Evaluation of Ammonium Nitrate(V) Morphology and Porosity Obtained by SEM and Tomography Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
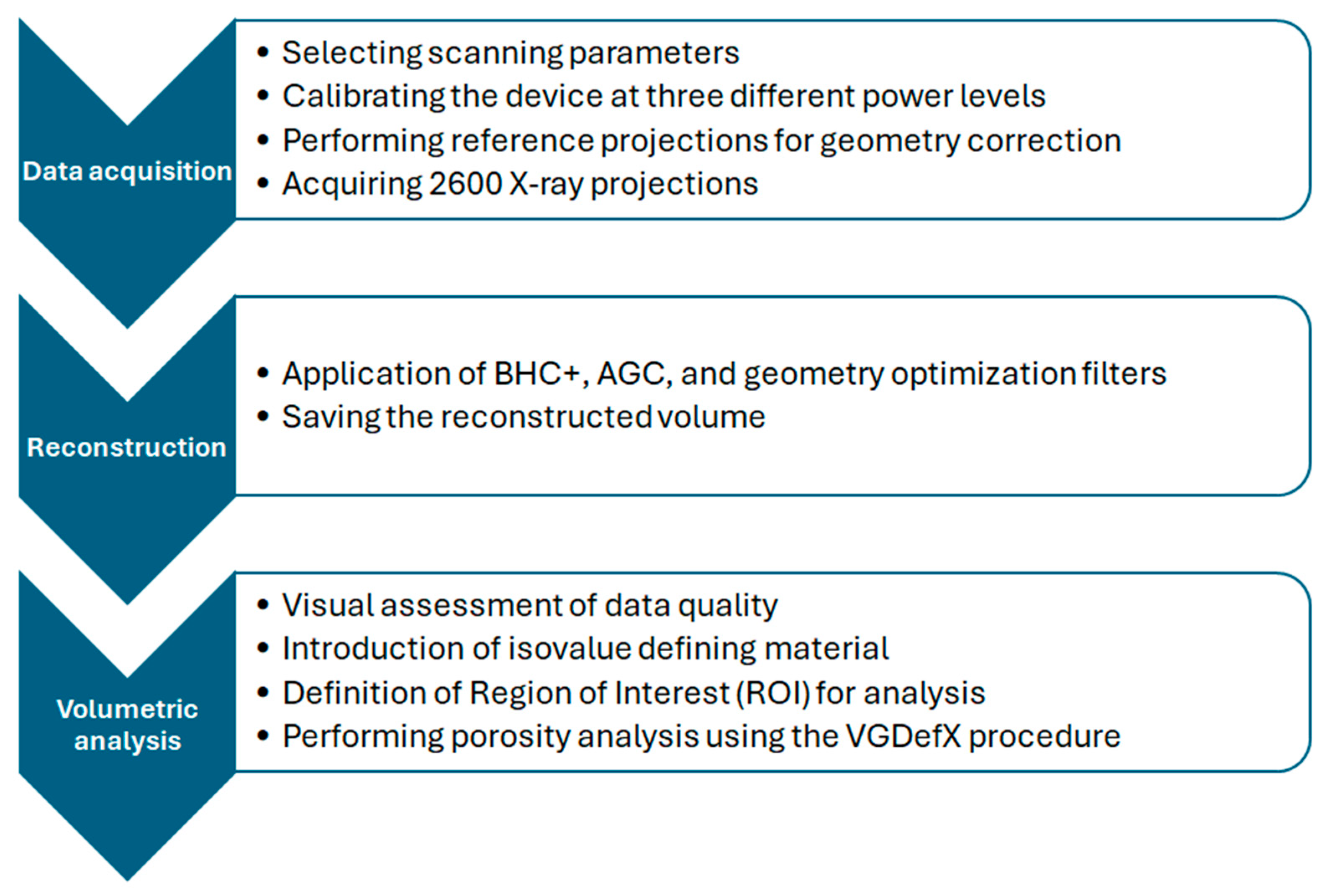
2.2. Methods
3. Results and Discussion
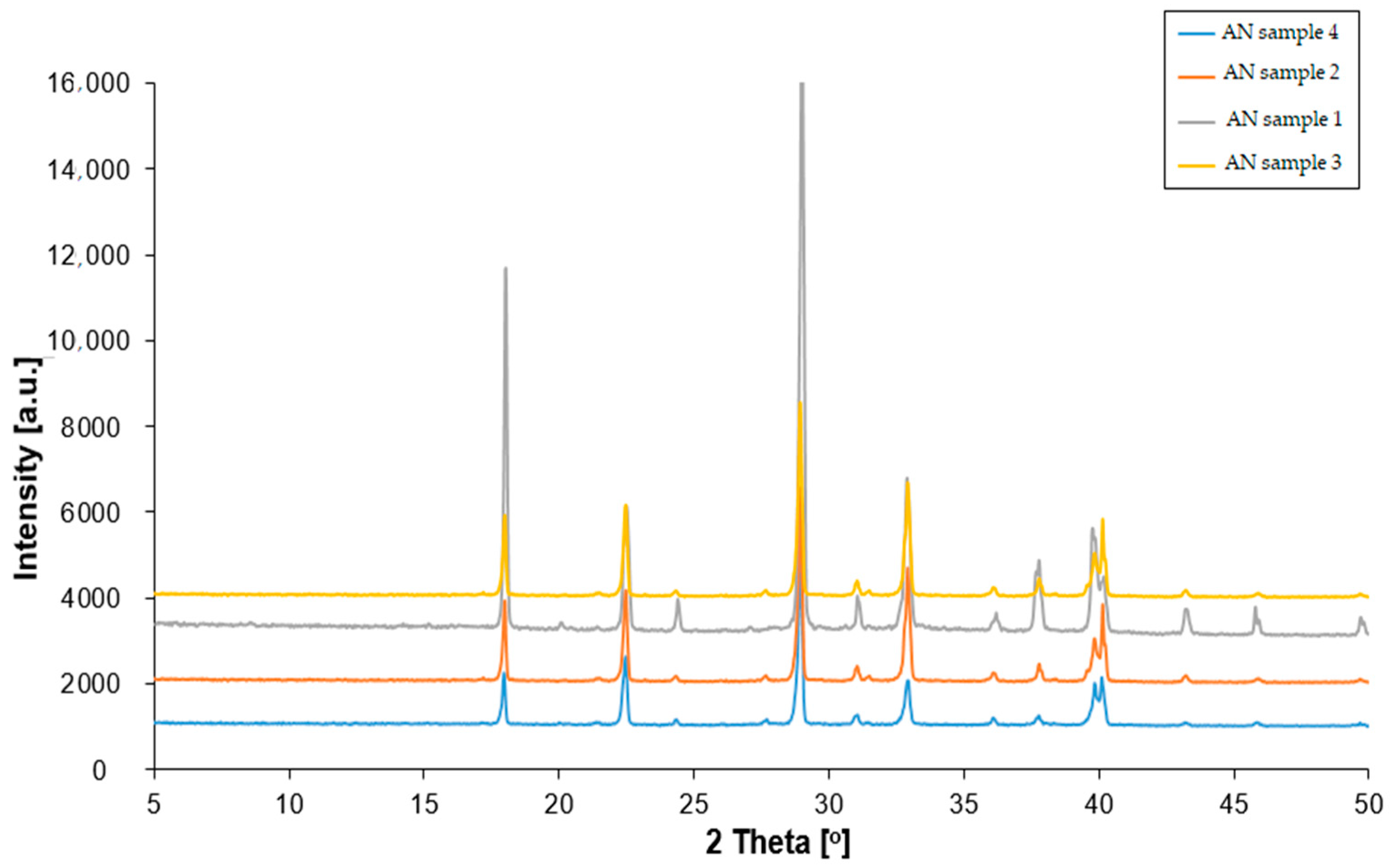
3.1. XRD Analysis
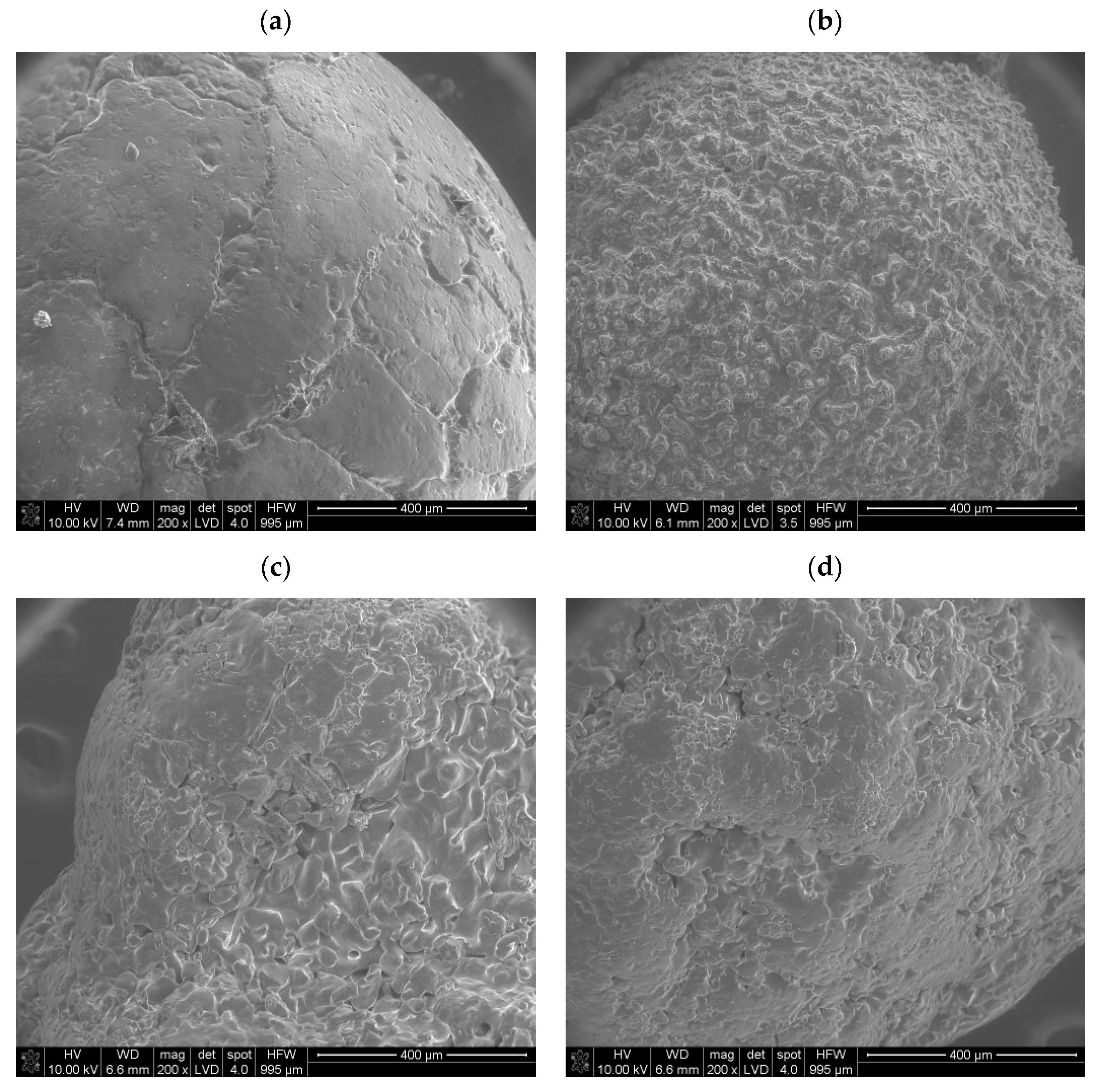
3.2. Results of SEM
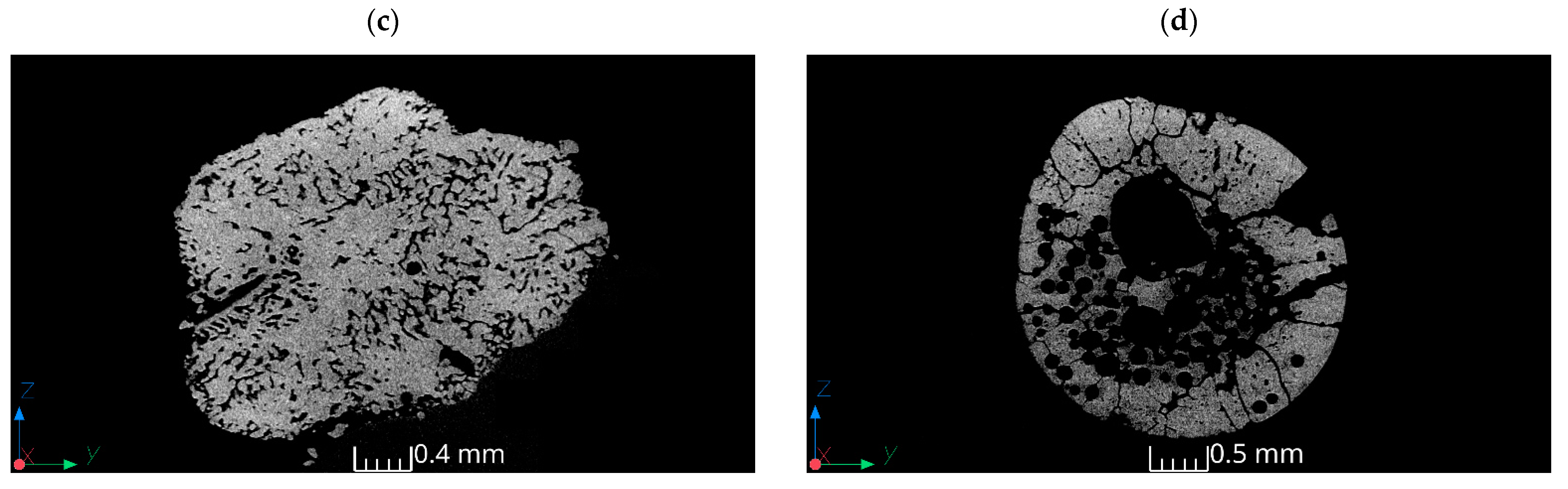
3.3. Results of Tomography
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pittman, W.; Han, Z.; Harding, B.; Rosas, C.; Jiang, J.; Pineda, A.; Mannan, M.S. Lessons to be learned from an analysis of ammonium nitrate disasters in the last 100 years. J. Hazard. Mater. 2014, 280, 472. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, P.; Dikovska, A.; Nikov, R.; Atanasova, G.; Grochowska, K.; Karczewski, J.; Fukata, N.; Jevasuwan, W.; Nedyalkov, N. Surface-Enhanced Raman Spectroscopy of Ammonium Nitrate Using Al Structures, Fabricated by Laser Processing of AlN Ceramic. Materials 2024, 17, 2254. [Google Scholar] [CrossRef] [PubMed]
- Léonard, F.; Zhang, Z.; Krebs, H.; Bruno, G. Structural and Morphological Quantitative 3D Characterisation of Ammonium Nitrate Prills by X-Ray Computed Tomography. Materials 2020, 13, 1230. