Mechanochemical Synthesis of Resveratrol–Piperazine Cocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Conventional Synthesis of the Cocrystal
2.3. Mechanochemical Synthesis
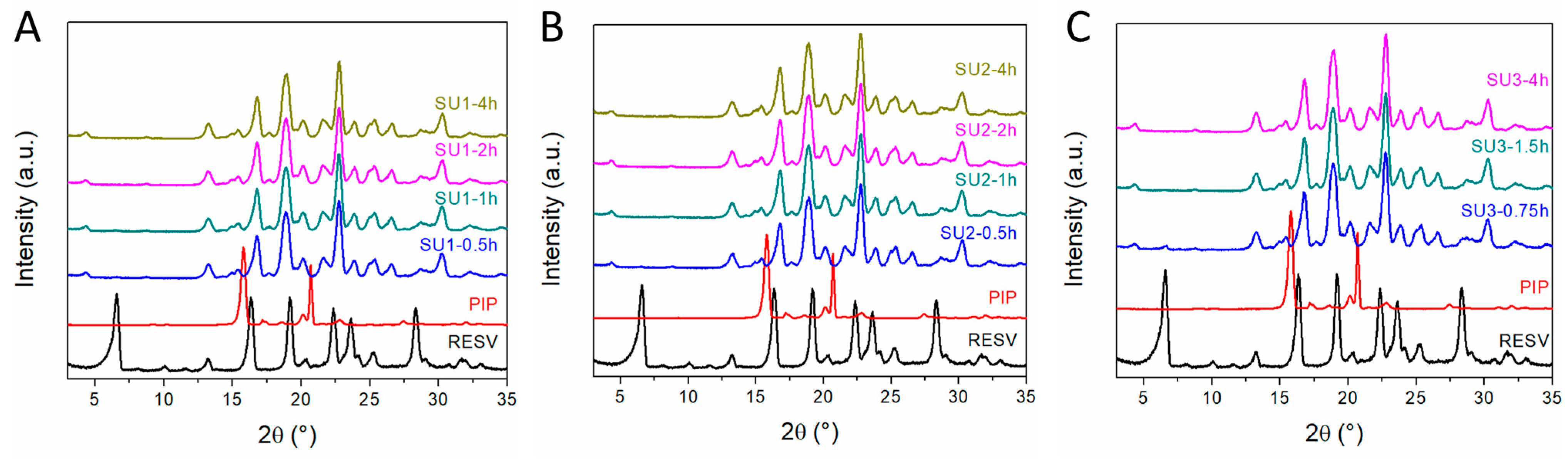
2.4. Mechanochemical Scale-Up
2.5. Characterization Methods
3. Results and Discussion
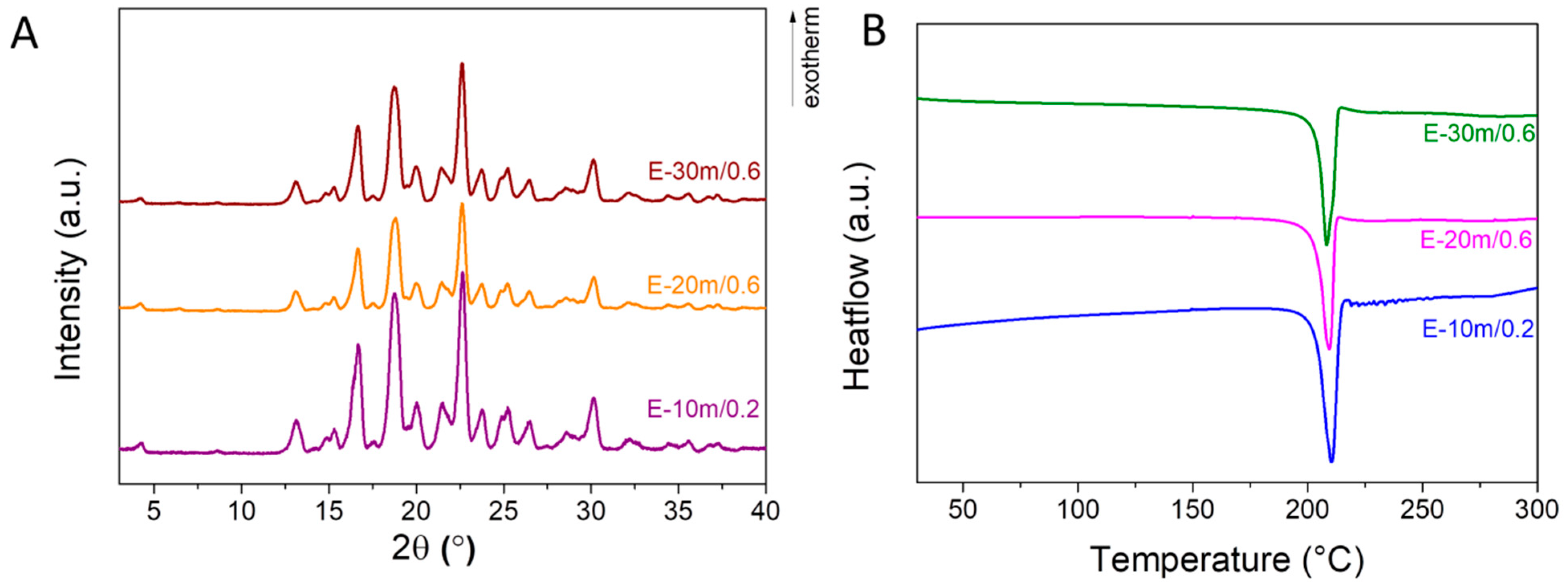
3.1. Influence of Solvent Addition
3.2. Thermodynamic Characterization of the Cocrystals
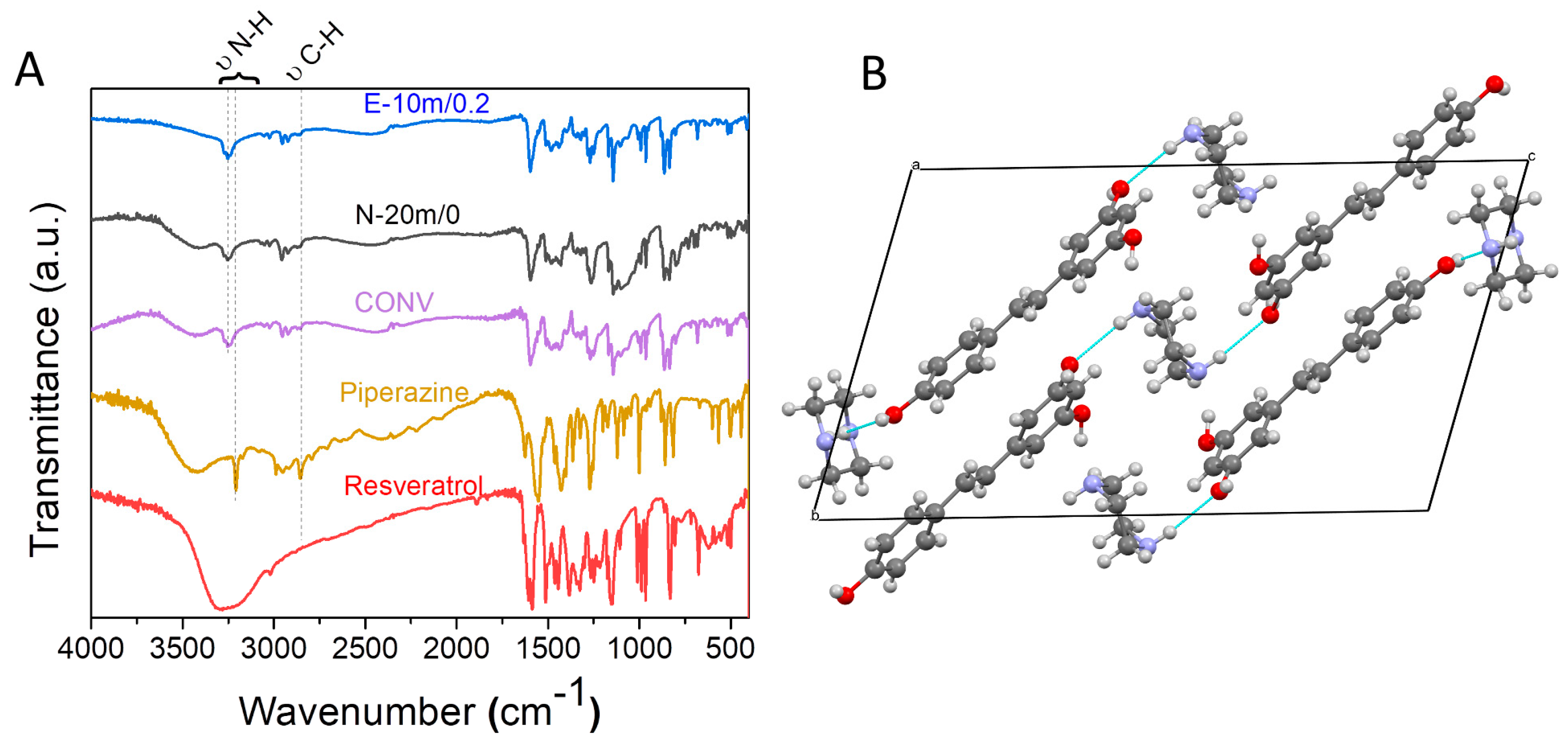
3.3. Physico-Chemical Characterization of the Resveratrol–Piperazine Cocrystal
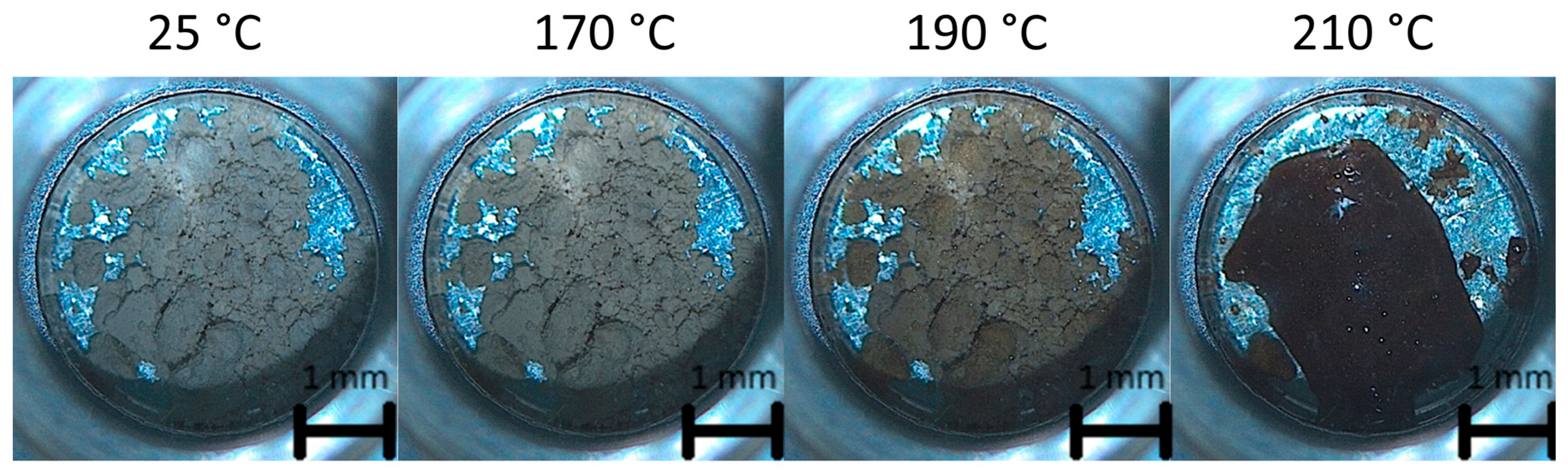
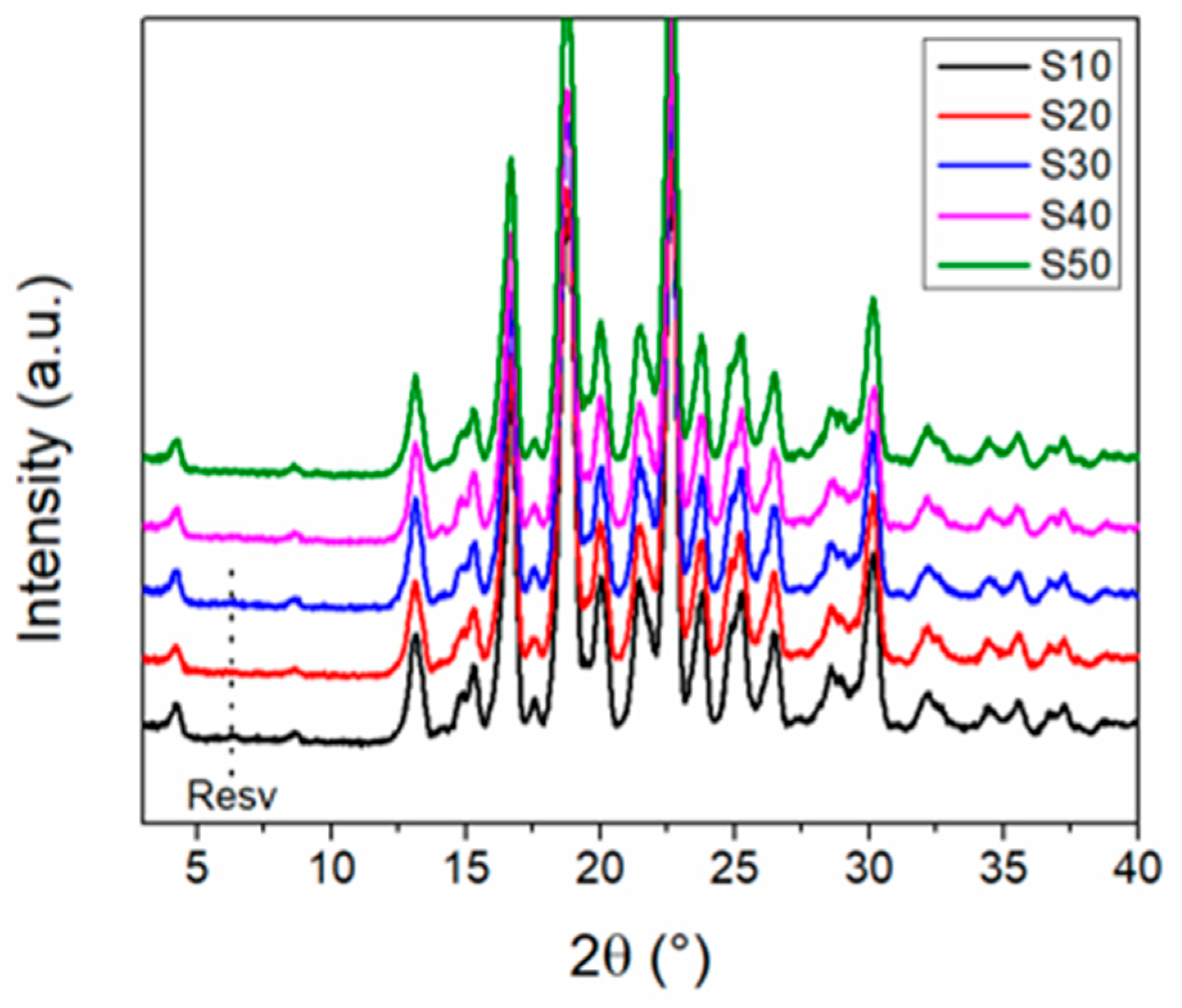
3.4. Scale-Up Experiments of the Resveratrol–Piperazine Cocrystal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Boeckel, T.P.V. Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Piao, M.; Tu, Y.; Zhang, N.; Diao, Q.; Bi, Y. Advances in the Application of Phytogenic Extracts as Antioxidants and Their Potential Mechanisms in Ruminants. Antioxidants. 2023, 12, 879. [Google Scholar] [CrossRef]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef]
