Effect of Different Surface Treatments as Methods of Improving the Mechanical Properties after Repairs of PMMA for Dentures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
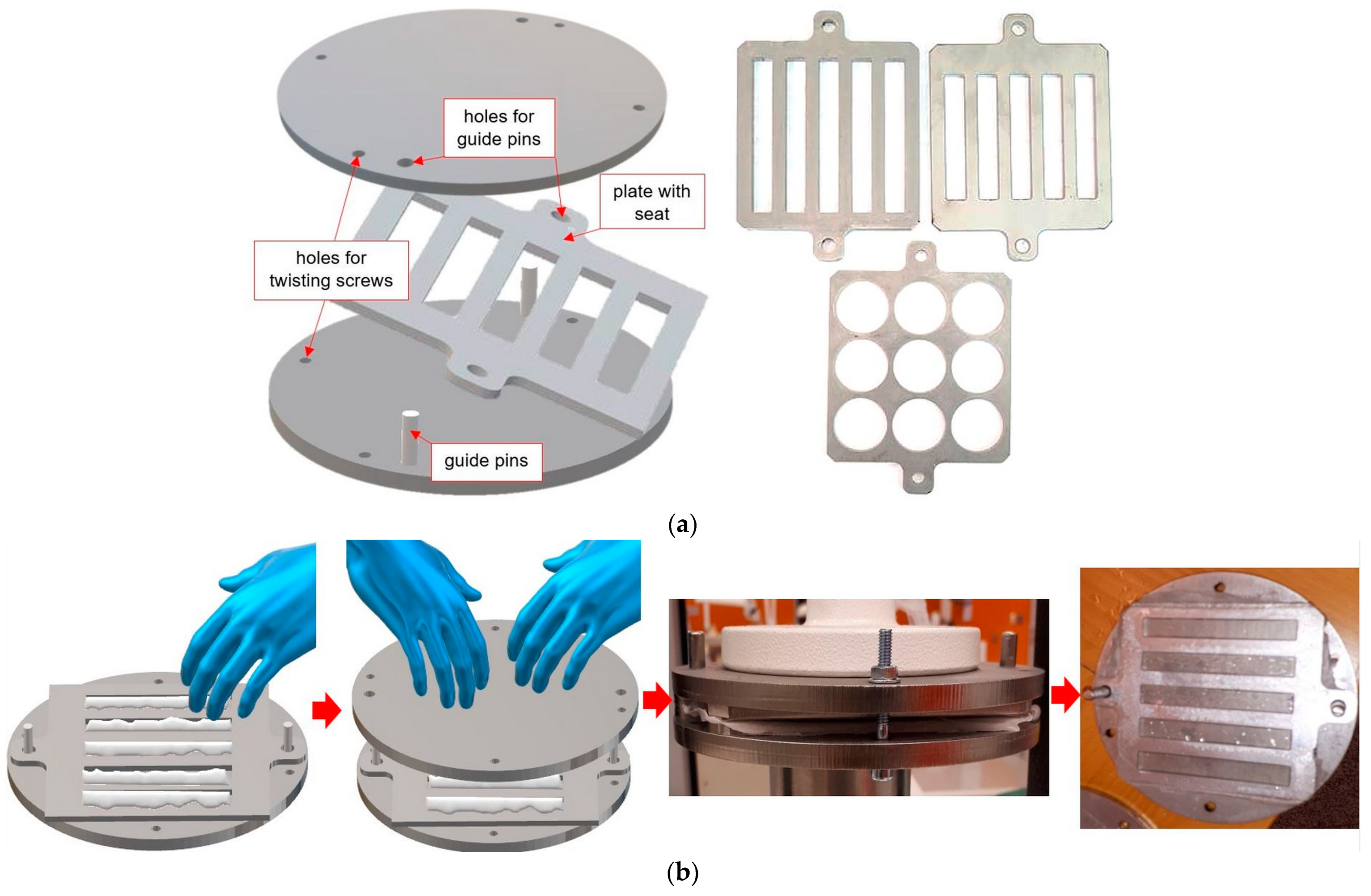
2.2. Samples Preparation
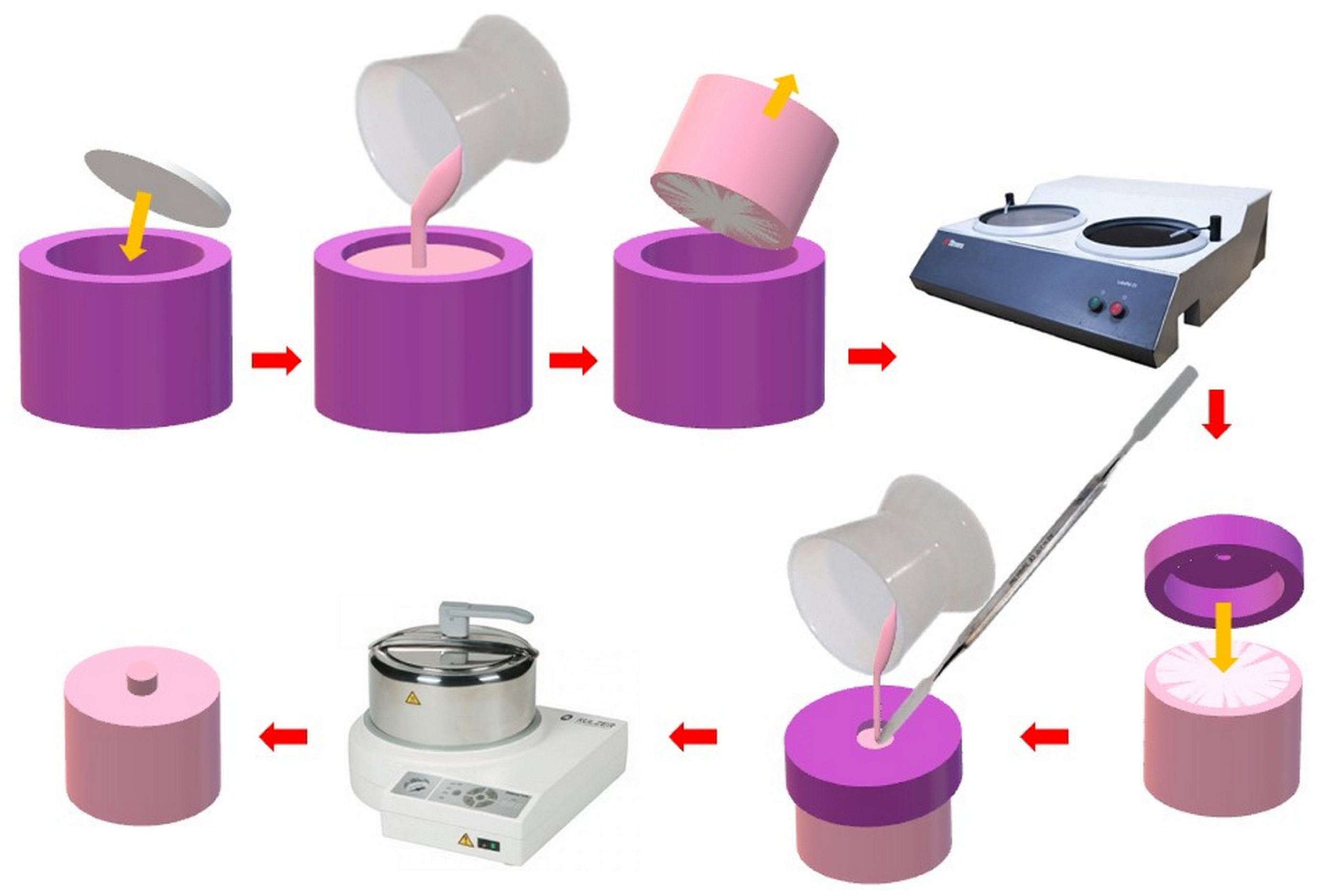
2.2.1. Polymerization of Heat-Polymerized PMMA Resin Samples
2.2.2. Parameters for Abrasive Blasting and Determining Exposure Times to Chemical Agents
2.2.3. Manufacturing of Samples Simulating Repair for Impact Strength and Flexural Strength Tests
2.2.4. Manufacturing of Samples Simulating Repair for Shear Strength Test
2.3. Methods
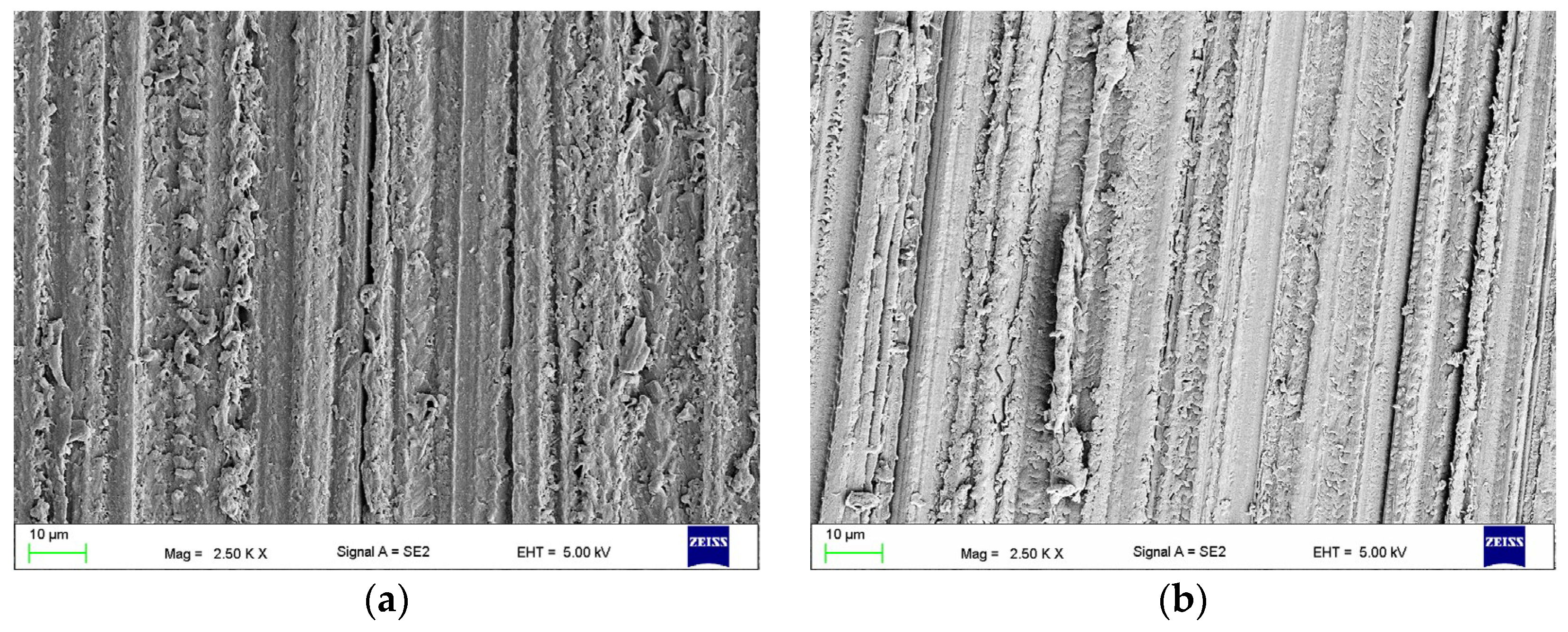
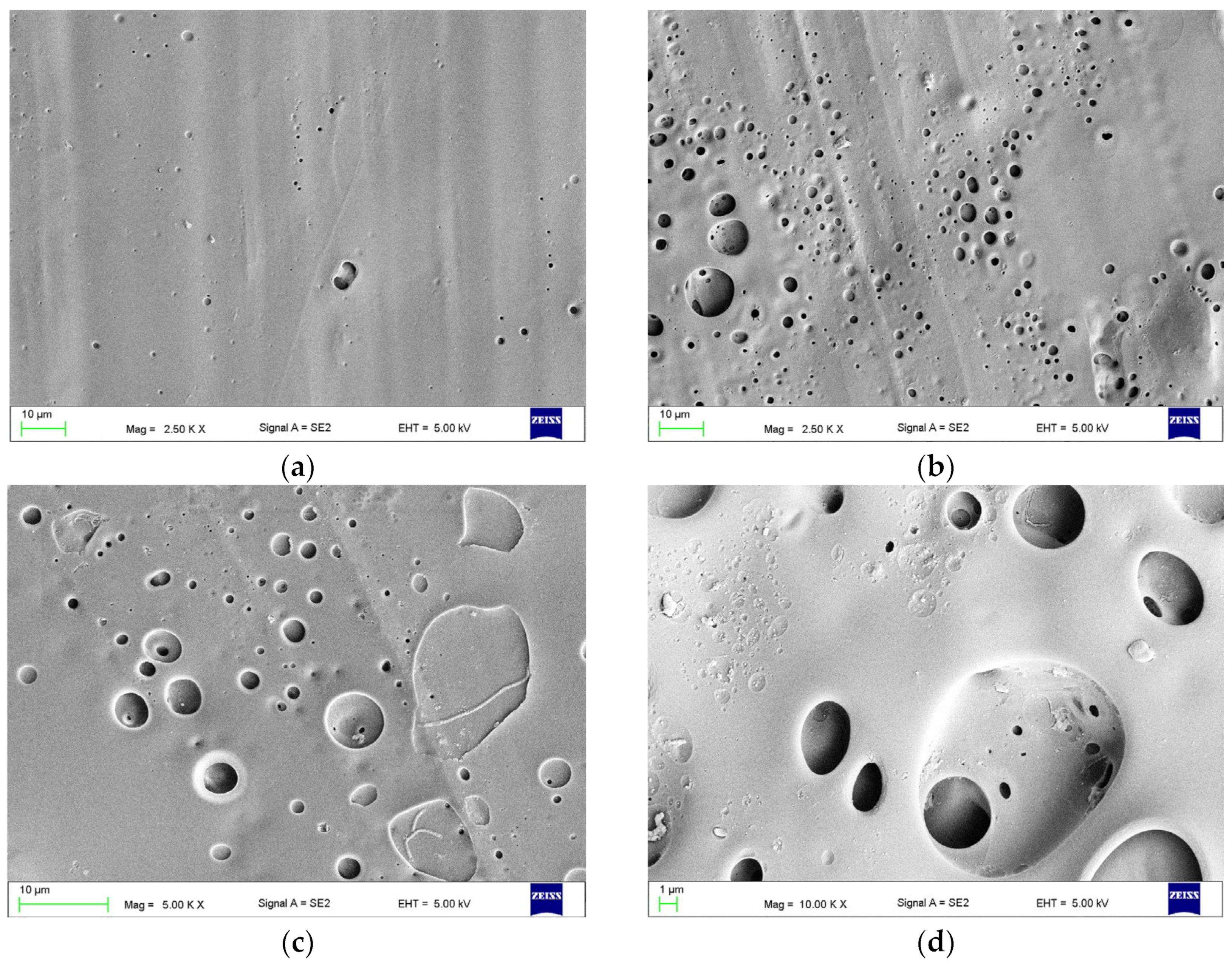
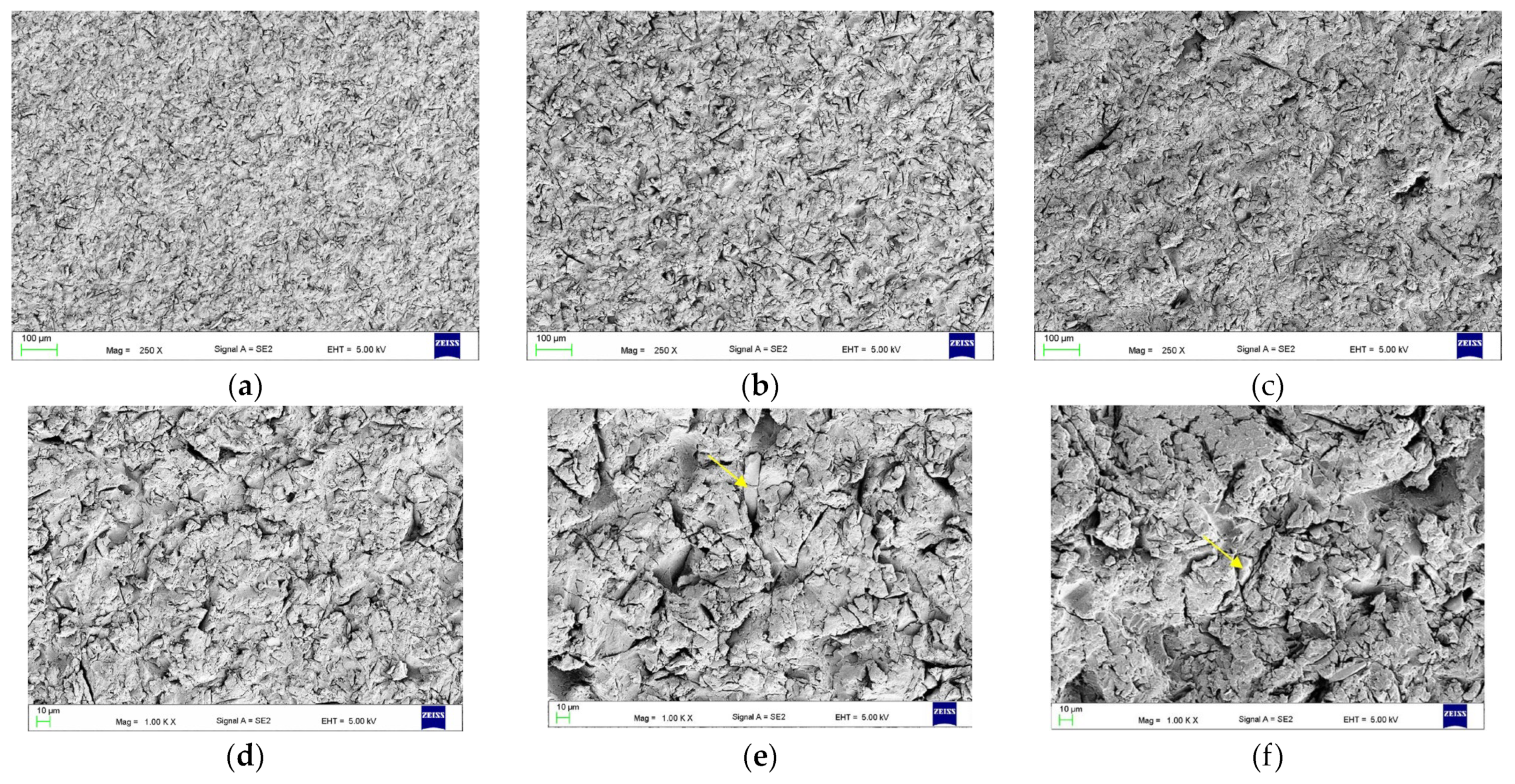
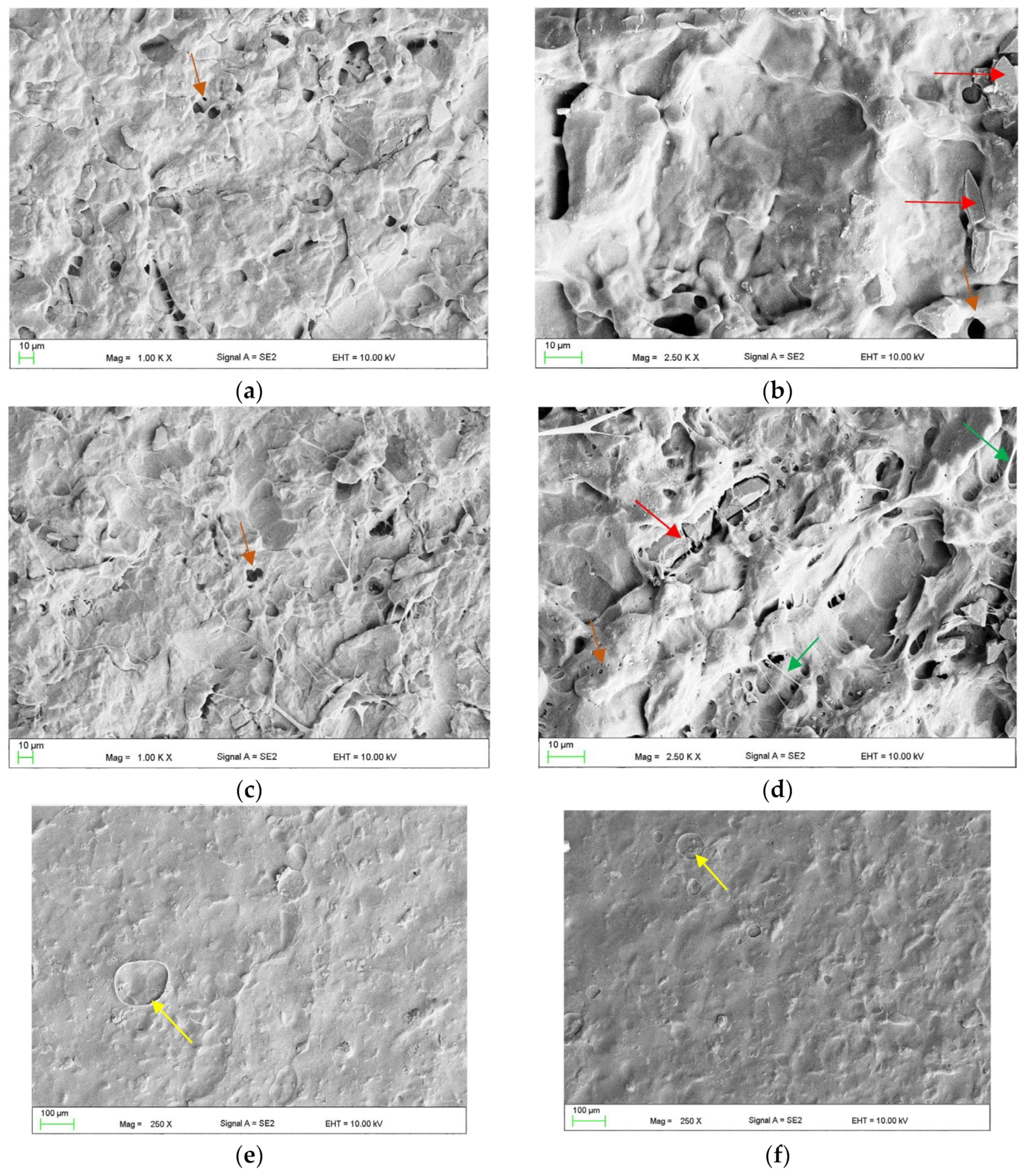
2.3.1. The Surface Morphologies after Treatments (Scanning Electron Microscope Observations)
2.3.2. Strength of “Repaired” Samples during a Flexural Strength Test
- adhesive type (A)—the material used to perform the repair was separated from the base material (repaired);
- cohesive type (C)—there was destruction of the base and/or repair material without adhesive areas—the materials were destroyed without tearing the connection zone;
- mixed type (M)—areas of type A and C occurred simultaneously.
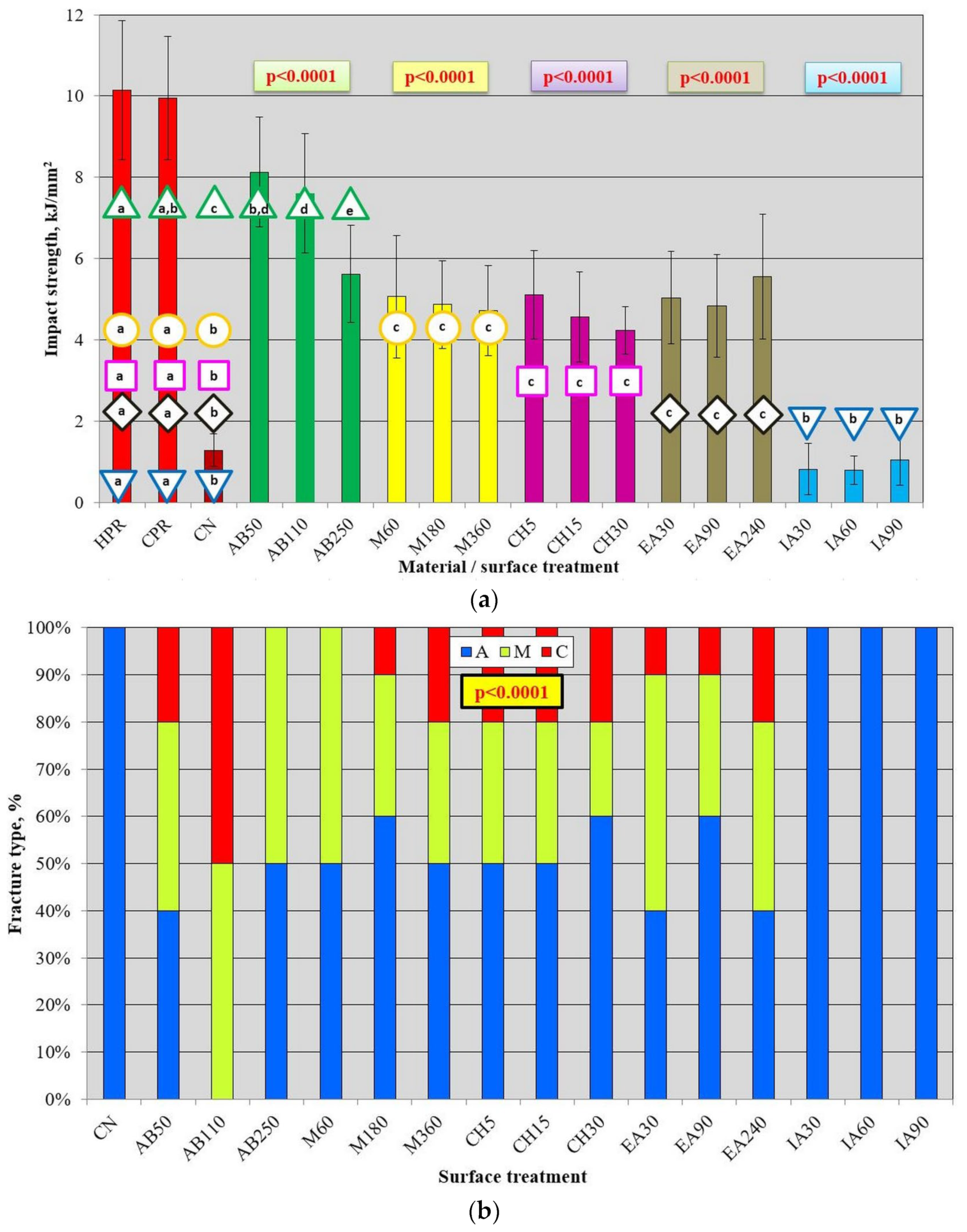
2.3.3. Charpy Impact Strength
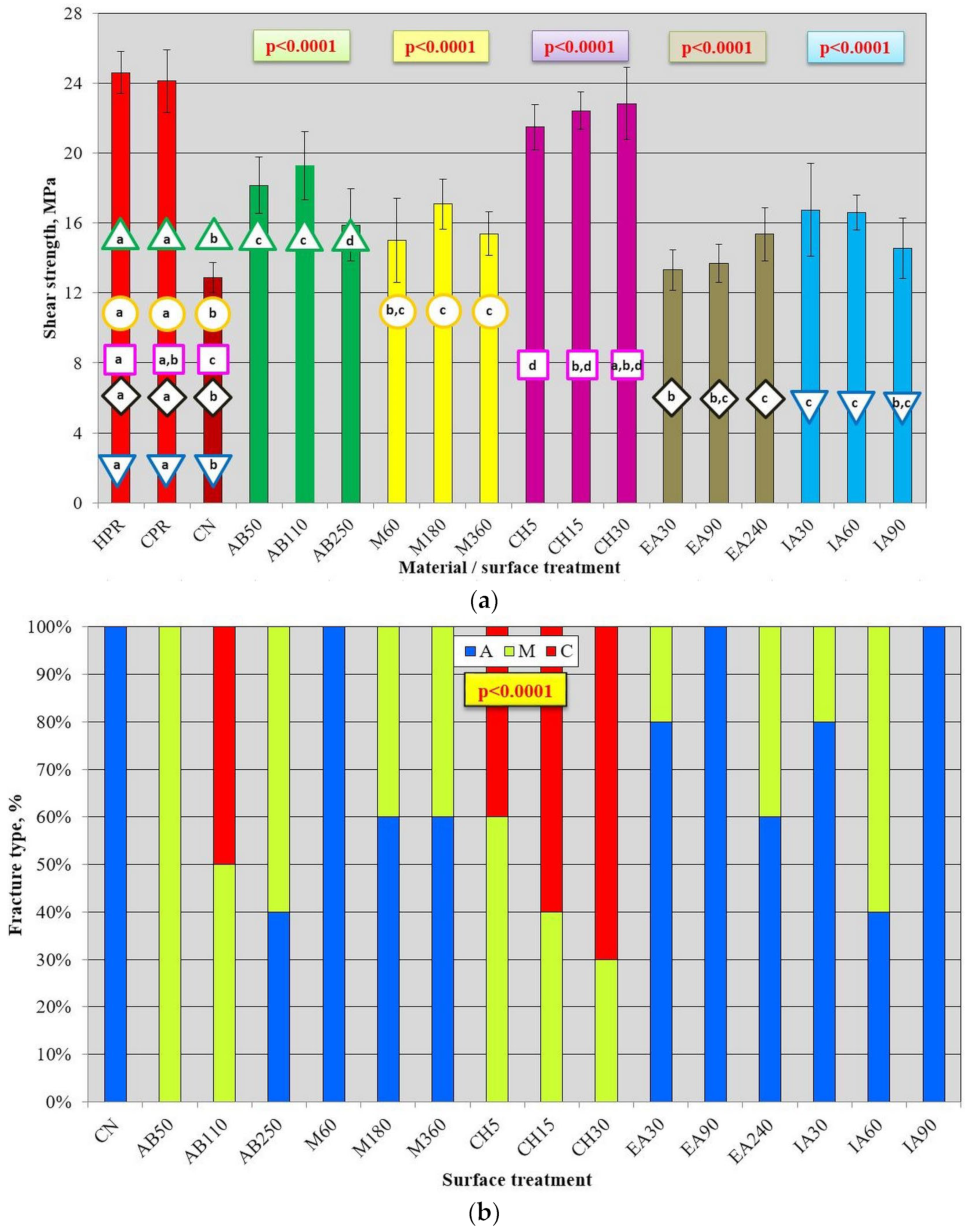
2.3.4. Shear Bond Strength
2.4. Statistical Analysis
3. Results
3.1. The Effect of Abrasive Blasting and Chemical Agent Treatment on Surface Morphology
3.2. Mechanical Properties after AB and Chemical Treatment
3.3. The Effect of Combined Treatment on Surface Morphology
3.4. Mechanical Properties after Combined Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osica, I.; Szlązak, K.; Kapłan, T.; Cierech, M.; Wróbel, K.; Mierzwińska-Nastalska, E.; Bucki, J.J.; Święszkowski, W. Badanie porowatości i nasiąkliwości wybranych tworzyw akrylowych stosowanych w protetyce stomatologicznej w wykonawstwie płyt protez. Przetw. Tworz. 2013, 19, 32–36. [Google Scholar]
- Sakaguchi, R.; Powers, J. Craig’s Restorative Dental Materials, 13th ed.; Elsevier Mosby: Philadelphia, PA, USA, 2012; ISBN 978-0-323-08108-5. [Google Scholar]
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006148-4. [Google Scholar]
- Peltzer, K.; Hewlett, S.; Yawson, A.E.; Moynihan, P.; Preet, R.; Wu, F.; Guo, G.; Arokiasamy, P.; Snodgrass, J.J.; Chatterji, S.; et al. Prevalence of Loss of All Teeth (Edentulism) and Associated Factors in Older Adults in China, Ghana, India, Mexico, Russia and South Africa. Int. J. Environ. Res. Public Health 2014, 11, 11308–11324. [Google Scholar] [CrossRef]
