Preparation and Properties of Elastic Mullite Fibrous Porous Materials with Excellent High-Temperature Resistance and Thermal Stability
Abstract
1. Introduction
2. Materials and Methods
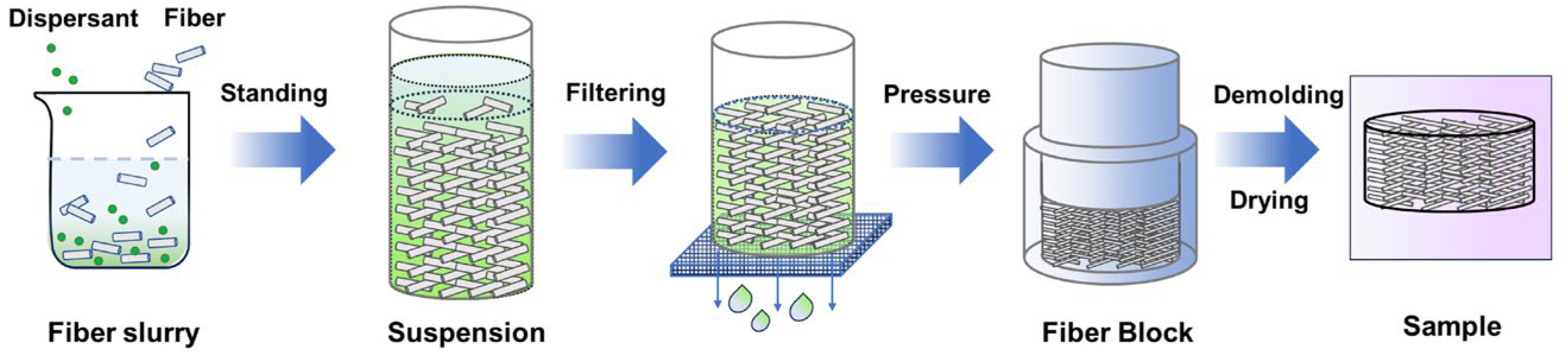
2.1. Materials and Experiment Process
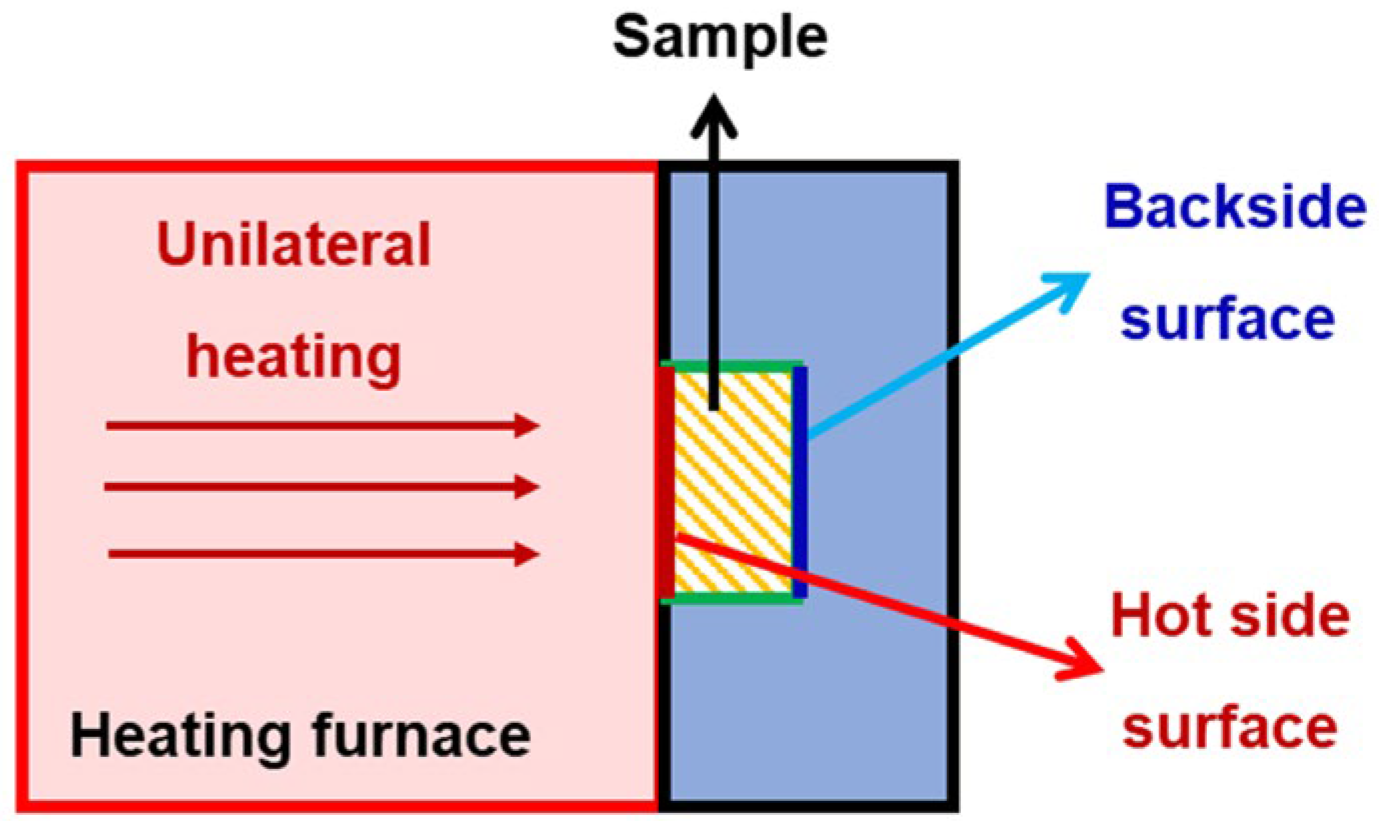
2.2. Characterization
3. Results
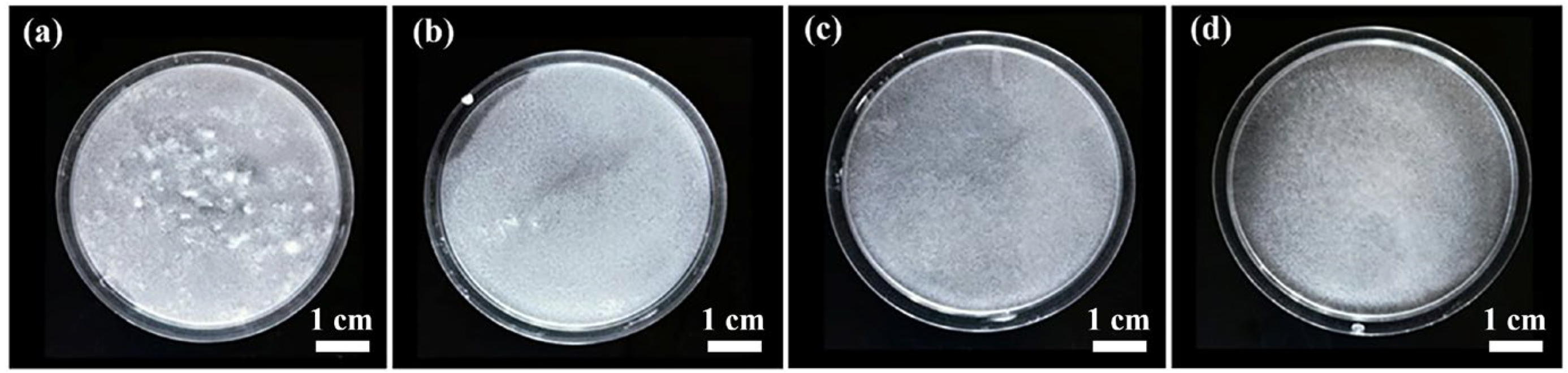
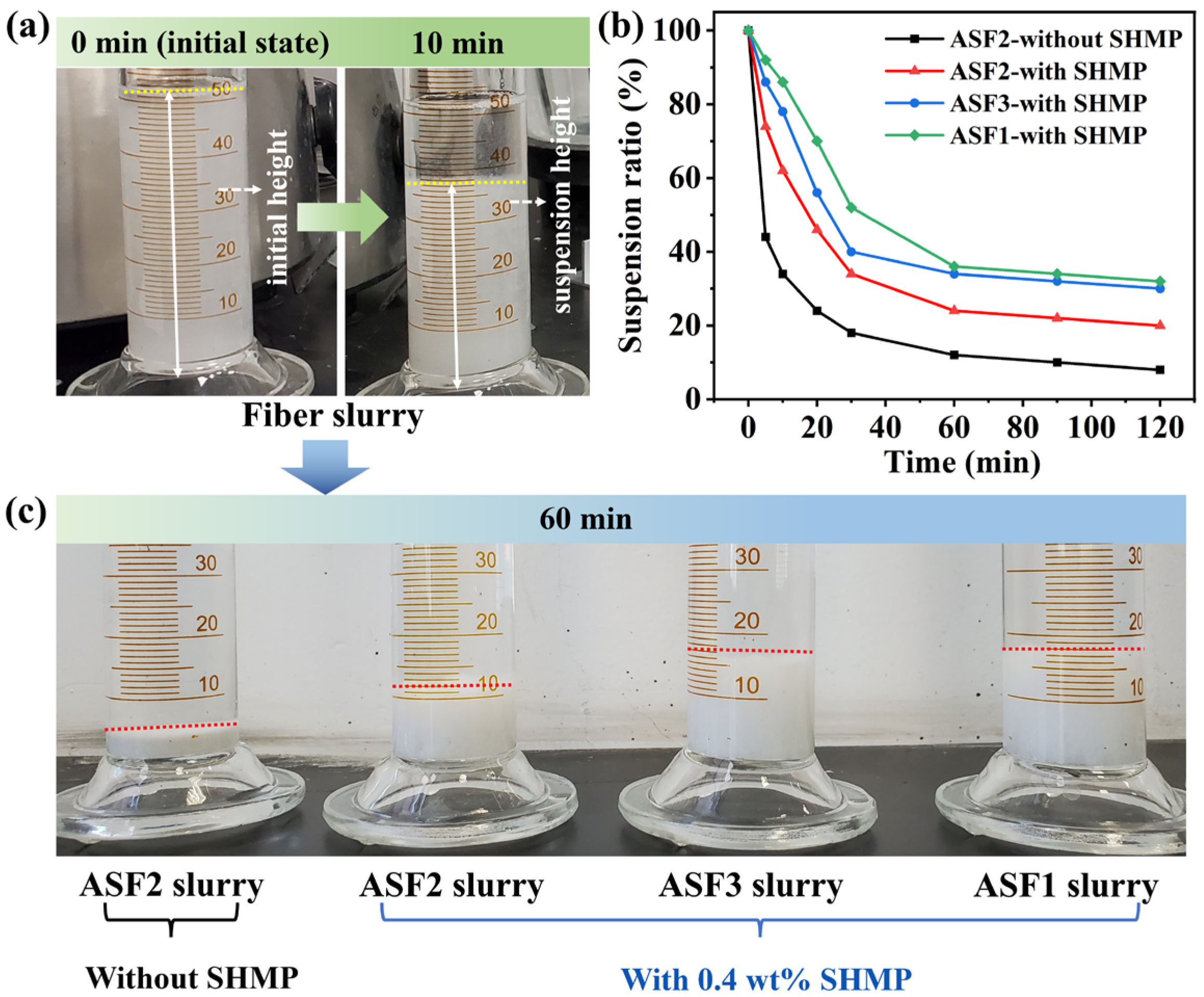
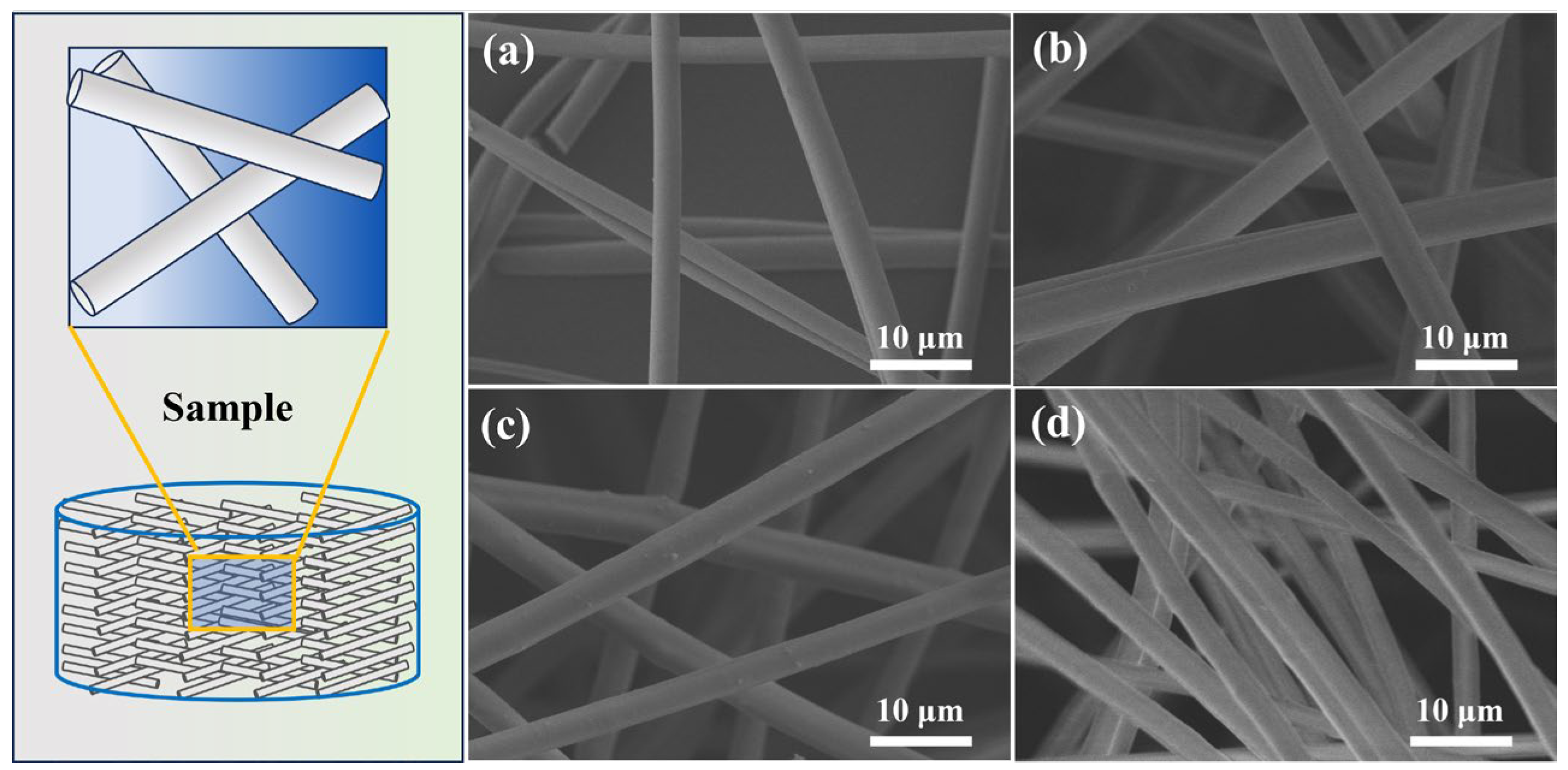
3.1. Dispersibility, Bulk Density, and Thermal Conductivity of Alumina–Silica Fibers
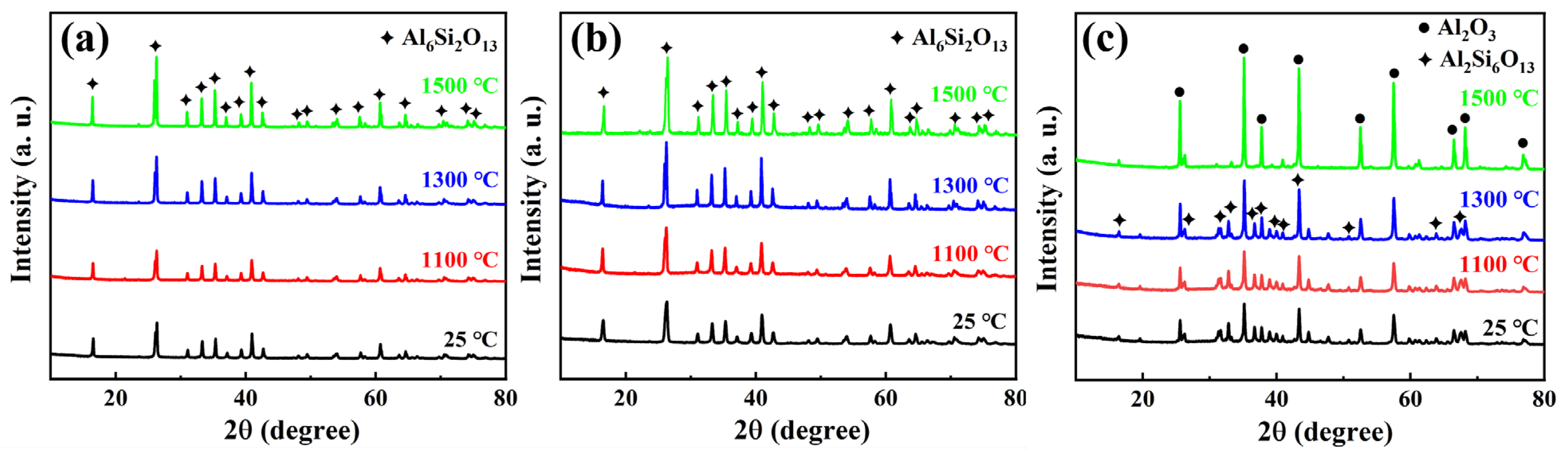
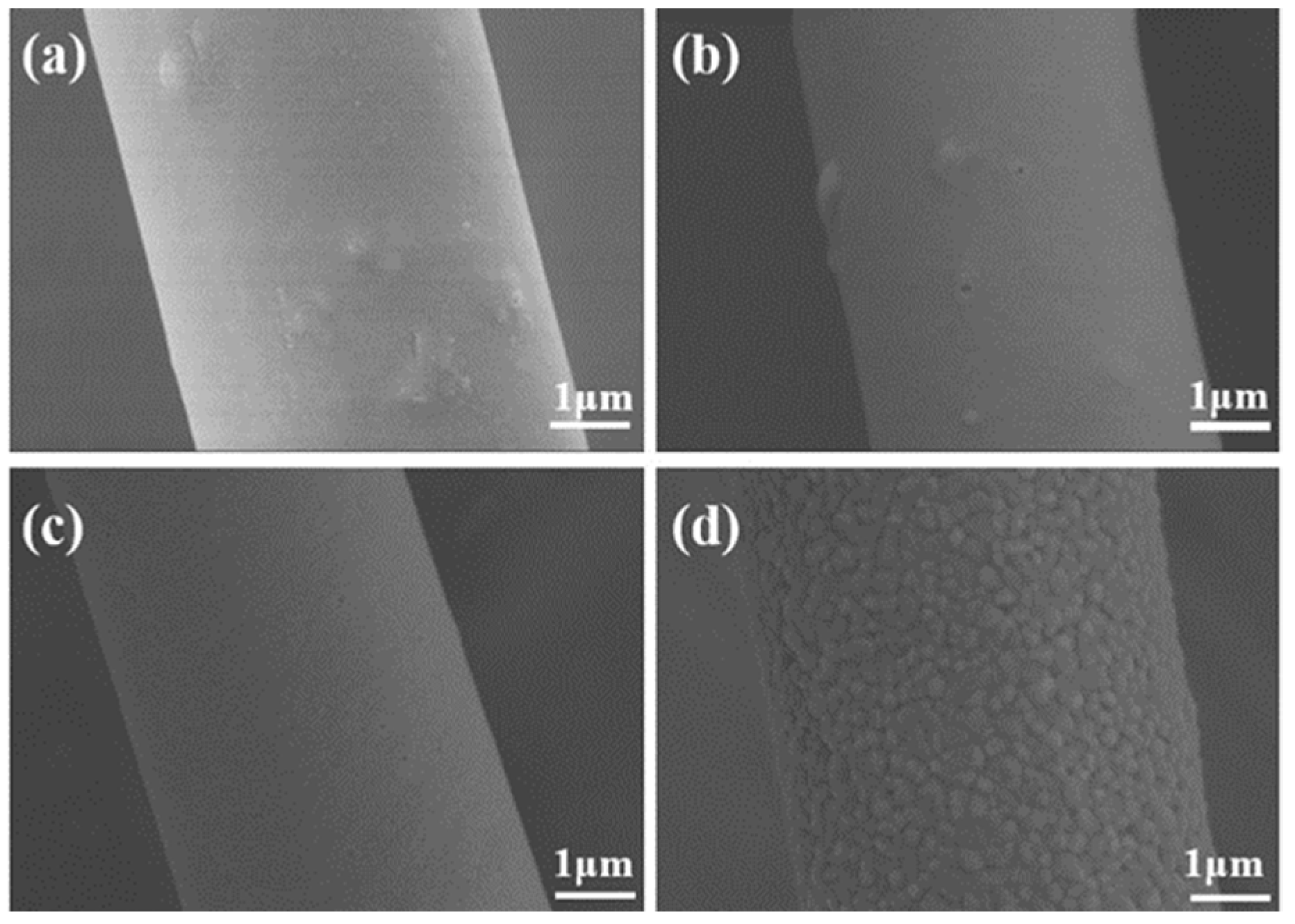
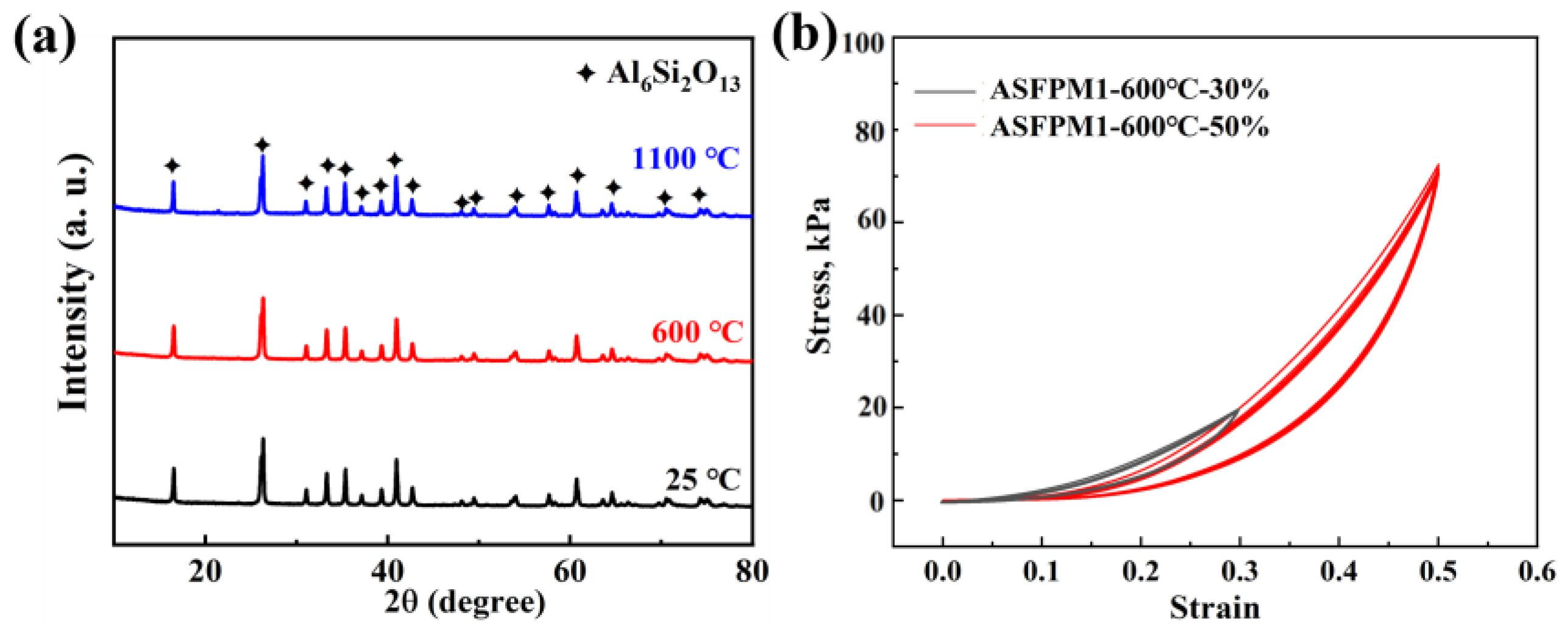
3.2. Evolution of the Composition, Structure, and High-Temperature Resistance and Thermal Stability of ASFPM1, ASFPM2, and ASFPM3
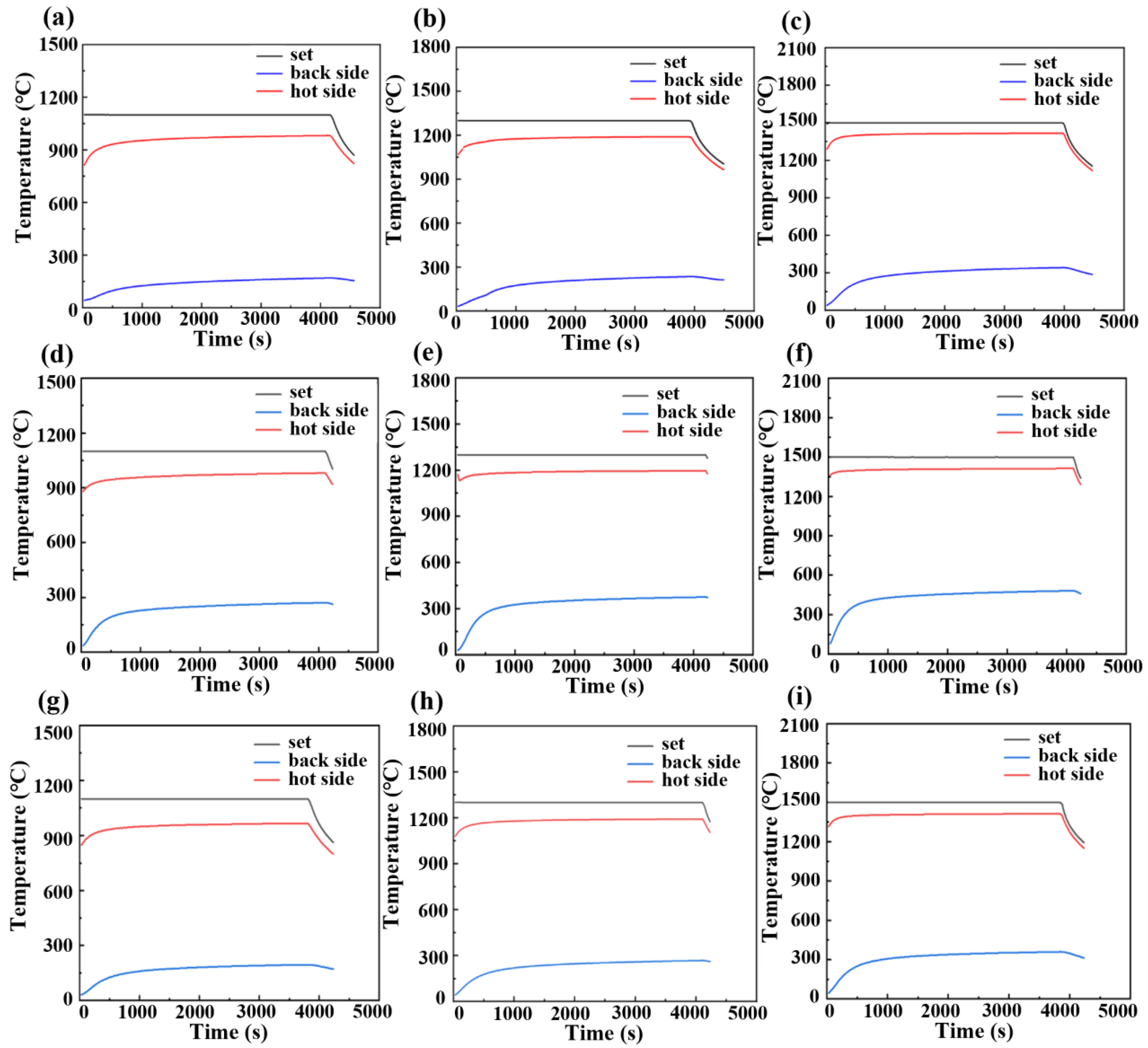
3.3. Thermal Insulation Performance and Compressive Rebound Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, R.Q.; Tsourdos, A.; Savvaris, A.; Chai, S.C.; Xia, Y.Q.; Philip Chen, C.L. Review of advanced guidance and control algorithms for space/aerospace vehicles. Prog. Aerosp. Sci. 2021, 122, 100696. [Google Scholar] [CrossRef]
- Uyanna, O.; Najafi, H. Thermal protection systems for space vehicles: A review on technology development, current challenges and future prospects. Acta Astronaut. 2020, 176, 341–356. [Google Scholar] [CrossRef]
- Kim, H.S.; Kang, B.R.; Choi, S.M. Microstructure and mechanical properties of vacuum plasma sprayed HfC, TiC, and HfC/TiC ultra-high-temperature ceramic coatings. Materials 2019, 13, 124. [Google Scholar] [CrossRef]
- Korb, L.J.; Morant, C.A.; Calland, R.M.; Thatcher, C.S. The shuttle orbiter thermal protection system. Am. Ceramic Soc. Bull. 1981, 60, 1188. [Google Scholar]
