Effect of Discharge Energy on Micro-Arc Oxidation Coating of Zirconium Alloy
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization
3. Micro-Arc Oxidation Discharge Mechanism and Model
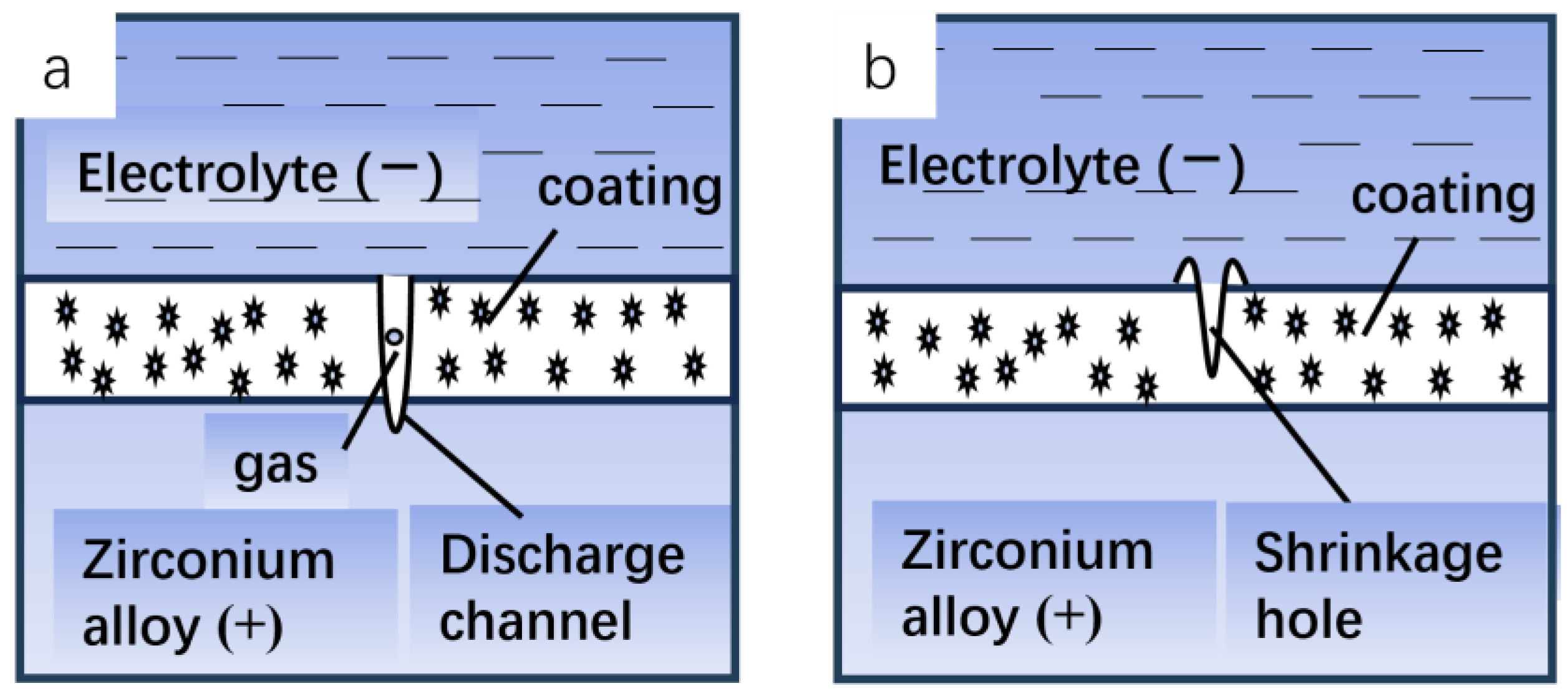
3.1. Discharge Mechanism
- U—the voltage of the power supply in the microarc oxidation process;
- I—the magnitude of the positive pulse current;
- R—the equivalent resistance of the pulse-generating discharge circuit;
- t—the discharge time of the power supply during processing;
- f—the frequency of the working power supply;
- D—the pulse duty cycle.
3.2. Discharge Model
- (1)
- When the power supply is connected and starts working, due to the small initial voltage, a thin anodic oxidation coating will be formed on the surface of the substrate. At the same time, due to the electrolytic reaction of the electrolyte, a part of the mixed gas with oxygen as the main component will be produced, including a small amount of hydrogen generated by the anodic oxidation reaction, and will be influenced by the composition of the electrolyte.
