Abstract
This study focuses on the efficient removal of Ni(II) from spent lithium-ion batteries (LIBs) to support environmental conservation and sustainable resource management. A composite material, known as molecular sieve (MS)-based metal–organic framework (MOF) composites (MMCs), consisting of a synthesized MS matrix with integrated MOFs, was developed for the adsorption of Ni(II). The structural and performance characteristics of the MMCs were evaluated using X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), and N2 adsorption–desorption isotherms (BET). Batch adsorption experiments were conducted to assess the Ni(II) adsorption performance of the MMCs. The results revealed that, under conditions of pH 8 and a temperature of 298 K, the MMCs achieved near-equilibrium Ni(II) adsorption within 6 h, with a maximum theoretical adsorption capacity of 204.1 mg/g. Further analysis of the adsorption data confirmed that the adsorption process followed a pseudo-second-order kinetic model and Langmuir isotherm model, indicating a spontaneous, endothermic chemical adsorption mechanism. Importantly, the MMCs exhibited superior Ni(II) adsorption compared to the MS. This study provides valuable insights into the effective recovery and recycling of Ni(II) from spent LIBs, emphasizing its significance for environmental sustainability and resource circularity.
1. Introduction
Nickel is an important non-ferrous metal with a wide range of applications in the electronics industry and battery production [1,2]. Especially in the production of ternary lithium batteries, nickel, as one of the main components of the positive electrode material, is widely used [3]. However, with the rapid development of the global electric vehicle market and the increasing demand for electronic products, nickel resources are facing increasingly severe supply pressures. According to statistics, the current global nickel reserves are limited, and nickel mining and production also face many problems, such as high costs, environmental impacts, etc., making the rational utilization of nickel resources particularly important [4,5].
Spent lithium-ion batteries (LIBs) contain a large amount of nickel [6]. If these nickels are not effectively recovered and reused, it will not only lead to a waste of resources but also pose significant pollution risks to the environment [7]. The treatment and disposal of spent LIBs often face many challenges, such as the difficulty of heavy metal recovery, potential threats to the surrounding environment and human health, etc. [2,8]. Therefore, it is crucial to develop efficient and economical techniques to effectively separate and recover nickel elements from spent LIBs. This can not only effectively solve the problem of waste battery treatment and disposal but also achieve resource recycling and reuse, achieving the dual purposes of resource recycling and environmental protection.
The separation of metal ions is a key issue in the fields of environmental protection and resource recovery, and adsorption technology, as a commonly used separation method, has significant advantages in separating metal ions [4]. Adsorption technology is characterized by simple operation, high efficiency, rapidity, and broad adsorption capabilities for various substances, making it widely used in the separation and enrichment processes of metal ions [9]. In addition to adsorption, methods for separating metal ions also include solvent extraction [10], membrane separation [11], precipitation, etc., but relatively speaking, adsorption is more emphasized in practical applications.
Common adsorption materials at present include carbon materials, biological materials, etc. Carbon materials such as activated carbon, carbon nanotubes, etc., with high surface area and pore structure, exhibit excellent performance in the adsorption of metal ions. Biological materials such as biopolymers, microorganisms, plant extracts, etc., possess natural adsorption properties and have a certain potential for the biological adsorption of metal ions. However, existing adsorption materials still have some drawbacks, such as limited adsorption capacity, low selectivity for specific metal ions, poor regenerability, etc., which restrict their application in the separation of metal ions.
Therefore, there is an urgent need to develop new adsorption materials to address these issues. Modified molecular sieves (MSs) are one of the important directions in current research [12]. The purpose of modifying MS is to enhance their adsorption performance, further expanding their application fields. Modification can be achieved by introducing new functional groups or coordinating atoms, controlling their crystal structure and pore structure, or improving their surface affinity [13,14]. These modification methods can significantly improve the adsorption capacity, selectivity, and regenerability of MSs, making them more suitable for various environmental governances, resource recoveries, and chemical industries [15,16].
In recent years, researchers have actively explored various new methods of modifying MSs, such as introducing functional groups, surface modification, synthesizing composite materials, etc. [17]. Among them, the synthesis of composite materials is an effective way. By combining MSs with other functional materials, composite materials with synergistic effects can be formed, overcoming the shortcomings of traditional MSs and endowing them with new properties [18]. This method not only enhances the adsorption performance and stability of MSs but also broadens their application range in different fields.
Therefore, modifying MSs to enhance their adsorption performance and functionalization has become one of the hotspots in current research. By continuously exploring new modification methods and material combinations, more efficient and environmentally friendly MS materials can be developed, making greater contributions to environmental protection, resource recovery, and the development of the chemical industry [12,13,16].
