Growth of Carbon Nanofibers and Carbon Nanotubes by Chemical Vapour Deposition on Half-Heusler Alloys: A Computationally Driven Experimental Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.1.1. Half-Heusler Alloys
2.1.2. CVD Process Description
2.1.3. Characterization of Produced Coatings
2.2. Computational Section
2.2.1. Governing Equations and Model Assumptions
2.2.2. Gas-Phase Reactions
3. Results
3.1. Characterization of the Catalytic Substrate
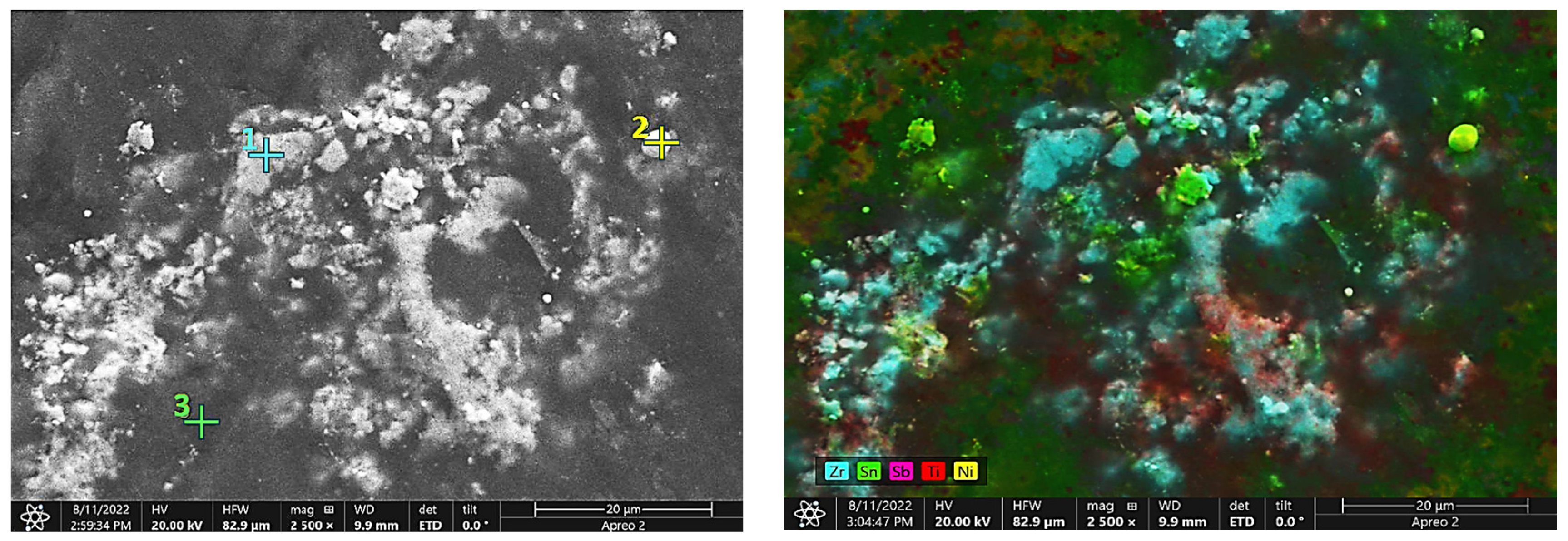
3.1.1. Elemental Analysis through SEM/EDS
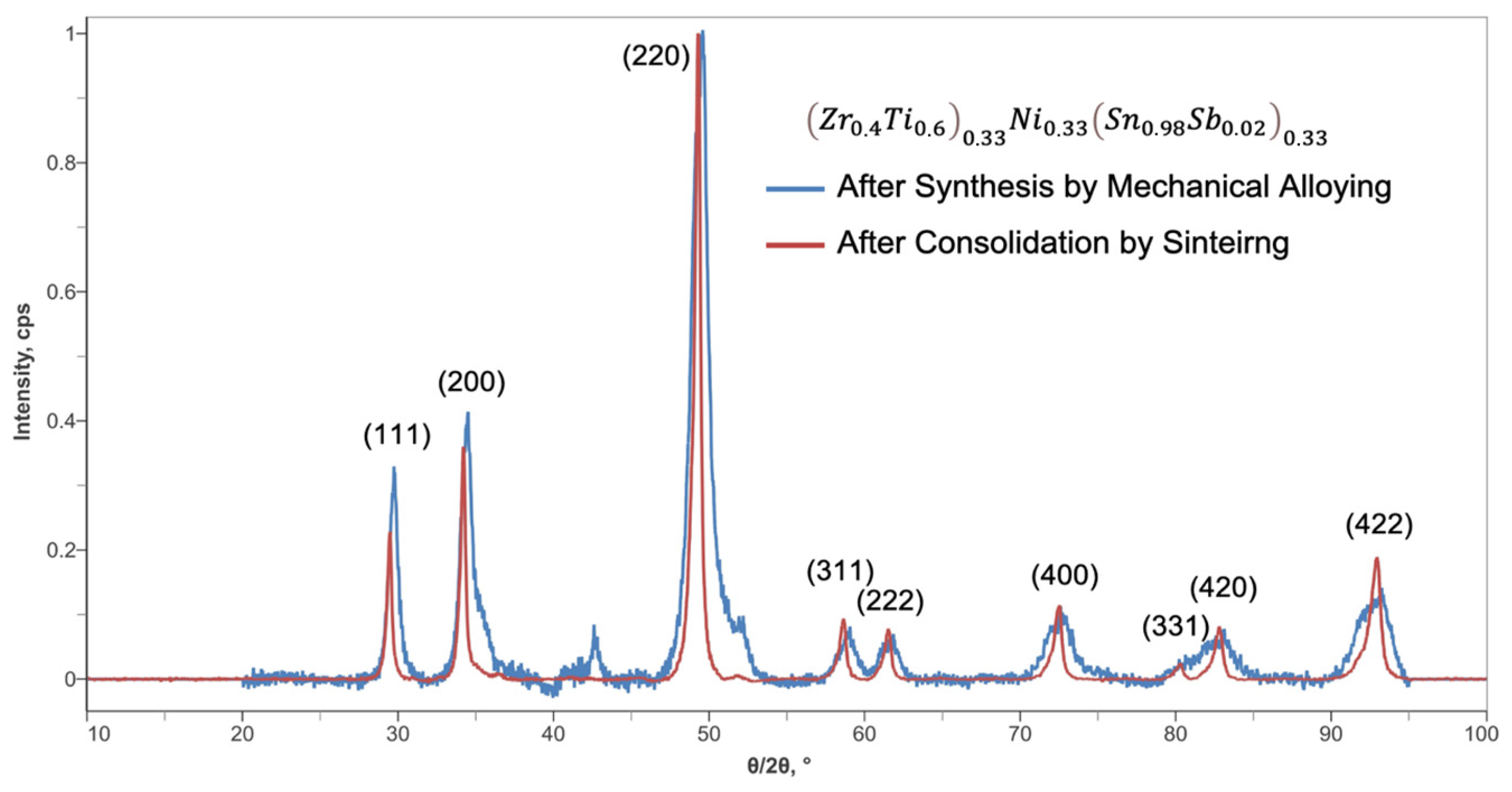
3.1.2. Crystallographic Structure of Half-Heusler Alloy
3.2. Numerical Analysis
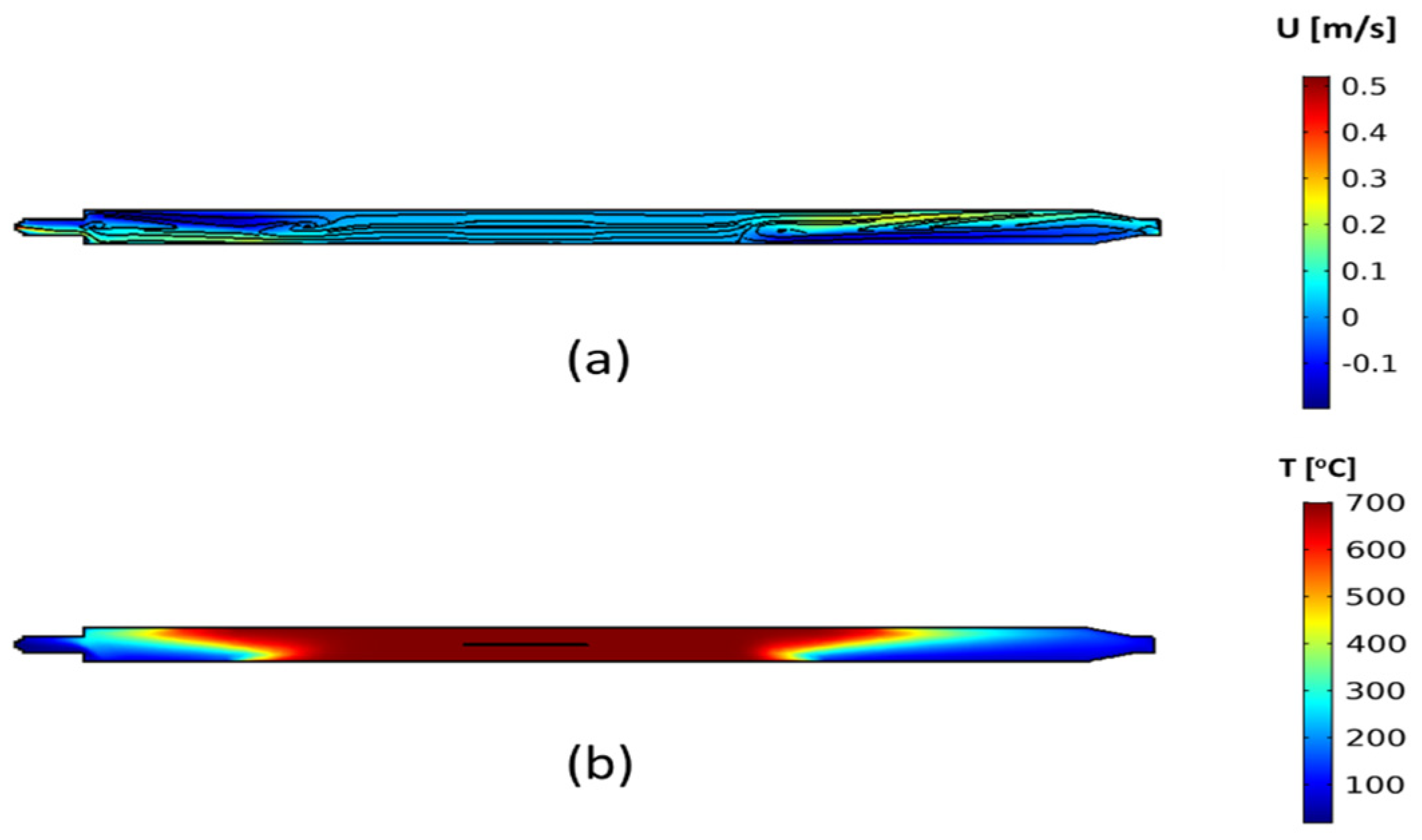
3.2.1. Flow and Temperature Field
3.2.2. Chemical Species
Effect of Process Temperature
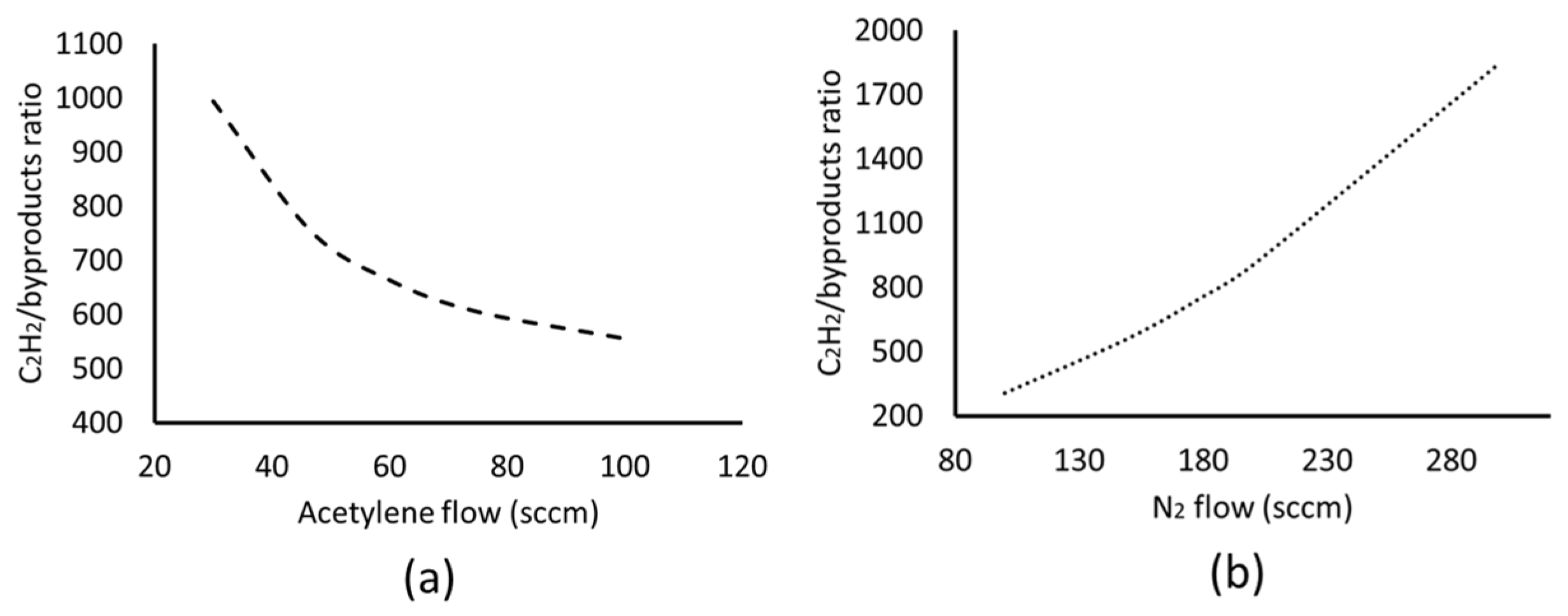
Effect of Inlet Flow
3.3. Deposited CNFs and CNTs on (ZrTi)Ni(SnSb)
3.3.1. Visual Observation
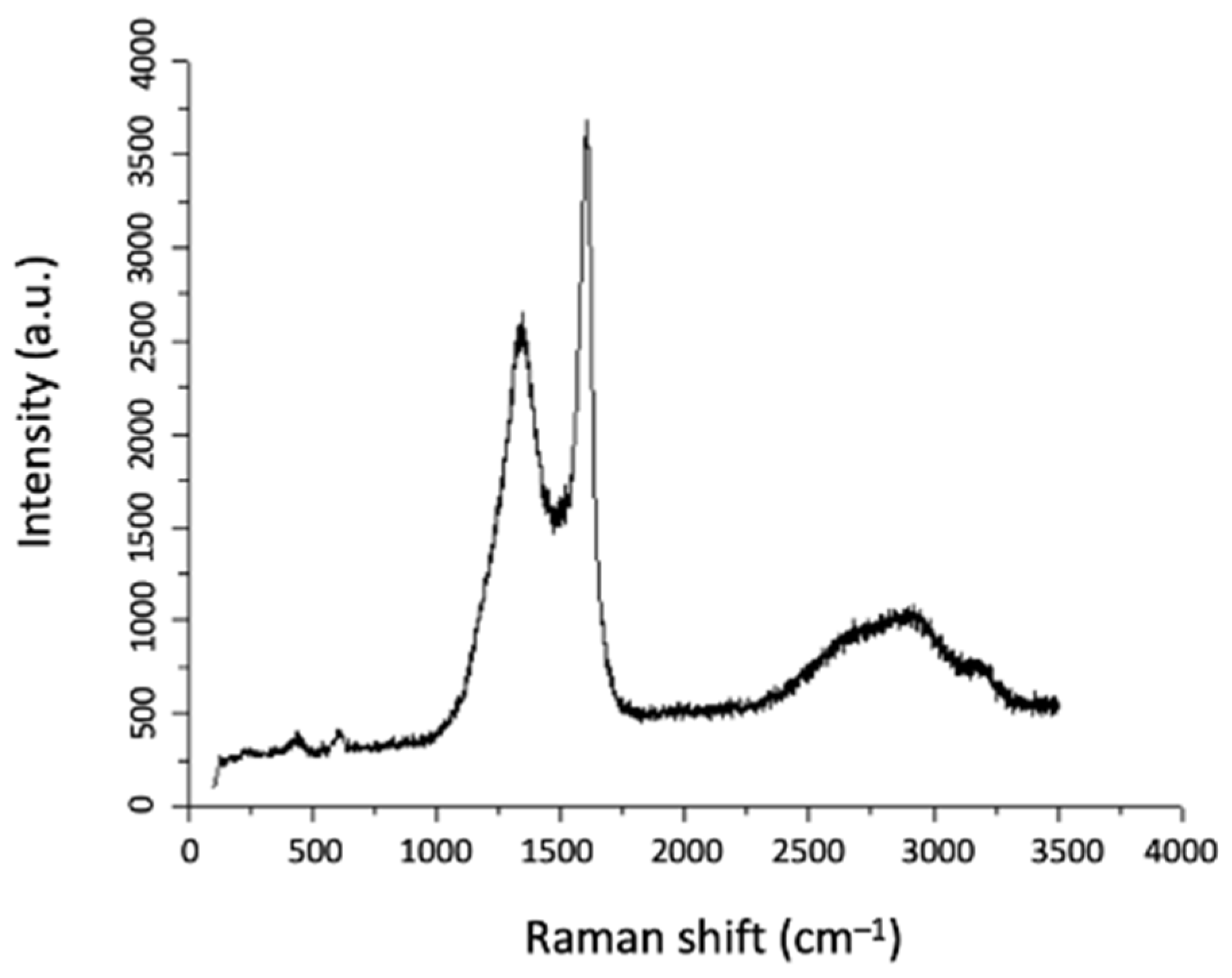
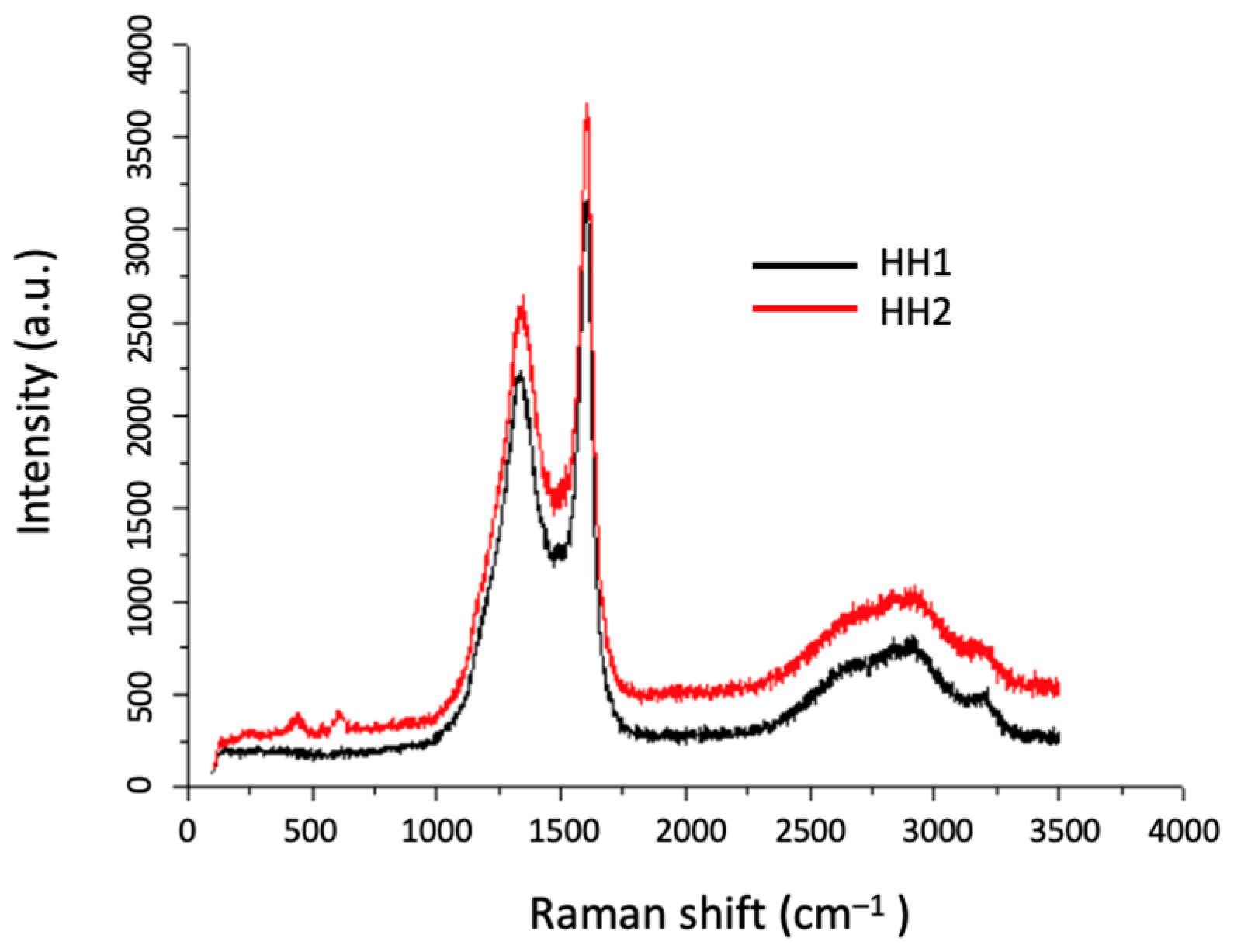
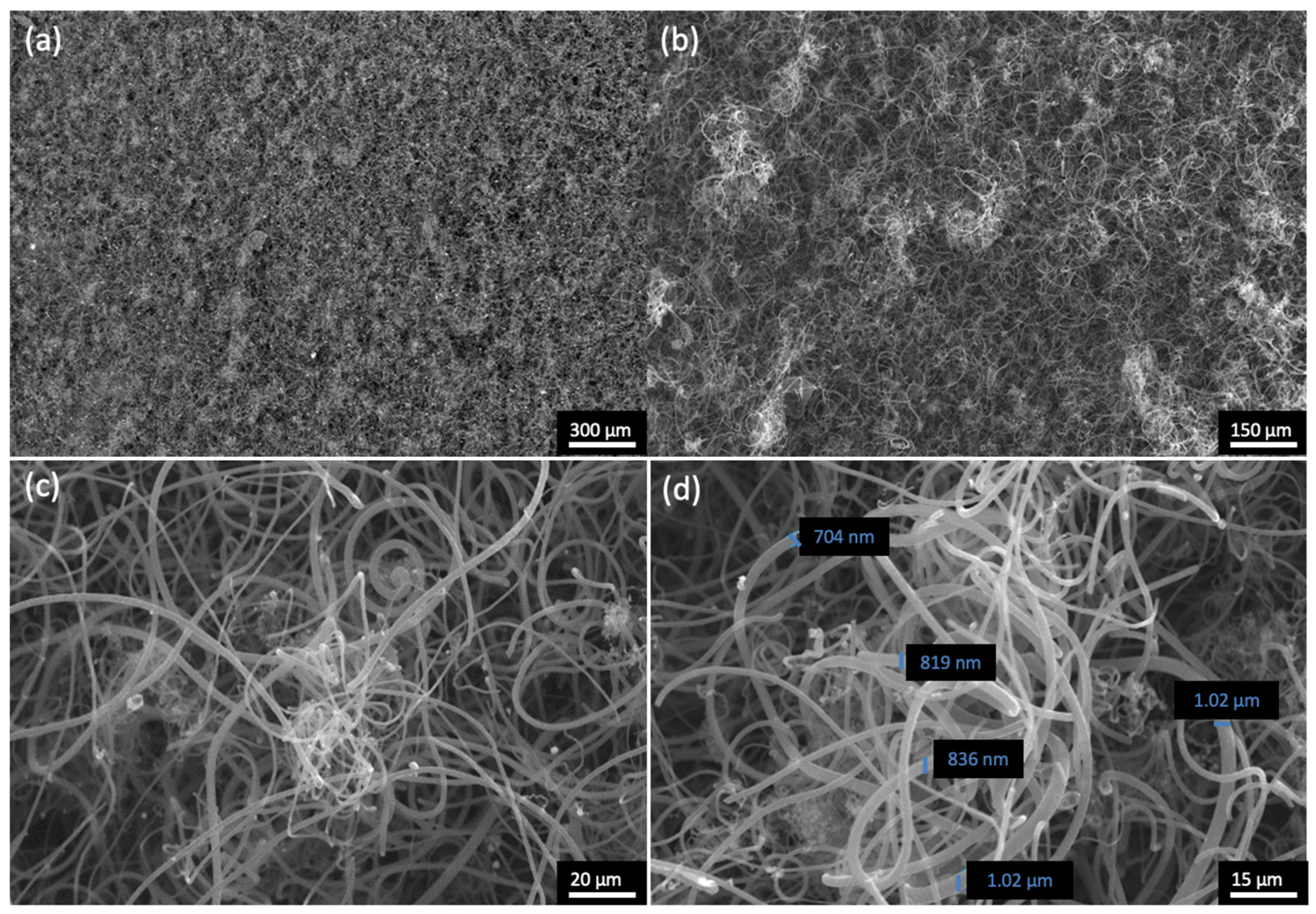
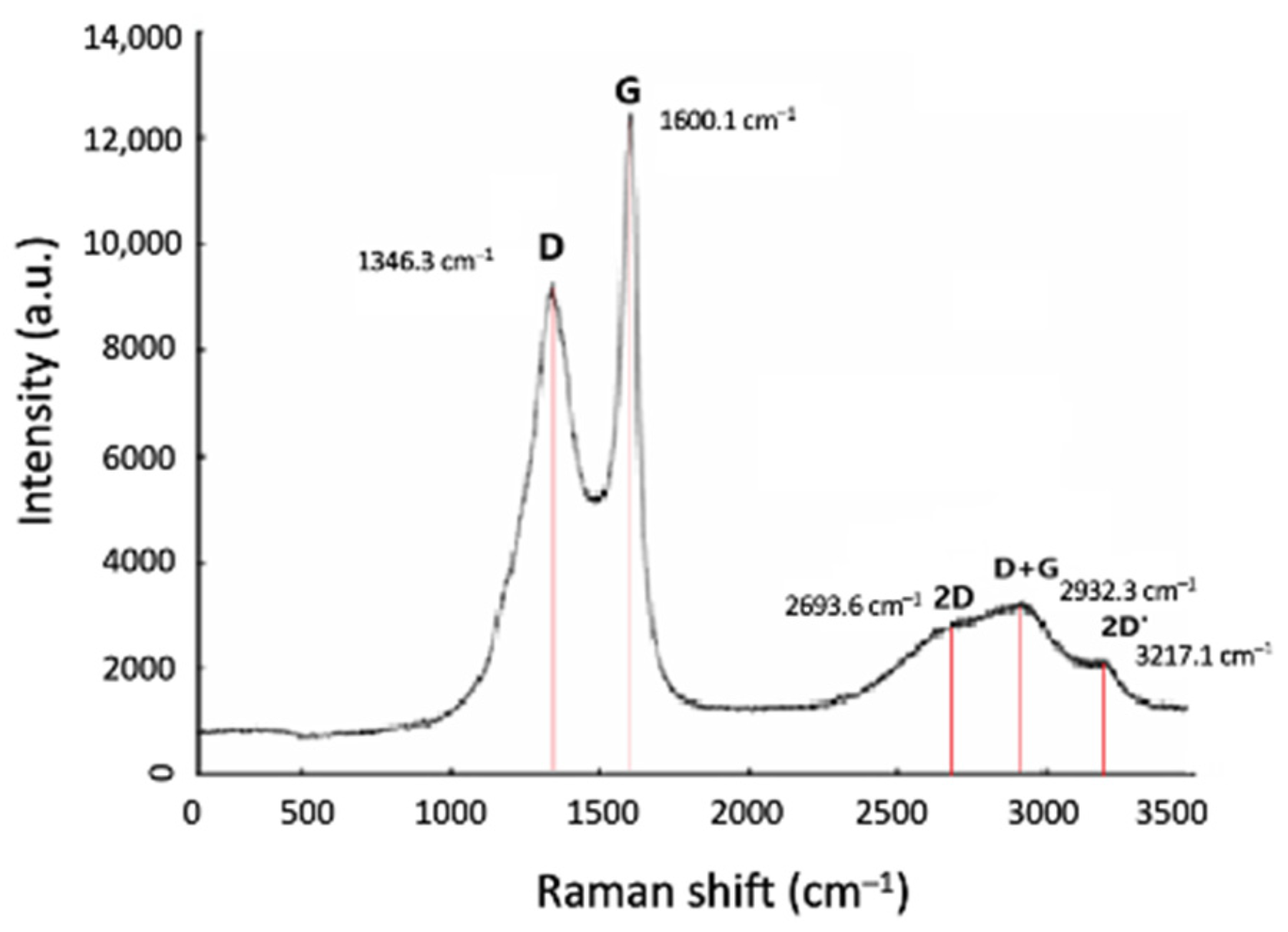
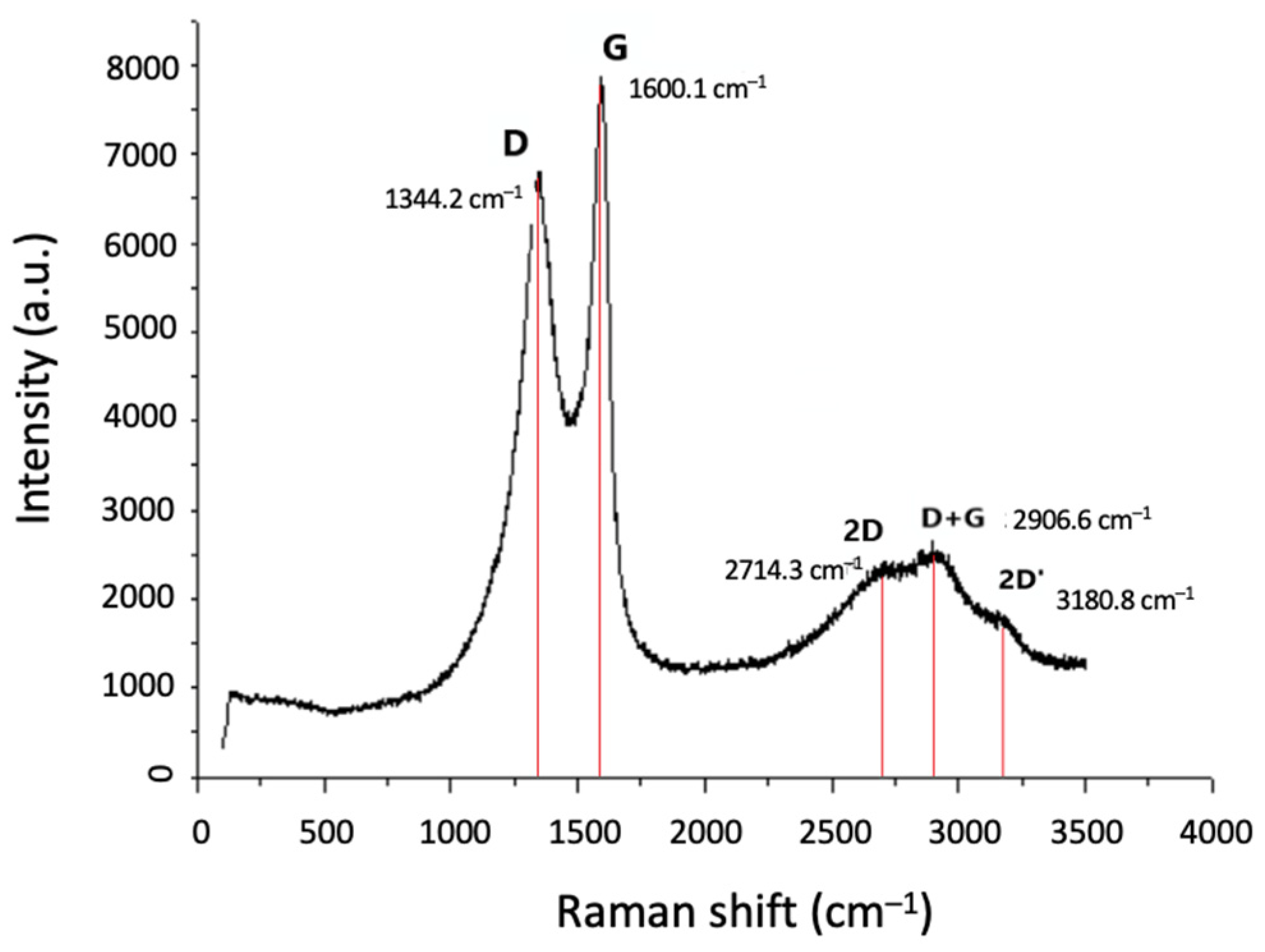
3.3.2. Morphological and Structural Characterisation of CNF/CNT Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Lukaszewicz, D. Automotive composite structures for crashworthiness. In Advanced Composite Materials for Automotive Applications: Structural Integrity