- Phillips, C.J.C.; Hosseintabar-Ghasemabad, B.; Gorlov, I.F.; Slozhenkina, M.I.; Mosolov, A.A.; Seidavi, A. Immunomodulatory Effects of Natural Feed Additives for Meat Chickens. Life 2023, 13, 1287. [Google Scholar] [CrossRef]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Tin, Y.-Y.; Soe, M.-T.-P.; Ko, B.; Park, S.; Lee, J. Recent technologies for amorphization of poorly water-soluble drugs. Pharmaceutics 2021, 13, 1318. [Google Scholar] [CrossRef]
- Knopp, M.M.; Nguyen, J.H.; Mu, H.; Langguth, P.; Rades, T.; Holm, R. Influence of copolymer composition on in vitro and in vivo performance of celecoxib-PVP/VA amorphous solid dispersions. AAPS J. 2016, 18, 416–423. [Google Scholar] [CrossRef]
- Băran, A.; Aricov, L.; Stîngă, G.; Iovescu, A.; Leontieş, A.-R.; Jerca, V.V. The effect of C12E6 nonionic surfactant on the solubilization of Eosin Y in unmodified- and hydrophobically modified poly(Acrylic acid) solutions. J. Mol. Liq. 2022, 346, 117103. [Google Scholar] [CrossRef]
- Gîfu, I.C.; Maxim, M.E.; Iovescu, A.; Aricov, L.; Simion, E.L.; Leontieş, A.R.; Anastasescu, M.; Munteanu, C.; Anghel, D.-F. Natural aging of multilayer films containing hydrophobically modified poly(acrylate)s or their complexes with surfactants. Appl. Surf. Sci. 2017, 412, 489–496. [Google Scholar] [CrossRef]