Metal–organic frameworks (MOFs) as a new type of material have shown great potential in the field of metal ion separation [19,20,21,22]. MOFs have a high specific surface area, controllable pore structure, and abundant functional groups, making them perform well in adsorption, separation, storage, etc. Especially in the process of metal ion separation, MOFs exhibit excellent adsorption performance and selectivity, providing new possibilities for solving the problems of metal ion separation and enrichment [23,24]. However, due to the relatively high cost of MOF preparation, its application range and promotion in practical applications are limited.
To overcome the problem of high cost in preparing MOFs and further leverage their advantages in metal ion separation, we propose a reasonable combination of MOFs with MSs. This combination can fully utilize the efficient adsorption performance of MOFs and the structural stability of MSs, complementing each other’s shortcomings, and achieving a more efficient metal ion separation process. By designing and constructing MOFs and MS composite materials reasonably, the advantages of both can be combined to increase the adsorption capacity and stability of the materials, thereby achieving effective separation and enrichment of metal ions.
In this study, we synthesized a composite material called MS-MOFs composites (MMCs), which consisted of a molecular sieve (MS) with aluminum ions as metal ion sources and added organic ligands of 1,3,5-benzenetricarboxylic acid (H3BDCH3BTC). This novel material was utilized for the separation of nickel ions, offering a promising solution for the depletion of nickel resources and the treatment of spent LIBs. The new composite material not only reduces the preparation cost of MOFs but also enhances its adsorption capacity for nickel ions. The development of these innovative composite materials provides a novel approach towards environmental protection and resource utilization, with significant practical and scientific implications.
2. Experimental Section
2.1. Materials
NaAlO2, SiO2 particle, and 1,3,5-benzenetricarboxylic acid (H3BTC) were obtained from Aladdin Reagent, Shanghai. N,N-dimethylformamide (DMF, 99.8%), ethanol, HCl, and NaOH were purchased from Chengdu KeLong Chemical Reagent Factory (Chengdu, China). All reagents were used without further purification.
2.2. Preparation of MMCs
In a 250 mL flask, 2 g of SiO2 were combined with a 30% NaOH solution and refluxed at 90 °C for 4 h. Around 2.7 g of NaAlO2 and deionized water were subsequently added. The mixture was stirred and refluxed for 24 h at a high temperature of 120 °C for crystallization. After reaching room temperature, the product was filtered and washed with deionized water until the pH of the filtrate dropped below 11. The resulting residue was dried overnight at 120 °C, yielding a white powder identified as the MS. Next, 2.00 g of the MS and 1.2 g of H3BTC were added to 100 mL of N,N-dimethylformamide in a reflux setup at 120 °C for 12 h. The solid product obtained underwent centrifugation, followed by three rinses with DMF and ethanol. It was then vacuum-dried overnight at 60 °C to yield the final solid product, MMCs.
2.3. Characterization of MMCs
Scanning electron microscope (SEM) analysis was performed using a JSM-5610LV microscope (STARJOY LIMITED, Tokyo, Japan). X-ray diffraction (XRD) was conducted by scanning from 5° to 60° with an XRD-7000 instrument (CuKα radiation at 40 kV and 30 mA, Shimadzu, Kyoto, Japan). Fourier transform infrared (FT-IR) spectra of the samples were obtained using a Fourier transform infrared spectrometer (Thermo Fisher, Waltham, MA, USA). The BET surface area was determined by N2 adsorption–desorption (Autosorb-iQ, Quantachrome, Boynton Beach, FL, USA).
2.4. Adsorption Experiment
Take 10 mg of the adsorbents MS and MMCs and place them separately in 50 mL simulated wastewater solutions with 10 mg/L of Ni(II), adjusting the pH using a 0.1 mol/L NaOH or HCl solution. After a specific duration of oscillation in a constant temperature shaking incubator, transfer a specific volume of the supernatant to a centrifuge tube, centrifuge at 10,000 rpm for 10 min, then extract a sample and determine the concentration of Ni(II) in the residual solution using ICP-MS. Calculate the adsorption capacity of Ni(II) by the MS and MMCs based on Equation (1) [25]. The sorption experiments were replicated three times.
where, qe represents the adsorption capacity (mg/g); V denotes the volume of the Ni(II)-containing wastewater (L); C0 and Ce represent the nickel ion concentrations in the solution before and after adsorption (mg/L); and m refers to the mass of the MS and MMCs (g).
3. Results and Discussion
3.1. Characterization
The XRD spectra of MS and MMCs are depicted in Figure 1a. The graph illustrates that MS is composed of a well-oriented crystallized structure, with main characteristic peaks observed at 2θ = 10.2, 24.6, 27.5, and 30 degrees, which are attributed to the four-membered ring structure [17]. Upon introducing the ligand H3BDC into the MS to construct MOF layers, the material exhibits prominent MIL-96 characteristic peaks. Concurrently, some characteristic peaks of the MS still persist, indicating the successful preparation of the material.