- Turton, B.; Alqunaybit, G.; Tembhe, A.; Qari, A.; Rawal, K.; Mandel, E.; Calabrese, J.; Henshaw, M. Estimation of Oral Disease Burden among Older Adults in LTC: A Scoping Review. Int. J. Environ. Res. Public Health 2024, 21, 248. [Google Scholar] [CrossRef]
- Oleiwi, J.K.; Hamad, Q.A. Studying the Mechanical Properties of Denture Base Materials Fabricated from Polymer Composite Materials. Al-Khwarizmi Eng. J. 2018, 14, 100–111. [Google Scholar] [CrossRef]
- Iftikhar, J.; Saleem, M.N.; Awais, F.; Naz, A.; Tuasene, A.; Saleem, Z.; Qamar, K. Frequency and Causes of Fracture of Acrylic Resin Complete Dentures in Edentulous Patients. Pak. J. Med. Health Sci. 2022, 16, 160–162. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Lassila, V.P.; Lappalainen, R. Evaluation of Damage to Removable Dentures in Two Cities in Finland. Acta Odontol. Scand. 1993, 51, 363–369. [Google Scholar] [CrossRef]
- Shakir, S.; Jalil, H.; Khan, M.A.; Qayum, B.; Qadeer, A. Causes and Types of Denture Fractures—A Study. Pak. Oral Dent. J. 2017, 37, 634–637. [Google Scholar]
- Takamiya, A.S.; Monteiro, D.R.; Marra, J.; Compagnoni, M.A.; Barbosa, D.B. Complete Denture Wearing and Fractures among Edentulous Patients Treated in University Clinics. Gerodontology 2012, 29, e728–e734. [Google Scholar] [CrossRef]
- Bhattacharya, S.R.; Ray, P.K.; Makhal, M.; Sen, S.K. Incidence and Causes of Fracture of Acrylic Resin Complete Denture. J. Evol. Med. Dent. Sci. 2014, 3, 14787–14793. [Google Scholar] [CrossRef]
- Choudhary, S. Complete Denture Fracture—A Proposed Classification System and Its Incidence in National Capital Region Population: A Survey. J. Indian Prosthodont. Soc. 2019, 19, 307–312. [Google Scholar] [CrossRef]
- Alkurt, M.; Yeşil Duymuş, Z.; Gundogdu, M. Effect of Repair Resin Type and Surface Treatment on the Repair Strength of Heat-Polymerized Denture Base Resin. J. Prosthet. Dent. 2014, 111, 71–78. [Google Scholar] [CrossRef]
- Deb, S.; Muniswamy, L.; Thota, G.; Thota, L.; Swarnakar, A.; Deepak, P.V.; Badiyani, B.K.; Kumar, A. Impact of Surface Treatment with Different Repair Acrylic Resin on the Flexural Strength of Denture Base Resin: An In Vitro Study. J. Contemp. Dent. Pr. 2020, 21, 1137–1140. [Google Scholar]
- AlQahtani, M.; Haralur, S.B. Influence of Different Repair Acrylic Resin and Thermocycling on the Flexural Strength of Denture Base Resin. Medicina 2020, 56, 50. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Leung, K.C.M.; Yu, O.Y.; Lam, W.Y.H.; Wong, A.W.Y.; Chu, C.H. Prosthodontic Rehabilitation and Follow-Up Using Maxillary Complete Conventional Immediate Denture. Clin. Cosmet. Investig. Dent. 2020, 12, 437–445. [Google Scholar] [CrossRef]