and Crashworthiness; Elmarakbi, A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tindi, A.; Carallo, G.A.; Greco, A.; Romero-Sánchez, M.D.; Vertuccio, L.; Guadagno, L. Effective Practical Solutions for De-Icing of Automotive Component. Nanomaterials 2022, 12, 2979. [Google Scholar] [CrossRef] [PubMed]
- Taherian, R.; Ghorbani, M.M.; Nasr, M.; Kiahosseini, S.R. Fabrication and investigation of polymer-based carbon composite as gas diffusion layer of proton exchange membrane of fuel cells. Mater. Sci. Adv. Comp. Mater. 2018, 1, 1–12. [Google Scholar] [CrossRef]
- Meng, X.; Deng, X.; Zhou, L.; Hu, B.; Tan, W.; Zhou, W.; Liu, M.; Shao, Z. A Highly Ordered Hydrophilic–Hydrophobic Janus Bi-Functional Layer with Ultralow Pt Loading and Fast Gas/Water Transport for Fuel Cells. Energy Environ. Mater. 2021, 4, 126–133. [Google Scholar] [CrossRef]
- Zasypkina, A.A.; Ivanova, N.A.; Spasov, D.D.; Mensharapov, R.M.; Alekseeva, O.K.; Vorobyeva, E.A.; Kukueva, E.V.; Fateev, V.N. Electrode with a Carbon Nanotube Array for a Proton Exchange Membrane Fuel Cell. Inorganics 2023, 11, 219. [Google Scholar] [CrossRef]
- Yin, M.; Hasier, L.; Nash, P. A review of phase equilibriain Heusler alloy systems containing Fe, Co or Ni. J. Mater. Sci. 2016, 51, 50–70. [Google Scholar] [CrossRef]