- Nicoud, L.; Licordari, F.; Myerson, A.S. Estimation of the solubility of metastable polymorphs: A critical review. Cryst. Growth Des. 2018, 18, 7228–7237. [Google Scholar] [CrossRef]
- Serajuddin, A.T. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef]
- Patole, T.; Deshpande, A. Co-crystallization-a technique for solubility enhancement. Int. J. Pharm. Sci. Res. 2014, 5, 3566. [Google Scholar]
- Singh, M.; Barua, H.; Jyothi, V.G.S.S.; Dhondale, M.R.; Nambiar, A.G.; Agrawal, A.K.; Kumar, P.; Shastri, N.R.; Kumar, D. Cocrystals by Design: A Rational Coformer Selection Approach for Tackling the API Problems. Pharmaceutics 2023, 15, 1161. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Sun, W.-J.; Lu, T.; Tong, H.H.Y.; Sun, C.C.; Zheng, Y. Resveratrol cocrystals with enhanced solubility and tabletability. Int. J. Pharm. 2016, 509, 391–399. [Google Scholar] [CrossRef]
- Pop, M.M.; Bulieris, P.V.; Pongratz, I.; Fruth-oprisan; Mitran, R.-A. Resveratrol-Piperazine Co-Crystals. U.S. Patent 11,560,358, 9 April 2019. [Google Scholar]