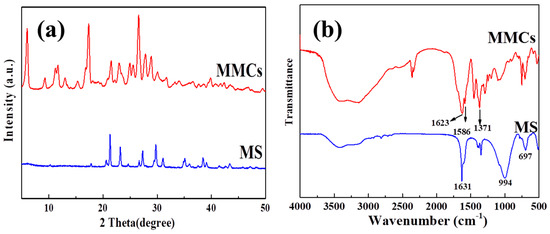
Figure 1.
The XRD patterns (a) and FT−IR spectrometry (b) of the MS and MMCs.
The FT-IR spectra of the MS and MMCs are shown in Figure 1b. The peak at 1631 cm−1 is associated with the bending vibration of the -OH group in the MS, while the peak at 997 cm−1 corresponds to the asymmetric stretching vibration characteristic peak of the Al-O tetrahedra, and the peak at 697 cm−1 is related to Si-O in the MS [18,26]. In the MMCs’ spectrum, the peaks at 1586 cm−1 and 1371 cm−1 are attributed to the asymmetric and symmetric stretching vibrations of carboxyl groups within the material [27]. Additionally, some characteristic peaks of the MS disappear or weaken, and some characteristic peaks of aluminum-based MOFs emerge, indicating the successful preparation of MMCs [20,28].
The nitrogen adsorption–desorption isotherms of the MS and MMCs are shown in Figure 2, and Table 1 presents the structural characteristics data of nitrogen adsorption–desorption for the two materials. The results indicate that the nitrogen adsorption−desorption isotherm of the MS and MMCs belongs to the type IV curve, and the type IV isotherm is characterized by the accompaniment of the H3 hysteresis loop after capillary condensation, suggesting that mesoporous structures were formed on the MOF NFs. Compared to the MS, the specific surface area and pore volume of MMCs have increased, attributed to the formation of MOFs, which enhances the pore structure.
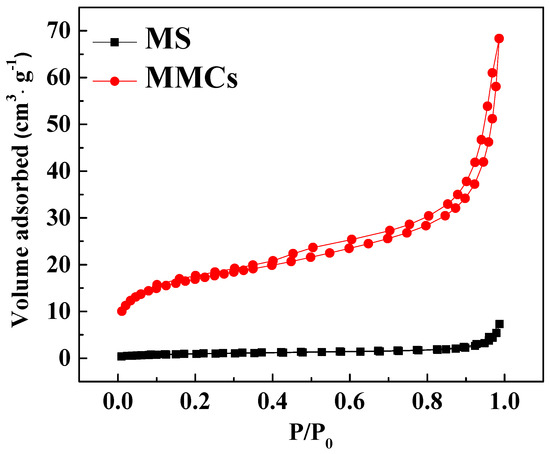
Figure 2.
N2 adsorption−desorption isotherms of MS and MMCs.

Table 1.
Pore structure parameters of MS and MMCs.
The specific surface area was calculated according to the BET equation, while the pore volume and pore diameter were determined using the BJH equation.
The SEM images of the MS and MMCs in Figure 3 reveal distinct morphological differences. The MS exhibits a spherical morphology, while the composite MMCs display a flaky structure, indicating that the formation of a multi-framework structure has influenced the physical appearance of the material.
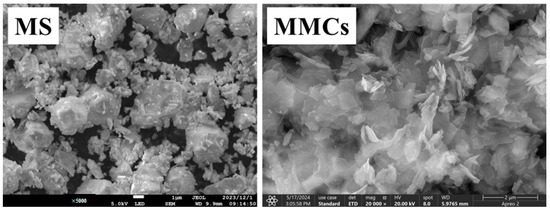
Figure 3.
SEM images of MS and MMCs.
3.2. Study on Adsorption Properties
3.2.1. Effect of pH
From Figure 4a, it can be observed that when the pH was in the range of 4–7, the adsorption of Ni(II) by the MS and MMCs was below 10 mg/g. This can be attributed to the higher concentration of hydrogen ions in acidic conditions, leading to protonation of the adsorption sites on the materials. As a result, hydrogen ions competed with Ni(II) for adsorption, resulting in lower adsorption of Ni(II) by the MS and MMCs. As the pH increased, the impact of hydrogen ions gradually decreased, leading to a rapid increase in the adsorption of Ni(II) by the MS and MMCs. At pH 8.0, the adsorption capacities of Ni(II) by the MS and MMCs were 27.9 and 39.5 mg/g, respectively. At pH 9.0, due to the formation of Ni(OH)2 precipitation [3,29], the adsorption capacities of Ni(II) by the two materials reached 42.7 mg/g and 44.7 mg/g. Since Ni(OH)2 is the primary form of Ni(II) at this pH, further studies were conducted under pH = 8.0 conditions.