- Graf, T.; Felser, C.; Parkin, S.S.P. Heusler compounds: Applications in spintronics. In Handbook of Spin-Tronics; Yongbing, X., Awschalom, D.D., Nitta, J., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 335–364. [Google Scholar] [CrossRef]
- Nishino, Y.; Kato, H.; Kato, M.; Mizutani, U. Effect of off-stoichiometry on the transport properties of the Heusler-type Fe2VAl compound. Phys. Rev. B 2001, 63, 233303. [Google Scholar] [CrossRef]
- Planes, A.; Mañosa, L.; Acet, M. Magnetocaloric effect and its relation to shape-memory properties in ferromagnetic Heusler alloys. J. Phys. Condens. Matter 2009, 21, 233201. [Google Scholar] [CrossRef] [PubMed]
- Rogl, G.; Rogl, P.F. Development of Thermoelectric Half-Heusler Alloys over the Past 25 Years. Crystals 2023, 13, 1152. [Google Scholar] [CrossRef]
- Zhu, H.; Li, W.; Nozariasbmarz, A.; Liu, N.; Zhang, Y.; Priya, S.; Poudel, B. Half-Heusler alloys as emerging high power density thermoelectric cooling materials. Nat. Commun. 2023, 14, 3300. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Kameoka, S.; Tsai, A.-P. The emergence of Heusler alloy catalysts. Sci. Technol. Adv. Mater. 2019, 20, 445–455. [Google Scholar] [CrossRef]
- Kojima, T.; Kameoka, S.; Tsai, A.-P. Catalytic Properties of Heusler Alloys for Steam Reforming of Methanol. ACS Omega 2019, 4, 21666–21674. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Kameoka, S.; Tsai, A.-P. Heusler Alloys: A Group of Novel Catalysts. ACS Omega 2017, 2, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Wakayama, T.; Oi, Y. Catalytic Characteristics of Component Elements in Heusler Alloys for Hydrogenation of Propyne. Mater. Trans. 2024, 65, 530–533. [Google Scholar] [CrossRef]