- Komine, A.; Kobayashi, A.S. Behavior of the Space Shuttle Thermal Protection System. J. Am. Ceram. Soc. 1983, 66, 641–644. [Google Scholar] [CrossRef]
- Davis, J.B.; Marshall, D.B.; Oka, K.S.; Housley, R.M.; Morgan, P.E.D. Ceramic composites for thermal protection systems. Compos. Part A-Appl. S. 1999, 30, 483–488. [Google Scholar] [CrossRef]
- Ungar, E.W. Ablation Thermal Protection Systems: Suitability of ablation systems to thermal protection depends on complex physical and chemical processes. Science 1967, 158, 740–744. [Google Scholar] [CrossRef]
- Wurster, K.E.; Riley, C.J.; Zhou, C.J.; Zoby, E.V. Engineering aerothermal analysis for X-34 thermal protection system design. J. Spacecr. Rockets 1999, 36, 216–228. [Google Scholar] [CrossRef][Green Version]
- Devapal, D.; Gopakumar, M.P.; Prabhakaran, P.V.; Packirisamy, S. Ceramic coating on flexible external insulation. Curr. Sci. 2018, 114, 137–143. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, L.Y.; Ma, X.H.; Xu, X.J.; Guo, A.R.; Hou, F.; Liu, J.C. Preparation of a flexible high emissivity coating on quartz fiber fabric for thermal protection. Ceram. Int. 2017, 43, 14292–14300. [Google Scholar] [CrossRef]
- Bardy, E.R.; Mollendorf, J.C.; Pendergast, D.R. Thermal Conductivity and Compressive Strain of Aerogel Insulation Blankets Under Applied Hydrostatic Pressure. J. Heat Transf. 2007, 129, 232–235. [Google Scholar] [CrossRef]
- Triantou, K.I.; Mergia, K.; Perez, B.; Florez, S.; Stefan, A.; Ban, C.; Pelin, G.; Ionescu, G.; Zuber, C.; Fischer, W.P.P.; et al. Thermal shock performance of carbon-bonded carbon fiber composite and ceramic matrix composite joints for thermal protection re-entry applications. Compos. Part. B-Eng. 2017, 111, 270–278. [Google Scholar] [CrossRef]
