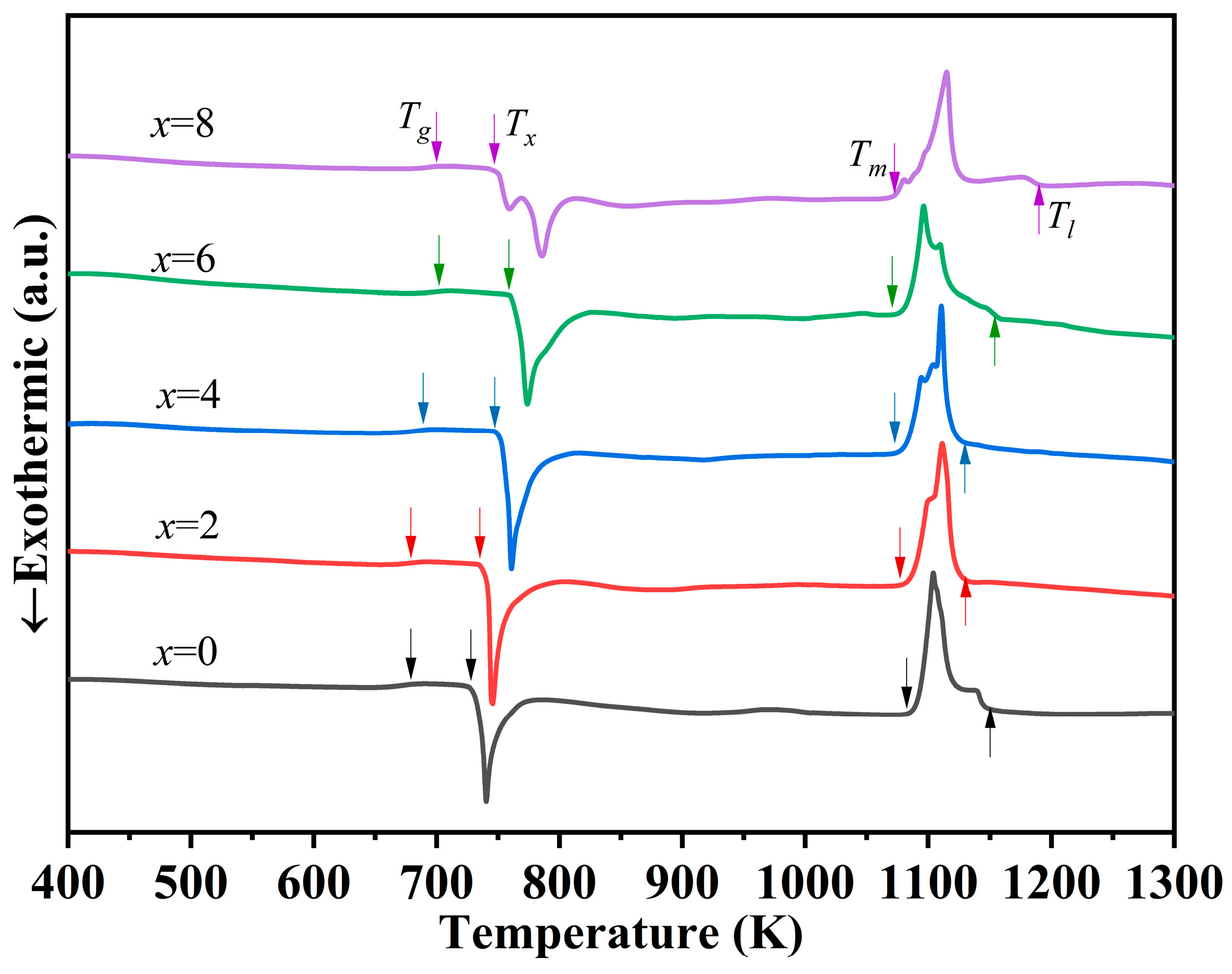




| Alloys | Dc (mm) | Tg (K) | Tx (K) | Tm (K) | Tl (K) | ΔT (K) | Trg | γ | ∆Hmix (kJ/mol) |
|---|---|---|---|---|---|---|---|---|---|
| x = 0 | 5 | 679 | 728 | 1080 | 1150 | 49 | 0.590 | 0.398 | −31.3 |
| x = 2 | 7 | 682 | 735 | 1076 | 1132 | 53 | 0.602 | 0.405 | −32.6 |
| x = 4 | 10 | 689 | 748 | 1072 | 1130 | 59 | 0.610 | 0.411 | −34.0 |
| x = 6 | 8 | 702 | 758 | 1071 | 1154 | 56 | 0.608 | 0.408 | −35.3 |
| x = 8 | 3 | 700 | 747 | 1069 | 1190 | 47 | 0.588 | 0.395 | −36.6 |
| Alloys | (s−1) | (MPa) | (MPa) | (%) |
|---|---|---|---|---|
| x = 0 | 1 × 10−3 | 1582 ± 31 | 1621 ± 27 | 1.06 ± 0.31 |
| 3 × 103 | - | 1146 ± 71 | - | |
| x = 2 | 1 × 10−3 | 1610 ± 27 | 1662 ± 23 | 1.95 ± 0.33 |
| 3 × 103 | - | 1202 ± 63 | - | |
| x = 4 | 1 × 10−3 | 1707 ± 19 | 1781 ± 18 | 2.68 ± 0.17 |
| 3 × 103 | - | 1294 ± 51 | - | |
| x = 6 | 1 × 10−3 | 1722 ± 36 | 1791 ± 44 | 3.45 ± 0.21 |
| 3 × 103 | - | 1310 ± 73 | - | |
| x = 8 | 1 × 10−3 | 1658 ± 33 | 1720 ± 35 | 2.16 ± 0.27 |
| 3 × 103 | - | 1217 ± 46 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Li, J.; Zhang, K.; Zhang, H.; Wang, H.; Fang, Y.; Ma, Y.; Wang, Z.; Zhang, X.; Gai, X. Glass-Forming Ability, Mechanical Properties, and Energetic Characteristics of ZrCuNiAlNbHfY Bulk Metallic Glasses. Materials 2024, 17, 3136. https://doi.org/10.3390/ma17133136
Yu X, Li J, Zhang K, Zhang H, Wang H, Fang Y, Ma Y, Wang Z, Zhang X, Gai X. Glass-Forming Ability, Mechanical Properties, and Energetic Characteristics of ZrCuNiAlNbHfY Bulk Metallic Glasses. Materials. 2024; 17(13):3136. https://doi.org/10.3390/ma17133136
Chicago/Turabian StyleYu, Xin, Jianbin Li, Kaichuang Zhang, Huijie Zhang, Hao Wang, Yuanhang Fang, Yusong Ma, Zhenxiong Wang, Xinggao Zhang, and Xiqiang Gai. 2024. "Glass-Forming Ability, Mechanical Properties, and Energetic Characteristics of ZrCuNiAlNbHfY Bulk Metallic Glasses" Materials 17, no. 13: 3136. https://doi.org/10.3390/ma17133136





