Zinc/Magnesium Ferrite Nanoparticles Functionalized with Silver for Optimized Photocatalytic Removal of Malachite Green
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
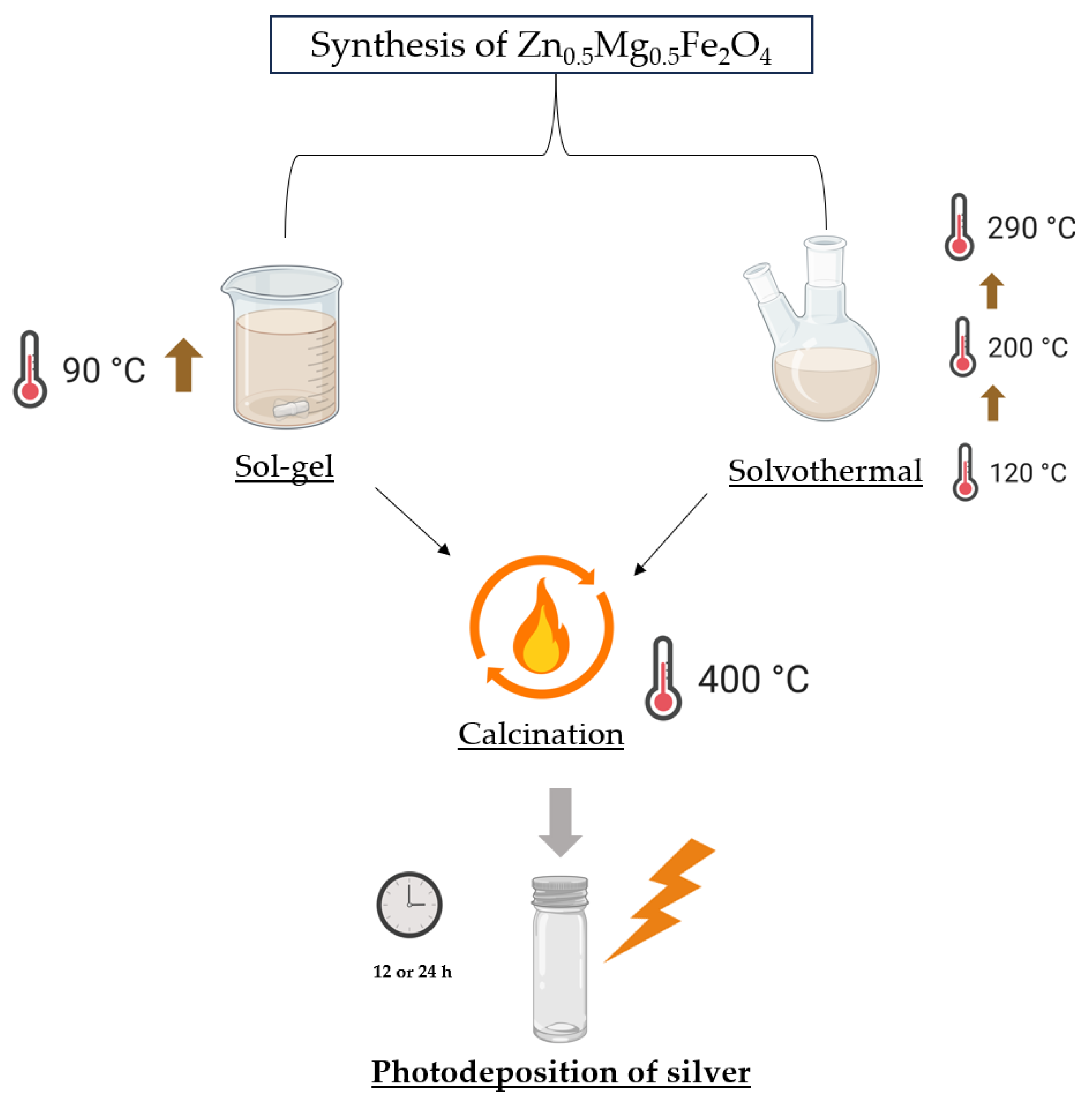
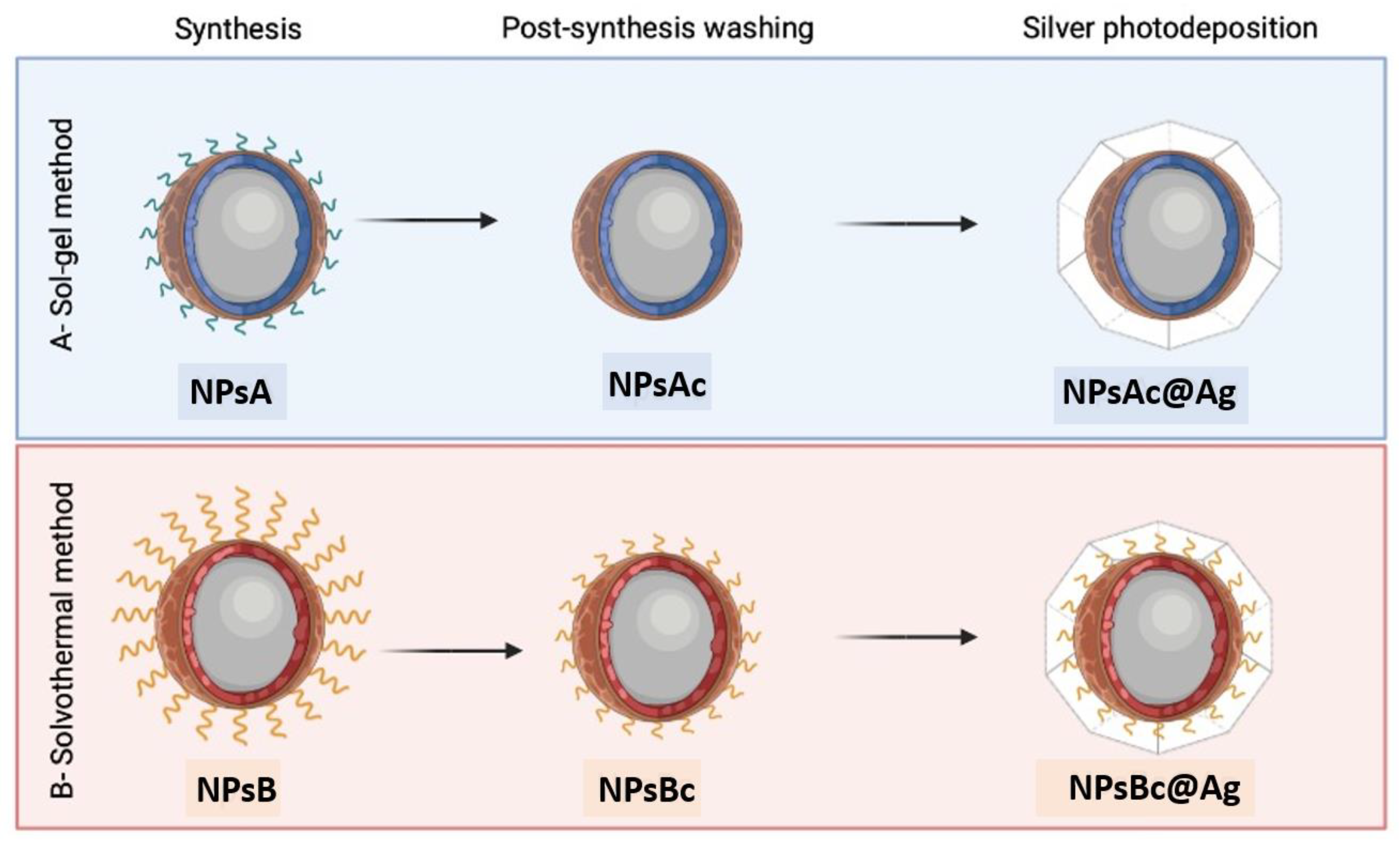
2.2. Synthesis of Zn0.5Mg0.5Fe2O4 Ferrites by Sol-Gel and Solvothermal Methods
2.3. Functionalization of Synthesized NPs with Silver
2.4. Structural Characterization of Synthesized NPs
2.5. Photocatalytic Assays
3. Results and Discussion
3.1. Characterization of NPs
3.1.1. UV/Vis Absorption Spectra
3.1.2. X-ray Diffraction (XRD)
3.1.3. Transmission Electron Microscopy (TEM)
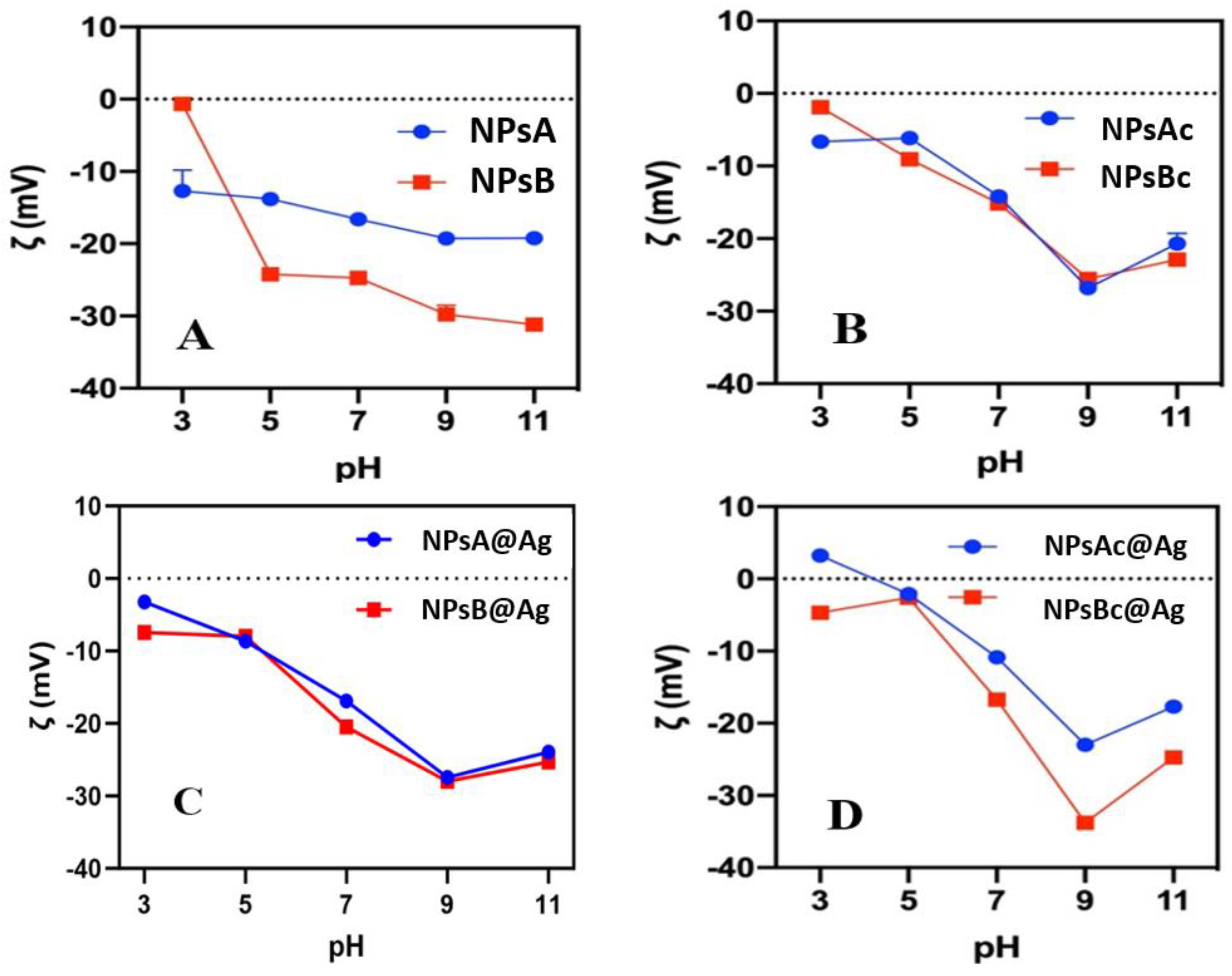
3.1.4. Zeta-Potential pH Profiles
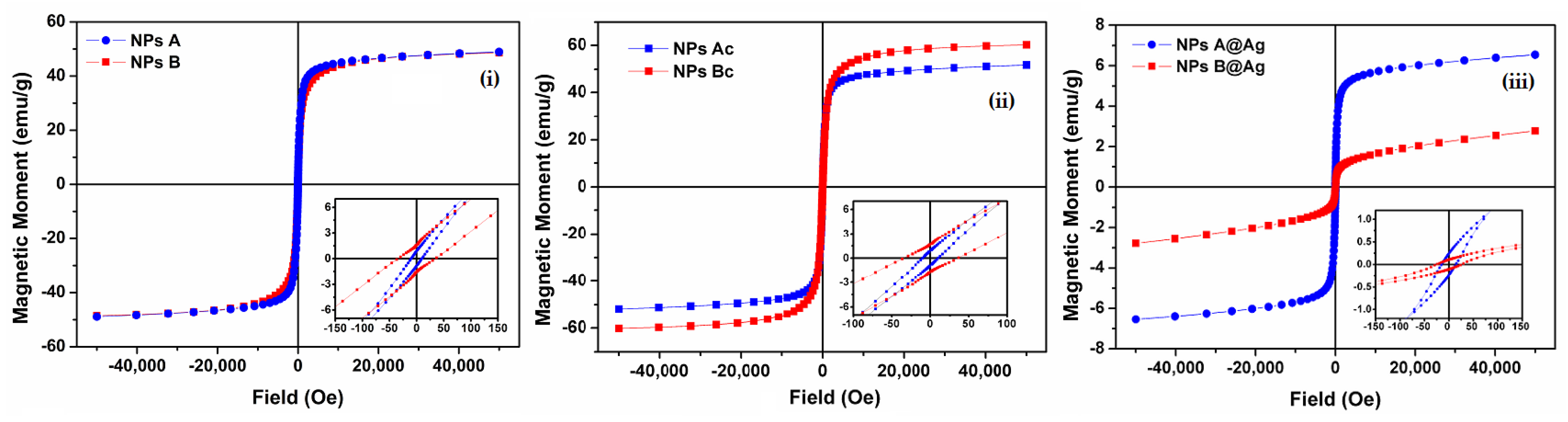
3.1.5. Magnetic Properties
3.2. Photocatalytic Assays
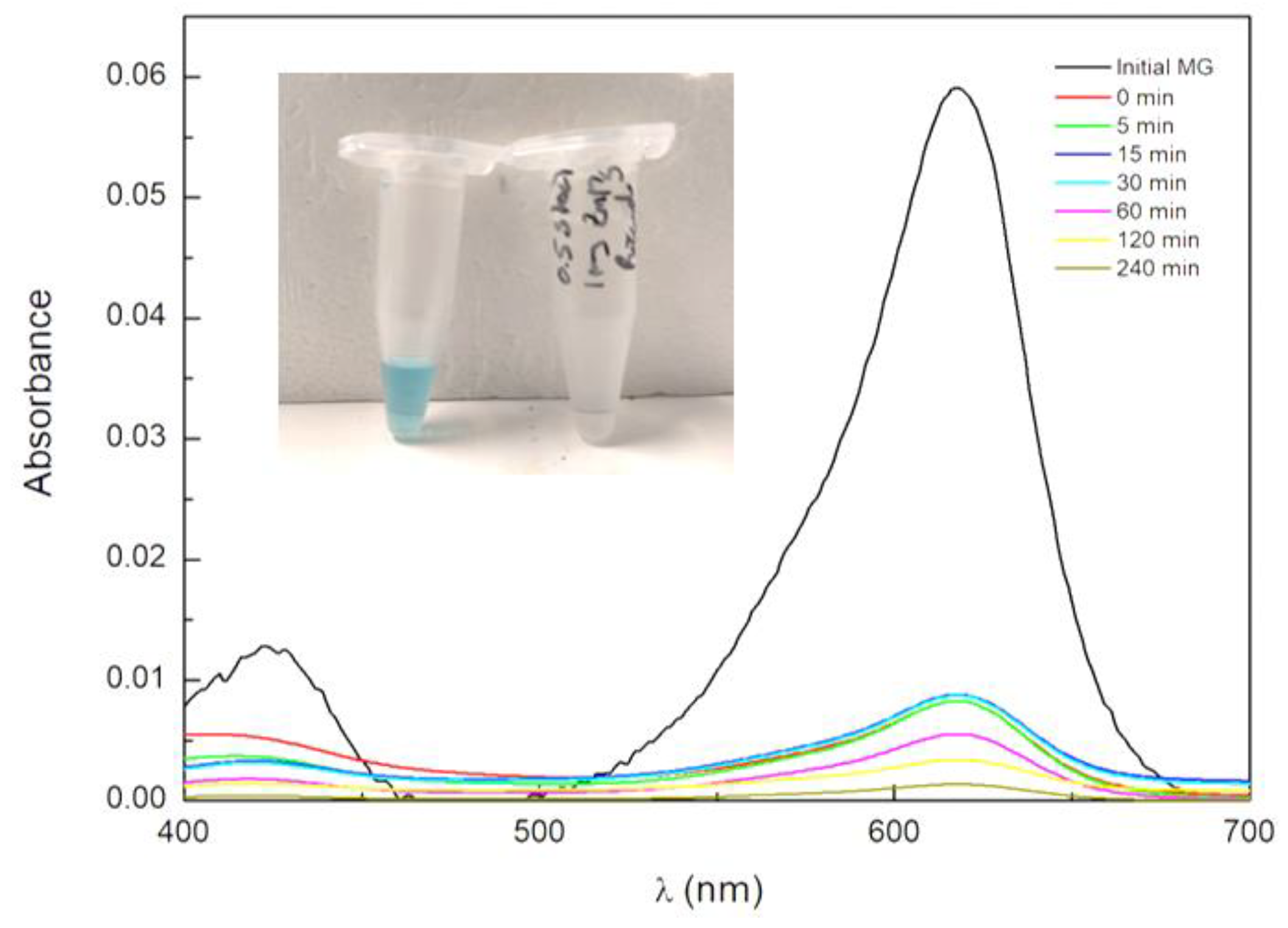
3.2.1. Adsorption Phenomena on NPs
3.2.2. Influence of Silver on NP Catalytic Activity
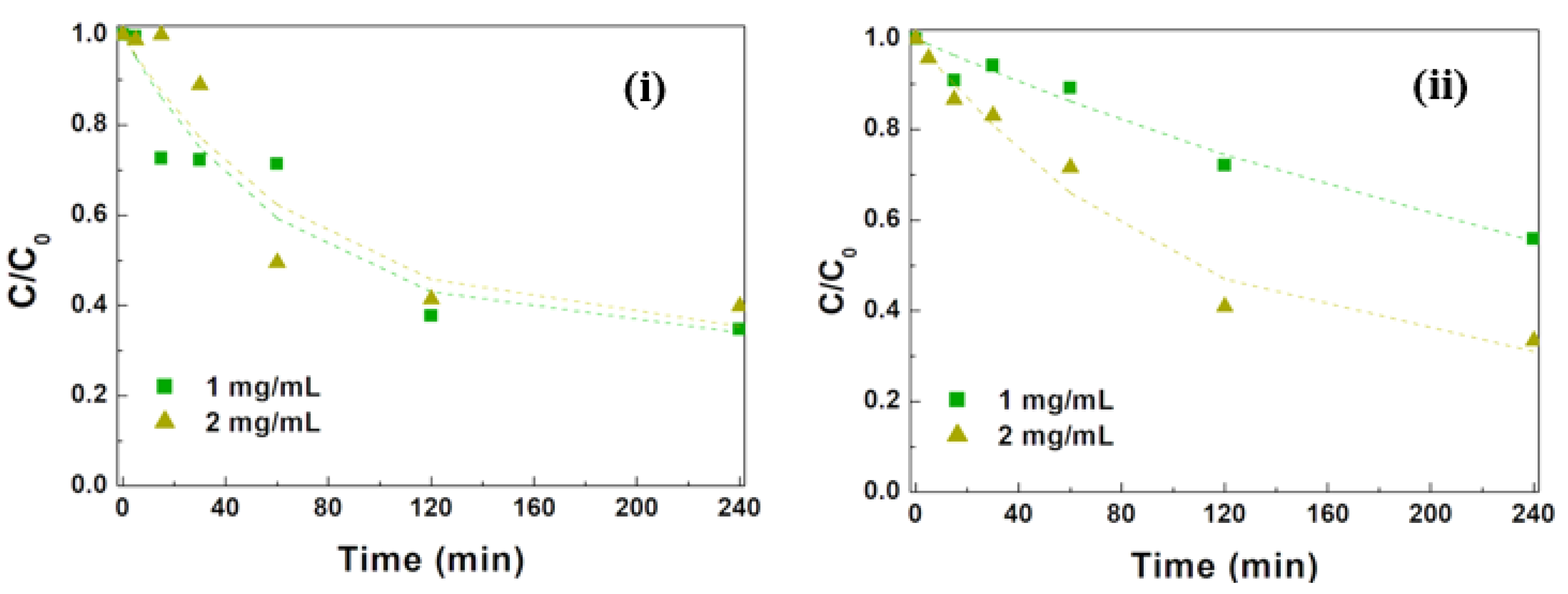
3.2.3. Influence of NP Concentration
3.2.4. Influence of Ag Photodeposition Time
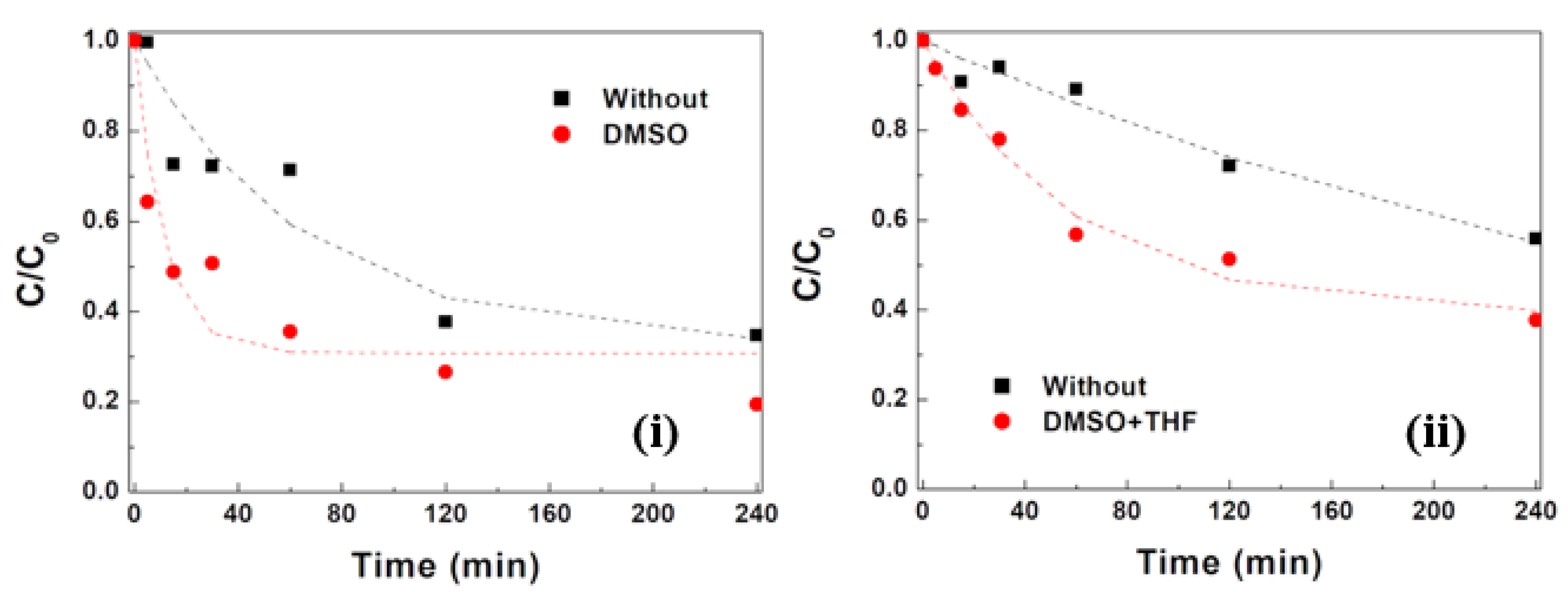
3.2.5. Influence of NP Surface Cleaning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Liu, X.; Ding, Y.; Liu, N. ‘Urban residents’ acceptance of recycled water: An improved innovation-decision model considering the needs satisfied and social characteristics. Sustain. Prod. Consum. 2022, 33, 1005–1017. [Google Scholar] [CrossRef]
- Gomes, K.; Guenther, E.; Morris, J.; Miggelbrink, J.; Caucci, S. Resource nexus oriented decision making along the textile value chain: The case of wastewater management. Curr. Res. Environ. Sustain. 2022, 4, 100153. [Google Scholar] [CrossRef]
- Iacob, V.-S. The Wastewater—A Problem of Integrated Urban Water Management. Procedia Econ. Financ. 2013, 6, 436–443. [Google Scholar] [CrossRef]
- Priyadarshini, I.; Alkhayyat, A.; Obaid, A.J.; Sharma, R. Water pollution reduction for sustainable urban development using machine learning techniques. Cities 2022, 130, 103970. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Hüesker, F.; Lepenies, R. Why does pesticide pollution in water persist? Environ. Sci. Policy 2021, 128, 185–193. [Google Scholar] [CrossRef]