- Mouchon, E.; Colomban, P. Oxide ceramic matrix/oxide fibre woven fabric composites exhibiting dissipative fracture behaviour. Composites 1995, 26, 175–182. [Google Scholar] [CrossRef]
- Li, Y.T.; Guo, A.R.; Xu, X.J.; Xue, Y.J.; Yan, L.W.; Hou, F.; Liu, J.C. Preparation and Properties of Highly Elastic, Lightweight, and Thermally Insulating SiO2 Fibrous Porous Materials. Materials 2022, 15, 3069. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, E.V.; Maksimov, V.G.; Ivakhnenko, Y.A. Internal defects of multifilament threads made of oxide refractory fibers. Refract. Ind. Ceram. 2022, 63, 100–104. [Google Scholar] [CrossRef]
- Kamiuto, K. Two-parameter formula for the total effective thermal conductivities of ceramic-fiber insulations. Energy 1991, 16, 701–706. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Y.S.; Deng, Z.Z.; Peng, Y.; Dong, J.H.; Zhu, Z.; Ma, D.H.; Zhu, L.Y.; Zhang, G.H.; Wang, X.Q. Modification of YSZ fiber composites by Al2TiO5 fibers for high thermal shock resistance. J. Adv. Ceram. 2022, 11, 922–934. [Google Scholar] [CrossRef]
- Jalouli, A.; Khuje, S.; Sheng, A.; Islam, A.; Luigi, M.D.; Petit, D.; Li, Z.; Zhuang, C.G.; Kester, L.; Armstrong, J.; et al. Flexible Copper–Graphene Nanoplates on Ceramic Supports for Radiofrequency Electronics with Electromagnetic Interference Shielding and Thermal Management Capacity. ACS Appl. Nano Mater. 2021, 4, 11841–11848. [Google Scholar] [CrossRef]