- Al Nakash, S.; Kadhum, I. The Influence of Different Chemical Surface Treatment on Transverse Strength of Repaired Heat Cure Acrylic Resins. J. Al Rafidain Univ. Coll. 2013, 2013, 1. [Google Scholar]
- Yadav, N.S.; Khare, S.; Mishra, S.K.; Vyas, R.; Mahajan, H.; Chitumalla, R. In-Vitro Evaluation of Transverse Strength of Repaired Heat Cured Denture Base Resins without Surface Treatment and with Chemical and Mechanical Surface Treatment. J. Int. Oral Health 2015, 7, 89–92. [Google Scholar]
- Sarac, Y.S.; Sarac, D.; Kulunk, T.; Kulunk, S. The Effect of Chemical Surface Treatments of Different Denture Base Resins on the Shear Bond Strength of Denture Repair. J. Prosthet. Dent. 2005, 94, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, M.; Rezaei, S.; Zareeian, L. Effect of Chemical Surface Treatments and Repair Material on Transverse Strength of Repaired Acrylic Denture Resin. Indian J. Dent. Res. 2008, 19, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Thunyakitpisal, N.; Thunyakitpisal, P.; Wiwatwarapan, C. The Effect of Chemical Surface Treatments on the Flexural Strength of Repaired Acrylic Denture Base Resin. J. Prosthodont. 2011, 20, 195–199. [Google Scholar] [CrossRef]
- Pereira, R.d.P.; Delfino, C.S.; Butignon, L.E.; Vaz, M.A.K.; Arioli-Filho, J.N. Influence of Surface Treatments on the Flexural Strength of Denture Base Repair. Gerodontology 2012, 29, e234–e238. [Google Scholar] [CrossRef]
- Arun Kumar, P.; Iniyan, K.; Balasubramaniam, R.; Viswanathan, M.; Hines, P.A.J.; Monnica, V. The Effect of Surface Treatments on the Shear Bond Strength of Acrylic Resin Denture Base With Different Repair Acrylic Resin: An In Vitro Study. J. Pharm. Bioallied Sci. 2019, 11, S380–S384. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Abualsaud, R.; Al-Thobity, A.M.; Akhtar, S.; Helal, M.A.; Al-Harbi, F.A. Impact of Different Surface Treatments and Repair Material Reinforcement on the Flexural Strength of Repaired PMMA Denture Base Material. Dent. Mater. J. 2020, 39, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Alqutaibi, A.Y.; Baik, A.; Almuzaini, S.A.; Farghal, A.E.; Alnazzawi, A.A.; Borzangy, S.; Aboalrejal, A.N.; AbdElaziz, M.H.; Mahmoud, I.I.; Zafar, M.S. Polymeric Denture Base Materials: A Review. Polymers 2023, 15, 3258. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, E2299. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Thakur, A. Applications of Poly(Methyl Methacrylate) Polymer in Dentistry: A Review. Mater. Today Proc. 2022, 50, 1619–1625. [Google Scholar] [CrossRef]