- Mehta, B.K.; Singh, S.S.; Chaturvedi, S.; Wahajuddin, M.; Thakur, T.S. Rational Coformer Selection and the Development of New Crystalline Multicomponent Forms of Resveratrol with Enhanced Water Solubility. Cryst. Growth Des. 2018, 18, 1581–1592. [Google Scholar] [CrossRef]
- Martin, R.J. Modes of action of anthelmintic drugs. Vet. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Lu, J.; Rohani, S. Preparation and characterization of theophylline−nicotinamide cocrystal. Org. Process Res. Dev. 2009, 13, 1269–1275. [Google Scholar] [CrossRef]
- Haskins, M.M.; Zaworotko, M.J. Screening and Preparation of Cocrystals: A Comparative Study of Mechanochemistry vs. Slurry Methods. Cryst. Growth Des. 2021, 21, 4141–4150. [Google Scholar] [CrossRef]
- Weyna, D.R.; Shattock, T.; Vishweshwar, P.; Zaworotko, M.J. Synthesis and structural characterization of cocrystals and pharmaceutical cocrystals: Mechanochemistry vs. slow evaporation from solution. Cryst. Growth Des. 2009, 9, 1106–1123. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, C.; Hu, X.; Chen, K.; Qi, L.; Cui, P.; Zhou, L.; Yin, Q. Mechanochemical Synthesis of Cocrystal: From Mechanism to Application. Cryst. Growth Des. 2023, 23, 4680–4700. [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la OContreras, C.M.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M. Mechanochemistry: A Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef]
- Hugo, K.C. Confinement effects on freezing and melting. J. Phys. Condens. Matter 2001, 13, R95. [Google Scholar] [CrossRef]
- Glasgow, A.R.; Ross, G.S.; Horton, A.T.; Enagonio, D.; Dixon, H.D.; Saylor, C.P.; Furukawa, G.T.; Reilly, M.L.; Henning, J.M. Comparison of cryoscopic determinations of purity of benzene by thermometric and calorimetric procedures. Anal. Chim. Acta 1957, 17, 54–79. [Google Scholar] [CrossRef]
- Van’t Hoff, J., XII. The function of osmotic pressure in the analogy between solutions and gases. Lond. Edinb. Philos. Mag. J. Sci. 1888, 26, 81–105. [Google Scholar] [CrossRef]
- Juère, E.; Florek, J.; Bouchoucha, M.; Jambhrunkar, S.; Wong, K.Y.; Popat, A.; Kleitz, F. In Vitro Dissolution, Cellular Membrane Permeability, and Anti-Inflammatory Response of Resveratrol-Encapsulated Mesoporous Silica Nanoparticles. Mol. Pharm. 2017, 14, 4431–4441. [Google Scholar] [CrossRef]