- Rahbek, D.B.; Roberson, G.E.; Johnsen, B.B. Improved ballistic limit velocity from filament-wound fibres as composite cover on ceramic tiles. Compos. Struct. 2023, 323, 117452. [Google Scholar] [CrossRef]
- Reurings, C.; Koussios, S.; Bergsma, O.K.; Vergote, K.; Paeshuyse, L.; Benedictus, R. Experimental method for investigating wear of porous thermal insulation systems exposed to realistic, hot, turbulent gas flow. Wear 2020, 203536, 466–467. [Google Scholar] [CrossRef]
- Trujillo, B.M.; Meyer, R.R.; Sawko, P.M. In-flight load testing of advanced thermal protection systems. AIAA J. 1983, 2704, 2704. [Google Scholar]
- Tsukahara, A.; Yamao, H.; Miho, K. Advanced Thermal Protection Systems for Reusable Launch Vehicles. AIAA J. 2001, 1909, 1909. [Google Scholar]
- Hasan, M.A.; Rashmi, S.; Esther, A.C.M.; Bhavanisankar, P.Y.; Sherikar, B.N.; Sridhara, N.; Dey, A. Evaluations of Silica Aerogel-Based Flexible Blanket as Passive Thermal Control Element for Spacecraft Applications. J. Mater. Eng. Perform. 2018, 27, 1265–1273. [Google Scholar] [CrossRef]
- Beck, R.A.S.; Driver, D.M.; Wright, M.J.; Hwang, H.H.; Edquist, K.T.; Sepka, S.A. Development of the mars science laboratory heatshield thermal protection system. J. Spacecr. Rockets 2014, 51, 1139–1150. [Google Scholar] [CrossRef]
- Behrens, B.; Mueller, M. Technologies for thermal protection systems applied on re-usable launcher. Acta Astronaut. 2004, 55, 529–536. [Google Scholar] [CrossRef]
- Adám, P.; Temesi, O.; Dankáazi, Z.; Voniatis, C.; Rohonczy, J.; Sinkó, K. Various colloid systems for drawing of aluminum oxide fibers. Ceram. Int. 2022, 48, 5499–5508. [Google Scholar] [CrossRef]
- Timurkutluk, C.; Toruntay, F.; Onbilgin, S.; Atalmis, G.; Timurkutluk, B. Development of ceramic fiber reinforced glass ceramic sealants for microtubular solid oxide fuel cells. Ceram. Int. 2023, 48, 15703–15710. [Google Scholar] [CrossRef]