- Vuksic, J.; Pilipovic, A.; Pericic, T.P.; Kranjcic, J. The Influence of Contemporary Denture Base Fabrication Methods on Residual Monomer Content, Flexural Strength and Microhardness. Materials 2024, 17, 1052. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, G.; Donmez, M.B.; Akay, C.; Abou-Ayash, S.; Schimmel, M.; Yilmaz, B. Effect of Thermal Cycling on the Flexural Strength and Hardness of New-Generation Denture Base Materials. J. Prosthodont. 2023, 32, 81–86. [Google Scholar] [CrossRef]
- Mohd Farid, D.A.; Zahari, N.A.H.; Said, Z.; Ghazali, M.I.M.; Hao-Ern, L.; Mohamad Zol, S.; Aldhuwayhi, S.; Alauddin, M.S. Modification of Polymer Based Dentures on Biological Properties: Current Update, Status, and Findings. Int. J. Mol. Sci. 2022, 23, 10426. [Google Scholar] [CrossRef] [PubMed]
- Arora, O.; Ahmed, N.; Nallaswamy, D.; Ganapathy, D.; Srinivasan, M. Denture Base Materials: An In Vitro Evaluation of the Mechanical and Color Properties. J. Dent. 2024, 145, 104993. [Google Scholar] [CrossRef]
- Özatik, Ş.; Bural Alan, C. Flexural Strength of Repaired Denture Base Materials Manufactured for the CAD-CAM Technique. J. Oral Sci. 2024, 66, 120–124. [Google Scholar] [CrossRef]
- Goodacre, B.J. 3D Printing of Complete Dentures: A Narrative Review. Int. J. Prosthodont. 2024, 37, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Alqarawi, F.K.; Gad, M.M. Tendency of Microbial Adhesion to Denture Base Resins: A Systematic Review. Front. Oral Health 2024, 5, 1375186. [Google Scholar] [CrossRef] [PubMed]
- Aldegheishem, A.; AlDeeb, M.; Al-Ahdal, K.; Helmi, M.; Alsagob, E.I. Influence of Reinforcing Agents on the Mechanical Properties of Denture Base Resin: A Systematic Review. Polymers 2021, 13, 3083. [Google Scholar] [CrossRef]
- Kaul, S.; Ahmed, S.; Nandini, V.V.; Lathief, J.; Boruah, S. Evaluation of Physical Properties of Denture Base Resins Containing Silver Nanoparticles of Aloe Barbadensis Miller, Morinda Citrifolia, and Boesenbergia Rotunda and Its Anti-Microbial Effect: An In Vitro Study. Cureus 2023, 15, e48260. [Google Scholar] [CrossRef] [PubMed]
- Palaskar, J.N.; Hindocha, A.D.; Mishra, A.; Gandagule, R.; Korde, S. Evaluating the Antifungal Effectiveness, Leaching Characteristics, Flexural Strength, and Impact Strength of Polymethyl Methacrylate Added with Small-Scale Silver Nanoparticles—An in Vitro Study. J. Indian Prosthodont. Soc. 2024, 24, 165–174. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Rahoma, A.; Al-Thobity, A.M.; Akhtar, S.; Fouda, S.M. Double-Layered Acrylic Resin Denture Base with Nanoparticle Additions: An In Vitro Study. J. Prosthet. Dent. 2022, 127, 174–183. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Evans, J.L.; Hamlet, S.; Love, R.M. Overview of Incorporation of Inorganic Antimicrobial Materials in Denture Base Resin: A Scoping Review. J. Prosthet. Dent. 2023, 130, 202–211. [Google Scholar] [CrossRef]