- (2)
- With the increase in oxidation time and the increase in voltage, the coating will continue to thicken, and the precipitated gas will continue to accumulate in the discharge channel. When the voltage reaches the breakdown voltage, the gas will be ionized by the voltage breakdown to form a plasma, which will trigger the discharge effect under the action of the electric field strength. At the same time, denser electric sparks can be observed on the surface of the substrate.
- (3)
- In the process of spark discharge in the micro-zone, concentrated energy will be released, causing the temperature in the micro-zone to rise instantly, and the local high temperature will melt the metal matrix to form molten oxide. Due to the discharge pressure generated in the discharge channel and the pressure of gas ejecting outward, the molten oxide with higher temperatures will be ejected outward through the discharge channel. As the coating surface is in contact with the electrolyte, it cools rapidly, the volume shrinks, and a jet vent similar to a crater is formed on the surface of the film layer; and finally, a certain thickness of the oxidation coating is obtained.
4. Results and Discussion
4.1. Thickness of MAO Coating
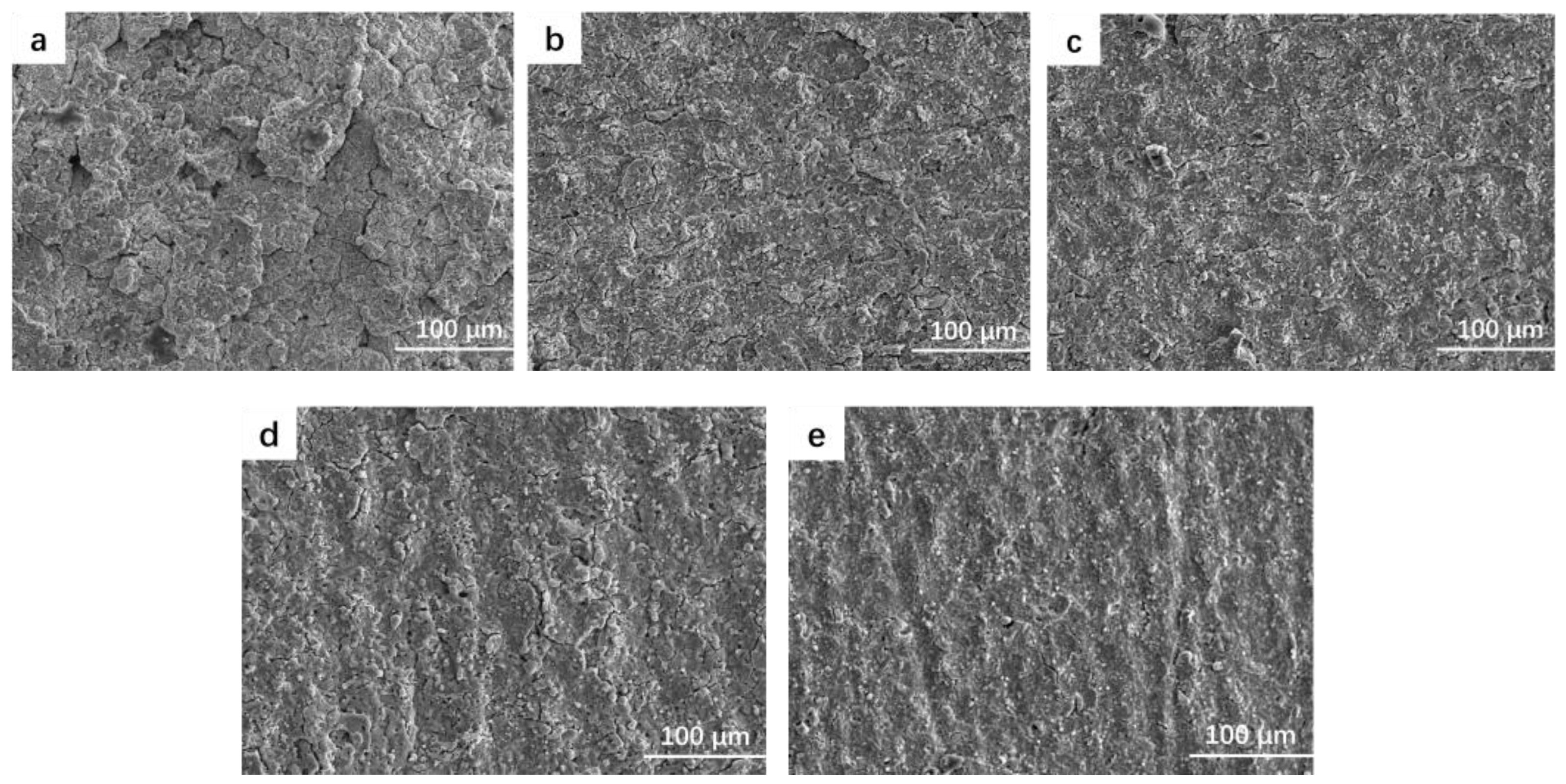
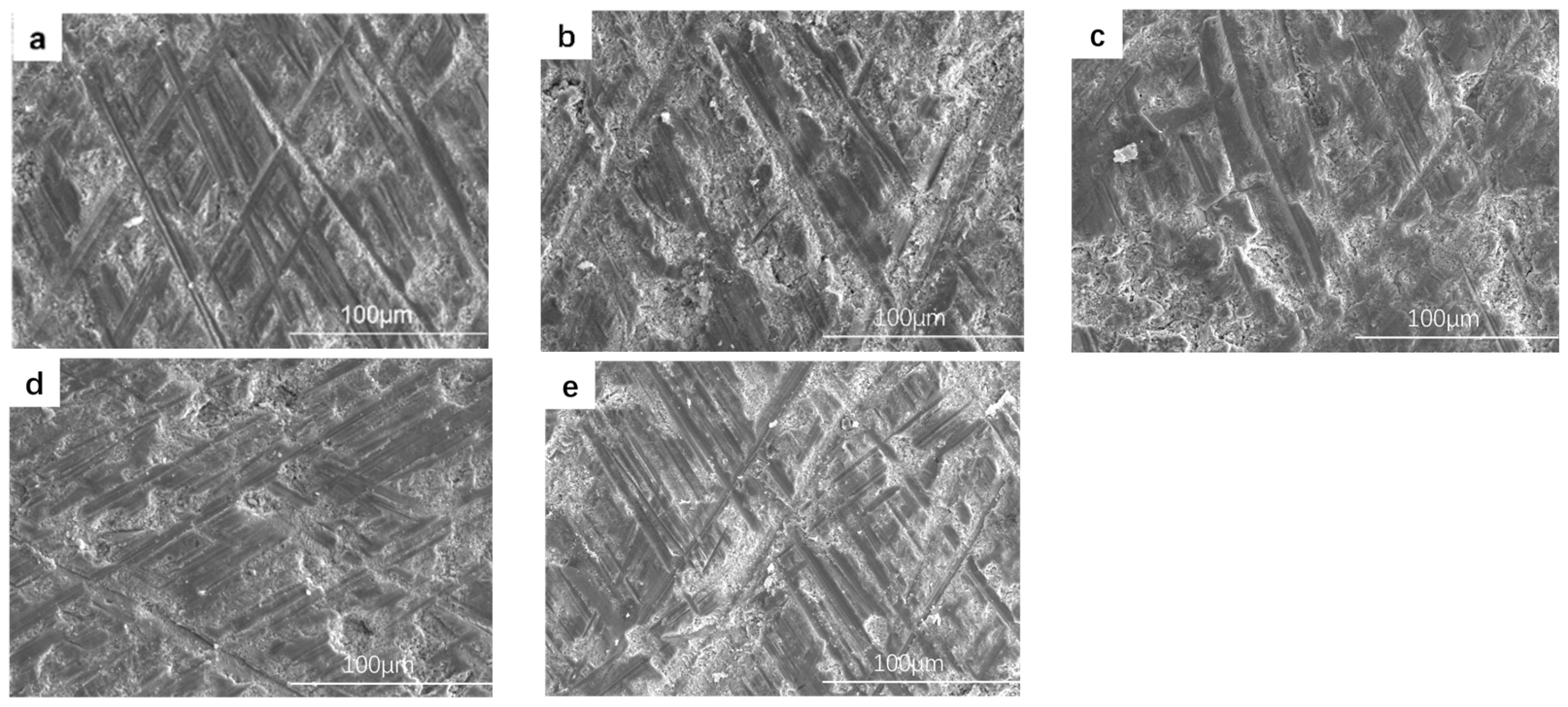
4.2. Coating Micromorphology