- Kojima, T.; Nakajima, S.; Tate, S. Intermetallic Catalysts Consisting of Early 3d Transition Metals and p-Block Metals for Ammonia Decomposition. Mater. Trans. 2024, 65, 218–523. [Google Scholar] [CrossRef]
- Mushtaq, M.; Algethami, N.; Khan, M.A.R.; Ayesh, A.I.; Mateen, M.; Laref, A.; Abdelmohsen, S.A.; Hossain, M.K. Adsorption of NOx (x = 1, 2) Molecules on the CoFeMnSi(001) Surface: First-Principles Insights. ACS Omega 2023, 8, 14005–14012. [Google Scholar] [CrossRef]
- Trompeta, A.F.; Koklioti, M.A.; Perivoliotis, D.K.; Lynch, I.; Charitidis, C.A. Towards a holistic environmental impact assessment of carbon nanotube growth through chemical vapour deposition. J. Clean. Prod. 2016, 129, 384–394. [Google Scholar] [CrossRef]
- Gakis, G.P.; Termine, S.; Trompeta, A.F.A.; Aviziotis, I.G.; Charitidis, C.A. Unraveling the mechanisms of carbon nanotube growth by chemical vapor deposition. Chem. Eng. J. 2022, 445, 136807. [Google Scholar] [CrossRef]
- Gakis, G.P.; Skountzos, E.N.; Aviziotis, I.G.; Charitidis, C.A. Multi-parametric analysis of the CVD of CNTs: Effect of reaction temperature, pressure and acetylene flow rate. Chem. Eng. Sci. 2023, 267, 118374. [Google Scholar] [CrossRef]