- Zhang, L.S.; Baima, M.; Andrew, T.L. Enhancing thermal stability of oxide ceramic matrix composites via matrix doping. J. Eur. Ceram. Soc. 2022, 42, 3282–3289. [Google Scholar]
- Ramachandran, K.; Leelavinodhan, S.; Antao, C.; Copti, A.; Mauricio, C.; Jyothi, Y.L.; Jayaseelan, D.D. Analysis of failure mechanisms of Oxide-Oxide ceramic matrix composites. J. Eur. Ceram. Soc. 2022, 42, 1626–1634. [Google Scholar] [CrossRef]
- Yada, M.; Tanaka, G.; Isono, K.; Kamochi, N.; Ichinose, H. Ultra-reduction of drying and firing shrinkage on pottery slip casting by adding mullite fiber. J. Eur. Ceram. Soc. 2024, 44, 2677–2684. [Google Scholar] [CrossRef]
- Feng, Z.J.; Wang, M.C.; Lu, R.Y.; Xu, W.C.; Zhang, T.; Tong, W.; Zhang, J.F.; Liao, Y.L. A composite structural high-temperature-resistant adhesive based on in-situ grown mullite whiskers. Mater. Today Commun. 2020, 23, 100944. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Yang, N.; Hou, Y.; Chen, D.; Liu, J.; Cai, G.; Wang, M. The Effect of Different Diluents and Curing Agents on the Performance of Epoxy Resin-Based Intumescent Flame-Retardant Coatings. Materials 2024, 17, 348. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.W.; Xu, J.; Guo, J.; Meng, X.Y.; Zhang, P.; Fan, F.Y.; Qu, Y.N.; Gao, F. High wave transmittance and low thermal conductivity Y–α-SiAlON porous ceramics for high-temperature radome applications. J. Adv. Ceram. 2023, 12, 1273–1287. [Google Scholar] [CrossRef]