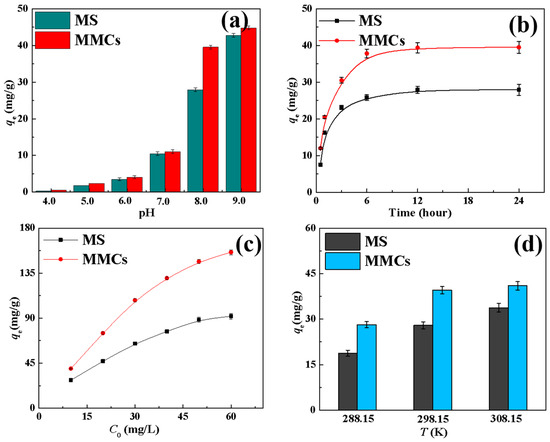
Figure 4.
Effect of various parameters on adsorption efficiency of MS and MMCs for Ni(II): (a) pH; (b) contact time; (c) initial concentration of nickel ions; and (d) temperature.
At pH 8.0, the adsorption capacity of Ni(II) by the MS was 27.9 mg/g, indicating that the prepared MS exhibited good adsorption performance. Upon the formation of MMCs by composite with MOFs, the adsorption capacity of Ni(II) further increased to 35.9 mg/g. This phenomenon suggests that the surface area of MMCs was enhanced after MOFs composite, thereby directly increasing the material’s capability to adsorb metal ions. Therefore, composite formation with MOFs is necessary and meaningful for enhancing the adsorption capacity of MSs.
3.2.2. Effect of Contact Time
Figure 4a illustrates the impact of adsorption time on the Ni(II) adsorption performance of MSs and MMCs. It can be observed from the graph that the adsorption capacities of Ni(II) by the MS and MMCs both increase with prolonged adsorption time. In the initial stages of the adsorption reaction, the adsorption of Ni(II) rapidly increases with time but later slows down as a result of the decrease in Ni(II) concentration and diminishing reactive sites until reaching saturation. The adsorption capacities of Ni(II) by the MS and MMCs reach equilibrium at around 6 h, with adsorption amounts of 27.9 and 39.5 mg/g, respectively.
Figure 5a,b depict the fitting of pseudo-first-order (PFO, Equation (2)) and pseudo-second-order (PSO, Equation (3)) kinetic models [17,30] to the adsorption dynamics of Ni(II) by the MS and MMCs, along with the fitting parameters of the PFO and PSO models (Table 2). The two models are as follows:
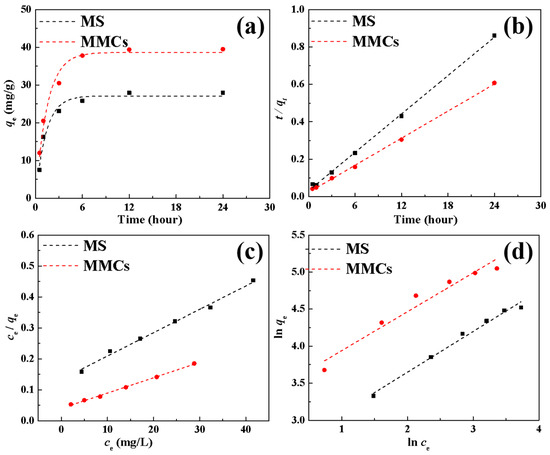
Figure 5.
The kinetic and isotherm model for Ni(II) adsorption on the MS and MMCs. (a) PFO model, (b) PSO model, (c) Langmuir model, and (d) Freundlich model.

Table 2.
The kinetic model parameters for MS and MMCs adsorption on Ni(II).
In this context, qt and qe represent the adsorption amount at time t and at equilibrium (mg/g), respectively. Additionally, k1 and k2 are the rate constants in the PFO and PSO models.
Compared to the PFO model, the adsorption of Ni(II) by the MS and MMCs aligns well with the PSO model. Furthermore, the calculated qe,cal based on the PSO model is closer to the experimental values, indicating that the adsorption process of Ni(II) by the MS and MMCs still predominantly occurs through chemical adsorption.