- Chladek, G.; Pakieła, K.; Pakieła, W.; Żmudzki, J.; Adamiak, M.; Krawczyk, C. Effect of Antibacterial Silver-Releasing Filler on the Physicochemical Properties of Poly(Methyl Methacrylate) Denture Base Material. Materials 2019, 12, 4146. [Google Scholar] [CrossRef] [PubMed]
- Jagini, A.S.; Marri, T.; Jayyarapu, D.; Kumari, R.; D, V.; K, M. Effect of Long-Term Immersion in Water and Artificial Saliva on the Flexural Strength of Two Heat Cure Denture Base Resins. J. Contemp. Dent. Pr. 2019, 20, 341–346. [Google Scholar] [CrossRef]
- Takahashi, Y.; Chai, J.; Kawaguchi, M. Equilibrium Strengths of Denture Polymers Subjected to Long-Term Water Immersion. Int. J. Prosthodont. 1999, 12, 348–352. [Google Scholar]
- Chandrahari, N.; Kumar, C.R.; Abdul R, S.; N, P.; Singh, M.; Singh, S. Comparison of Fracture Resistance of Heat Cure Resins Polymerized by Conventional and Microwave Methods after Immersion in Artificial Saliva. J. Contemp. Dent. Pr. 2019, 20, 71–77. [Google Scholar]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Łysik, D.; Mystkowska, J.; Markiewicz, G.; Deptuła, P.; Bucki, R. The Influence of Mucin-Based Artificial Saliva on Properties of Polycaprolactone and Polylactide. Polymers 2019, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Niemirowicz-Laskowska, K.; Mystkowska, J.; Łysik, D.; Chmielewska, S.; Tokajuk, G.; Misztalewska-Turkowicz, I.; Wilczewska, A.Z.; Bucki, R. Antimicrobial and Physicochemical Properties of Artificial Saliva Formulations Supplemented with Core-Shell Magnetic Nanoparticles. Int. J. Mol. Sci. 2020, 21, 1979. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Rahoma, A.; Abualsaud, R.; Al-Thobity, A.M.; Fouda, S.M. Effect of Repair Gap Width on the Strength of Denture Repair: An In Vitro Comparative Study. J. Prosthodont. 2019, 28, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.A.; Abualsaud, R.; Al-Thobity, A.M.; Almaskin, D.F.; AlZaher, Z.A.; Abushowmi, T.H.; Qaw, M.S.; Akhtar, S.; Al-Harbi, F.A. Effect of SiO2 Nanoparticles Addition on the Flexural Strength of Repaired Acrylic Denture Base. Eur. J. Dent. 2020, 14, 19–23. [Google Scholar] [CrossRef]
- Beyli, M.S.; von Fraunhofer, J.A. Repair of Fractured Acrylic Resin. J. Prosthet. Dent. 1980, 44, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Ikuyama, T.; Hayakawa, E.; Tsue, F.; Takahashi, Y. Effect of Surface Preparation Using Ethyl Acetate on the Repair Strength of Denture Base Resin. Acta Odontol. Scand. 2006, 64, 159–163. [Google Scholar] [CrossRef]