- Giari, L.; Guerranti, C.; Perra, G.; Cincinelli, A.; Gavioli, A.; Lanzoni, M.; Castaldelli, G. PFAS levels in fish species in the Po River (Italy): New generation PFAS, fish ecological traits and parasitism in the foreground. Sci. Total Environ. 2023, 876, 162828. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; Bezerra, C.O.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Chormare, R.; Kumar, M.A. Environmental health and risk assessment metrics with special mention to biotransfer, bioaccumulation and biomagnification of environmental pollutants. Chemosphere 2022, 302, 134836. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, J.; Sun, F.; Liu, Y.; Tang, M.; Yang, Y. Historical development and prospect of intimately coupling photocatalysis and biological technology for pollutant treatment in sewage: A review. Sci. Total Environ. 2022, 835, 155482. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Mazari, S.A.; Karri, R.R.; Abdullah, E.C. Emerging pollutants and their removal using visible-light responsive photocatalysis—A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 106643. [Google Scholar] [CrossRef]
- Kim, D.Y.; Patel, S.K.S.; Rasool, K.; Lone, N.; Bhatia, S.K.; Seth, C.S.; Ghodake, G.S. Bioinspired silver nanoparticle-based nanocomposites for effective control of plant pathogens: A review. Sci. Total Environ. 2024, 908, 168318. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Gupta, R.K.; Karuppanan, K.K.; Padhi, D.K.; Ranganathan, S.; Paramanantham, P.; Lee, J.K. Trametes versicolor Laccase-Based Magnetic Inorganic-Protein Hybrid Nanobiocatalyst for Efficient Decolorization of Dyes in the Presence of Inhibitors. Materials 2024, 17, 1790. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health impact of silver nanoparticles: A review of the biodistribution and toxicity following various routes of exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, D.; Amal, R.; Low, G.; McEvoy, S. Role of nanoparticles in photocatalysis. J. Nanoparticle Res. 1999, 1, 439–458. [Google Scholar] [CrossRef]