- Gakis, G.P.; Vergnes, H.; Scheid, E.; Vahlas, C.; Boudouvis, A.G.; Caussat, B. Detailed investigation of the surface mechanisms and their interplay with transport phenomena in alumina atomic layer deposition from TMA and water. Chem. Eng. Sci. 2019, 195, 399–412. [Google Scholar] [CrossRef]
- Gkinis, P.A.; Aviziotis, I.G.; Koronaki, E.D.; Gakis, G.P.; Boudouvis, A.G. The effects of flow multiplicity on GaN deposition in a rotating disk CVD reactor. J. Cryst. Growth 2017, 458, 140–148. [Google Scholar] [CrossRef]
- Koronaki, E.D.; Gakis, G.P.; Cheimarios, N.; Boudouvis, A.G. Efficient tracing and stability analysis of multiple stationary and periodic states with exploitation of commercial CFD software. Chem. Eng. Sci. 2016, 150, 26–34. [Google Scholar] [CrossRef]
- COMSOL Multiphysics. COMSOL AB; COMSOL Multiphysics: Stockholm, Sweden, 2019. [Google Scholar]
- Kee, R.J.; Rupley, F.M.; Miller, J.A.; Coltrin, M.E.; Grcar, J.F.; Meeks, E.; Moffat, H.K.; Lutz, A.E.; Dixon-Lewis, G.; Smooke, M.D.; et al. CHEMKIN Collection, Release 3.6; Reaction Design, Inc.: San Diego, CA, USA, 2000. [Google Scholar]
- Rokstad, O.A.; Lindvaag, O.A.; Holmen, A. Acetylene pyrolysis in tubular reactor. Int. J. Chem. Kinet. 2014, 46, 104–115. [Google Scholar] [CrossRef]
- Colket, M.B. Kinetic Mechanism for Pyrolysis of Acetylene near 1000K. In Proceedings of the American Chemical Society National Meeting, New Orleans, LA, USA, 30 August–4 September 1987; pp. 417–427. [Google Scholar]