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, S.; Fernández-Pérez, M.; Villafranca-Sánchez, M.; González-Pradas, E.; Flores-Céspedes, F. Controlled Release of Ammonium Nitrate from Ethylcellulose Coated Formulations. Ind. Eng. Chem. Res. 2007, 46, 3304–3311. [Google Scholar] [CrossRef]
- Tzika, M.; Alexandridou, S.; Kiparissides, C. Evaluation of the morphological and release characteristics of coated fertilizer granules produced in a Wurster fluidized bed. Powder Technol. 2003, 132, 16. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Jarosiewicz, A. Polysulfone coating with starch addition in CRF formulation. Desalination 2004, 163, 247–252. [Google Scholar] [CrossRef]
- Rao, K.V.R.; Hariharan, P.L.; Jagannathan, K.; Yoganarasimhan, S.R. Scanning electron microscopy of ammonium nitrate prills in relations to their application in ammonium nitrate-fuel oil systems. Fuel 1989, 68, 1118–1122. [Google Scholar]
- Maranda, A.; Czerwińska, A.; Paszula, J.; Jóźwik, P. Modification of detonation parameters of emulsion explosives by the addition of an oxidizer-fuel system. Przem. Chem. 2020, 19, 40–45. [Google Scholar]
- Zygmunt, B.; Buczkowski, D. Influence of Ammonium Nitrate Prills’ Properties on Detonation Velocity of ANFO. PropellantsExplos. Pyrotech. 2007, 32, 411–414. [Google Scholar] [CrossRef]