- Kokkinos, P.; Mantzavinos, D.; Venieri, D. Current trends in the application of nanomaterials for the removal of emerging micropollutants and pathogens from water. Molecules 2020, 25, 2016. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Tran, M.L.; Van Tran, T.T.; Juang, R.S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Aslam, S.; Noor, A.E.; Jini, D.; Majeed, S.; Velusamy, P.; Alothman, A.A.; Alshgari, R.A.; Mushab, M.S.S.; et al. Hydrothermally synthesized Ag-TiO2 nanofibers (NFs) for photocatalytic dye degradation and antibacterial activity. Chemosphere 2023, 321, 138077. [Google Scholar] [CrossRef]
- Nuengmatcha, P.; Kuyyogsuy, A.; Porrawatkul, P.; Pimsen, R.; Chanthai, S.; Nuengmatcha, P. Efficient degradation of dye pollutants in wastewater via photocatalysis using a magnetic zinc oxide/graphene/iron oxide-based catalyst. Water Sci. Eng. 2023, 16, 243–251. [Google Scholar] [CrossRef]
- Khatri, A.; Rana, P.S. Visible light assisted photocatalysis of Methylene Blue and Rose Bengal dyes by iron doped NiO nanoparticles prepared via chemical co-precipitation. Phys. B Condens. Matter 2020, 579, 411905. [Google Scholar] [CrossRef]
- Du, F.; Yang, D.; Kang, T.; Ren, Y.; Hu, P.; Song, J.; Teng, F.; Fan, H. SiO2/Ga2O3 nanocomposite for highly efficient selective removal of cationic organic pollutant via synergistic electrostatic adsorption and photocatalysis. Sep. Purif. Technol. 2022, 295, 121221. [Google Scholar] [CrossRef]
- Michel, C.R.; Martínez-Preciado, A.H. Photocatalytic performance of β-Ga2O3 microcubes towards efficient degradation of malachite green. Ceram. Int. 2022, 48, 9746–9752. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Ebrahim, S.E. Recent advances in nano-semiconductors photocatalysis for degrading organic contaminants and microbial disinfection in wastewater: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100666. [Google Scholar] [CrossRef]
- Shetty, V. Solar light active biogenic titanium dioxide embedded silver oxide (AgO/Ag2O@TiO2) nanocomposite structures for dye degradation by photocatalysis. Mater. Sci. Semicond. Process. 2021, 132, 105923. [Google Scholar] [CrossRef]
- Fernandes, R.J.C.; Magalhães, C.A.B.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetic nanoparticles of zinc/calcium ferrite decorated with silver for photodegradation of dyes. Materials 2019, 12, 3582. [Google Scholar] [CrossRef]
- Salih, S.J.; Mahmood, W.M. Review on magnetic spinel ferrite (MFe2O4) nanoparticles: From synthesis to application. Heliyon 2023, 6, e16601. [Google Scholar] [CrossRef]