3.2.3. Effect of Initial Concentration of Ni(II)
The initial concentration plays a crucial role in determining the mass transfer efficiency of pollutants between the solid and liquid phases, making it another key factor influencing the adsorption capacity. By increasing the initial concentration of metal ions, the removal capacity of the adsorbent can be effectively enhanced. The effective removal of Ni(II) by the MS and MMCs follows this trend, as shown in Figure 4c. As the initial concentration of Ni(II) is raised from 10 mg/L to 60 mg/L, the adsorption capacity of the MS and MMCs for Ni(II) increases from 27.9 mg/g and 39.5 mg/g to 91.8 mg/g and 155.8 mg/g, respectively. This increase in adsorption is primarily attributed to the higher Ni(II) concentration, which boosts the concentration gradient between the adsorbate and the adsorbent, leading to improved mass transfer dynamics and facilitating more migration of Ni(II) to the adsorbent surface. Moreover, the higher Ni(II) concentration increases the likelihood of collision with active sites on the adsorbent. The isotherm fitting of Ni(II) adsorption on the MS and MMCs using Langmuir (Equation (4)) and Freundlich (Equation (5)) models [31,32] is displayed in Figure 5c,d, respectively, with the fitting parameters listed in Table 3. The Langmuir and Freundlich equations are as follows:
where Ce (mg/L) is the equilibrium Ni(II) concentration, qe and qm (mg g−1) are the equilibrium and maximum theoretical adsorption of Ni(II), respectively, KL (L mg−1) is the Langmuir adsorption equilibrium constant, and KF (mg g−1(L mg−1)1/n) and n are the Freundlich constants related to the adsorption capacity and adsorption strength, respectively.

Table 3.
The isothermal model parameters of Ni(II) adsorption on the MS and MMCs.
It is evident that the adsorption of Ni(II) by the MS and MMCs conforms well to the Langmuir model, with higher R2 values (Table 3) indicating a monolayer adsorption process for Ni(II). The maximum adsorption capacities of Ni(II) by the MS and MMCs are 133.3 mg/g and 204.1 mg/g, respectively, aligning well with the experimental trends. Furthermore, the significantly higher adsorption capacity of MMCs compared to MSs indicates the successful modification of MSs. Table 4 compares the adsorption capacities of different adsorbents for Ni(II), revealing that MMCs demonstrate a significantly higher adsorption capacity for Ni(II), indicating their substantial potential in the separation of Ni(II).

Table 4.
A comparison of Ni(II) sorption from the literature using different adsorbents.
3.2.4. Effect of Temperature
Figure 4d illustrates the impact of temperature on the adsorption of nickel ions by the MS and MMCs. It can be seen that as the temperature rises from 288 K to 308 K, the adsorption capacity of the MS and MMCs for nickel ions gradually increases. This indicates that the adsorption of nickel ions by both adsorbents is an endothermic process, and the increase in temperature promotes the adsorption of nickel ions by the MS and MMCs. To further investigate the adsorption mechanism of MSs and MMCs for nickel ions, the enthalpy change (ΔH0), entropy change (ΔS0), and Gibbs free energy (ΔG0) of the adsorption process of Ni(II) by the MS and MMCs were calculated according to Equations (6) and (7) [39]. The results are shown in Table 5. Since ΔH0 > 0 and ΔG0 < 0 for the adsorption of nickel ions by the MS and MMCs, it indicates that the adsorption of nickel ions by both materials is a spontaneous endothermic process.

Table 5.
The thermodynamic parameters of Ni(II)adsorption on the MS and MMCs.
3.2.5. Study on Cycle Stability
The repeated use of adsorbent materials is an extremely important factor in determining their adsorption performance. The cyclic regeneration performance of MSs and MMCs was studied through adsorption–desorption cycles. After adsorption of Ni(II) by the two materials, they were repeatedly washed with 1.0 M hydrochloric acid to desorb Ni(II) until no Ni(II) was detected in the wash solution. Subsequently, the MS and MMCs were used again for adsorption experiments in the same concentration of Ni(II) solution. The adsorption and desorption cycles were repeated six times for the MS and MMCs, and the adsorption capacity for each cycle was recorded, as shown in Figure 6. After six adsorption–desorption cycles, the adsorption capacity of the MS and MMCs remained at over 90% of the original adsorption capacity. Therefore, the desorption process did not alter the structure and chemical properties of the MS and MMCs, indicating that they possess good stability and regeneration performance.
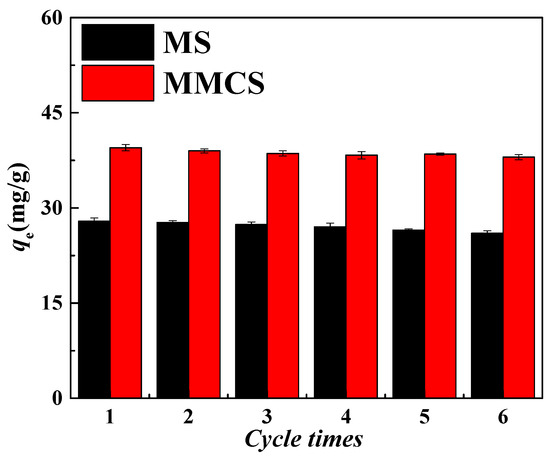
Figure 6.
Study on cyclic stability of MS and MMCs.