- Ma, H.; Pan, L.; Nakayama, Y. Modelling the growth of carbon nanotubes produced by chemical vapor deposition. Carbon 2011, 49, 854–861. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Lett. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Puretzky, A.A.; Geohegan, D.B.; Jesse, S.; Ivanov, I.N.; Eres, G. In situ measurements and modeling of carbon nanotube array growth kinetics during chemical vapor deposition. Appl. Phys. A Mater. Sci. Process. 2005, 81, 223–240. [Google Scholar] [CrossRef]
- Frenklach, M.; Taki, S.; Durgaprasad, M.B.; Matula, R.A. Soot formation in shock-tube pyrolysis of acetylene, allene, and 1,3-butadiene. Combust. Flame 1983, 54, 81–101. [Google Scholar] [CrossRef]
- Musso, S.; Porro, S.; Rovere, M.; Giorcelli, M.; Tagliaferro, A. Fluid dynamic analysis of gas flow in a thermal-CVD system designed for growth of carbon nanotubes. J. Cryst. Growth 2008, 310, 477–483. [Google Scholar] [CrossRef]
- Picher, M.; Anglaret, E.; Jourdain, V. High temperature activation and deactivation of single-walled carbon nanotube growth investigated by in situ Raman measurements. Diam. Relat. Mater. 2010, 19, 581–585. [Google Scholar] [CrossRef]
