Synthesis of HAp by Means of Sonoprecipitation Method
Abstract
1. Introduction
- tissue engineering: HAp-coated implants are widely used in dentistry and orthopedics because HAp promotes the growth of a new bone towards the implant;
- drug/antigens/proteins delivery platforms, ensuring slow release rate and the protection of an active ingredient from degradation;
2. Calculations’ Basis
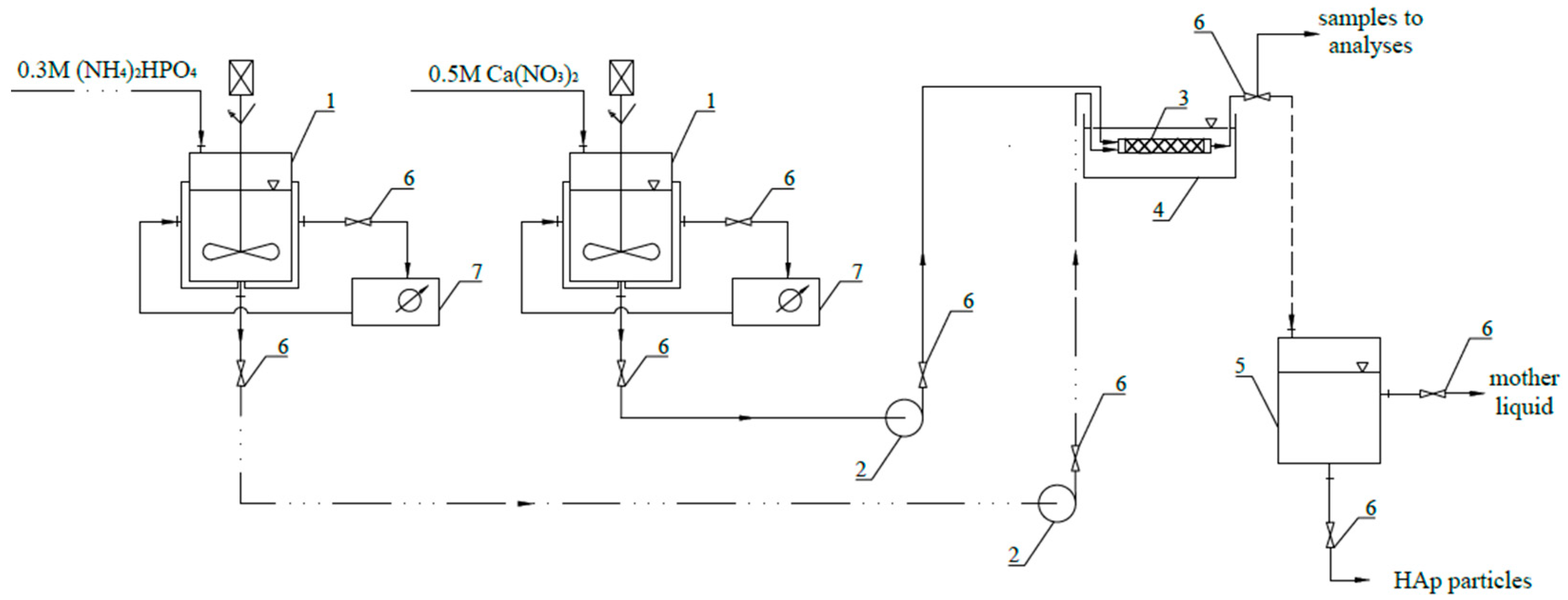
3. Materials and Methods
4. Results and Discussion
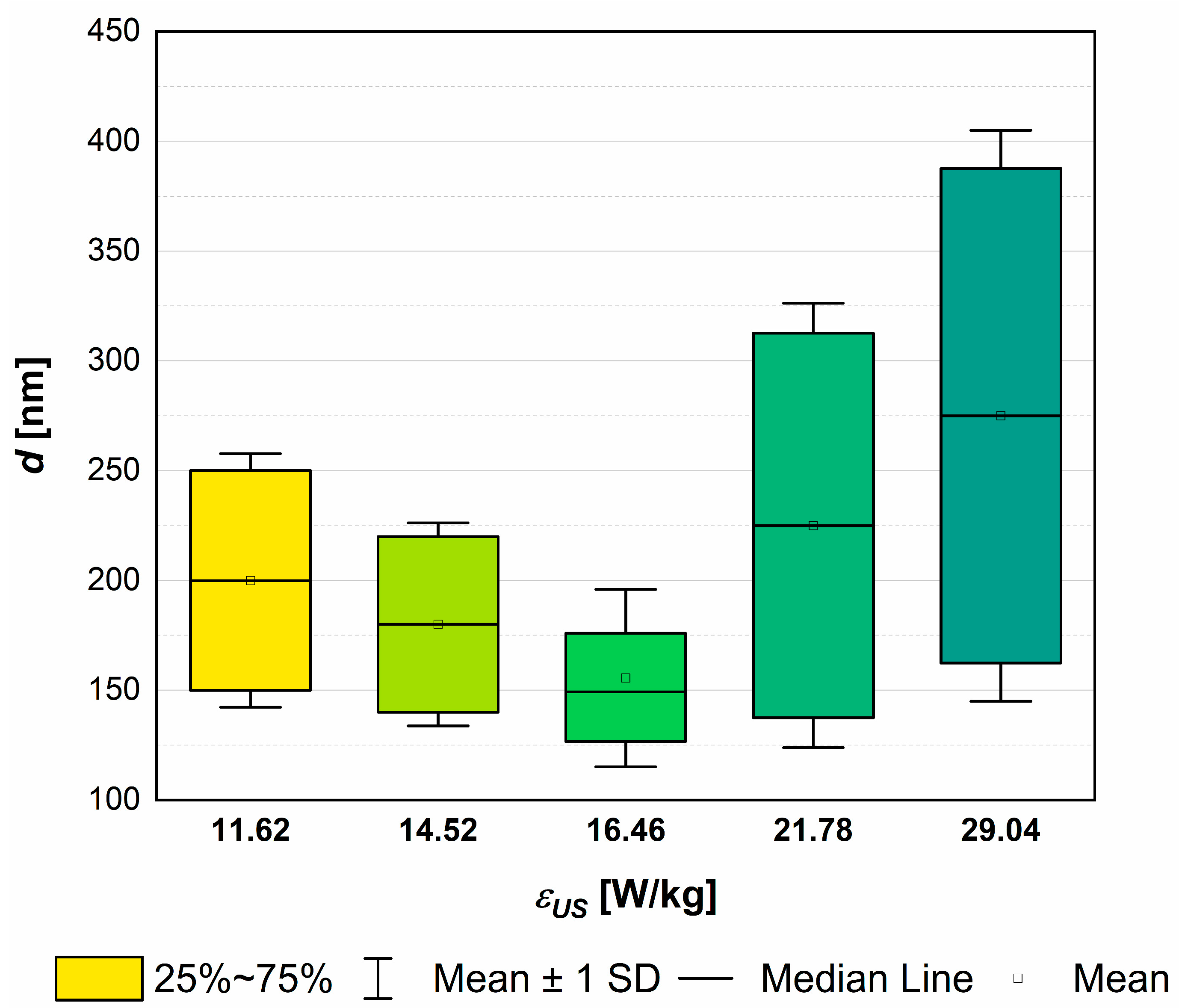
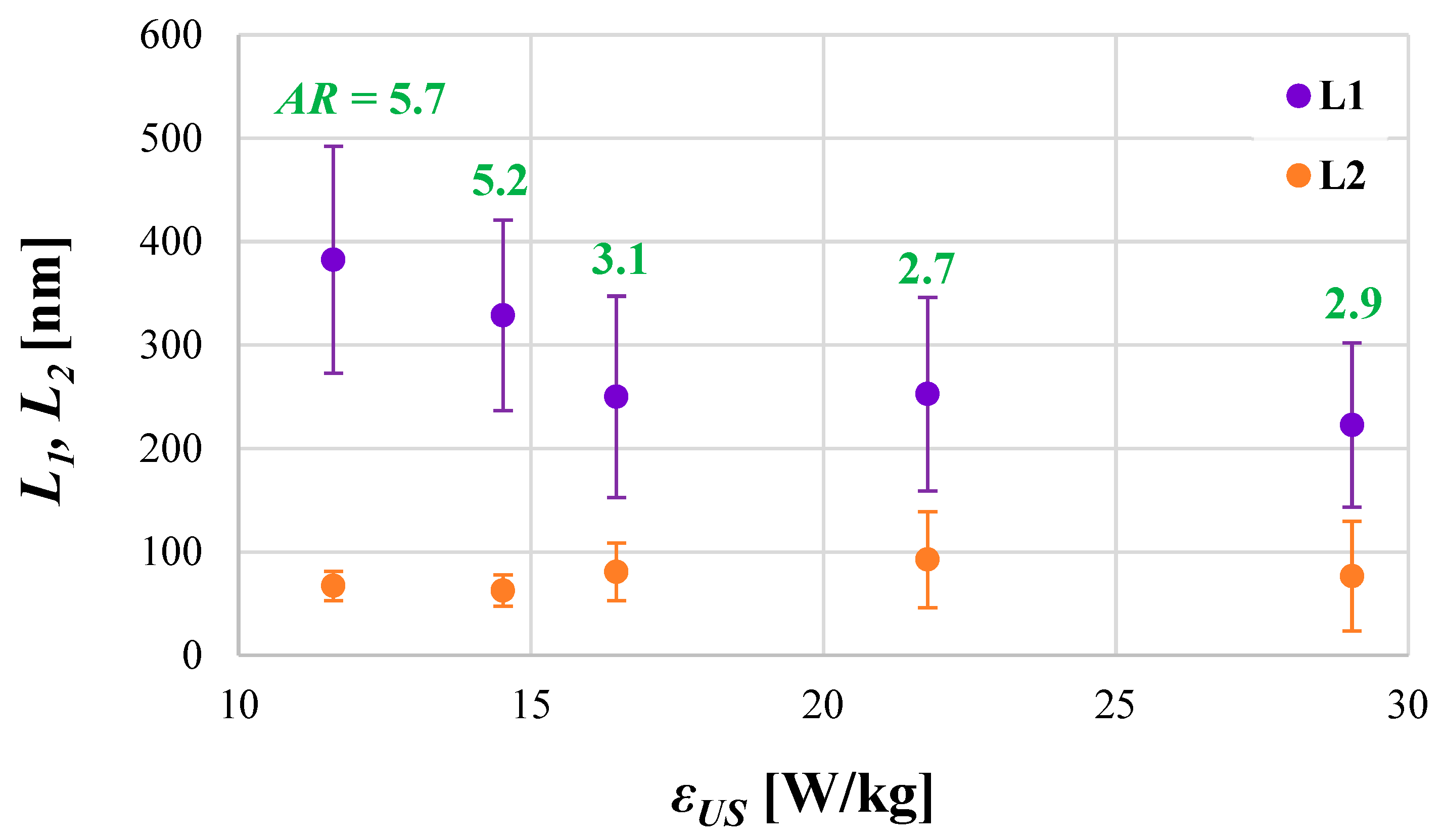
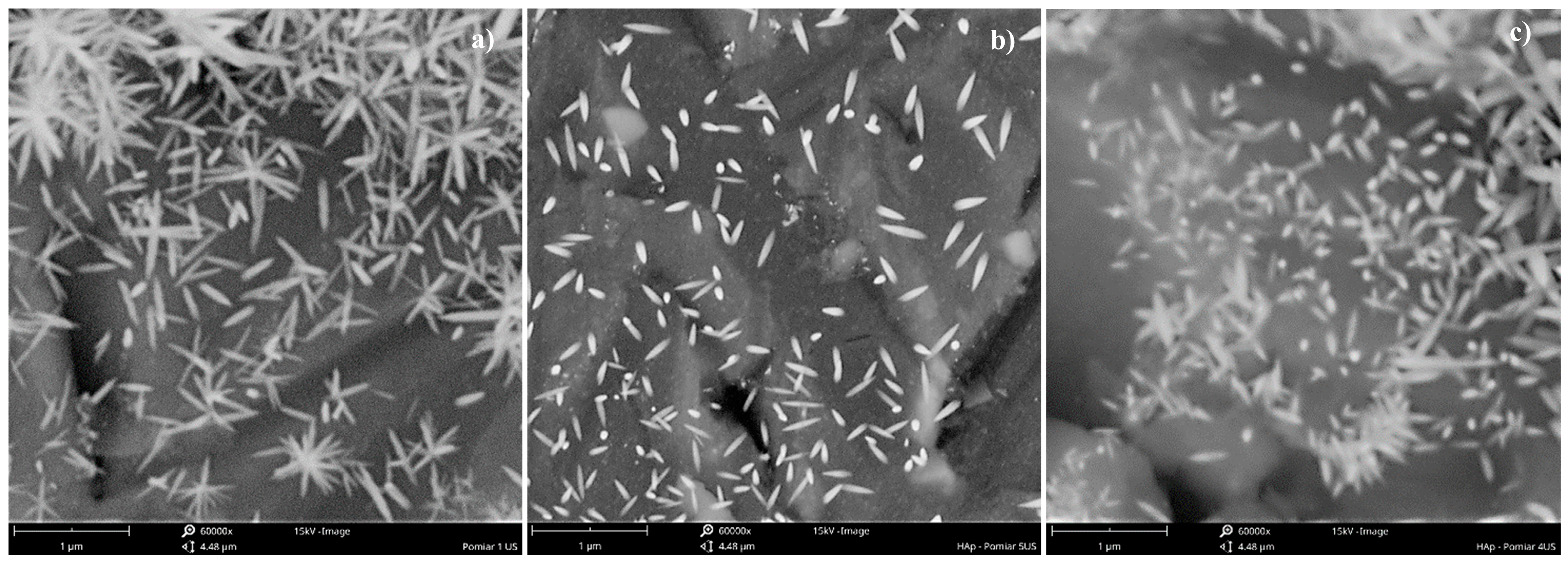