- Bural, C.; Bayraktar, G.; Aydin, I.; Yusufoğlu, İ.; Uyumaz, N.; Hanzade, M. Flexural Properties of Repaired Heat-Polymerising Acrylic Resin after Wetting with Monomer and Acetone. Gerodontology 2010, 27, 217–223. [Google Scholar] [CrossRef]
- Thummawanich, W.; Wiwatwarrapan, C.; Sirichompun, C. Mechanical and Physical Properties of an MMA-Based Orthodontic Base-Plate Material after Ultrasonic Treatment in Water. J. Clin. Diagn. Res. 2018, 12, ZC09–ZC012. [Google Scholar] [CrossRef]
- Gad, M.M.; Albazroun, Z.; Aldajani, F.; Elakel, A.M.; El Zayat, M.; Akhtar, S.; Khan, S.Q.; Ali, S.; Rahoma, A.M. Repair Bond Strength of Conventionally and Digitally Fabricated Denture Base Resins to Auto-Polymerized Acrylic Resin: Surface Treatment Effects In Vitro. Materials 2022, 15, 9062. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R. Effect of Repair and Surface Treatments on the Strength of Digitally Fabricated Resin-Based Dental Prostheses: A Systematic Review of In Vitro Studies. J. Dent. 2024, 141, 104806. [Google Scholar] [CrossRef]
- Gibreel, M.; Perea-Lowery, L.; Garoushi, S.; Wada, J.; Lassila, L.; Vallittu, P. Effect of Different Surface Treatments on Shear Bond Strength of Autopolymerizing Repair Resin to Denture Base Materials Processed with Different Technologies. J. Prosthodont. Res. 2024. [Google Scholar] [CrossRef]
- Sahin, Z.; Ozer, N.E.; Akan, T.; Kılıcarslan, M.A.; Karaagaclıoglu, L. The Effect of Various Surface Treatments on the Repair Bond Strength of Denture Bases Produced by Digital and Conventional Methods. Odontology 2023. [Google Scholar] [CrossRef]
- Li, P.; Krämer-Fernandez, P.; Klink, A.; Xu, Y.; Spintzyk, S. Repairability of a 3D Printed Denture Base Polymer: Effects of Surface Treatment and Artificial Aging on the Shear Bond Strength. J. Mech. Behav. Biomed. Mater. 2021, 114, 104227. [Google Scholar] [CrossRef]
- Viotto, H.E.D.C.; Silva, M.D.D.; Nunes, T.S.B.S.; Coelho, S.R.G.; Pero, A.C. Effect of Repair Methods and Materials on the Flexural Strength of 3D-Printed Denture Base Resin. J. Adv. Prosthodont. 2022, 14, 305–314. [Google Scholar] [CrossRef]
- Sahin, Z.; Ozer, N.E.; Akan, T.; Kılıcarslan, M.A.; Karaagaclıoglu, L. The Impact of Different Surface Treatments on Repair Bond Strength of Conventionally, Subtractive-, and Additive-Manufactured Denture Bases. J. Esthet. Restor. Dent. 2024. early view. [Google Scholar] [CrossRef]