3.2.6. Study on Adsorption Mechanism
To investigate the adsorption mechanism of nickel ions by MMCs, XPS scan spectroscopy analysis was conducted before and after the adsorption of nickel ions on MMCs, as shown in Figure 7. The XPS spectra of MMCs before and after nickel ion adsorption are shown in Figure 7a. From the spectra, a new peak with a binding energy of 856.2 eV appeared on the surface of MMCs, indicating the successful adsorption of nickel ions on the surface of MMCs [1,4]. The O 1s spectrum of MMCs (Figure 7c) shows three peaks at 531.4 eV, 532.2 eV, and 533.1 eV, corresponding to the oxygen atoms in Al-OBTC, Si-O-Si, and Si-O-H structures, respectively [6,8,40]. After the adsorption of nickel ions by MMCs, a chemical shift to the left occurred in the binding energy of Si-O-H, indicating a chemical transformation in the chemical form of oxygen atoms. This is due to the oxygen atoms providing lone pair electrons to the nickel ions to form coordination bonds.
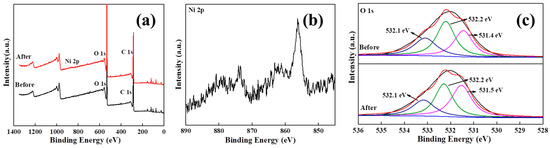
Figure 7.
XPS spectra of full spectrum: (a) Ni 2p, (b) O 1s, and (c) before and after adsorption of MMCs.
4. Conclusions
In summary, in this study, MS-based MOF composite materials (MMCs) were prepared using H3BDC as a ligand. These materials were utilized for the separation of nickel ions, and the results indicated that adsorption equilibrium could be achieved after 6 h at pH 8, with equilibrium adsorption capacities of 27.9 mg/g and 39.5 mg/g for the MS and MMCs, respectively. The adsorption of nickel by the MS and MMCs followed the Langmuir model and pseudo-second-order kinetic model, with a theoretical maximum adsorption capacity of 133.3 mg/g and 204.1 mg/g. Furthermore, the adsorption capacity of nickel ions by MMCs was higher than that of the MS, indicating the successful preparation of the composite material. The adsorption process was mainly driven by a coordination mechanism. This study demonstrates that the utilization of MMCs for the adsorption and separation of nickel ions is effective and holds significant implications for the treatment and disposal of spent LIBs. Future research could further explore different combinations of ligands and matrices to enhance the adsorption performance of composite materials for nickel ions. Additionally, consideration could be given to optimizing the preparation process to improve the stability and regenerability of the composite material. Furthermore, exploring the application of this composite material for the adsorption and separation of other metal ions could expand its use in environmental remediation and resource recovery. Through ongoing research and improvements, the adsorption and separation performance of the composite material can be further enhanced, providing a more effective solution for the disposal of spent LIBs and related issues.
Author Contributions
Conceptualization, Z.L., L.Y. and C.Y.; investigation, experimental: Z.L., L.Y., Y.L., L.D. and C.Y.; writing—review and editing: L.Y. and C.Y.; funding acquisition: Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Research Project of Chongqing Municipal Education Commission (KJQN202104701).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Milevskii, N.A.; Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Separation of Li(I), Co(II), Ni(II), Mn(II), and Fe(III) from hydrochloric acid solution using a menthol-based hydrophobic deep eutectic solvent. Hydrometallurgy 2022, 207, 105777. [Google Scholar] [CrossRef]
- Chan, K.H.; Malik, M.; Azimi, G. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour. Conserv. Recycl. 2022, 178, 106076. [Google Scholar] [CrossRef]
- Choubey, P.K.; Dinkar, O.S.; Panda, R.; Kumari, A.; Jha, M.K.; Pathak, D.D. Selective extraction and separation of Li, Co and Mn from leach liquor of discarded lithium ion batteries (LIBs). Waste Manag. 2021, 121, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Zante, G.; Masmoudi, A.; Barillon, R.; Trébouet, D.; Boltoeva, M. Separation of lithium, cobalt and nickel from spent lithium-ion batteries using TBP and imidazolium-based ionic liquids. J. Ind. Eng. Chem. 2020, 82, 269–277. [Google Scholar] [CrossRef]
- Li, C.; Dai, G.; Liu, R.; Wang, C.; Wang, S.; Ju, Y.; Jiang, H.; Jiao, S.; Duan, C. Separation and recovery of nickel cobalt manganese lithium from waste ternary lithium-ion batteries. Sep. Purif. Technol. 2023, 306, 122559. [Google Scholar] [CrossRef]