4.1. The Role of Ultrasonic Power εUS
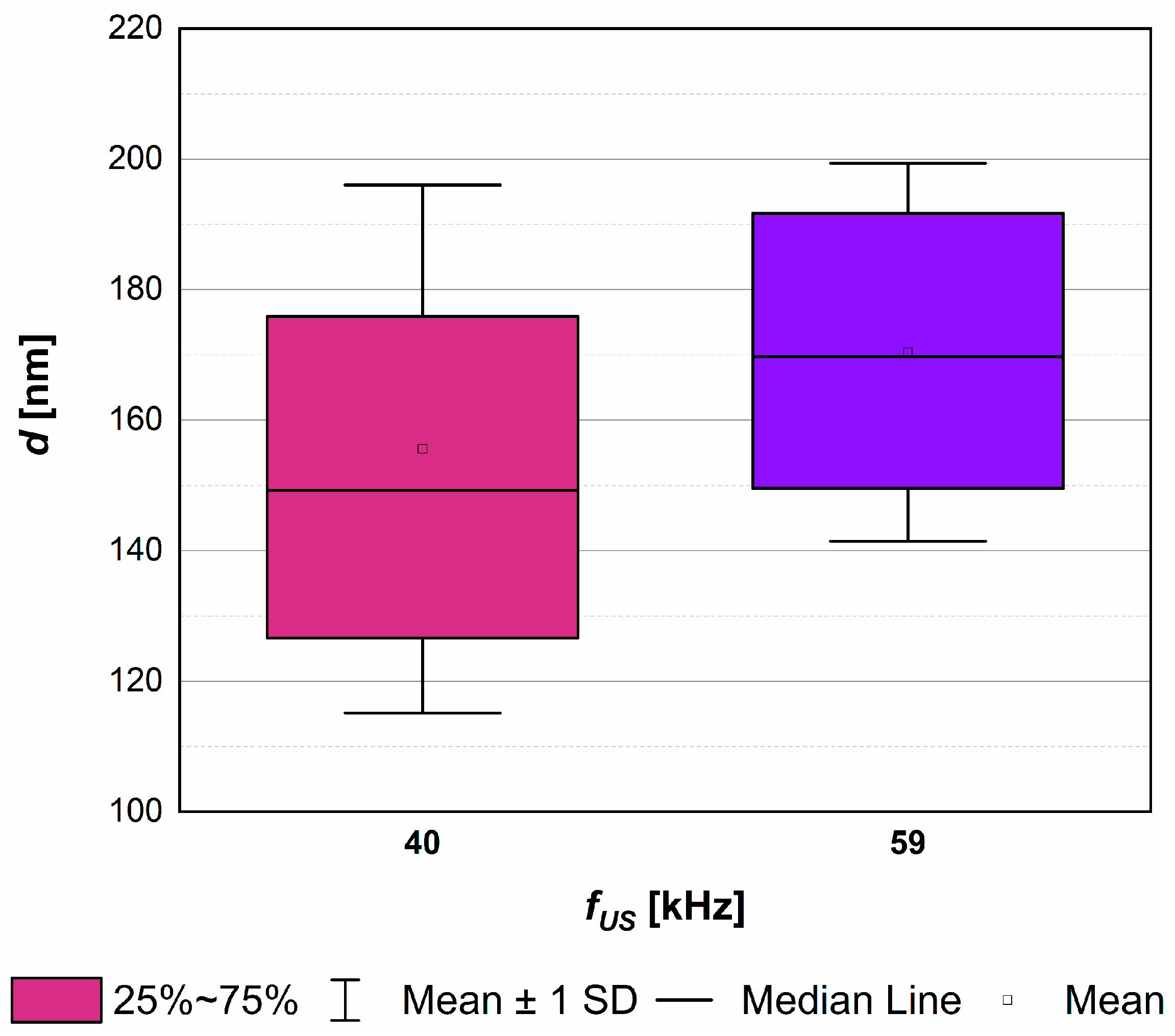
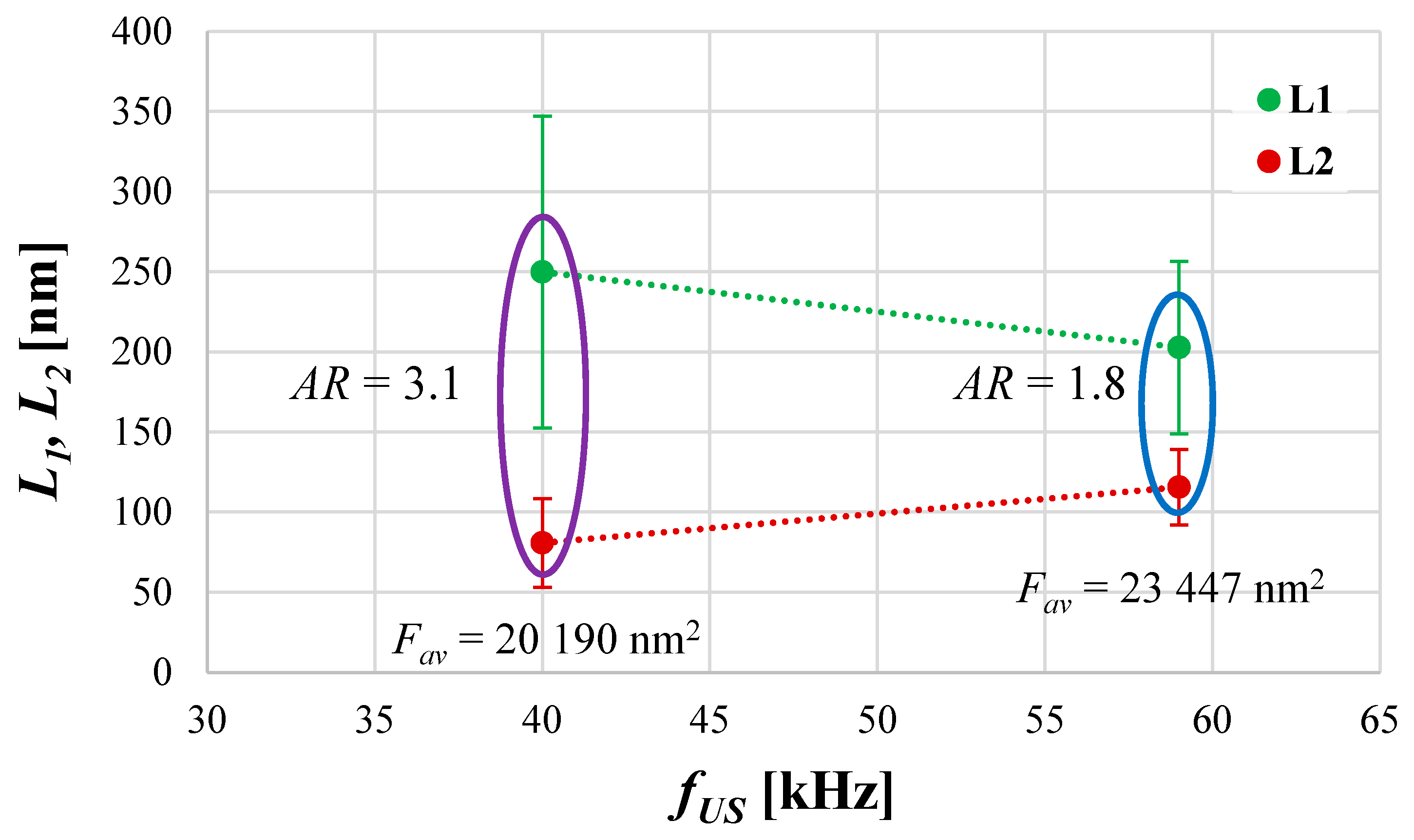
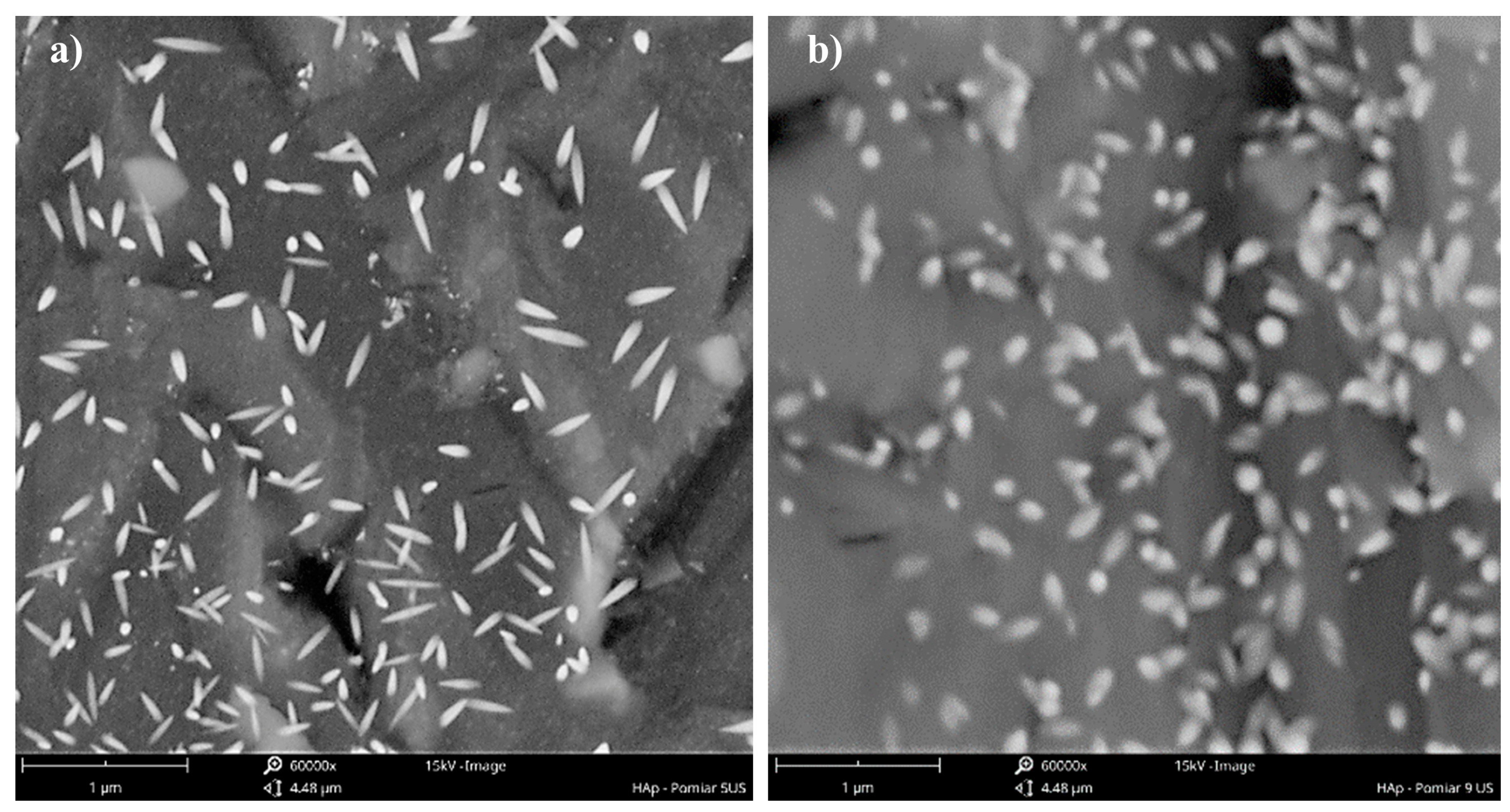
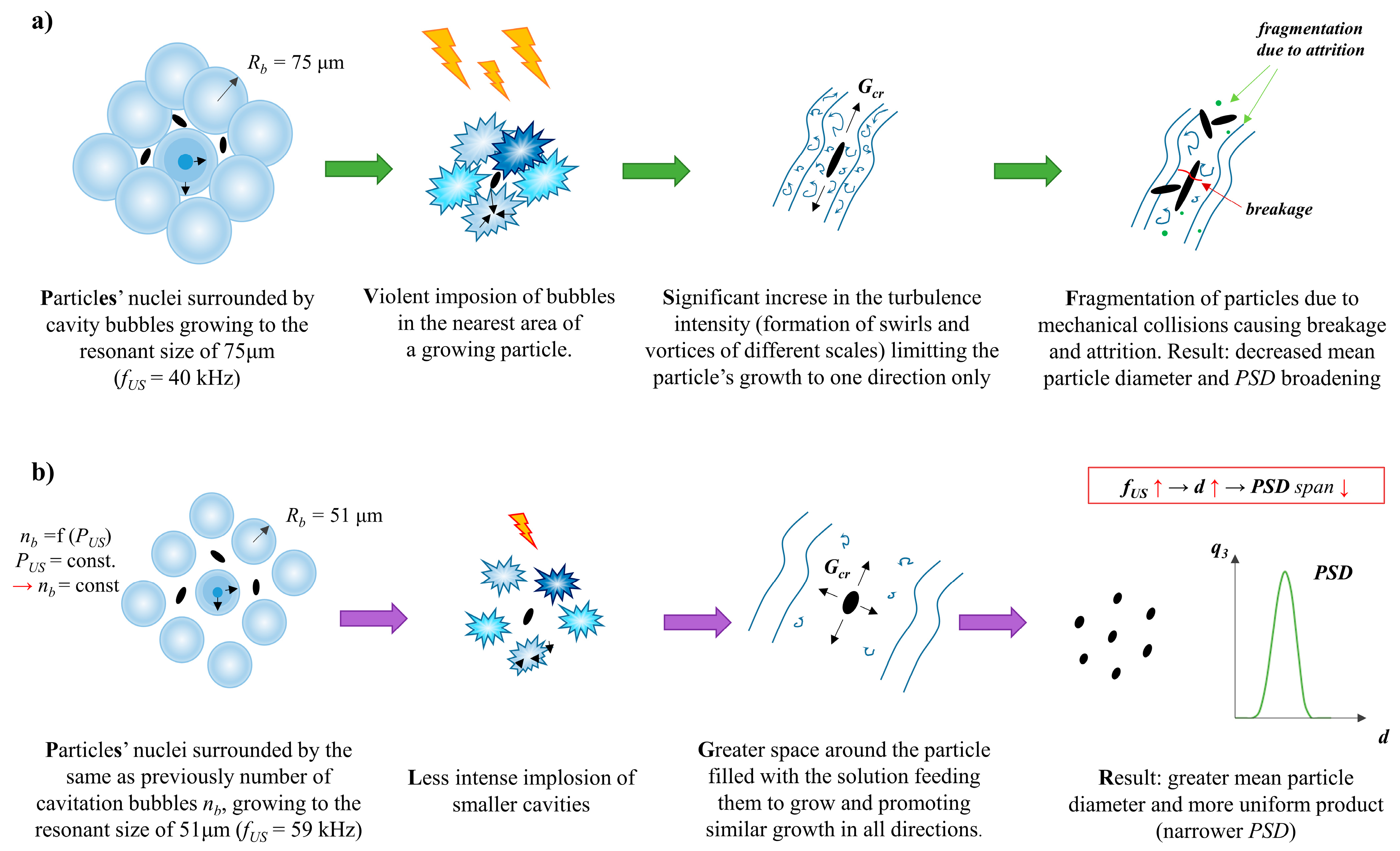
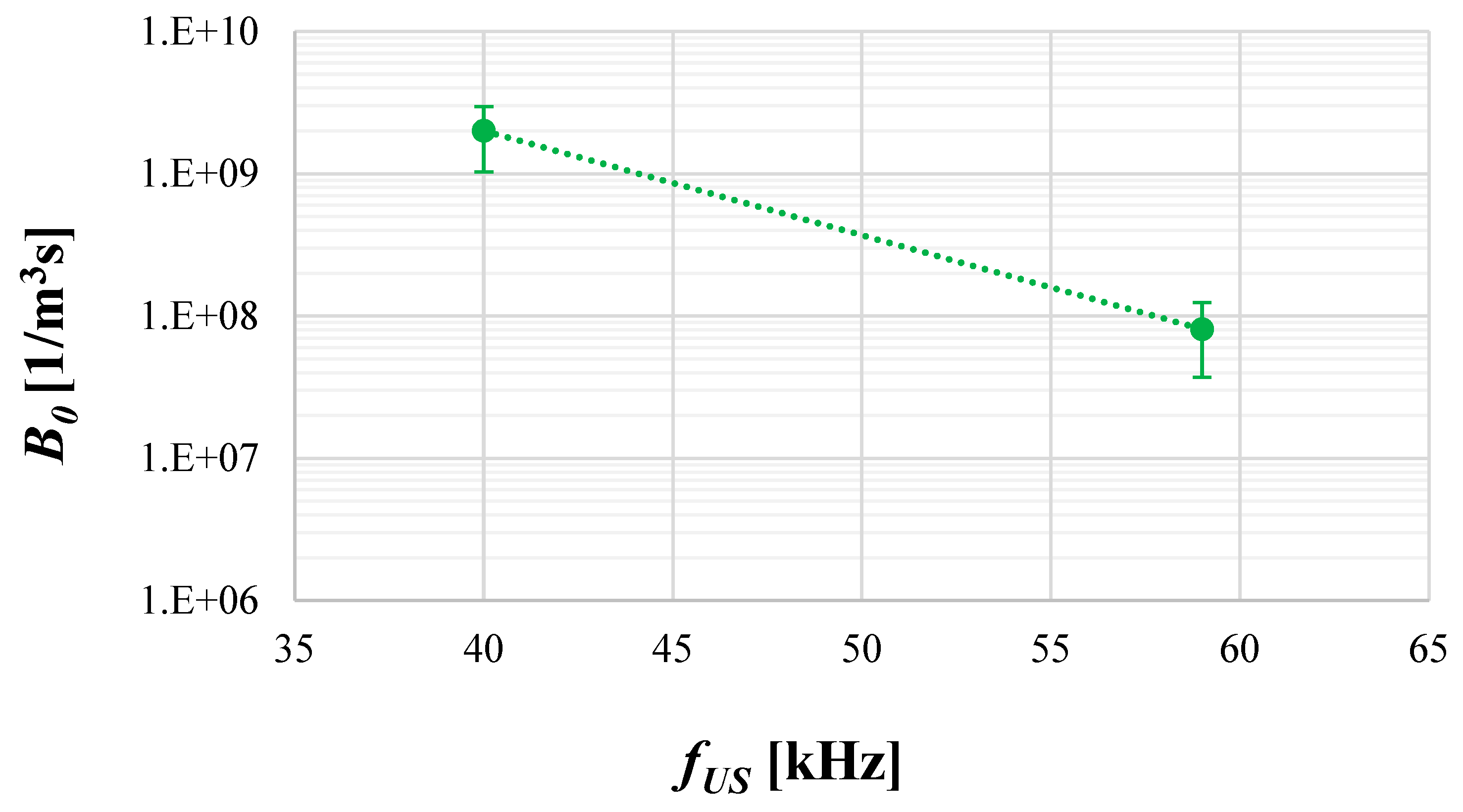
4.2. The Role of Ultrasonic Frequency fUS
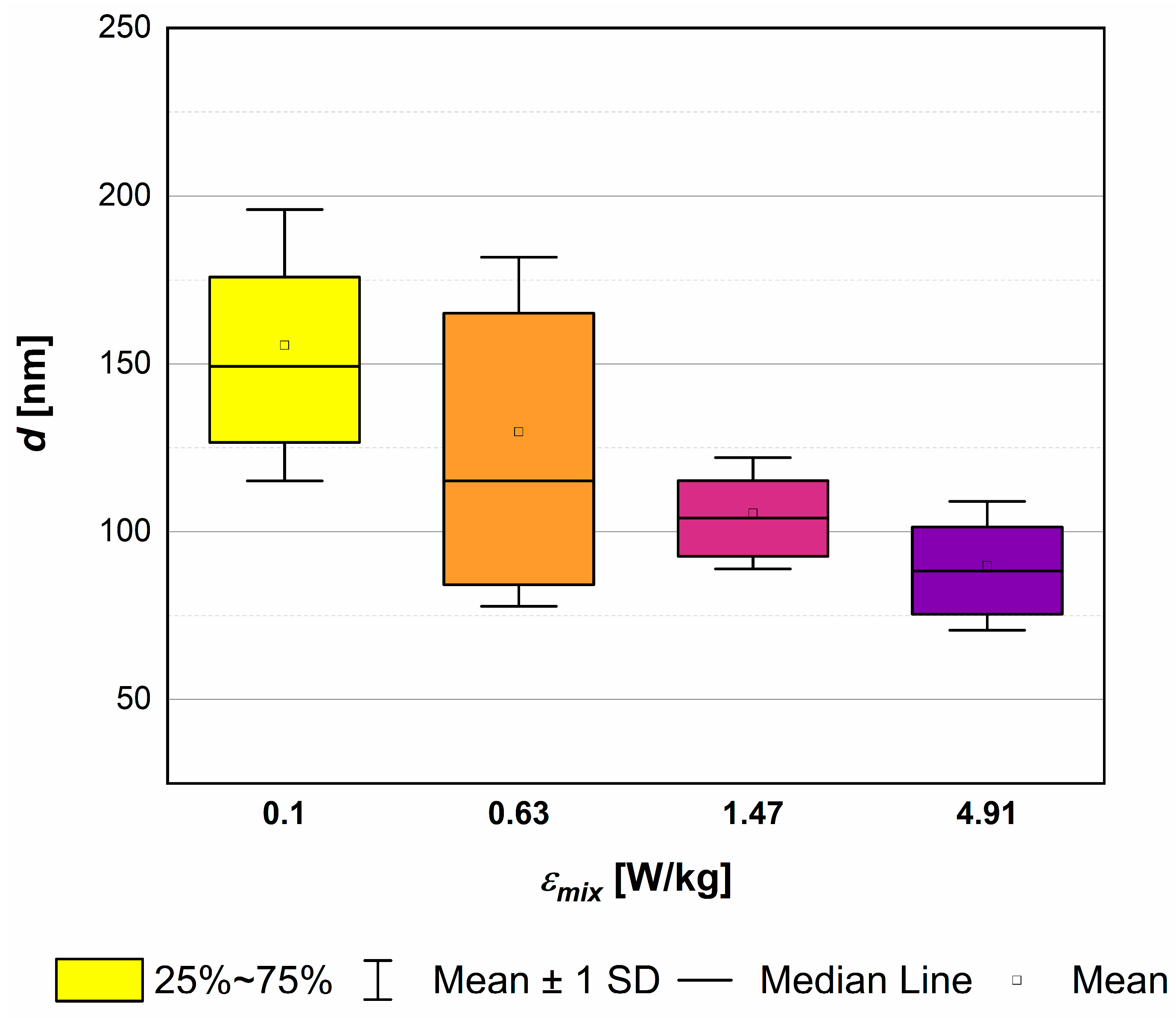