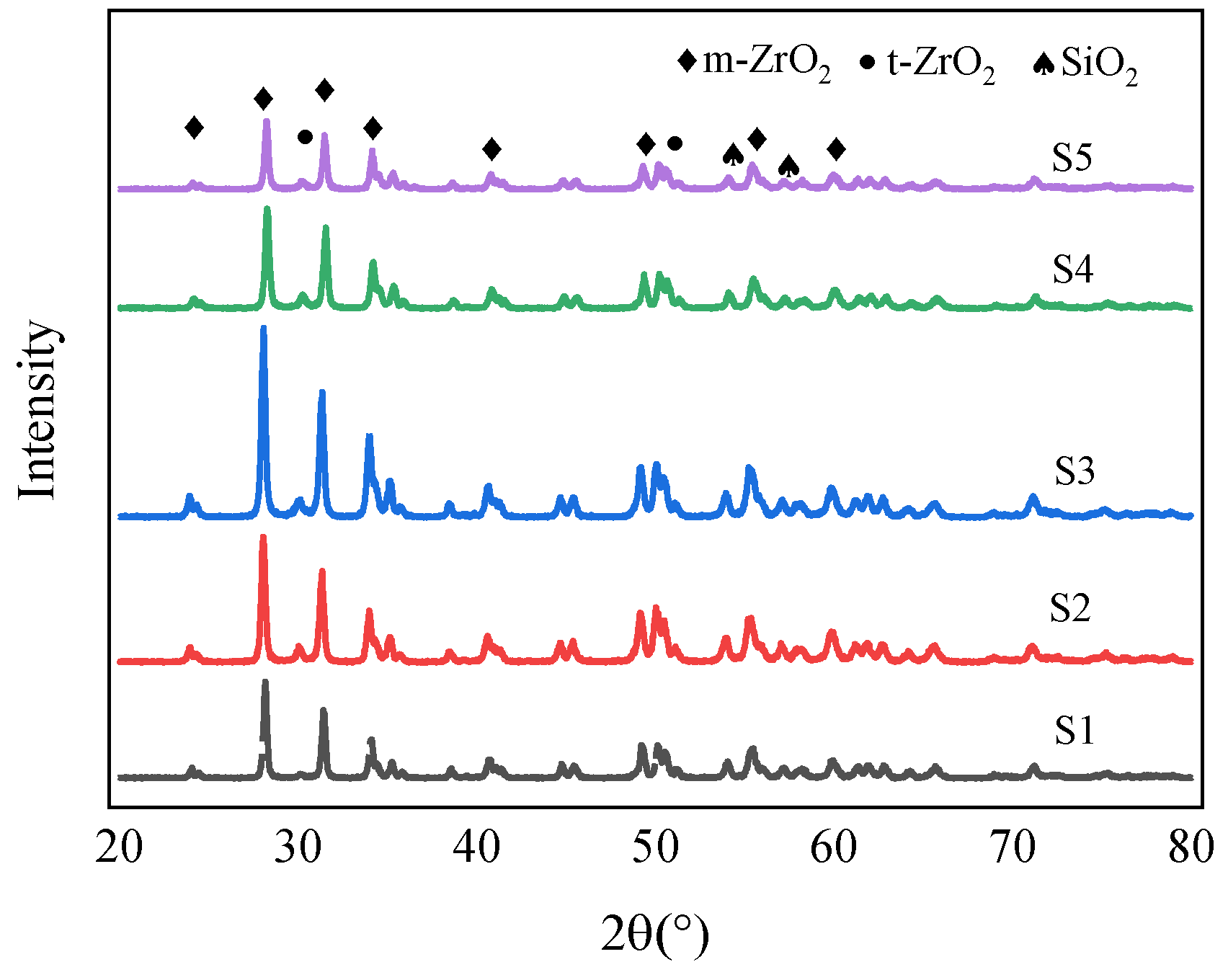
4.3. Coating Phase Composition
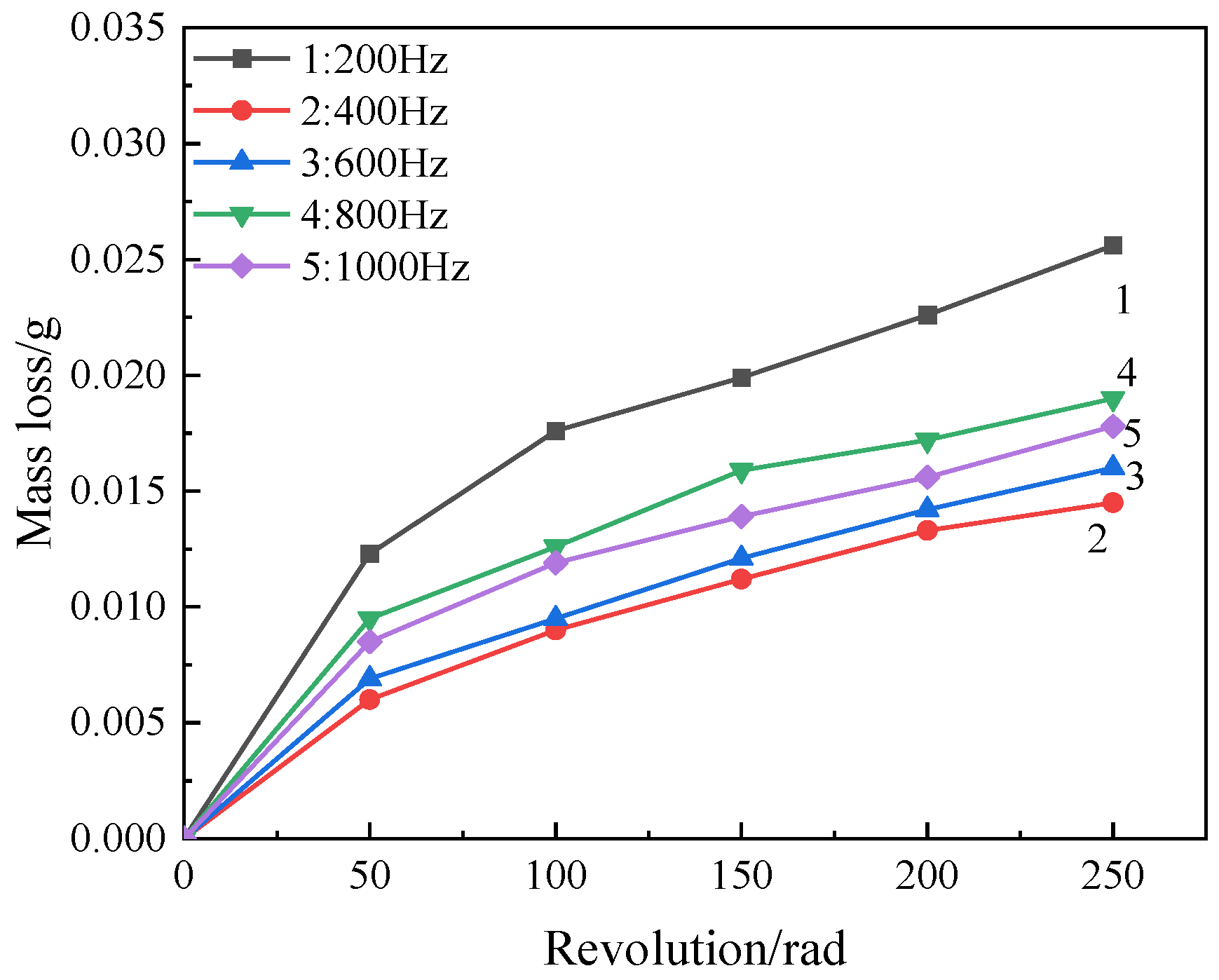
4.4. Coating Wear Resistance
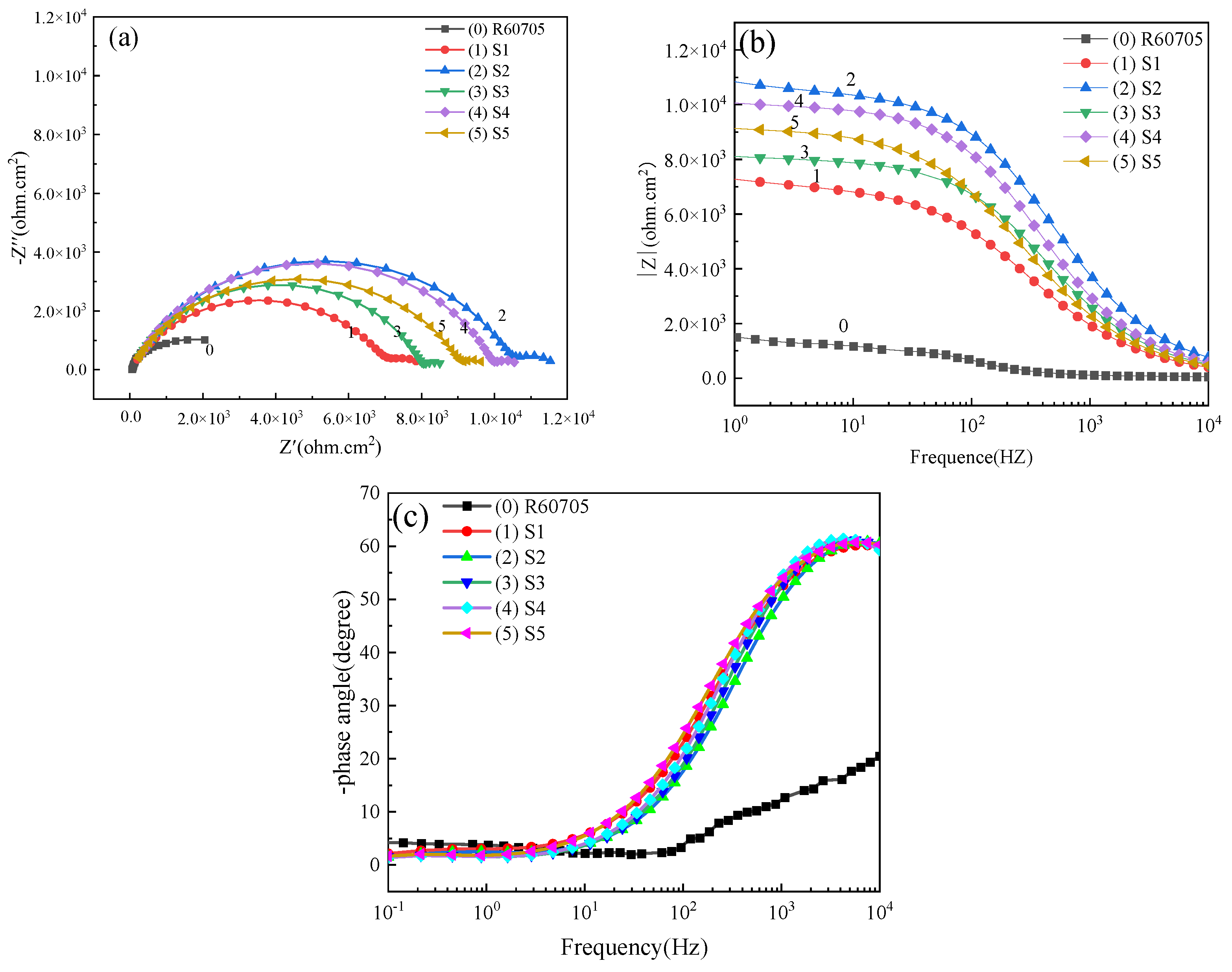
4.5. Corrosion Resistance of Coating
5. Conclusions
- (1)
- The low frequency has little effect on the thickness of the coating. With the increase in frequency, the monopulse discharge energy continues to decrease, and the coating thickness decreases significantly, and it reaches the lowest level at 1000 Hz. With the increase in frequency, the pore size of the discharge on the surface of the coating decreases and the surface tends to be smooth and flat.
- (2)
- The coating is mainly composed of the monoclinic phase (m-ZrO2), tetragonal phase (t-ZrO2), and SiO2 phase. The binding energies of Zr are 181.38 eV (3d3/2) and 183.58 eV (3d5/2). The oxidation coating is mainly composed of the Zr, O, and Si elements.