- Miyake, A.; Ogawa, T. Influence of Physical Properties of Ammonium Nitrate on the Detonation Velocities of ANFO. In Proceedings of the 24th International Pyrotechnic Seminar, Monterey, CA, USA, 27–31 July 1998; pp. 383–390. [Google Scholar]
- Jackson, S.I.; Kiyanda, C.B.; Short, M. Experimental observations of detonation in ammonium-nitrate-fuel-oil (ANFO) surrounded by a high-sound-speed, shockless, aluminum confiner. Proc. Combust. Inst. 2011, 33, 2219–2226. [Google Scholar] [CrossRef]
- Johansson, C.H.; Persson, P.A. Detonics of High Explosives; Academic Press: Cambridge, MA, USA, 1970. [Google Scholar]
- Biessikirski, A.; Pytlik, M.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Napruszewska, B.D. Influence of the Ammonium Nitrate(V) Porous Prill Assortments and Absorption Index on Ammonium Nitrate Fuel Oil Blasting Properties. Energies 2020, 13, 3763. [Google Scholar] [CrossRef]
- Lotspeich, E.; Petr, V. The characterization of ammonium nitrate mini-prills. In Dynamic Behavior of Materials; Song, B., Casem, D., Kimberley, J., Eds.; Conference Proceedings of the Society for Experimental Mechanics Series; Springer: Cham, Switzerland, 2015; Volume 1, pp. 319–325. [Google Scholar]