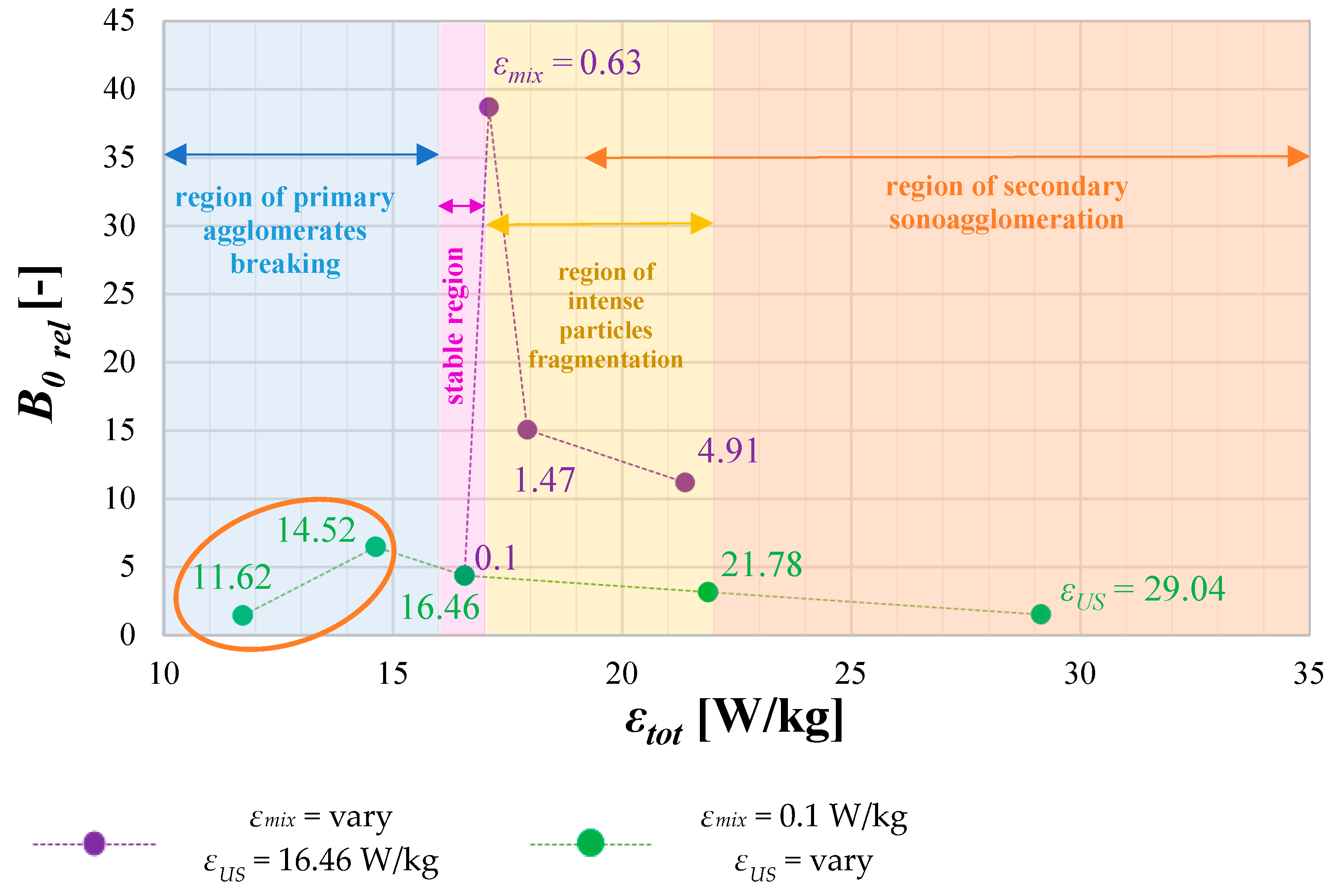
4.3. The Role of Unit Mixing Power εmix
- reduce the agglomeration phenomenon (if the destruction stress, resulting from mixing on static elements or a combination of mixing and the additional use of US, exceeds the agglomerates’ tensile strength);
- lower the mean particle size;
- change the shape of the particles (which can either be considered an advantage or a disadvantage);
- alter the nucleation kinetics,
- excessive particle destruction (due to intense breakage);
- reduction of product purity due to the secondary sonoagglomeration phenomenon.