- (3)
- With the increase in frequency, the wear resistance of oxide coating first increases and then decreases. The corrosion resistance increases first and then decreases. The corrosion resistance is best at 400 Hz, when the corrosion potential is −0.215 V and the corrosion current density is 2.546 × 10−8 A·cm−2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Gong, F.; Hao, M.; Wu, L.; Yin, C.; Sun, Z.; Xiao, R. Enhanced thermal transport and corrosion resistance by coating vertically-aligned graphene on zirconium alloy for nuclear reactor applications. Appl. Surf. Sci. 2022, 582, 152484. [Google Scholar] [CrossRef]
- Slobodyan, M. Resistance, electron- and laser-beam welding of zirconium alloys for nuclear applications: A review. Nucl. Eng. Technol. 2020, 53, 1049–1078. [Google Scholar] [CrossRef]
- Slobodyan, M. High-energy surface processing of zirconium alloys for fuel claddings of water-cooled nuclear reactors. Nucl. Eng. Des. 2021, 382, 111364. [Google Scholar] [CrossRef]
- Nelson, M.; Samuha, S.; Kamerman, D.; Hosemann, P. Temperature-Dependent Mechanical Anisotropy in Textured Zircaloy Cladding. J. Nucl. Mater. 2024, 595, 155045. [Google Scholar] [CrossRef]
- Colins, K.D. Inferential-Statistical Reevaluation of Spent Fuel Zircaloy Cladding Integrity. Nucl. Technol. 2023, 209, 582–594. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, H.; McAuliffe, R.D.; Yan, Y.; Curlin, S. Hydrogen effects on thermal diffusivity and electrical resistivity of zircaloy cladding. J. Nucl. Mater. 2023, 574, 154213. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.; Zhao, X.; Wu, J.; Zhao, B.; Zhao, H.; Wu, J.; Zhang, Y.; Liu, C. Zirconium based neutron absorption material with outstanding corrosion resistance and mechanical properties. J. Nucl. Mater. 2022, 567, 153763. [Google Scholar] [CrossRef]
- Wang, M.; Lv, K.; Du, Z.; Chen, W.; Ji, P.; Wang, W.; Pang, Z. Study on Growth Mechanism and Characteristics of Zirconium Alloy Micro-Arc Oxidation Film. Metals 2023, 13, 935. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Huang, H.; He, M.; Wangyang, P.; Gu, L. Effect of micro-groove on microstructure and performance of MAO ceramic coating fabricated on the surface of aluminum alloy. J. Alloys Compd. 2019, 777, 94–101. [Google Scholar] [CrossRef]
- Lu, J.P.; Cao, G.P.; Quan, G.F.; Wang, C.; Zhuang, J.J.; Song, R.G. Effects of Voltage on Microstructure and Corrosion Resistance of Micro-arc Oxidation Ceramic Coatings Formed on KBM10 Magnesium Alloy. J. Mater. Eng. Perform. 2018, 27, 147–154. [Google Scholar] [CrossRef]
- Zhu, M.; Lan, X.; Wang, P.; Luo, Q.; Sun, F. Effect of cobalt oxide on characteristics of micro-arc oxidation coatings on TC11 alloy. Int. J. Appl. Ceram. Technol. 2024, 21, 1778–1788. [Google Scholar] [CrossRef]
- Zhang, R.; Lv, K.; Du, Z.; Chen, W.; Ji, P.; Wang, M. Effects of Graphene on the Wear and Corrosion Resistance of Micro-Arc Oxidation Coating on a Titanium Alloy. Metals 2021, 12, 70. [Google Scholar] [CrossRef]
- Jiao, Z.J.; Yu, C.; Wang, X.M.; Zhou, Y.F.; Guo, L.; Xia, Y.; Zhang, B.C.; Zeng, R.C. Corrosion resistance enhanced by an atomic layer deposited Al2O3/micro-arc oxidation coating on magnesium alloy AZ31. Ceram. Int. 2024, 50, 5541–5551. [Google Scholar] [CrossRef]