- Feng, X.; Liu, K.; Xie, X.; Zhou, R.; Zhang, L.; Li, Q.; Fan, S.; Jiang, K. Thermal analysis study of the growth kinetics of carbon nanotubes and epitaxial graphene layers on them. J. Phys. Chem. C 2009, 113, 9623–9631. [Google Scholar] [CrossRef]
- Tan, L.-L.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Growth of carbon nanotubes over non-metallic based catalysts: A review on the recent developments. Catal. Today 2013, 217, 1–12. [Google Scholar] [CrossRef]
- Chen, C.-M.; Dai, Y.-M.; Huang, J.G.; Jehng, J.-M. Intermetallic catalyst for carbon nanotubes (CNTs) growth by thermal chemical vapor deposition method. Carbon 2006, 44, 1808–1820. [Google Scholar] [CrossRef]
- Surya, V.J.; Iyakutti, K.; Venkataramanan, N.; Mizuseki, H.; Kawazoe, Y. The role of Li and Ni metals in the adsorbate complex and their effect on the hydrogen storage capacity of single walled carbon nanotubes coated with metal hydrides, LiH and NiH2. Int. J. Hydrogen Energy 2010, 35, 2368–2376. [Google Scholar] [CrossRef]
- Jianwei, R.; Shijun, L.; Junmin, L. Hydrogen storage of multiwalled carbon nanotubes coated with Pd-Ni nanoparticles under moderate conditions. Chin. Sci. Bull. 2006, 51, 2959–2963. [Google Scholar] [CrossRef]






| ID | C2H2 Flow (mL/min) | N2 Flow (mL/min) | Reaction Time (min) | Reaction Temperature (°C) | Extra Steps |
|---|---|---|---|---|---|
| HH1 | 46.4 | 180 | 30 | 700 | - |
| HH2 (repeated) | 46.4 | 180 | 30 | 700 | - |
| HH3 | 46.4 | 200 | 30 | 700 | H2 flow 100 mL/min |