4.4. The Role of Operating Conditions on Nucleation Intensity B0
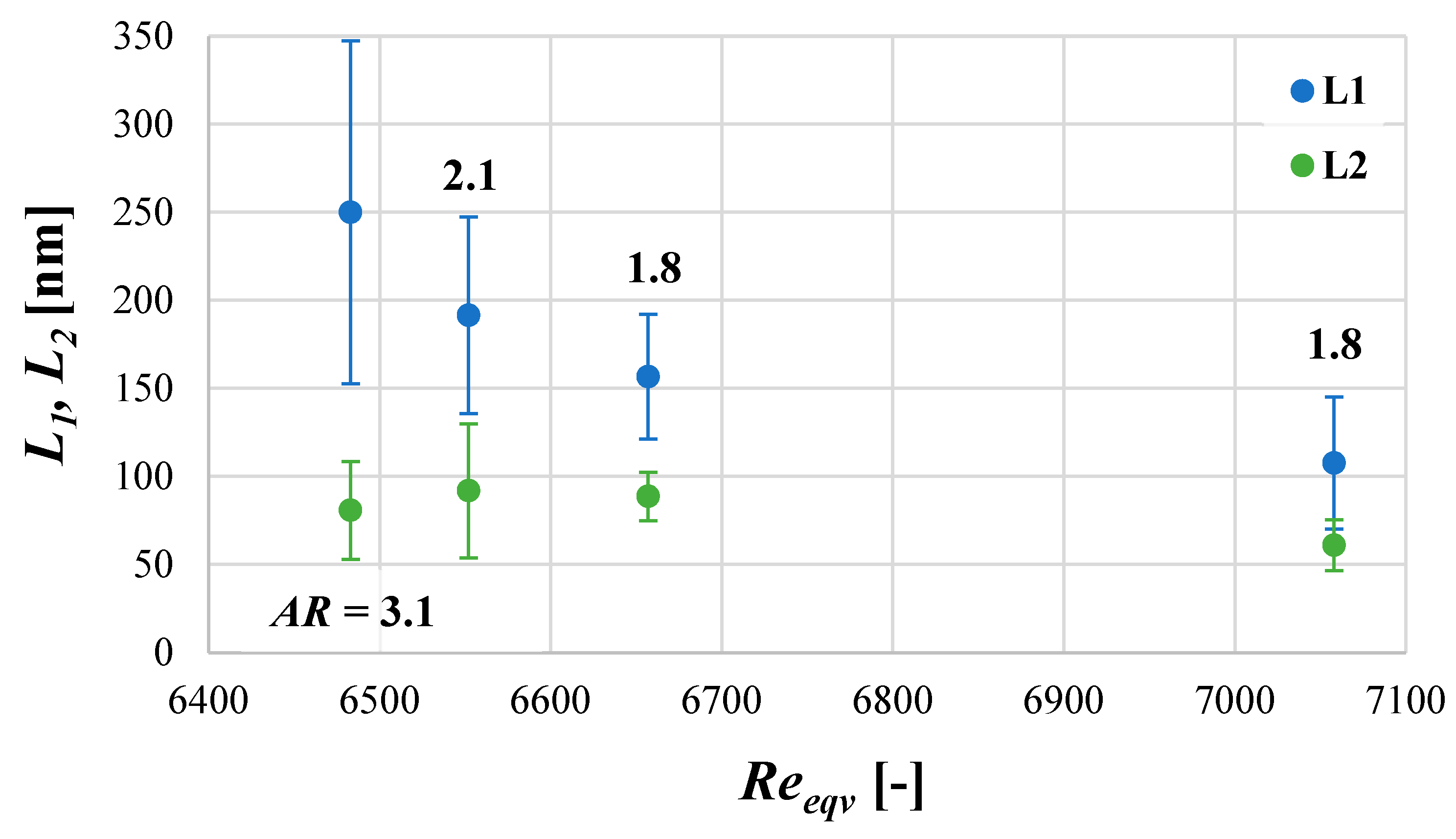
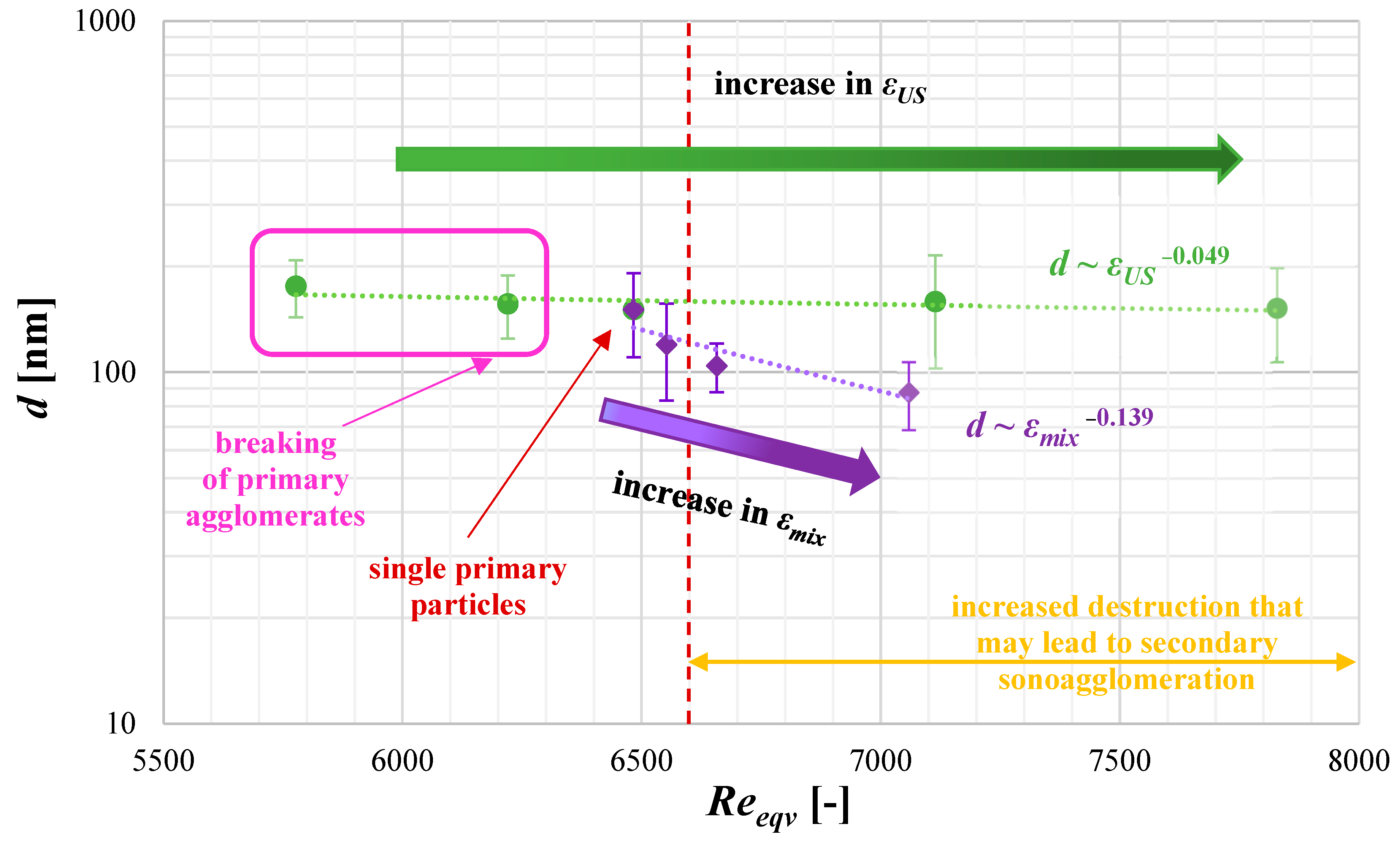
4.5. The Mean Product Size d as a Function of Turbulence Intensity
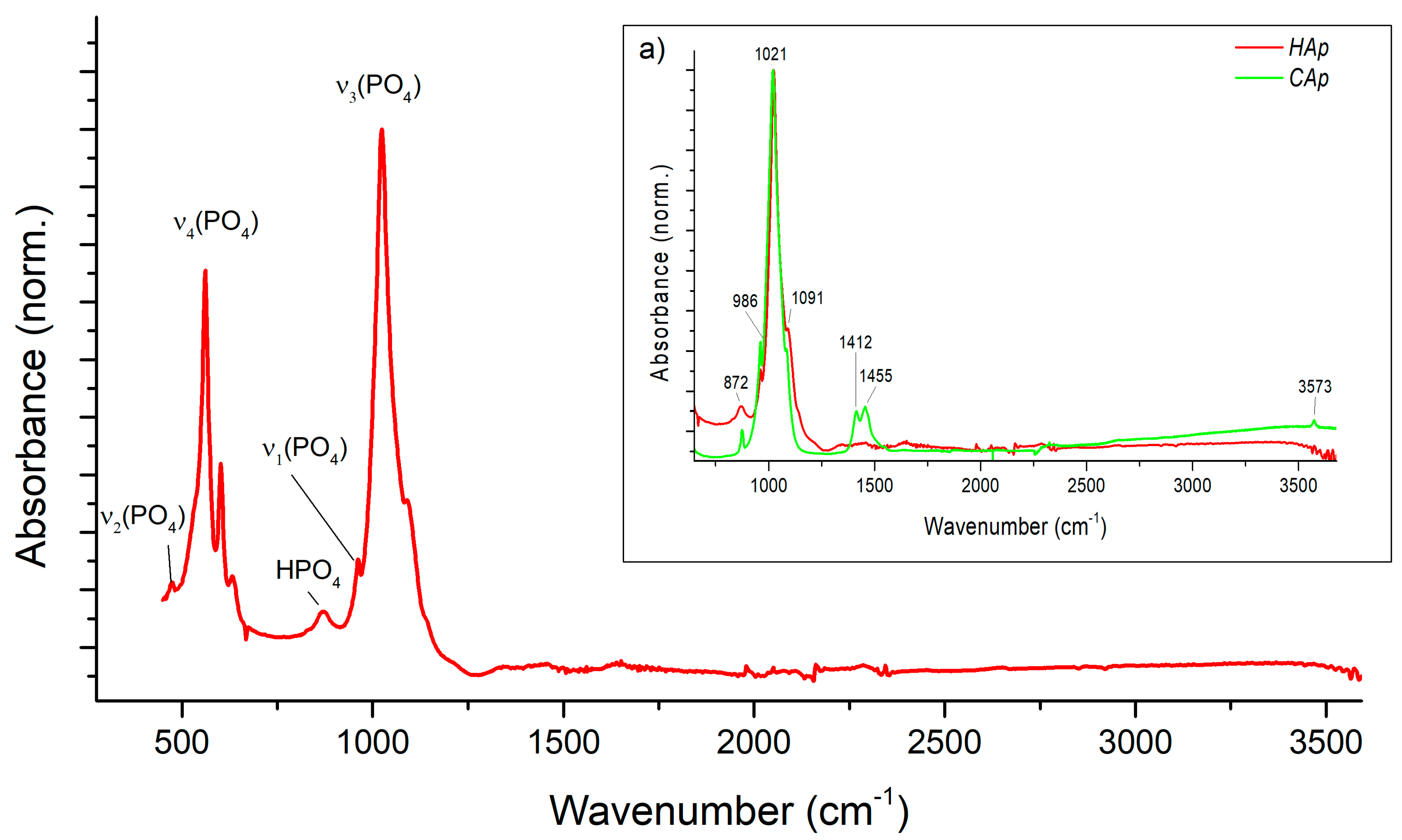
4.6. Powder Characterization—Chemical Composition and Product Purity
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| nucleation rate [m−3s−1] | |
| relative nucleation rate [-] | |
| equivalent Reynolds number | |
| equivalent diameter of a static mixer [m] | |
| total unit power input [W/kg] | |
| unit power input resulting from the ultrasounds’ use [W/kg] | |
| unit power input resulting from mixing in the static mixer [W/kg] | |
| B0mix | nucleation rate during experiments in silent conditions [m−3s−1] |
| B0US | nucleation rate during experiments with US-assistance [m−3s−1] |
| d | mean particle size [m] |
| dm | internal mixer diameter [m] |
| Fav | average area of particles’ surface [nm2] |
| fUS | ultrasounds frequency [kHz] |
| Lm | device length [m] |
| L1 | particle’s length [m] |
| L2 | particle’s width [m] |
| nb | number of cavity bubbles |
| n0 | nuclei number density per unit volume [1/m4] |
| PUS | power of ultrasounds [W] |
| Δp | pressure drop [Pa] |
| Rb | resonant size of cavity bubble [μm] |
| tm | residence time in STM [s] |
| VSTM | volume of STM (excluding static inserts) [m3] |
| VUB | volume of the ultrasonic bath [m3] |
| volumetric flow rate [l/h] | |
| Greek symbols | |
| εmix | unit power input resulting from mixing [W/kg] |
| εtot | total unit power input [W/kg] |
| εUS | unit power input resulting from the ultrasounds’ use [W/kg] |
| η | fluid dynamic viscosity [Pa·s] |
| ρ | fluid density [kg/m3] |
| τUS | sonication time [s] |
| Acronyms | |
| AR | aspect ratio |
| DTGS | deuterated triglycine sulfate detector |
| FT-IR | Fourier-transform infrared spectroscopy |
| PSD | particle size distribution |
| SEM | scanning electron microscopy |
| STM | static mixer |
| US | ultrasounds |
| XRD | X-ray diffraction |
References
- Kantharia, N.; Naik, S.; Apte, S.; Kheur, M.; Kheur, S.; Kale, B. Nano-Hydroxyapatite and Its Contemporary Applications. J. Dent. Res. Sci. Dev. 2014, 1, 15. [Google Scholar] [CrossRef]
- Kalita, S.J.; Bhardwaj, A.; Bhatt, H.A. Nanocrystalline Calcium Phosphate Ceramics in Biomedical Engineering. Mater. Sci. Eng. C 2007, 27, 441–449. [Google Scholar] [CrossRef]
- Khalid, H.; Chaudhry, A.A. Basics of Hydroxyapatite-Structure, Synthesis, Properties, and Clinical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081028346. [Google Scholar]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Morgan, T.T.; Muddana, H.S.; Altinoǧlu, E.I.; Rouse, S.M.; Tabaković, A.; Tabouillot, T.; Russin, T.J.; Shanmugavelandy, S.S.; Butler, P.J.; Eklund, P.C.; et al. Encapsulation of Organic Molecules in Calcium Phosphate Nanocomposite Particles for Intracellular Imaging and Drug Delivery. Nano Lett. 2008, 8, 4108–4115. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis Methods for Nanosized Hydroxyapatite with Diverse Structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.Y.; Park, S.J. The Facile and Low Temperature Synthesis of Nanophase Hydroxyapatite Crystals Using Wet Chemistry. Mater. Sci. Eng. C 2014, 36, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Donadel, K.; Laranjeira, M.C.M.; Gonçalves, V.L.; Fávere, V.T.; De Lima, J.C.; Prates, L.H.M. Hydroxyapatites Produced by Wet-Chemical Methods. J. Am. Ceram. Soc. 2005, 88, 2230–2235. [Google Scholar] [CrossRef]
- Latocha, J.; Wojasiński, M.; Jurczak, K.; Gierlotka, S.; Sobieszuk, P.; Ciach, T. Precipitation of Hydroxyapatite Nanoparticles in 3D-Printed Reactors. Chem. Eng. Process.-Process Intensif. 2018, 133, 221–233. [Google Scholar] [CrossRef]