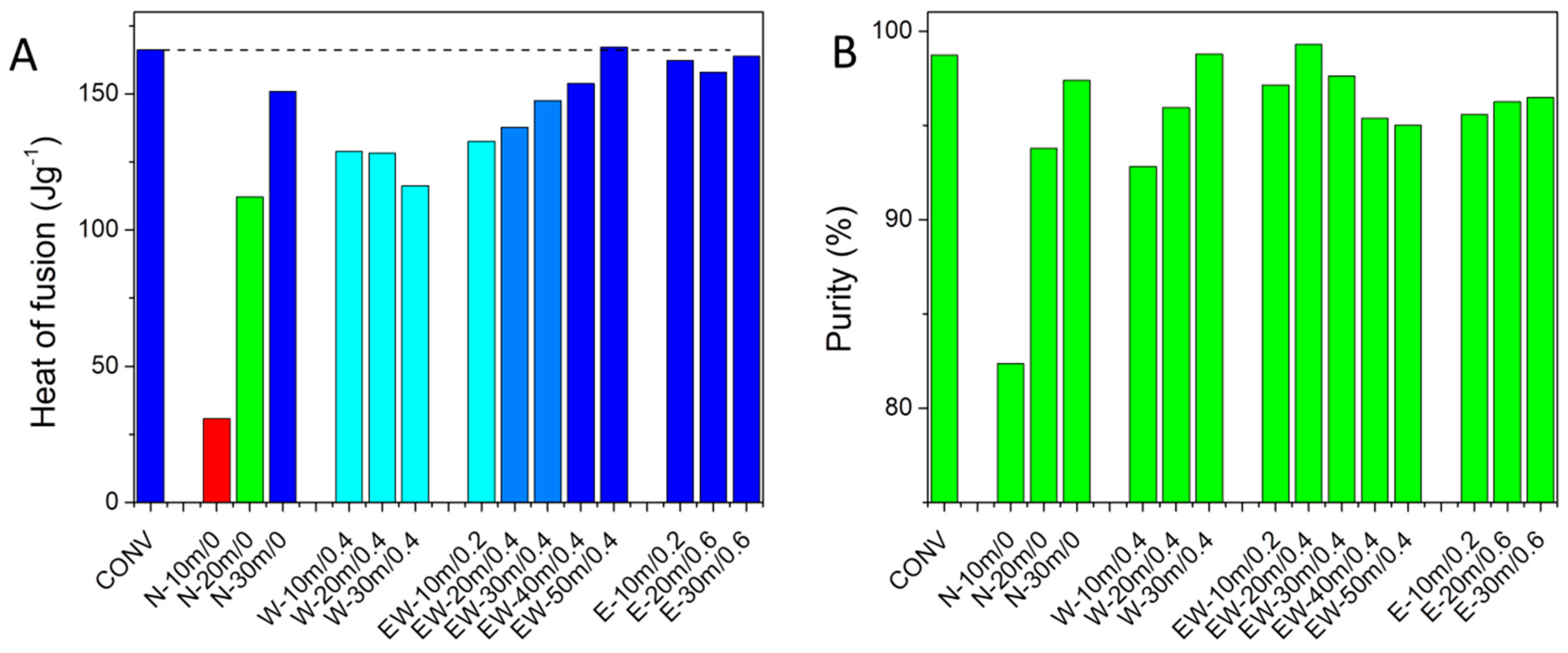
| Sample | Solvent | Time (min) | Vsolvent/mcocrystal (mL g−1) | ΔH (Jg−1) |
|---|---|---|---|---|
| Conv | - | - | - | 166.2 |
| N-10m/0 | None | 10 | 0 | 30.7 |
| N-20m/0 | 20 | 0 | 112.2 | |
| N-30m/0 | 30 | 0 | 150.8 | |
| W-10m/0.4 | H2O | 10 | 0.4 | 128.8 |
| W-20m/0.4 | 20 | 0.4 | 128.2 | |
| W-30m/0.4 | 30 | 0.4 | 116.3 | |
| EW-10m/0.2 | EtOH:H2O 1:1 (V/V) | 10 | 0.4 | 132.6 |
| EW-20m/0.4 | 20 | 0.4 | 137.7 | |
| EW-30m/0.4 | 30 | 0.4 | 147.5 | |
| EW-40m/0.4 | 40 | 0.4 | 153.8 | |
| EW-50m/0.4 | 50 | 0.4 | 167.1 | |
| E-10m/0.2 | EtOH | 10 | 0.6 | 162.2 |
| E-20m/0.6 | 20 | 0.6 | 158.0 | |
| E-30m/0.6 | 30 | 0.6 | 163.8 |
| Sample | Solubility in PBS | |
|---|---|---|
| (μg mL−1) | (%) | |
| Resveratrol | 63.0 ± 0.5 | 100 ± 1.1 |
| N-10m/0 | 69.9 ± 2.9 | 111 ± 4.7 |
| E-20m/0.6 | 73.2 ± 0.9 | 116.2 ± 1.7 |
| CONV | 67.0± 0.6 | 106.3 ± 1.3 |
| Sample | mRESV (g) | mPIP (g) | Vsolvent/mcocrystal (mL g−1) |
| SU1 | 25.0 | 9.53 | 0.6 |
| SU2 | 18.24 | 6.95 | 1.4 |