- Biessikirski, A.; Kuterasiński, Ł.; Pyra, J.; Dworzak, M. Comparison of properties of ammonium nitrates used of fertilizers and explosive materials. Przem. Chem. 2016, 95, 1381–1384. [Google Scholar]
- Biessikirski, A.; Kuterasiński, Ł. Research on Morphology and Topology of ANFO Based on Various Types of Oxygen Component; Wydawnictwa AGH: Kraków, Poland, 2018. [Google Scholar]
- Miyake, A.; Takahara, K.; Ogawa, T.; Ogata, Y.; Wada, Y.; Arai, H. Influence of physical properties of ammonium nitrate on the detonation behavior of ANFO. J. Loss Prev. Process Ind. 2001, 14, 533–538. [Google Scholar] [CrossRef]
- Viktorov, S.D.; Frantov, A.E.; Lapikov, I.N.; Andreev, V.V.; Starshinov, A.V. Effect of the microstructure of ammonium nitrate granules on the detonability of composite propellants based on it. Combust. Explos. Shock Waves 2016, 52, 727–731. [Google Scholar] [CrossRef]
- Onederra, I.; Araos, M. Blasting without Nitrogen Oxide Hazard. Altern. Explos. Developed. CRC Min. News 2015, 30, 5. [Google Scholar]
- Landucci, G.; Reniers, G.; Cozzani, V.; Salzano, E. Vulnerability of industrial facilities to attacks with imporivised devices aimed at triggering domino scenarios. Reliab. Eng. Syst. Saf. 2015, 143, 53–62. [Google Scholar] [CrossRef]