- Pham, T.T.T.; Nguyen, T.P.; Pham, T.N.; Vu, T.P.; Tran, D.L.; Thai, H.; Dinh, T.M.T. Impact of Physical and Chemical Parameters on the Hydroxyapatite Nanopowder Synthesized by Chemical Precipitation Method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035014. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; O’Brien, F.J.; Partap, S.; Levingstone, T.J.; Stanton, K.T.; Dickson, G.R. The Synthesis and Characterization of Nanophase Hydroxyapatite Using a Novel Dispersant-Aided Precipitation Method. J. Biomed. Mater. Res.-Part A 2010, 95, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, J.; Wang, J.; Yang, S.; Chen, Y. Synthesis of Nanorod and Needle-like Hydroxyapatite Crystal and Role of PH Adjustment. J. Cryst. Growth 2009, 311, 4740–4746. [Google Scholar] [CrossRef]
- Wojasiński, M.; Duszyńska, E.; Ciach, T. Lecithin-Based Wet Chemical Precipitation of Hydroxyapatite Nanoparticles. Colloid Polym. Sci. 2015, 293, 1561–1568. [Google Scholar] [CrossRef]
- Pon-On, W.; Meejoo, S.; Tang, I.M. Formation of Hydroxyapatite Crystallites Using Organic Template of Polyvinyl Alcohol (PVA) and Sodium Dodecyl Sulfate (SDS). Mater. Chem. Phys. 2008, 112, 453–460. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Yang, D.; Li, Y.; Yang, Z.; Lu, W.W. Synthesis of Calcium Phosphate/Calcium Sulphate Powder. Mater. Chem. Phys. 2004, 88, 285–289. [Google Scholar] [CrossRef]
- Brito, L.; Carlos, J.; Gomes De Queiroz Diaz, M.M.; Tenedório Matos Da Silva, V.M.; Quadros De Oliveira e Santos, P.A.; Mendes Monteiro, F.J.; Da Cunha Mateus, A. Production Method for Calcium Phosphate Nano- Particles with High Purity and Their Use. U.S. Patent Application 12/159,696, 22 October 2009. [Google Scholar]
- Silva, V.M.T.M.; Quadros, P.A.; Laranjeira, P.E.M.S.C.; Dias, M.M.; Lopes, J.C.B. A Novel Continuous Industrial Process for Producing Hydroxyapatite Nanoparticles. J. Dispers. Sci. Technol. 2008, 29, 542–547. [Google Scholar] [CrossRef]
- Ribeiro, A.; Manrique, Y.A.; Barreiro, F.; Lopes, J.C.B.; Dias, M.M. Continuous Production of Hydroxyapatite Pickering Emulsions Using a Mesostructured Reactor. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 616, 126365. [Google Scholar] [CrossRef]
- Rouhani, P.; Taghavinia, N.; Rouhani, S. Rapid Growth of Hydroxyapatite Nanoparticles Using Ultrasonic Irradiation. Ultrason. Sonochem. 2010, 17, 853–856. [Google Scholar] [CrossRef]
- Hazar Yoruç, A.B.; Ipek, Y. Sonochemical Synthesis of Hydroxyapatite Nanoparticles with Different Precursor Reagents. Acta Phys. Pol. A 2012, 121, 230–232. [Google Scholar] [CrossRef]
- Wang, J.; Shaw, L.L. Nanocrystalline Hydroxyapatite with Simultaneous Enhancements in Hardness and Toughness. Biomaterials 2009, 30, 6565–6572. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, A.; Dasgupta, S.; Bandyopadhyay, A. Synthesis, Processing, Mechanical, and Biological Property Characterization of Hydroxyapatite Whisker-Reinforced Hydroxyapatite Composites. J. Am. Ceram. Soc. 2009, 92, 323–330. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dubey, A.; Lahiri, D. The Influence of Bioactive Hydroxyapatite Shape and Size on the Mechanical and Biodegradation Behaviour of Magnesium Based Composite. Ceram. Int. 2020, 46, 27205–27218. [Google Scholar] [CrossRef]
- Stec, M.; Synowiec, P.M. Solid Features Modification by the Reactor Selection and US Support during Reactive Crystallization. Materials 2022, 15, 7419. [Google Scholar] [CrossRef]
- Stec, M.; Synowiec, P.M. The Use of Ultrasound-Assisted Precipitation for the Production of Micro-Sized Crystals with a Narrow CSD. Chem. Eng. Process.-Process Intensif. 2022, 173, 108830. [Google Scholar] [CrossRef]
- Stec, M.; Synowiec, P.M. Intensification of Precipitation Process by Means of Ultrasound-Assisted Methods and the Selection of Reactor Type. Chem. Eng. Process.-Process Intensif. 2021, 163, 108379. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carrera, I.; Frise, E.; Verena, K.; Mark, L.; Tobias, P.; Stephan, P.; Curtis, R.; Stephan, S.; Benjamin, S.; et al. Fiji: An Open Platform for Biological Image Analysis. Nat. Methods 2009, 9, 676–682. [Google Scholar] [CrossRef]
