WO3/BiOBr S-Scheme Heterojunction Photocatalyst for Enhanced Photocatalytic CO2 Reduction
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of the Photocatalyst
2.1.1. Materials
2.1.2. Preparation of BiOBr
2.1.3. Preparation of WO3/BiOBr
2.2. Photocatalytic CO2 Reduction
2.3. Characterization
2.4. Photoelectrochemical Measurements
3. Results and Discussion
3.1. Structure and Morphology
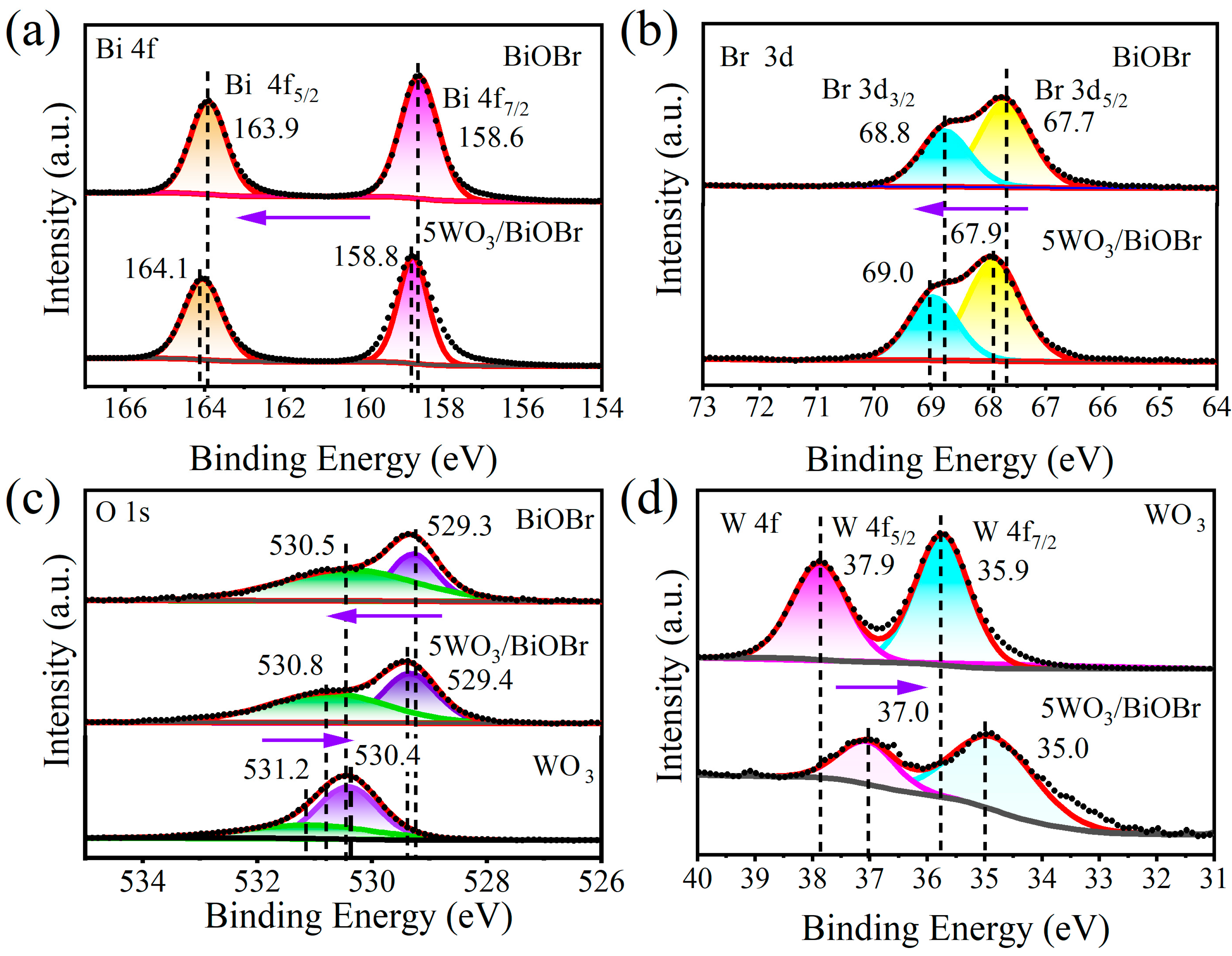
3.2. Surface Chemical State
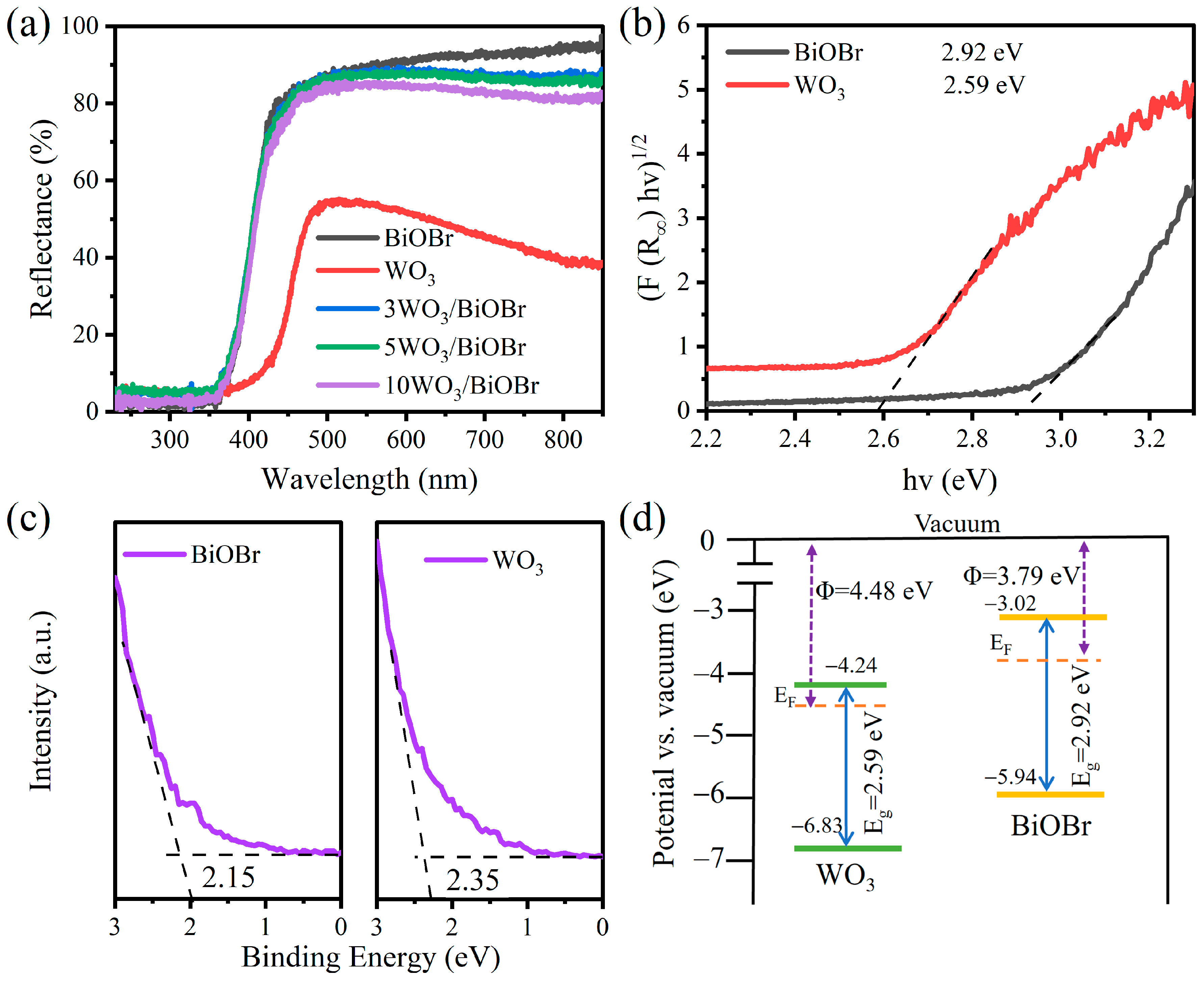
3.3. Light Absorption Capacity and Band Structure
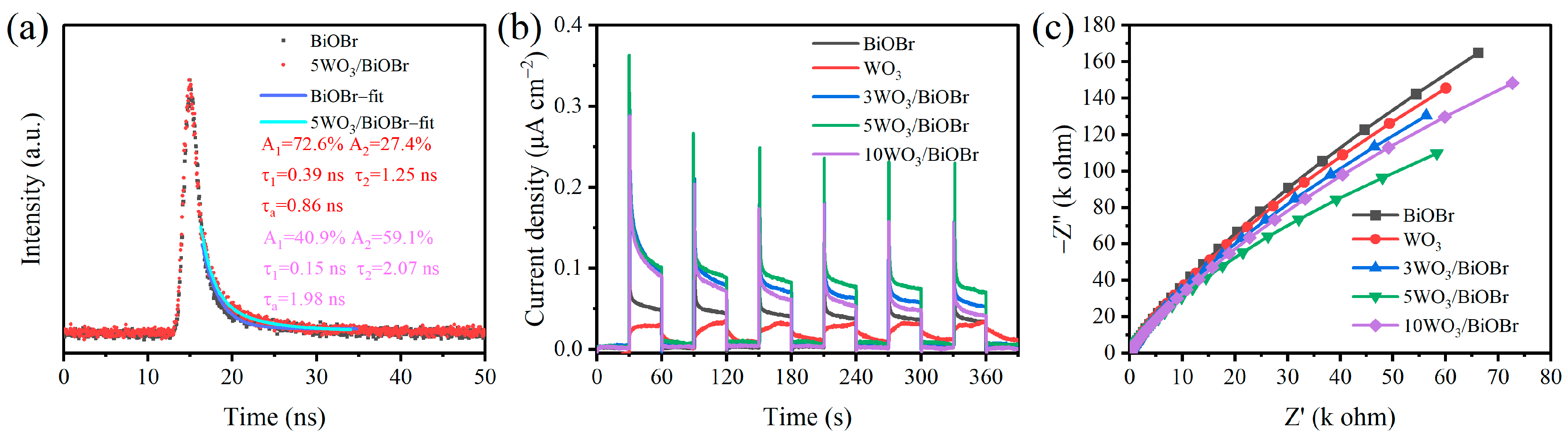
3.4. Charge Transfer and Separation
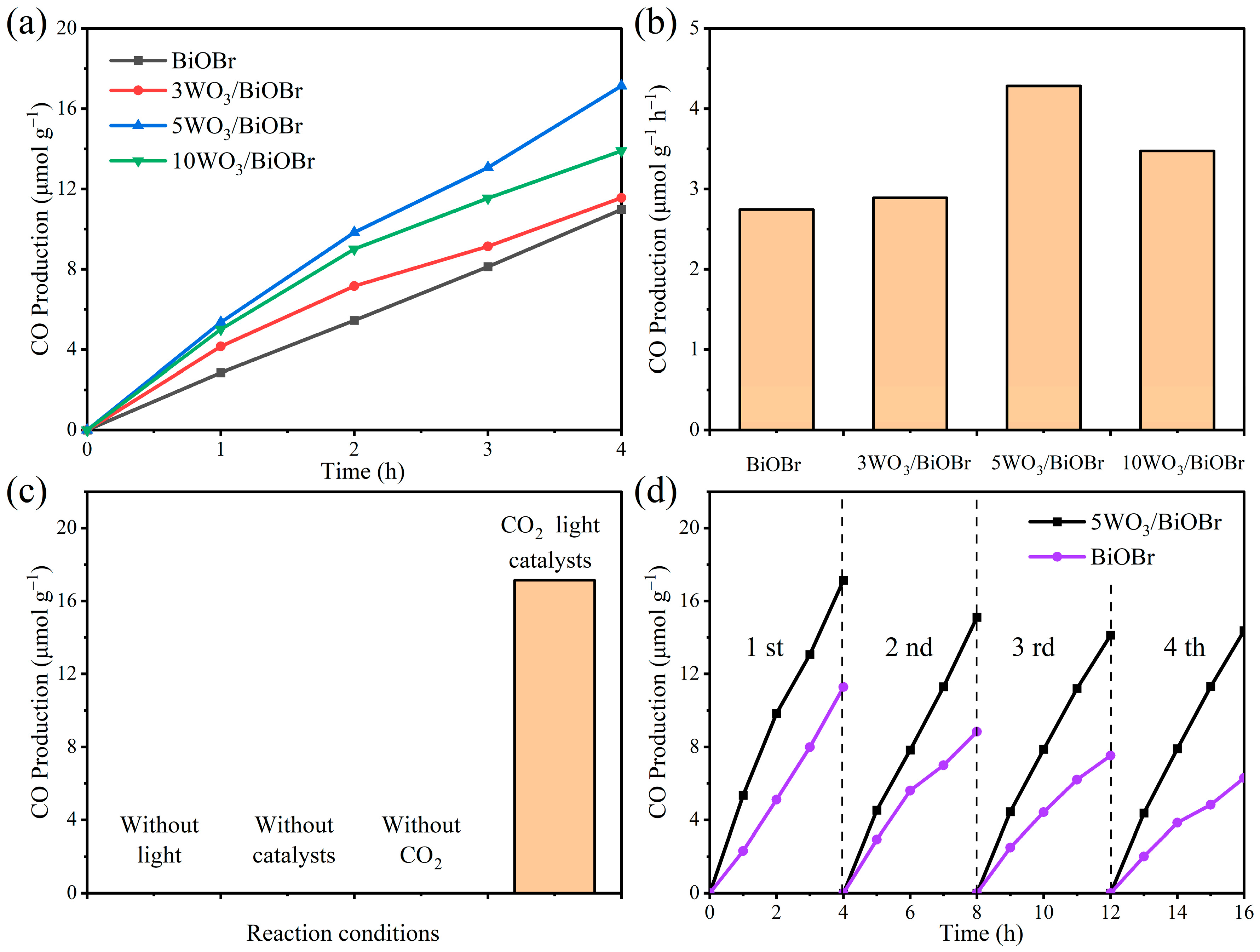
3.5. Photocatalytic CO2 Reduction
3.6. In Situ FTIR Spectra
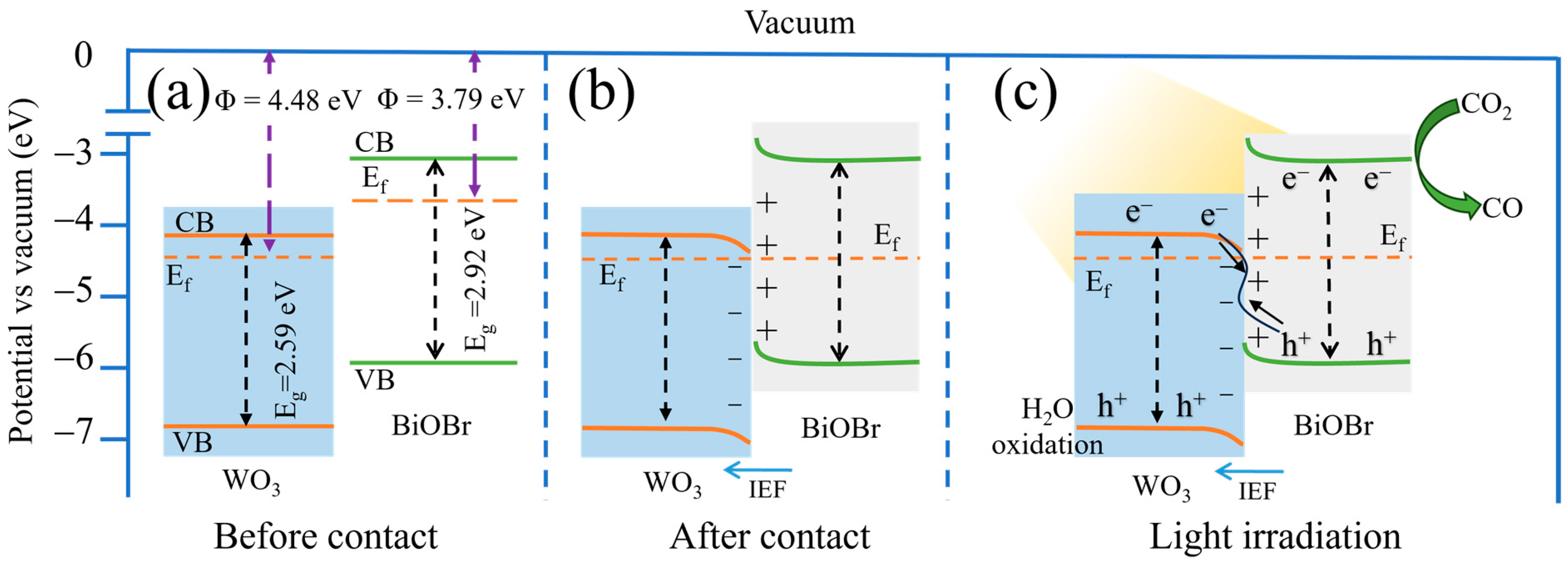
3.7. Possible Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Dang, Y.; Cui, X.; Bu, X.; Li, J.; Li, S.; Sun, Y.; Gao, P. Selective synthesis of olefins via CO2 hydrogenation over transition-metal-doped iron-based catalysts. Appl. Catal. B 2023, 321, 122050. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B 2020, 272, 119006. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Zhang, Y.; Tian, N.; Ma, T.; Huang, H. Inside-and-Out Semiconductor Engineering for CO2 Photoreduction: From Recent Advances to New Trends. Small Struct. 2020, 2, 2000061. [Google Scholar] [CrossRef]
- Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated Bilayer MOF Enables High-Selectivity Photocatalytic Reduction of CO2 to CO. Adv. Mater. 2023, 35, 2209814. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, P.; Li, Y.; Tang, S.; Lin, X.; Zhang, H.; Zhu, Z.; Chen, F. Graphene aerogel-based NiAl-LDH/g-C3N4 with ultratight sheet-sheet heterojunction for excellent visible-light photocatalytic activity of CO2 reduction. Appl. Catal. B 2022, 306, 121065. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Li, H.; Li, H.; Qi, W.; Zhang, Q.; Zhu, J.; Zhao, P.; Yang, S. Accelerating photogenerated charge kinetics via the g-C3N4 Schottky junction for enhanced visible-light-driven CO2 reduction. Appl. Catal. B 2022, 318, 121863. [Google Scholar] [CrossRef]
- Jo, Y.; Kim, C.; Moon, G.-H.; Lee, J.; An, T.; Choi, W. Activation of peroxymonosulfate on visible light irradiated TiO2 via a charge transfer complex path. Chem. Eng. J. 2018, 346, 249–257. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- Li, L.; Ma, D.; Xu, Q.; Huang, S. Constructing hierarchical ZnIn2S4/g-C3N4 S-scheme heterojunction for boosted CO2 photoreduction performance. Chem. Eng. J. 2022, 437, 135153. [Google Scholar] [CrossRef]