- Lyu, Y.; Du, B.H.; Chen, G.Q.; Zhao, G.D.; Cheng, Y.; Zhou, S.B.; Lv, Q.R.; Zhang, X.H.; Han, W.B. Microstructural regulation, oxidation resistance, and mechanical properties of Cf/SiC/SiHfBOC composites prepared by chemical vapor infiltration with precursor infiltration pyrolysis. J. Adv. Ceram. 2021, 11, 120–135. [Google Scholar] [CrossRef]
- Cai, G.S.; Wu, J.X.; Guo, J.Y.; Wan, Y.G.; Zhou, Q.J.; Zhang, P.Y.; Yu, X.L.; Wang, M.C. A Novel Inorganic Aluminum Phosphate-Based Flame Retardant and Thermal Insulation Coating and Performance Analysis. Materials 2023, 16, 4498. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Cai, G.S.; Wan, Y.G.; Zhang, R.Y.; Li, J.C.; Zhang, J.F.; Zhang, H.J.; Liu, H.L.; Yu, X.L.; Wang, M.C. High anti-ablative epoxy resin-based flame retardant and thermal insulation coating based on spontaneous Ceramization and vitrification. Ceram. Int. 2024, 50, 24233–24251. [Google Scholar] [CrossRef]
- Yazdani, A.; Manesh, H.D.; Zebarjad, S.M. Piezoelectric properties and damping behavior of highly loaded PZT/polyurethane particulate composites. Ceram. Int. 2023, 49, 4055–4063. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.; Lee, S.; Kim, S. Reduced graphene oxide-based wearable and bio-electrolyte triggered pressure sensor with tunable sensitivity. Ceram. Int. 2021, 47, 17702–17710. [Google Scholar] [CrossRef]
- Wang, M.C.; Liang, Z.L.; Yan, S.Q.; Tao, X.; Zou, Y.L.; Li, J.T.; Zhou, X.M.; Zhang, H.J. The preparation and property analysis of B4C modified inorganic amorphous aluminum phosphates-based intumescent flame retardant coating. Constr. Build. Mater. 2022, 359, 129480. [Google Scholar] [CrossRef]
- Kwon, Y.R.; Kim, H.C.; Moon, S.K.; Kim, J.S.; Chang, Y.W.; Kim, D.H. Facile preparation and characterization of low-gloss waterborne polyurethane coatings using amine-based chain extenders. Polym. Int. 2022, 72, 54–60. [Google Scholar] [CrossRef]