| SU3 | 101.72 | 38.8 | 1.0 |
| Sample | Time (min) | Onset (°C) | ΔH (Jg−1) |
|---|---|---|---|
| S10 | 10 | 204.9 | 146.6 |
| S20 | 20 | 202.9 | 142.1 |
| S30 | 30 | 204.4 | 144.0 |
| S40 | 40 | 204.7 | 145.5 |
| S50 | 50 | 203.4 | 148.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, R.-A.; Ioniţă, S.; Lincu, D.; Soare, E.M.; Atkinson, I.; Rusu, A.; Pandele-Cuşu, J.; Iordache, C.; Pongratz, I.; Pop, M.M.; et al. Mechanochemical Synthesis of Resveratrol–Piperazine Cocrystals. Materials 2024, 17, 3145. https://doi.org/10.3390/ma17133145
Mitran R-A, Ioniţă S, Lincu D, Soare EM, Atkinson I, Rusu A, Pandele-Cuşu J, Iordache C, Pongratz I, Pop MM, et al. Mechanochemical Synthesis of Resveratrol–Piperazine Cocrystals. Materials. 2024; 17(13):3145. https://doi.org/10.3390/ma17133145
Chicago/Turabian StyleMitran, Raul-Augustin, Simona Ioniţă, Daniel Lincu, Elena Mirabela Soare, Irina Atkinson, Adriana Rusu, Jeanina Pandele-Cuşu, Coca Iordache, Ingemar Pongratz, Mihaela Maria Pop, and et al. 2024. "Mechanochemical Synthesis of Resveratrol–Piperazine Cocrystals" Materials 17, no. 13: 3145. https://doi.org/10.3390/ma17133145