- Liapun, V.; Hanif, M.B.; Sihor, M.; Vislocka, X.; Pandiaraj, S.; Unnikrishnan, V.K.; Thirunavukkarasu, G.K.; Edelmannová, M.F.; Reli, M.; Monfort, O.; et al. Versatile application of BiVO4/TiO2 S-scheme photocatalyst: Photocatalytic CO2 and Cr(VI) reduction. Chemosphere 2023, 337, 139397. [Google Scholar] [CrossRef]
- Yue, X.; Cheng, L.; Fan, J.; Xiang, Q. 2D/2D BiVO4/CsPbBr3 S-scheme heterojunction for photocatalytic CO2 reduction: Insights into structure regulation and Fermi level modulation. Appl. Catal. B 2022, 304, 120979. [Google Scholar] [CrossRef]
- Ye, L.; Deng, Y.; Wang, L.; Xie, H.; Su, F. Bismuth-Based Photocatalysts for Solar Photocatalytic Carbon Dioxide Conversion. ChemSusChem 2019, 12, 3671–3701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xia, Y.; Wang, X.; Wen, N.; Li, H.; Jiao, X.; Chen, D. Promoted photocarriers separation in atomically thin BiOCl/Bi2WO6 heterostructure for solar-driven photocatalytic CO2 reduction. Chem. Eng. J. 2022, 449, 137874. [Google Scholar] [CrossRef]
- Zhao, J.; Miao, Z.; Zhang, Y.; Wen, G.; Liu, L.; Wang, X.; Cao, X.; Wang, B. Oxygen vacancy-rich hierarchical BiOBr hollow microspheres with dramatic CO2 photoreduction activity. J. Colloid Interface Sci. 2021, 593, 231–243. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.; Li, Y.; Wu, X.; Zhang, G. Promoted charge separation from nickel intervening in [Bi2O2]2+ layers of Bi2O2S crystals for enhanced photocatalytic CO2 conversion. Appl. Catal. B 2021, 294, 120249. [Google Scholar] [CrossRef]
- Meng, L.; Qu, Y.; Jing, L. Recent advances in BiOBr-based photocatalysts for environmental remediation. Chinese Chem. Lett. 2021, 32, 3265–3276. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Shi, W.; Ling, P.; Sun, Y.; Jiao, X.; Gao, S.; Liang, L.; Xu, J.; Yan, W.; et al. Efficient Visible-Light-Driven CO2 Reduction Mediated by Defect-Engineered BiOBr Atomic Layers. Angew Chem. Int. 2018, 57, 8719–8723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, M.; Zhang, L.; Bingham, P.A.; Tanaka, M.; Li, W.; Kubuki, S. BiOBr/MoS2 catalyst as heterogenous peroxymonosulfate activator toward organic pollutant removal: Energy band alignment and mechanism insight. J. Colloid Interface Sci. 2021, 594, 635–649. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Ding, D.; Yan, J.; Wang, C.; Carabineiro, S.A.C.; Liu, Y.; Lv, K. Effect of oxygen vacancies on the photocatalytic activity of flower-like BiOBr microspheres towards NO oxidation and CO2 reduction. Sep. Purif. Technol. 2023, 309, 123054. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, M.; Xia, F.; Cao, N.; Zhang, F.; Ni, W.; Yue, X.; Yan, K.; He, Y.; Shi, Y.; et al. Atomically Dispersed Electron Traps in Cu Doped BiOBr Boosting CO2 Reduction to Methanol by Pure H2O. Small 2023, 19, 2207581. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, B.; Zhang, L.; Yu, J.; Tan, H. BiOBr/NiO S-Scheme Heterojunction Photocatalyst for CO2 Photoreduction. Sol. Rrl 2021, 6, 2100587. [Google Scholar] [CrossRef]
- Yan, C.; Xu, M.; Cao, W.; Chen, Q.; Song, X.; Huo, P.; Zhou, W.; Wang, H. Fabricated S-scheme BiOBr/Cu2O heterojunction photocatalyst for adjusting conversion of CO2 to CH4. J. Environ. Chem. Eng. 2023, 11, 111479. [Google Scholar] [CrossRef]
- Zhang, T.; Maihemllti, M.; Okitsu, K.; Talifur, D.; Tursun, Y.; Abulizi, A. In situ self-assembled S-scheme BiOBr/pCN hybrid with enhanced photocatalytic activity for organic pollutant degradation and CO2 reduction. Appl. Surf. Sci. 2021, 556, 149828. [Google Scholar] [CrossRef]
- Meng, A.; Cheng, B.; Tan, H.; Fan, J.; Su, C.; Yu, J. TiO2/polydopamine S-scheme heterojunction photocatalyst with enhanced CO2-reduction selectivity. Appl. Catal. B 2021, 289, 120039. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, A.; Sharma, G.; Dhiman, P.; Garcia-Penas, A.; Stadler, F.J. Recent advances in perovskite-based Z-scheme and S-scheme heterojunctions for photocatalytic CO2 reduction. Chemosphere 2023, 339, 139765. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, B.; Zhang, J.; Ghasemi, J.B.; Mousavi, M.; Yu, J. S-scheme heterojunction photocatalysts for CO2 reduction. Matter 2022, 5, 4187–4211. [Google Scholar] [CrossRef]
- Li, X.; Song, X.; Ma, C.; Cheng, Y.; Shen, D.; Zhang, S.; Liu, W.; Huo, P.; Wang, H. Direct Z-Scheme WO3/Graphitic Carbon Nitride Nanocomposites for the Photoreduction of CO2. ACS Appl. Nano Mater. 2020, 3, 1298–1306. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Song, X.; Huang, B.; Ma, X.; Xing, R. CdS/WO3 S-scheme heterojunction with improved photocatalytic CO2 reduction activity. J. Photochem. Photobiol. B 2022, 233, 112480. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ding, Y.; Zheng, S.; Ye, Y.; Li, Z.; Xu, L. In-Situ Generated CsPbBr3 Nanocrystalson O-Defective WO3 for Photocatalytic CO2 Reduction. ChemSusChem 2022, 15, e202102295. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.Y.; Li, J.B.; Fu, F.Q.; Yao, J.; Jiang, S.T.; Zeng, G.M. Fabrication of novel magnetically separable BiOBr/CoFe2O4 microspheres and its application in the efficient removal of dye from aqueous phase by an environment-friendly and economical approach. Appl. Surf. Sci. 2016, 364, 604–612. [Google Scholar] [CrossRef]
- Shan, W.; Hu, Y.; Bai, Z.; Zheng, M.; Wei, C. In situ preparation of g-C3N4/bismuth-based oxide nanocomposites with enhanced photocatalytic activity. Appl. Catal. B Environ. 2016, 188, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Lai, C.; Li, B.; Huang, D.; Zeng, G.; Xu, P.; Qin, L.; Liu, S.; Liu, X.; Yi, H.; et al. Rational design 2D/2D BiOBr/CDs/g-C3N4 Z-scheme heterojunction photocatalyst with carbon dots as solid-state electron mediators for enhanced visible and NIR photocatalytic activity: Kinetics, intermediates, and mechanism insight. J. Cata 2019, 369, 469–481. [Google Scholar] [CrossRef]
- Gao, Z.; Yao, B.; Yang, F.; Xu, T.; He, Y. Preparation of BiOBr-Bi heterojunction composites with enhanced photocatalytic properties on BiOBr surface by in-situ reduction. Mat. Sci. Semiconr. Proc. 2020, 108, 104882. [Google Scholar] [CrossRef]
- Li, W.; Zou, Y.; Geng, X.; Xiao, F.; An, G.; Wang, D. Constructing highly catalytic oxidation over BiOBr-based hierarchical microspheres: Importance of redox potential of doped cations. Mol. Cat. 2017, 438, 19–29. [Google Scholar] [CrossRef]
- Thiyagarajan, K.; Muralidharan, M.; Sivakumar, K. Defects Induced Magnetism in WO3 and Reduced Graphene Oxide-WO3 Nanocomposites. J. Supercond. Nov. Magn. 2017, 31, 117–125. [Google Scholar] [CrossRef]
- Du, C.; Nie, S.; Feng, W.; Zhang, J.; Qi, M.; Liang, Y.; Wu, Y.; Feng, J.; Dong, S.; Liu, H.; et al. Hydroxyl regulating effect on surface structure of BiOBr photocatalyst toward high-efficiency degradation performance. Chemosphere 2022, 287, 132246. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Yang, M.J.; Yang, S.Y.; Xu, Y.H. Enhanced photocatalytic degradation of glyphosate over 2D CoS/BiOBr heterojunctions under visible light irradiation. J. Hazard. Mater. 2021, 407, 124798. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Tang, Q.; Hu, J.; Wang, Z.; Jiang, B.; Xiao, Y.; Song, R.; Guo, S. Construction of a hierarchical BiOBr/C3N4 S-scheme heterojunction for selective photocatalytic CO2 reduction towards CO. J. Mater. Chem. A 2023, 11, 24999–25007. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Zhang, D.; Liu, C.; Zhou, X.; Yang, H.; Qu, J.; He, D. Efficient photocatalytic water disinfection by a novel BP/BiOBr S-scheme heterojunction photocatalyst. Chem. Eng. J. 2023, 468, 143581. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Zhang, J.; Xu, K.; Wang, Z. In situ construction of WO3 nanoparticles decorated Bi2MoO6 microspheres for boosting photocatalytic degradation of refractory pollutants. J. Colloid. Interf. Sci. 2019, 556, 335–344. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Z.; Feng, Y.; Huang, J.; Hu, J.; Hao, A.; Ca, Y. A small organic molecule strategy for remedying oxygen vacancies by bismuth defects in BiOBr nanosheet with excellent photocatalytic CO2 reduction. Nano Res. 2024, 17, 297–306. [Google Scholar] [CrossRef]
- Jin, Y.; Li, F.; Li, T.; Xing, X.; Fan, W.; Zhang, L.; Hu, C. Enhanced internal electric field in S-doped BiOBr for intercalation, adsorption and degradation of ciprofloxacin by photoinitiation. Appl. Catal. B 2022, 302, 120824. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Y.; Zhang, L.; Shen, J.; Meng, L.; Wang, X. Restructuring surface frustrated Lewis acid-base pairs of BiOBr through isomorphous Sn doping for boosting photocatalytic CO2 reduction. Chem. Eng. J. 2023, 464, 142536. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Al-Hajji, L.A.; Ismail, A.A. Minimizing CO2 emissions by photocatalytic CO2 reduction to CH3OH over Li2MnO3/WO3 heterostructures under visible illumination. Environ. Res. 2024, 241, 117573. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yang, Q.; Ye, H.; Xie, Y.; Shen, Z.; Guo, Y.; Deng, Y.; Ling, Y.; Yu, J.; Luo, G.; et al. Novel 2D/2D BiOBr/Zn(OH)2 photocatalysts for efficient photoreduction CO2. Sep. Purif. Technol. 2023, 306, 122721. [Google Scholar] [CrossRef]
- Yaseen, M.; Jiang, H.; Li, J.; Yu, X.; Ashfaq Ahmad, M.; Nauman Ali, R.; Wang, L.; Yang, J.; Liu, Q. Synergistic effect of Z-scheme and oxygen vacancy of CeO2/WO3 heterojunction for enhanced CO2 reduction activity. Appl. Surf. Sci. 2023, 631, 157360. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.-g.; Wang, Y.; Hao, Y.-j.; Liu, R.-h.; Li, F.-t. One-step synthesis of defected Bi2Al4O9/β-Bi2O3 heterojunctions for photocatalytic reduction of CO2 to CO. Green Energy Environ. 2021, 6, 244–252. [Google Scholar] [CrossRef]