- Fernandes, R.J.C.; Magalhães, C.A.B.; Rodrigues, A.R.O.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Photodeposition of silver on zinc/calcium ferrite nanoparticles: A contribution to efficient effluent remediation and catalyst reutilization. Nanomaterials 2021, 11, 831. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chakraborty, N.; Mondal, S.; Pal, M. Band gap engineered Sn-doped bismuth ferrite nanoparticles for visible light induced ultrafast methyl blue degradation. Ceram. Int. 2022, 48, 37253–37263. [Google Scholar] [CrossRef]
- Hu, B.; Cui, Y.; Yang, X.; Xu, X.; Janani, B.J.; Fakhri, A. Fabrication of novel rational Ti-Sn doped Cu-ferrite nanoparticles for robust photocatalysis reaction, magnetic resonance imaging, and chemo-magneto-photo-thermal therapy. Surf. Interfaces 2022, 33, 102226. [Google Scholar] [CrossRef]
- Kalikeri, S.; Kodialbail, V.S. Visible light active Bismuth ferrite embedded TiO2 nanocomposite structures for dye mineralization by photocatalysis—A strategy to harness solar energy for remediation of water contaminated with mixture of dyes. Surf. Interfaces 2023, 36, 102492. [Google Scholar] [CrossRef]
- Kousar, T.; Aadil, M.; Zulfiqar, S.; Somaily, H.H.; Hassan, W.; Sabeeh, H.; Mahmood, F. Temperature controlled synthesis of Co-Ni mixed ferrite nanostructure for the mineralization of azo dye: A novel and facile approach. J. Alloys Compd. 2022, 923, 166224. [Google Scholar] [CrossRef]
- Din, S.H.U.; Arshed, M.H.; Ullah, S.; Agboola, P.O.; Shakir, I.; Irshad, A.; Shahid, M. Ag-doped nickel ferrites and their composite with rGO: Synthesis, characterization, and solar light induced degradation of coloured and colourless effluents. Ceram. Int. 2022, 48, 15629–15639. [Google Scholar] [CrossRef]
- Lopis, A.D.; Choudhari, K.S.; Kanakikodi, K.S.; Maradur, S.P.; Kulkarni, S.D. Selective, conformal deposition of silver on heterojunction under direct sunlight: Plasmon enhanced photocatalysis. Mater. Res. Bull. 2022, 154, 111929. [Google Scholar] [CrossRef]
- Abu-Hussien, S.H.; Hemdan, B.A.; Alzahrani, O.M.; Alswat, A.S.; Alatawi, F.A.; Alenezi, M.A.; Darwish, D.B.E.; Bafhaid, H.S.; Mahmoud, S.F.; Ibrahim, M.F.M.; et al. Microbial Degradation, Spectral analysis and Toxicological Assessment of Malachite Green Dye by Streptomyces exfoliatus. Molecules 2022, 17, 6456. [Google Scholar] [CrossRef] [PubMed]