- Rached, R.N.; Powers, J.M.; Del Bel Cury, A.A. Repair Strength of Autopolymerizing, Microwave, and Conventional Heat-Polymerized Acrylic Resins. J. Prosthet. Dent. 2004, 92, 79–82. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Substitutes for Methylene Chloride as Dental Softening Agent. Eur. J. Oral Sci. 2000, 108, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Barton, A.F.M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; Routledge: Boca Raton, FL, USA, 2017; ISBN 978-1-315-14057-5. [Google Scholar]
- Fink, J.K. Chapter 12—Terpene Resins. In Reactive Polymers Fundamentals and Applications, 2nd ed.; Fink, J.K., Ed.; Plastics Design Library; William Andrew Publishing: Oxford, UK, 2013; pp. 303–315. ISBN 978-1-4557-3149-7. [Google Scholar]
- Dooher, T.; Dixon, D. Multiwalled Carbon Nanotube/Polysulfone Composites: Using the Hildebrand Solubility Parameter to Predict Dispersion. Polym. Compos. 2011, 32, 1895–1903. [Google Scholar] [CrossRef]
- Evchuk, I.Y.; Musii, R.I.; Makitra, R.G.; Pristanskii, R.E. Solubility of Polymethyl Methacrylate in Organic Solvents. Russ. J. Appl. Chem. 2005, 78, 1576–1580. [Google Scholar] [CrossRef]
- Vallittu, P.; Lassila, V.; Lappalainen, R. Wetting the Repair Surface with Methyl Methacrylate Affects the Transverse Strength of Repaired Heat-Polymerized Resin. J. Prosthet. Dent. 1994, 72, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Rahoma, A.; Abualsaud, R.; Al-Thobity, A.M.; Akhtar, S.; Siddiqui, I.A.; Al-Harbi, F.A. Influence of Artificial Aging and ZrO2 Nanoparticle-Reinforced Repair Resin on the Denture Repair Strength. J. Clin. Exp. Dent. 2020, 12, e354–e362. [Google Scholar] [CrossRef] [PubMed]
- Seaton, P. Mechanics of Tensile and Shear Stress Generation in Fixed Partial Denture Retainers. J. Prosthet. Dent. 1994, 71, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.A.; Jamayet, N.; Lynch, E. Husein Biomechanical Stress in Removable Complete Dental Prostheses: A Narrative Review of Finite Element Studies. J. Int. Oral Health 2020, 5, 413–419. [Google Scholar] [CrossRef]
- Thean, H.P.; Chew, C.L.; Goh, K.I.; Norman, R.D. An Evaluation of Bond Strengths of Denture Repair Resins by a Torsional Method. Aust. Dent. J. 1998, 43, 5–8. [Google Scholar] [CrossRef]
- Prombonas, A.E.; Vlissidis, D.S. Comparison of the Midline Stress Fields in Maxillary and Mandibular Complete Dentures: A Pilot Study. J. Prosthet. Dent. 2006, 95, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.B.M.; Shaik, S.; Sachdeva, H.; Khare, S.; Haralur, S.B.; Roopa, K.T. Effect of Reinforcement Using Stainless Steel Mesh, Glass Fibers, and Polyethylene on the Impact Strength of Heat Cure Denture Base Resin—An In Vitro Study. J. Int. Oral Health 2015, 7, 71–79. [Google Scholar]