- Xiao, Y.; Maimaitizi, H.; Okitsu, K.; Tursun, Y.; Abulizi, A. Sonochemical Fabrication of S-scheme Hierarchical CdS/BiOBr Heterojunction Photocatalyst with High Performance for Carbon Dioxide Reduction. Part. Part. Syst. Charact. 2022, 39, 2200019. [Google Scholar] [CrossRef]
- Chico-Vecino, M.; Murillo-Sierra, J.C.; Pino-Sandoval, D.A.; Hinojosa-Reyes, L.; Maya-Treviño, M.L.; Contreras, D.; Hernández-Ramírez, A. Preparation of WO3/In2O3 heterojunctions and their performance on the CO2 photocatalytic conversion in a continuous flow reactor. J. Environ. Chem. Eng. 2023, 11, 110372. [Google Scholar] [CrossRef]
- Huang, W.; Hua, X.; Zhao, Y.; Li, K.; Tang, L.; Zhou, M.; Cai, Z. Enhancement of visible-light-driven photocatalytic performance of BiOBr nanosheets by Co2+ doping. J. Mater. Sci. Mater. Electron. 2019, 30, 14967–14976. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Cai, J.; Zhong, L.; Lang, Y.; Ma, Q. Z-scheme g-C3N4/ZnS heterojunction photocatalyst: One-pot synthesis, interfacial structure regulation, and improved photocatalysis activity for bisphenol A. Colloids Surf. A 2022, 653, 130027. [Google Scholar] [CrossRef]
- Kong, W.; Zhu, D.; Zhang, Y.; Luo, R.; Ma, J.; Lei, J.; Ju, H. Electron Donor Coordinated Metal-Organic Framework to Enhance Photoelectrochemical Performance. Angew. Chem. Int. 2023, 62, 202308514. [Google Scholar] [CrossRef] [PubMed]
- Radha, R.; Kulangara, R.V.; Elaiyappillai, E.; Sridevi, J.; Balakumar, S. Modulation in the Band Dispersion of Bi2WO6 Nanocrsytals Using the Electronegativity of Transition Elements for Enhanced Visible Light Photocatalysis. Cryst. Growth Des. 2019, 19, 6224–6238. [Google Scholar] [CrossRef]
- Yu, T.; Liu, Q.; Zhu, Z.; Wu, W.; Liu, L.; Zhang, J.; Gao, C.; Yang, T. Construction of a photocatalytic fuel cell using a novel Z-scheme MoS2/rGO/Bi2S3 as electrode degraded antibiotic wastewater. Sep. Purif. Technol. 2021, 277, 119276. [Google Scholar] [CrossRef]
- Gaopeng, L.; Lin, W.; Bin, W.; Xingwang, Z.; Jinman, Y.; Pengjun, L.; Wenshuai, Z.; Ziran, C.; Jiexiang, X. Synchronous activation of Ag nanoparticles and BiOBr for boosting solar-driven CO2 reduction. Chin. Chem. Lett. 2023, 34, 107962. [Google Scholar]