- Fabin, M.; Jarosz, T. Improving ANFO: Effect of Additives and Ammonium Nitrate Morphology on Detonation Parameters. Materials 2021, 14, 5745. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Q.S.M.; Jones, D.E.G. Thermodesorption studies of ammonium nitrate prills by high-resolution thermogravimetry. J. Therm. Anal. Calorim. 2003, 74, 57–63. [Google Scholar] [CrossRef]
- Zawadzka-Małota, I.; Maranda, A. The effect of chemical and physical properties and particle morphology of granulated ammonium nitrate on the composition of post-explosion gases and detonation velocity of ammonium nitrate fuel oils. Mater. Wysokoenergetyczne 2021, 13, 157. [Google Scholar]
- Vidal, F.P.; Mitchell, I.T.; Létang, J.M. Use of fast realistic simulations on GPU to extract CAD models from microtomographic data in the presence of strong CT artefacts. Precis. Eng. 2022, 74, 110. [Google Scholar] [CrossRef]
- Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Tatko, M.; Napruszewska, B.D. On the Influence of the Ammonium Nitrate(V) Provenance on Its Usefulness for the Manufacture of ANFO Type Explosives. Energies 2020, 13, 4942. [Google Scholar] [CrossRef]
- Kritikos, M. Porosity Measurement by X-ray Computed Tomography: Different Porosity Analysis Application. In Digital Conversion on the Way to Industry 4.0; Durakbasa, N.M., Gençyılmaz, M.G., Eds.; ISPR 2020. Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Kucharska, M.; Dybeł, P. Bond conditions in wall-type steel-reinforced self-compacting concrete element. J. Build. Eng. 2023, 75, 106944. [Google Scholar] [CrossRef]
- Kaczmarczyk, G.P.; Wałach, D.; Natividade-Jesus, E.; Ferreira, R. Change of the Structural Properties of High-Performance Concretes Subjected to Thermal Effects. Materials 2022, 15, 5753. [Google Scholar] [CrossRef] [PubMed]
- Tyc, A.; Nieweś, D.; Pankalla, E.; Huculak-Mączka, M.; Hoffmann, K.; Hoffmann, J. Anti-Caking Coatings for Improving the Useful Properties of Ammonium Nitrate Fertilizers with Composition Modeling Using Box–Behnken Design. Materials 2021, 14, 5761. [Google Scholar] [CrossRef] [PubMed]
- Maranda, A.; Paszula, J.; Zawadzka-Małota, I.; Kuczyńska, B.; Witkowski, W.; Nikolczuk, K.; Wilk, Z. Aluminum Powder Influence on ANFO Detonation Parameters. Cent. Eur. J. Energ. Mater. 2011, 8, 279–292. [Google Scholar]
- Arslan, I.; Talin, A.A.; Wang, G.T. Three-Dimensional Visualization of Surface Defects in Core−Shell Nanowires. J. Phys. Chem. 2008, 112, 11093. [Google Scholar] [CrossRef]