- Thompson, D.L.; Pateli, I.M.; Lei, C.; Jarvis, A.; Abbott, A.P.; Hartley, J.M. Separation of nickel from cobalt and manganese in lithium ion batteries using deep eutectic solvents. Green Chem. 2022, 24, 4877–4886. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of cobalt, nickel, and manganese in leach solutions of waste lithium-ion batteries using Dowex M4195 ion exchange resin. Hydrometallurgy 2021, 206, 105757. [Google Scholar] [CrossRef]
- Yuan, G.; Li, Y.; Yu, Y.; Lei, Y.; Liu, F.; Liu, D.; Pu, X.; Xiong, W. Facile construction of a core-shell structured metal-organic frameworks nanofiber membrane for removing Co(II) from simulated radioactive wastewater. Sep. Purif. Technol. 2024, 336, 126295. [Google Scholar] [CrossRef]
- Lei, S.; Yang, C.; Cao, X.; Wei, S.; Weng, Y.; Yue, Y. Separation of lithium and transition metals from leachate of spent lithium-ion batteries by solvent extraction method with Versatic 10. Sep. Purif. Technol. 2020, 250, 117258. [Google Scholar]
- Peng, Y.; Pan, T.; Chen, C.; Zhang, Y.; Yuan, G.; Liu, D.; Pu, X.; Xiong, W. In Situ Synthesis of NH2-MIL-53-Al/PAN Nanofibers for Removal Co(II) through an Electrospinning Process. Langmuir 2024, 40, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Júnior, C.J.; de Alcântara Pessoa Filho, P. Modeling and simulation of an industrial adsorption process of dehydration of natural gas in 4A molecular sieves: Effect of adsorbent aging. Results Eng. 2023, 18, 101144. [Google Scholar] [CrossRef]
- Rahimalimamaghani, A.; Pacheco Tanaka, D.A.; Llosa Tanco, M.A.; Neira D’Angelo, F.; Gallucci, F. New hydrophilic carbon molecular sieve membranes for bioethanol dehydration via pervaporation. Chem. Eng. J. 2022, 435, 134891. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Zhang, C.; Huang, X.; Ma, K.; Zhang, Y. Preparation of graphene oxide/4A molecular sieve composite and evaluation of adsorption performance for Rhodamine B. Sep. Purif. Technol. 2022, 286, 120400. [Google Scholar] [CrossRef]
- Kretzschmar, A.; Selmert, V.; Kungl, H.; Tempel, H.; Eichel, R.-A. Application of a tailorable carbon molecular sieve to evaluate concepts for the molecular dimensions of gases. Micropor. Mesopor. Mat. 2022, 343, 112156. [Google Scholar] [CrossRef]
- Hou, M.; Li, L.; Song, J.; Xu, R.; He, Z.; Lu, Y.; Pan, Z.; Song, C.; Wang, T. Polyimide-derived carbon molecular sieve membranes for high-efficient hydrogen purification: The development of a novel phthalide-containing polyimide precursor. Sep. Purif. Technol. 2022, 301, 121982. [Google Scholar] [CrossRef]
- Goyal, N.; Gao, P.; Wang, Z.; Cheng, S.; Ok, Y.S.; Li, G.; Liu, L. Nanostructured chitosan/molecular sieve-4A an emergent material for the synergistic adsorption of radioactive major pollutants cesium and strontium. J. Hazard. Mater. 2020, 392, 122494. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, R. Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite. J. Hazard. Mater. 2017, 326, 157–164. [Google Scholar] [CrossRef]
- Yu, L.; Lan, T.; Yuan, G.; Duan, C.; Pu, X.; Liu, N. Synthesis and Application of a Novel Metal-Organic Frameworks-Based Ion-Imprinted Polymer for Effective Removal of Co(II) from Simulated Radioactive Wastewater. Polymers 2023, 15, 2150. [Google Scholar] [CrossRef]
- Rostami, M.S.; Khodaei, M.M. Investigation of permeability, hydrophilicity, and pollutant removal properties of PES/MIL-53(Al)@MWCNT nanofiltration membrane. Chem. Eng. J. 2023, 475, 146074. [Google Scholar] [CrossRef]
- Peng, Y.; Pan, T.; Chen, C.; Zhou, M.; Liu, D.; Feng, J.; Xiong, W.; Liu, N.; Yuan, G. Efficient removal of Co(II) from aqueous solution by one-step preparation of heterocyclic ligand-functionalized MOFs: Study on adsorption properties and irradiation stability. J. Radioanal. Nucl. Chem. 2023, 332, 4167–4177. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, D.; He, B.; Yu, B.; Pu, X.; Liu, D.; Xiong, W.; Liu, N.; Yuan, G. Facile preparation of UiO-66 derivatives for the removal of Co(II) from aqueous solution: Study on adsorption properties and irradiation stability. J. Radioanal. Nucl. Chem. 2023, 332, 4047–4056. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Cui, K.; Li, H.; Feng, J.; Pu, X.; Xiong, W.; Liu, N.; Yuan, G. Novel MOFs-based ion-imprinted polymer for selective separation of cobalt ions from waste battery leaching solution. Inorg. Chim. Acta 2022, 536, 120922. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Y.; Andrews, C.B.; Zheng, C. One-step construction of hierarchical porous channels on electrospun MOF/polymer/graphene oxide composite nanofibers for effective arsenate removal from water. Chem. Eng. J. 2022, 435, 134830. [Google Scholar] [CrossRef]