- Yulianingrum, A.S.; Surya, R.M.; Apriandanu, D.O.B.; Aqoma, H.; Yulizar, Y. Visible-light ZnO/In2Cu2O5 nanocomposite with increased photocatalytic activity for degradation of malachite green. Vacuum 2024, 226, 113317. [Google Scholar] [CrossRef]
- Yulizar, Y.; Abdullah, I.; Surya, R.M.; Alifa, N.L. Green synthesis of novel YMnO3-doped TiO2 for enhanced visible-light- driven photocatalytic degradation of malachite green. J. Environ. Manag. 2023, 342, 118139. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.D.; Rodrigues, A.R.O.; Bañobre-López, M.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetoliposomes based on shape anisotropic calcium/magnesium ferrite nanoparticles as nanocarriers for doxorubicin. Pharmaceutics 2021, 13, 1248. [Google Scholar] [CrossRef]
- Berbeć, S.; Żołądek, S.; Kulesza, P.J.; Pałys, B. Silver nanoparticles stabilized by polyoxotungstates. Influence of the silver—Polyoxotungstate molar ratio on UV/Vis spectra and SERS characteristics. J. Electroanal. Chem. 2019, 854, 113537. [Google Scholar] [CrossRef]
- Kumari, S.; Dhanda, N.; Thakur, A.; Gupta, V.; Singh, S.; Kumar, S.; Hameed, S.; Thakur, P. Nano Ca–Mg–Zn ferrites as tuneable photocatalyst for UV light-induced degradation of rhodamine B dye and antimicrobial behavior for water purification. Ceram. Int. 2023, 49, 12469–12480. [Google Scholar] [CrossRef]
- Mishra, B.; Munisha, B.; Nanda, J.; Sankaran, K.J.; Suman, S. Hydrothermally Synthesized Magnesium doped Zinc Ferrite Nanoparticles: An extensive study on structural, optical, magnetic, and dielectric properties. Mater. Chem. Phys. 2022, 292, 126791. [Google Scholar] [CrossRef]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystal. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Friedel, P.; Kleeberg, R. IUCr Commission on Powder Diffraction Newsletter; International Union of Crystallography: Chester, UK, 1998; pp. 5–8. [Google Scholar]
- Tatarchuk, T.; Myslin, M.; Lapchuk, I.; Shyichuk, A.; Murthy, A.P.; Gargula, R.; Kurzydio, P.; Bogacz, B.F.; Pedziwiatre, A.T. Magnesium-zinc ferrites as magnetic adsorbents for Cr(VI) and Ni(II) ions removal: Cation distribution and antistructure modeling. Chemosphere 2021, 270, 129414. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, N.; Mahmoudi Chenari, H.; Ghodsi, F.E. Rietveld refinement, morphology analysis, optical and magnetic properties of magnesium-zinc ferrite nanofibers. J. Magn. Magn. Mater. 2018, 468, 132–140. [Google Scholar] [CrossRef]