- Sun, M.; Zhu, C.; Wei, S.; Chen, L.; Ji, H.; Su, T.; Qin, Z. Phosphorus-Doped Hollow Tubular g-C3N4 for Enhanced Photocatalytic CO2 Reduction. Materials 2023, 16, 6665. [Google Scholar] [CrossRef] [PubMed]
- Juntrapirom, S.; Anuchai, S.; Thongsook, O.; Pornsuwan, S.; Meepowpan, P.; Thavornyutikarn, P.; Phanichphant, S.; Tantraviwat, D.; Inceesungvorn, B. Photocatalytic activity enhancement of g-C3N4/BiOBr in selective transformation of primary amines to imines and its reaction mechanism. Chem. Eng. J. 2020, 394, 124934. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.; Li, Q.; Feng, Y.-N.; Chen, F.-F.; Yu, Y. Spatial distribution of ZnIn2S4 nanosheets on g-C3N4 microtubes promotes photocatalytic CO2 reduction. Chem. Eng. J. 2021, 418, 129476. [Google Scholar] [CrossRef]
- Guo, H.; Wan, S.; Wang, Y.; Ma, W.; Zhong, Q.; Ding, J. Enhanced photocatalytic CO2 reduction over direct Z-scheme NiTiO3/g-C3N4 nanocomposite promoted by efficient interfacial charge transfer. Chem. Eng. J. 2021, 412, 128646. [Google Scholar] [CrossRef]
- Zhao, M.; Qin, J.; Wang, N.; Zhang, Y.; Cui, H. Reconstruction of surface oxygen vacancy for boosting CO2 photoreduction mediated by BiOBr/CdS heterojunction. Sep. Purif. Technol. 2024, 329, 125179. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Y.; Ling, Y.; Si, J.; Li, X.; Wang, J.; Ye, H.; Zhao, J.; Li, S.; Zhao, Q.; et al. One-step synthesis and Gd3+ decoration of BiOBr microspheres consisting of nanosheets toward improving photocatalytic reduction of CO2 into hydrocarbon fuel. Chem. Eng. J. 2020, 400, 125944. [Google Scholar] [CrossRef]
- Yin, S.; Zhao, X.; Jiang, E.; Yan, Y.; Zhou, P.; Huo, P. Boosting water decomposition by sulfur vacancies for efficient CO2 photoreduction. Energy Environ. Sci. 2022, 15, 1556–1562. [Google Scholar] [CrossRef]
- Meng, J.; Duan, Y.; Jing, S.; Ma, J.; Wang, K.; Zhou, K.; Ban, C.; Wang, Y.; Hu, B.; Yu, D.; et al. Facet junction of BiOBr nanosheets boosting spatial charge separation for CO2 photoreduction. Nano Energy 2022, 92, 106671. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Chen, H.; Weng, Y.-X.; Tang, H.; Chen, Z.; Zhu, W.; She, Y.; Xia, J.; Li, H. Unique Z-scheme carbonized polymer dots/Bi4O5Br2 hybrids for efficiently boosting photocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 293, 120182. [Google Scholar] [CrossRef]