- Li, X.; Niu, S.X.; Wang, D.S.; Li, J.; Jiao, Q.; Guo, X.L.; Xu, X.Q. Microstructure and Crystallization Kinetics of Silica-Based Ceramic Cores with Enhanced High-Temperature Property. Materials 2023, 16, 606. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Wan, Y.G.; Xiao, B.; Li, J.C.; Hu, Z.M.; Zhang, R.Y.; Hu, X.X.; Liu, J.C.; Cai, G.S.; Liu, H.L.; et al. The preparation and performance analysis of zirconium-modified aluminum phosphate-based high-temperature (RT-1500 °C) resistant adhesive for joining alumina in extreme environment. J. Adv. Ceram. 2024. [CrossRef]
- Gao, Y.H.; Xu, G.G.; Zhao, P.; Liu, L.L.; Zhang, E.L. One step co-sintering synthesis of gradient ceramic microfiltration membrane with mullite/alumina whisker bi-layer for high permeability oil-in-water emulsion treatment. Sep. Purif. Technol. 2023, 305, 122400. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Zhang, Y.L.; Yan, H.; Li, J.H.; Yin, X.M.; Sun, J.; Fu, Q.G.; Riedel, R. Microstructure and evolution of hafnium carbide whiskers via polymer-derived ceramics: A novel formation mechanism. J. Adv. Ceram. 2023, 12, 578–586. [Google Scholar] [CrossRef]
- Lee, M.; Kim, K.; Chung, C.W.; Kim, W.; Jeong, Y.; Lee, J. Mechanical characterization of recycled-PET fiber reinforced mortar composites treated with nano-SiO2 and mixed with seawater. Constr. Build. Mater. 2023, 392, 131882. [Google Scholar] [CrossRef]



| Sample | Mass Fraction of Al2O3 (%) | Mass Fraction of SiO2 (%) | Fiber Diameter (μm) | Phase Composition |
|---|---|---|---|---|
| ASF1 | 81.7 | 17.8 | 3.0–5.0 | Mullite |