- Zhang, Z. Study on the influence of magnesium doping on the magnetic properties of spinel Zn-Mg ferrite. Mater. Today Commun. 2021, 26, 101734. [Google Scholar] [CrossRef] [PubMed]
- Moradi, O.; Panahandeh, S. Fabrication of different adsorbents based on zirconium oxide, graphene oxide, and dextrin for removal of green malachite dye from aqueous solutions. Environ. Res. 2022, 214, 114042. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Mitan, M.; Kim, H.; Vaya, D. Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. J. Phys. Chem. Solids. 2021, 155, 110125. [Google Scholar] [CrossRef]
- Gouasmia, A.; Zouaoui, E.; Mekkaoui, A.A.; Haddad, A.; Bousba, D. Highly efficient photocatalytic degradation of malachite green dye over copper oxide and copper cobaltite photocatalysts under solar or microwave irradiation. Inorg. Chem. Commun. 2022, 145, 110066. [Google Scholar] [CrossRef]
- Surya, R.M.; Mauliddiyah, S.; Apriandanu, D.O.B.; Yulizar, Y. SmMnO3-decorated ZnO in a hexane-water interface for enhancing visible light-driven photocatalytic degradation of malachite green. Chemosphere 2022, 304, 135125. [Google Scholar] [CrossRef]
- Meena, S.; Vaya, D.; Das, B.K. Photocatalytic degradation of Malachite Green dye by modified ZnO nanomaterial. Bull. Mater. Sci. 2016, 7, 1735–1743. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Kahtani, A.A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Ahmed, S.A.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.; Rabie, A.M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef]
- Jasrotia, R.; Verma, A.; Verma, R.; Godara, S.K.; Ahmed, J.; Mehtab, A.; Ahmad, T.; Puri, P.; Kalia, S. Photocatalytic degradation of malachite green pollutant using novel dysprosium modified Zn–Mg photocatalysts for wastewater remediation. Ceram. Int. 2022, 48, 29111–29120. [Google Scholar] [CrossRef]
- Wald, L. Basics in Solar Radiation at Earth Surface. Revised Version 2, 2019, Tables 6.2 and 6.3. Available online: https://hal.science/hal-02175988 (accessed on 17 June 2024).