| HH4 | 46.4 | 360 | 15 | 700 | Thermal annealing at 400 °C |
| HH5 | 46.4 | 360 | 15 | 750 | - |
| Element | Point 1 | Point 2 | Point 3 |
|---|---|---|---|
| C | 13.8 | 2.9 | 0.0 |
| Ti | 6.6 | 0.4 | 25.1 |
| Ni | 1.4 | 23.7 | 3.6 |
| Zr | 74.0 | 0.0 | 27.1 |
| Sn | 4.2 | 73.0 | 44.2 |
| Sb | 0.0 | 0.0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aviziotis, I.G.; Manasi, A.; Ntziouni, A.; Gakis, G.P.; Trompeta, A.-F.A.; Li, X.; Dong, H.; Charitidis, C.A. Growth of Carbon Nanofibers and Carbon Nanotubes by Chemical Vapour Deposition on Half-Heusler Alloys: A Computationally Driven Experimental Investigation. Materials 2024, 17, 3144. https://doi.org/10.3390/ma17133144
Aviziotis IG, Manasi A, Ntziouni A, Gakis GP, Trompeta A-FA, Li X, Dong H, Charitidis CA. Growth of Carbon Nanofibers and Carbon Nanotubes by Chemical Vapour Deposition on Half-Heusler Alloys: A Computationally Driven Experimental Investigation. Materials. 2024; 17(13):3144. https://doi.org/10.3390/ma17133144
Chicago/Turabian StyleAviziotis, Ioannis G., Apostolia Manasi, Afroditi Ntziouni, Georgios P. Gakis, Aikaterini-Flora A. Trompeta, Xiaoying Li, Hanshan Dong, and Costas A. Charitidis. 2024. "Growth of Carbon Nanofibers and Carbon Nanotubes by Chemical Vapour Deposition on Half-Heusler Alloys: A Computationally Driven Experimental Investigation" Materials 17, no. 13: 3144. https://doi.org/10.3390/ma17133144