- Yuan, G.; Yu, Y.; Li, J.; Jiang, D.; Gu, J.; Tang, Y.; Qiu, H.; Xiong, W.; Liu, N. Facile fabrication of a noval melamine derivative-doped UiO-66 composite for enhanced Co(II) removal from aqueous solution. J. Mol. Liq. 2021, 328, 115484. [Google Scholar] [CrossRef]
- Yamane, Y.; Miyahara, M.T.; Tanaka, H. High-Performance Carbon Molecular Sieves for the Separation of Propylene and Propane. ACS Appl. Mater. Interfaces 2022, 14, 17878–17888. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.R.; Abid, H.R.; Tade, M.O.; Periasamy, V.; Sun, H.; Wang, S. Cascade applications of robust MIL-96 metal organic frameworks in environmental remediation: Proof of concept. Chem. Eng. J. 2018, 341, 262–271. [Google Scholar] [CrossRef]
- Singh, N.; Dalakoti, S.; Sharma, A.; Chauhan, R.; Murali, R.S.; Divekar, S.; Dasgupta, S. Shaping of MIL-53-Al and MIL-101 MOF for CO2/CH4, CO2/N2 and CH4/N2 separation. Sep. Purif. Technol. 2024, 341, 126820. [Google Scholar]
- Schaeffer, N.; Passos, H.; Gras, M.; Rodriguez Vargas, S.J.; Neves, M.C.; Svecova, L.; Papaiconomou, N.; Coutinho, J.A.P. Selective Separation of Manganese, Cobalt, and Nickel in a Fully Aqueous System. ACS Sustain. Chem. Eng. 2020, 8, 12260–12269. [Google Scholar] [CrossRef]
- Ying, Y.; Pourrahimi, A.M.; Sofer, Z.; Matejkova, S.; Pumera, M. Radioactive Uranium Preconcentration via Self-Propelled Autonomous Microrobots Based on Metal-Organic Frameworks. ACS Nano 2019, 13, 11477–11487. [Google Scholar] [CrossRef]
- Yin, C.; Peng, Y.; Li, H.; Yang, G.; Yuan, G. Facile construction of ZIF-94/PAN nanofiber by electrospinning for the removal of Co(II) from wastewater. Sci. Rep. 2024, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, T.; Feng, J.; Yu, B.; Xiong, W.; Yuan, G. Facile preparation of UiO-66-Lys/PAN nanofiber membrane by electrospinning for the removal of Co(II) from simulated radioactive wastewater. Sci. Total Environ. 2024, 914, 169725. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, X.; Peng, J. Highly selective removal and recovery of Ni(II) from aqueous solution using magnetic ion-imprinted chitosan nanoparticles. Carbohydr. Polym. 2021, 271, 118435. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Karimi-Jashni, A. Optimization of Ni(II) adsorption onto Cloisite Na(+) clay using response surface methodology. Chemosphere 2020, 246, 125710. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, R.; Yang, R.; Yang, F.; Chen, J.; Li, Y.; Zhou, R.; Xu, J. Synthesis of amino-functionalized biochar/spinel ferrite magnetic composites for low-cost and efficient elimination of Ni(II) from wastewater. Sci. Total Environ. 2020, 722, 137822. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Motamedi, M. Adsorption of Nickel, Ni(II), in Aqueous Solution by Modified Zeolite as a Cation-Exchange Adsorbent. J. Chem. Eng. Data 2019, 65, 185–197. [Google Scholar] [CrossRef]
- Liu, D.; Deng, S.; Vakili, M.; Du, R.; Tao, L.; Sun, J.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Fast and high adsorption of Ni(II) on vermiculite-based nanoscale hydrated zirconium oxides. Chem. Eng. J. 2019, 360, 1150–1157. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafting of cellulose with N-isopropylacrylamide and glycidyl methacrylate for efficient removal of Ni(II), Cu(II) and Pd(II) ions from aqueous solution. Sep. Purif. Technol. 2019, 219, 249–259. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Bahi, A.; Wuttke, S.; Kamkar, M.; Rojas, O.J.; Ko, F.; Arjmand, M. Electrospun nanofibers of chitosan/polyvinyl alcohol/UiO-66/nanodiamond: Versatile adsorbents for wastewater remediation and organic dye removal. Chem. Eng. J. 2023, 457, 141176. [Google Scholar] [CrossRef]
- Verma, A.; Johnson, G.H.; Corbin, D.R.; Shiflett, M.B. Separation of Lithium and Cobalt from LiCoO2: A Unique Critical Metals Recovery Process Utilizing Oxalate Chemistry. ACS Sustain. Chem. Eng. 2020, 8, 6100–6108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).