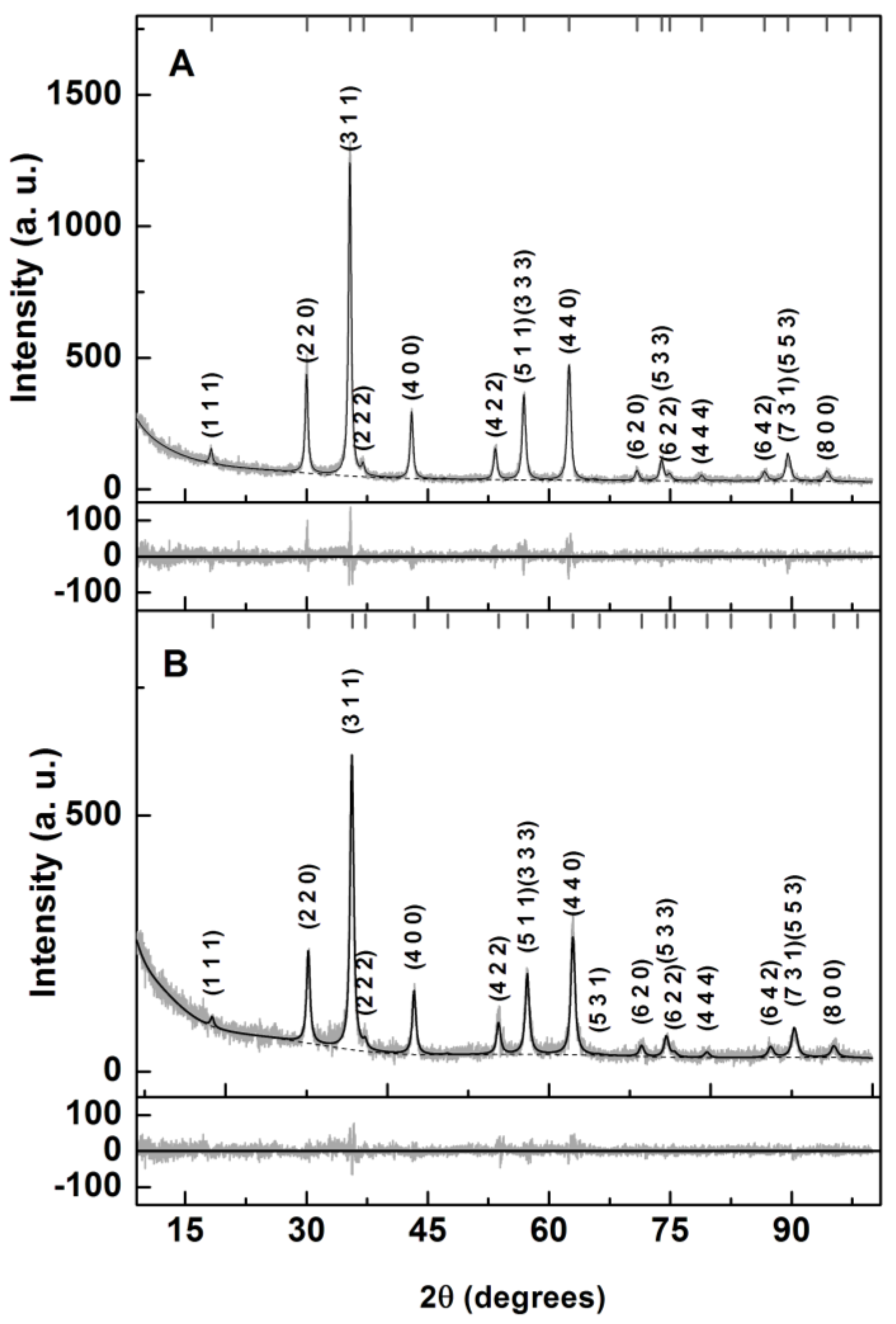
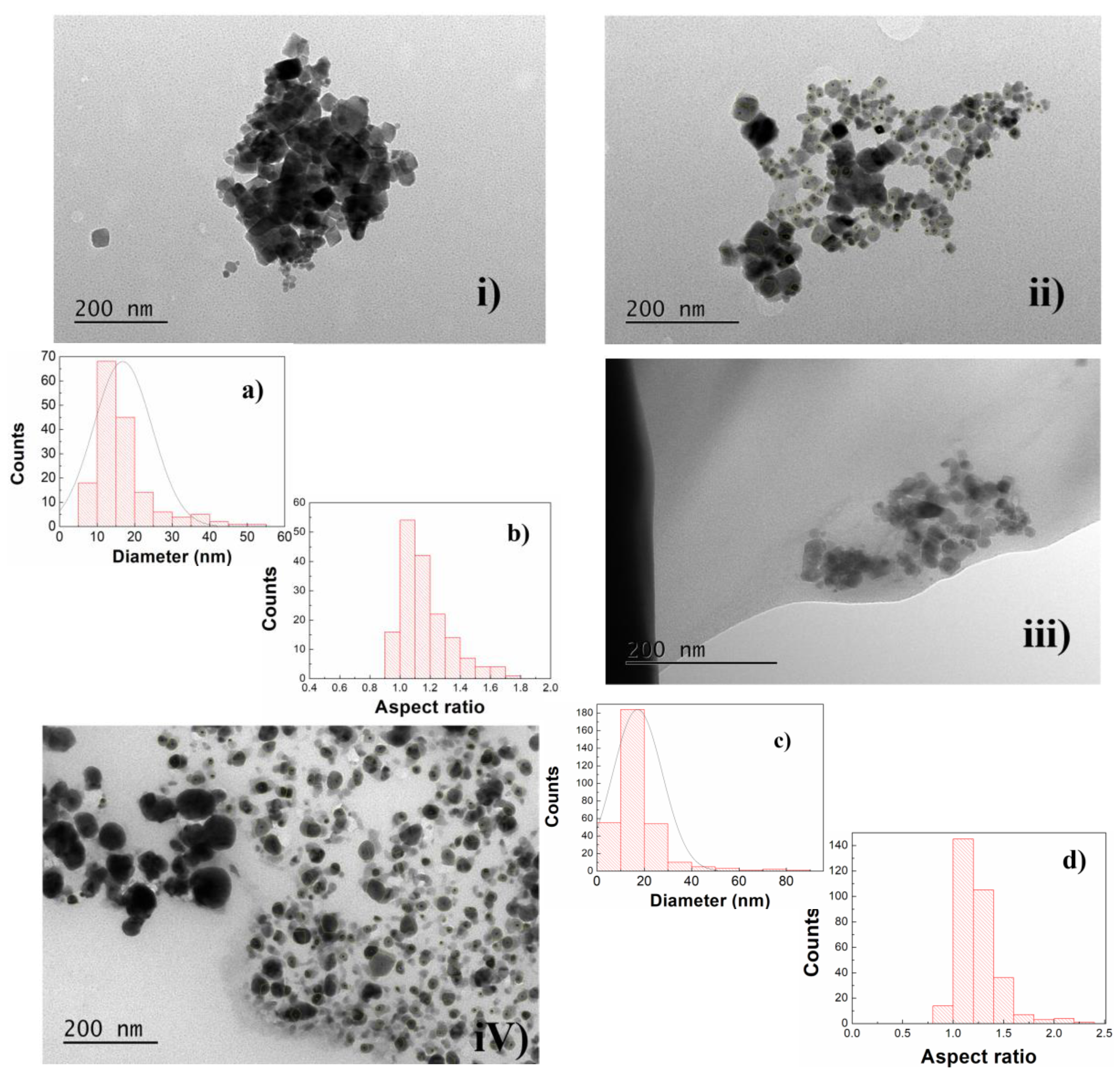



| Sample | Ox,y,z (*) | i (**) | Phase Size (nm) Lattice Constant (nm) Zn, Mg Ferrite | RP | χ2 |
|---|---|---|---|---|---|
| NPsA | 0.3783 | 1 (+) | 21.5 0.8401 | 9.2 | 1.3 |
| NPsB | 0.3760 | 1 (+) | 16.6 0.8344 | 10.8 | 1.5 |
| NPs Samples | Ms (emu g−1) | Mr (emu g−1) | Hc (Oe) | Mr/Ms |
|---|---|---|---|---|
| NPsA | 48.98 | 0.91 | 9.57 | 0.02 |
| NPsB | 48.53 | 1.61 | 33.94 | 0.03 |
| NPsAc | 51.82 | 0.91 | 10.26 | 0.02 |
| NPsBc | 60.17 | 1.63 | 36.18 | 0.03 |
| NPsA@Ag | 6.57 | 0.24 | 15.02 | 0.04 |
| NPsB@Ag | 2.79 | 0.11 | 26.65 | 0.04 |
| Sample | Synthesis | Light Condition | [NPs] (mg mL−1) | Initial Adsorption (30 min Dark) (%) | Average Initial Adsorption | Removal Activity under Dark/Visible Conditions (%) | Time (min) | Overall Removal Efficiency (%) | Rate Constant (min−1)|f∞ |
|---|---|---|---|---|---|---|---|---|---|
| Photolysis | - | Visible | - | n.a. | n.a. | 8.7 | 240 | 8.7 | 0.0004 |
| NPsA | Sol-gel method | Dark | 1 | 86.7 | 86.5 | 84.2 | 240 | 97.9 | n.a. |
| Visible | 86.2 | 77.4 | 96.9 | 0.0063 | |||||
| NPsB | Solvothermal method | Dark | 1 | 50.8 | 48.2 | 84.4 | 120 | 92.3 | n.a. |
| Visible | 45.6 | 86.2 | 92.5 | 0.0118 | |||||
| NPsA@Ag | Sol-gel method, 12 h Ag photodeposition | Dark | 1 | 40.7 | 49.2 | 38.2 | 240 | 63.3 | n.a. |
| Visible | 1 | 57.8 | 65.4 | 85.5 | 0.0153|0.3230 | ||||
| Visible | 2 | 48.5 | n.a. | 60.1 | 79.5 | 0.0138|0.3297 | |||
| Sol-gel method, 24 h Ag photodeposition | Visible | 1 | 40.5 | n.a. | 73.0 | 83.9 | 0.0053 | ||
| Visible | 2 | 41.7 | n.a. | 80.0 | 88.3 | 0.0057 | |||
| Sol-gel method, cleaned, 12 h Ag photodeposition | Dark | 1 | 86.0 | 80.2 | 22.7 | 89.2 | n.a. | ||
| Visible | 1 | 84.4 | 80.5 | 95.0 | 0.0910 |0.3070 | ||||
| NPsB@Ag | Solvothermal method, 12 h Ag photodeposition | Dark | 1 | 24.7 | 36.3 | n.a. | 240 | n.a. | n.a. |
| Visible | 1 | 47.9 | 44.1 | 71.0 | 0.0025 | ||||
| Visible | 2 | 39.8 | n.a. | 66.7 | 79.9 | 0.0098|0.2410 | |||
| Solvothermal method, 24 h Ag photodeposition | Visible | 1 | 6.1 | n.a. | 8.4 | 14.0 | 0.0004 | ||