- Goiato, M.C.; Freitas, E.; dos Santos, D.; de Medeiros, R.; Sonego, M. Acrylic Resin Cytotoxicity for Denture Base—Literature Review. Adv. Clin. Exp. Med. 2015, 24, 679–686. [Google Scholar] [CrossRef]









| Type of Treatment | Exposure Time, s | Abbreviation |
|---|---|---|
| Control | - | CN |
| Alumina blasting 50 µm | - | AB50 |
| Alumina blasting 110 µm | - | AB110 |
| Alumina blasting 250 µm | - | AB250 |
| Methyl methacrylate | 60 | M60 |
| 120 | M120 | |
| 180 | M180 | |
| 240 | M240 | |
| 300 | M300 | |
| 60 | M60 | |
| Ethyl acetate | 30 | EA30 |
| 60 | EA60 | |
| 90 | EA90 | |
| 120 | EA120 | |
| 180 | EA180 | |
| 240 | EA240 | |
| Methylene chloride | 5 | CH5 |
| 15 | CH15 | |
| 30 | CH30 | |
| 45 | CH45 | |
| 60 | CH60 | |
| 90 | CH90 | |
| Isopropyl alcohol | 30 | IA30 |
| 45 | IA45 | |
| 60 | IA60 | |
| 75 | IA75 | |
| 90 | IA90 |
| Property | AB | M | CH | EA | IA |
|---|---|---|---|---|---|
| Impact strength, kJ/mm2 (p < 0.0001) | 7.1 ± 1.7 a | 4.9 ± 1.3 b | 4.6 ± 1.0 b | 5.1 ± 1.4 b | 0.9 ± 0.6 c |
| Shear strength, MPa (p < 0.0001) | 17.8 ± 2.4 a | 15.8 ± 2.0 b | 22.2 ± 1.6 c | 14.1 ± 1.6 d | 16.0 ± 2.2 b |
| Flexural strength, MPa (p < 0.0001) | 72.8 ± 12.3 a | 52.0 ± 11.0 b | 59.5 ± 6.8 c | 41.0 ± 6.5 d | 49.5 ± 10.1 b |
| Property | AB110-M180 | AB110-EA240 | AB110-CH5 | AB110-CH15 | |
|---|---|---|---|---|---|
| Impact strength | AB110 | N | N | N | S |
| M180 | S | N/A | N/A | N/A | |
| EA240 | N/A | S | N/A | N/A | |
| CH5 | N/A | N/A | S | N/A | |
| CH15 | N/A | N/A | N/A | N | |
| Shear strength | AB110 | S | S | S | S |
| M180 | S | N/A | N/A | N/A | |
| EA240 | N/A | S | N/A | N/A | |
| CH5 | N/A | N/A | S | N/A | |
| CH15 | N/A | N/A | N/A | N | |
| Flexural strength | AB110 | N | N | N | N |
| M180 | S | N/A | N/A | N/A | |
| EA240 | N/A | S | N/A | N/A | |
| CH5 | N/A | N/A | S | N/A | |
| CH15 | N/A | N/A | N/A | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chladek, G.; Adeeb, S.; Pakieła, W.; Coto, N.P. Effect of Different Surface Treatments as Methods of Improving the Mechanical Properties after Repairs of PMMA for Dentures. Materials 2024, 17, 3254. https://doi.org/10.3390/ma17133254
Chladek G, Adeeb S, Pakieła W, Coto NP. Effect of Different Surface Treatments as Methods of Improving the Mechanical Properties after Repairs of PMMA for Dentures. Materials. 2024; 17(13):3254. https://doi.org/10.3390/ma17133254
Chicago/Turabian StyleChladek, Grzegorz, Sandra Adeeb, Wojciech Pakieła, and Neide Pena Coto. 2024. "Effect of Different Surface Treatments as Methods of Improving the Mechanical Properties after Repairs of PMMA for Dentures" Materials 17, no. 13: 3254. https://doi.org/10.3390/ma17133254