- Bai, P.; Zhao, Y.; Li, Y. Efficient photocatalytic CO2 reduction coupled with selective styrene oxidation over a modified g-C3N4/BiOBr composite with high atom economy. Green Chem. 2024, 26, 2290–2299. [Google Scholar] [CrossRef]
- Lin, W.; Gaopeng, L.; Bin, W.; Xin, C.; Chongtai, W.; Zixia, L.; Jiexiang, X.; Huaming, L. Oxygen Vacancies Engineering–Mediated BiOBr Atomic Layers for Boosting Visible Light-Driven Photocatalytic CO2 Reduction. Solar Rrl 2020, 5, 2000480. [Google Scholar]
- Guan, C.; Hou, T.; Nie, W.; Zhang, Q.; Duan, L.; Zhao, X. Enhanced photocatalytic reduction of CO2 on BiOBr under synergistic effect of Zn doping and induced oxygen vacancy generation. J. Colloid Interface Sci. 2023, 633, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Jiayu, Z.; Yupei, L.; Xiaojing, W.; Jun, Z.; Yinsu, W.; Fatang, L. Simultaneous Phosphorylation and Bi Modification of BiOBr for Promoting Photocatalytic CO2 Reduction. ACS Sustain. Chem. Eng. 2019, 7, 14953–14961. [Google Scholar]
- Xin, F.; ChuYa, W.; Longpeng, Z.; Qi, Z.; Hengdeng, Z.; Qi, W.; Guangcan, Z. Efficient visible-light-driven CO2 reduction mediated by novel Au-doped BiOBr nanosheets. J. Environ. Chem. Eng. 2023, 11, 109986. [Google Scholar]
- Ying, K.X.; Cathie, L.W.P.; WeeJun, O.; SiangPiao, C.; Rahman, M.A. Oxygen-Deficient BiOBr as a Highly Stable Photocatalyst for Efficient CO2 Reduction into Renewable Carbon-Neutral Fuels. ChemCatChem 2016, 8, 3074–3081. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Lu, X.; Chen, L.; Xie, X.; Qin, Z.; Ji, H.; Su, T. WO3/BiOBr S-Scheme Heterojunction Photocatalyst for Enhanced Photocatalytic CO2 Reduction. Materials 2024, 17, 3199. https://doi.org/10.3390/ma17133199
Li C, Lu X, Chen L, Xie X, Qin Z, Ji H, Su T. WO3/BiOBr S-Scheme Heterojunction Photocatalyst for Enhanced Photocatalytic CO2 Reduction. Materials. 2024; 17(13):3199. https://doi.org/10.3390/ma17133199
Chicago/Turabian StyleLi, Chen, Xingyu Lu, Liuyun Chen, Xinling Xie, Zuzeng Qin, Hongbing Ji, and Tongming Su. 2024. "WO3/BiOBr S-Scheme Heterojunction Photocatalyst for Enhanced Photocatalytic CO2 Reduction" Materials 17, no. 13: 3199. https://doi.org/10.3390/ma17133199
APA StyleLi, C., Lu, X., Chen, L., Xie, X., Qin, Z., Ji, H., & Su, T. (2024). WO3/BiOBr S-Scheme Heterojunction Photocatalyst for Enhanced Photocatalytic CO2 Reduction. Materials, 17(13), 3199. https://doi.org/10.3390/ma17133199