| ASF2 | 83.6 | 15.2 | 3.0–5.0 | Mullite |
| ASF3 | 96.9 | 2.58 | 3.0–5.0 | Mullite, Al2O3 |
| Sample | Bulk Density (g·cm−3) | Fiber Volume Fraction (%) | Thermal Conductivity (W·m−1·K−1) |
|---|---|---|---|
| ASFPM1 | 0.11 | 3.4 | 0.0486 |
| ASFPM2 | 0.17 | 5.3 | 0.0721 |
| ASFPM3 | 0.13 | 4.1 | 0.0511 |
| Sample | 1100 °C | 1300 °C | 1500 °C |
|---|---|---|---|
| ASFPM1 | 170.5 °C | 237.9 °C | 342.5 °C |
| ASFPM2 | 272.4 °C | 376.0 °C | 482.5 °C |
| ASFPM3 | 196.5 °C | 268.0 °C | 361.6 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, X.; Wang, Z.; Xue, Y.; Guo, A.; Yan, L.; Hou, F.; Liu, J. Preparation and Properties of Elastic Mullite Fibrous Porous Materials with Excellent High-Temperature Resistance and Thermal Stability. Materials 2024, 17, 3235. https://doi.org/10.3390/ma17133235
Zhang X, Zhang X, Wang Z, Xue Y, Guo A, Yan L, Hou F, Liu J. Preparation and Properties of Elastic Mullite Fibrous Porous Materials with Excellent High-Temperature Resistance and Thermal Stability. Materials. 2024; 17(13):3235. https://doi.org/10.3390/ma17133235
Chicago/Turabian StyleZhang, Xiang, Xueying Zhang, Zhongyan Wang, Yunjia Xue, Anran Guo, Liwen Yan, Feng Hou, and Jiachen Liu. 2024. "Preparation and Properties of Elastic Mullite Fibrous Porous Materials with Excellent High-Temperature Resistance and Thermal Stability" Materials 17, no. 13: 3235. https://doi.org/10.3390/ma17133235
APA StyleZhang, X., Zhang, X., Wang, Z., Xue, Y., Guo, A., Yan, L., Hou, F., & Liu, J. (2024). Preparation and Properties of Elastic Mullite Fibrous Porous Materials with Excellent High-Temperature Resistance and Thermal Stability. Materials, 17(13), 3235. https://doi.org/10.3390/ma17133235