- Wang, D.D.; Liu, X.T.; Wang, Y.; Zhang, Q.; Li, D.L.; Liu, X.; Su, H.; Zhang, Y.; Yu, S.X.; Shen, D. Role of the electrolyte composition in establishing plasma discharges and coating growth process during a micro-arc oxidation. Surf. Coat. Technol. 2020, 402, 126349. [Google Scholar] [CrossRef]
- Ji, P.; Lü, K.; Chen, W.; Wang, M. Study on Preparation of Micro-Arc Oxidation Film on TC4 Alloy with Titanium Dioxide Colloid in Electrolyte. Coatings 2022, 12, 1093. [Google Scholar] [CrossRef]
- Kuramshin, R.A.; Torbin, A.P.; Svistun, M.I.; Zagidullin, M.V.; Chernyshov, A.K.; Mikheyev, P.A. Measurements of Rate Constants of Energy Transfer Processes in Ar/He Plasma of Pulse-Periodic Discharge. Bull. Lebedev Phys. Inst. 2023, 50, 207–213. [Google Scholar] [CrossRef]
- Burkov, A.A. The Effect of Discharge Pulse Energy in Electrospark Deposition of Amorphous Coatings. Prot. Met. Phys. Chem. Surf. 2022, 58, 1018–1027. [Google Scholar] [CrossRef]
- Wang, J.; Du, M.; Wang, J.; Cheng, X.; Han, F. Effects of the Pulse Energy on the Characteristics of Ceramic Coatings on Titanium Alloys Prepared by Micro-arc Oxidation. Electromach. Mould. 2014, 1, 47–50. [Google Scholar]
- Jing, Y.; Huiling, Z.; Wang, J.; Du, M.; Han, F. Research on Effect law of Electric Parameters on Properties of Micro-arc Oxidation Coatings on LY12 Aluminum Alloy. Electromach. Mould. 2013, 3, 26–31. [Google Scholar]
- Yao, Z.; Jiang, Z.; Sun, X.; Xin, S.; Wu, Z. Influenceof the frequencyon the structureandcorrosion resistanceof ceramiccoatings onTi 6AI–4Valloyproducedbymicro-plasmaoxidation. Mater. Chem. 2005, 92, 408–412. [Google Scholar]




| Element | Hf | Nb | Fe + Cr | C | N | H | O | Zr |
|---|---|---|---|---|---|---|---|---|
| Content% | 4.5 | 2~3 | 0.2 | 0.05 | 0.025 | 0.005 | 0.18 | margin |
| Specimen Code | Frequency (Hz) | Forward/Negative Voltage (V) | Duty Cycle (%) | Oxidation Time (min) |
|---|---|---|---|---|
| S1 | 200 | 350/140 | 50 | 15 |
| S2 | 400 | |||
| S3 | 600 | |||
| S4 | 800 | |||
| S5 | 1000 |
| Frequency/Hz | Ecoor/V vs. SCE | Jcorr/(A·cm−2) |
|---|---|---|
| R60705 | −0.529 | 5.60 × 10−6 |
| S1 | −0.276 | 1.02 × 10−6 |
| S2 | −0.215 | 2.55 × 10−8 |
| S3 | −0.228 | 6.23 × 10−8 |
| S4 | −0.220 | 3.39 × 10−8 |
| S5 | −0.215 | 4.81 × 10−8 |
| Frequency/Hz | Rs (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | Rp (Ω·cm2) |
|---|---|---|---|---|
| R60705 | 43.65 | - | 976 | 9.76 × 102 |
| S1 | 39 | 7002 | 1105 | 8.11 × 103 |
| S2 | 91.38 | 10,448 | 1212 | 1.17 × 104 |
| S3 | 85.6 | 7958 | 669.2 | 8.63 × 103 |
| S4 | 91.3 | 9892 | 743.7 | 1.06 × 104 |
| S5 | 47.6 | 9102 | 685.7 | 9.79 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lv, K.; Du, Z.; Chen, W.; Pang, Z. Effect of Discharge Energy on Micro-Arc Oxidation Coating of Zirconium Alloy. Materials 2024, 17, 3166. https://doi.org/10.3390/ma17133166
Wang W, Lv K, Du Z, Chen W, Pang Z. Effect of Discharge Energy on Micro-Arc Oxidation Coating of Zirconium Alloy. Materials. 2024; 17(13):3166. https://doi.org/10.3390/ma17133166
Chicago/Turabian StyleWang, Wei, Kai Lv, Zhaoxin Du, Weidong Chen, and Zhi Pang. 2024. "Effect of Discharge Energy on Micro-Arc Oxidation Coating of Zirconium Alloy" Materials 17, no. 13: 3166. https://doi.org/10.3390/ma17133166
APA StyleWang, W., Lv, K., Du, Z., Chen, W., & Pang, Z. (2024). Effect of Discharge Energy on Micro-Arc Oxidation Coating of Zirconium Alloy. Materials, 17(13), 3166. https://doi.org/10.3390/ma17133166