- Jordens, J.; Gielen, B.; Xiouras, C.; Hussain, M.N.; Stefanidis, G.D.; Thomassen, L.C.J.; Braeken, L.; Van Gerven, T. Sonocrystallisation: Observations, Theories and Guidelines. Chem. Eng. Process.-Process Intensif. 2019, 139, 130–154. [Google Scholar] [CrossRef]
- Jordens, J.; Appermont, T.; Gielen, B.; Van Gerven, T.; Braeken, L. Sonofragmentation: Effect of Ultrasound Frequency and Power on Particle Breakage. Cryst. Growth Des. 2016, 16, 6167–6177. [Google Scholar] [CrossRef]
- Kim, H.; Suslick, K. The Effects of Ultrasound on Crystals: Sonocrystallization and Sonofragmentation. Crystals 2018, 8, 280. [Google Scholar] [CrossRef]
- Nalesso, S.; Bussemaker, M.J.; Sear, R.P.; Hodnett, M.; Lee, J. A Review on Possible Mechanisms of Sonocrystallisation in Solution. Ultrason. Sonochem. 2019, 57, 125–138. [Google Scholar] [CrossRef]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Effect of Power and Frequency on Bubble-Size Distributions in Acoustic Cavitation. Phys. Rev. Lett. 2009, 102, 084302. [Google Scholar] [CrossRef] [PubMed]
- Luque de Castro, M.D.; Priego-Capote, F. Ultrasound-Assisted Crystallization (Sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Bu, X.; Ulusoy, U.; Xie, G. Effect of Particle Shape on Bubble-Particle Attachment Behavior: Roles of Surfaces, Edges, and Vertexes. J. Clean. Prod. 2023, 429, 139606. [Google Scholar] [CrossRef]
- Stec, M.; Synowiec, P.M.; Bunikowska, B. The Development of Precipitate Characteristics by the Selection of a Reactor Type and Fluid-Dynamic Conditions. Chem. Eng. J. 2020, 393, 124697. [Google Scholar] [CrossRef]
- Synowiec, P.M.; Stec, M. Analysis of CaF 2 Precipitation Process in the Selected Static Mixers. J. Chem. 2019, 2019, 6728492. [Google Scholar] [CrossRef]
- Jamshidi, R.; Rossi, D.; Saffari, N.; Gavriilidis, A.; Mazzei, L. Investigation of the Effect of Ultrasound Parameters on Continuous Sonocrystallization in a Millifluidic Device. Cryst. Growth Des. 2016, 16, 4607–4619. [Google Scholar] [CrossRef]
- Ren, F.; Leng, Y. Carbonated Apatite, Type-A or Type-B? Key Eng. Mater. 2012, 493–494, 293–297. [Google Scholar] [CrossRef]
- Kontonasaki, E.; Zorba, T.; Papadopoulou, L.; Pavlidou, E.; Chatzistavrou, X.; Paraskevopoulos, K.; Koidis, P. Hydroxy Carbonate Apatite Formation on Particulate Bioglass In Vitro as a Function of Time. Cryst. Res. Technol. 2002, 37, 1165–1171. [Google Scholar] [CrossRef]




| Parameter | Unit | Starting Solution | |
|---|---|---|---|
| 0.3 M (NH4)2HPO4 | 0.5 M Ca(NO3)2 | ||
| Density ρ (t = 20 °C) | kg/m3 | 1023.14 | 1058.0 |
| Dynamic viscosity η (t = 20 °C) | Pa·s | 1.102 × 10−3 | 1.071 × 10−3 |
| pH (t = 20 °C) | - | initial: 8.3 after correction: 9.5 | 5.3 |
| Lp. | [L/h] | tm = τUS * [s] | εmix [W/kg] | fUS [kHz] | PUS [W] | εUS [W/kg] | εtot [W/kg] | Reeqv |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 1.61 | 0.10 | 40 | 120 | 11.62 | 11.72 | 5777 |
| 2 | 150 | 14.52 | 14.62 | 6220 | ||||
| 3 | 225 | 21.78 | 21.88 | 7114 | ||||
| 4 | 300 | 29.04 | 29.14 | 7828 | ||||
| 5 | 170 | 16.46 | 16.56 | 6483 | ||||
| 6 | 59 | 16.56 | 6483 | |||||
| 7 | 75 | 0.88 | 0.63 | 40 | 17.09 | 6552 | ||
| 8 | 100 | 0.65 | 1.47 | 17.93 | 6657 | |||
| 9 | 150 | 0.43 | 4.91 | 21.37 | 7058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stec, M.; Synowiec, P.M.; Stolarczyk, A. Synthesis of HAp by Means of Sonoprecipitation Method. Materials 2024, 17, 3240. https://doi.org/10.3390/ma17133240
Stec M, Synowiec PM, Stolarczyk A. Synthesis of HAp by Means of Sonoprecipitation Method. Materials. 2024; 17(13):3240. https://doi.org/10.3390/ma17133240
Chicago/Turabian StyleStec, Magdalena, Piotr Maria Synowiec, and Agnieszka Stolarczyk. 2024. "Synthesis of HAp by Means of Sonoprecipitation Method" Materials 17, no. 13: 3240. https://doi.org/10.3390/ma17133240
APA StyleStec, M., Synowiec, P. M., & Stolarczyk, A. (2024). Synthesis of HAp by Means of Sonoprecipitation Method. Materials, 17(13), 3240. https://doi.org/10.3390/ma17133240