| Visible | 2 | 6.4 | n.a. | 2.5 | 8.7 | 0.0004 | |||
| Solvothermal method, cleaning step, 12 h Ag photodeposition | Dark | 1 | 77.4 | n.a. | 24.2 | 82.8 | n.a. | ||
| Visible | 1 | 67.3 | n.a. | 62.2 | 87.6 | 0.0170|0.3880 |
| Nanomaterial | MG Concentration (mg/L) | Concentration Photocatalyst (mg/mL) | Light Source | Degradation (%) | Time (min) | Rate (min−1) | Reference |
|---|---|---|---|---|---|---|---|
| ZnO/In2Cu2O5 | 2.19 | 0.17 | Visible light | 93.9 | 120 | 0.0226 | [37] |
| YMnO3-doped TiO2 | 2.19 | 0.2 | Visible light | 95.4 | 120 | 0.0228 | [38] |
| Citric acid-capped cobalt oxide | 3.65 | 0.5 | Simulated sunlight | 91.2 | 100 | 0.0128 | [49] |
| SmMnO3-ZnO | 2.19 | 0.12 | Visible light | 91.7 | 120 | 0.0190 | [51] |
| ZnO (ZEDTA) | 3.65 | 0.2 | Simulated sunlight | 94.1 | 41 | 0.0582 | [52] |
| rGO-Fe3O4/TiO2 | 5.5 | 0.15 | Visible light | 99 | 55 | 0.0224 | [53] |
| CH/ZnO | 5 | 0.5 | Visible light | 100 | 90 | - | [54] |
| CH/Ce-ZnO | 5 | 0.3 | Visible light | 100 | 60 | - | [55] |
| NPsAc@Ag | 10 | 1 | Visible light | 95 | 240 | 0.0910 | This work |
| NPsBc@Ag | 10 | 1 | Visible light | 87.6 | 240 | 0.0170 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, R.J.C.; Cardoso, B.D.; Rodrigues, A.R.O.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Pereira, L.; Coutinho, P.J.G. Zinc/Magnesium Ferrite Nanoparticles Functionalized with Silver for Optimized Photocatalytic Removal of Malachite Green. Materials 2024, 17, 3158. https://doi.org/10.3390/ma17133158
Fernandes RJC, Cardoso BD, Rodrigues ARO, Pires A, Pereira AM, Araújo JP, Pereira L, Coutinho PJG. Zinc/Magnesium Ferrite Nanoparticles Functionalized with Silver for Optimized Photocatalytic Removal of Malachite Green. Materials. 2024; 17(13):3158. https://doi.org/10.3390/ma17133158
Chicago/Turabian StyleFernandes, Ricardo J. C., Beatriz D. Cardoso, Ana Rita O. Rodrigues, Ana Pires, André M. Pereira, João P. Araújo, Luciana Pereira, and Paulo J. G. Coutinho. 2024. "Zinc/Magnesium Ferrite Nanoparticles Functionalized with Silver for Optimized Photocatalytic Removal of Malachite Green" Materials 17, no. 13: 3158. https://doi.org/10.3390/ma17133158