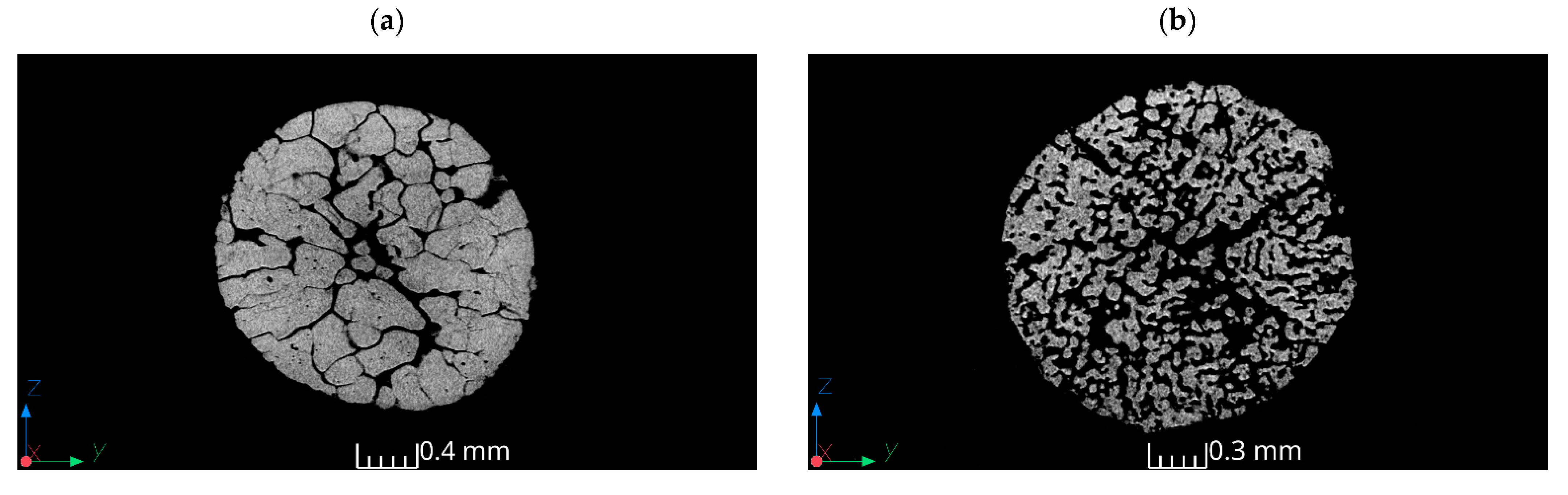
| Sample | Volume of the Material (mm3) | Surface Area of the Material (mm2) | Porosity (%) | Mean Diameter (mm) | Surface-to-Volume Ratio (mm⁻¹) |
|---|---|---|---|---|---|
| 1 | 5.48 | 115.99 | 48.87% | 2 | 21.17 |
| 2 | 2.1 | 144.74 | 62.93% | 1.6 | 68.92 |
| 3 | 8.65 | 358.05 | 13.45% | 3 | 41.39 |
| 4 | 9.1 | 329.6 | 70.63% | 3.2 | 36.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biessikirski, A.; Kaczmarczyk, G.P.; Kuterasiński, Ł.; Kolano, M.; Pytlik, M. Evaluation of Ammonium Nitrate(V) Morphology and Porosity Obtained by SEM and Tomography Imaging. Materials 2024, 17, 3156. https://doi.org/10.3390/ma17133156
Biessikirski A, Kaczmarczyk GP, Kuterasiński Ł, Kolano M, Pytlik M. Evaluation of Ammonium Nitrate(V) Morphology and Porosity Obtained by SEM and Tomography Imaging. Materials. 2024; 17(13):3156. https://doi.org/10.3390/ma17133156
Chicago/Turabian StyleBiessikirski, Andrzej, Grzegorz Piotr Kaczmarczyk, Łukasz Kuterasiński, Malwina Kolano, and Mateusz Pytlik. 2024. "Evaluation of Ammonium Nitrate(V) Morphology and Porosity Obtained by SEM and Tomography Imaging" Materials 17, no. 13: 3156. https://doi.org/10.3390/ma17133156








