Characterization of NiCuOxNy Coatings Obtained via RF Sputtering: Structure, Morphology, and Optical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition of NiCuOxNy Coatings
2.2. Characterization Techniques
3. Results and Discussion
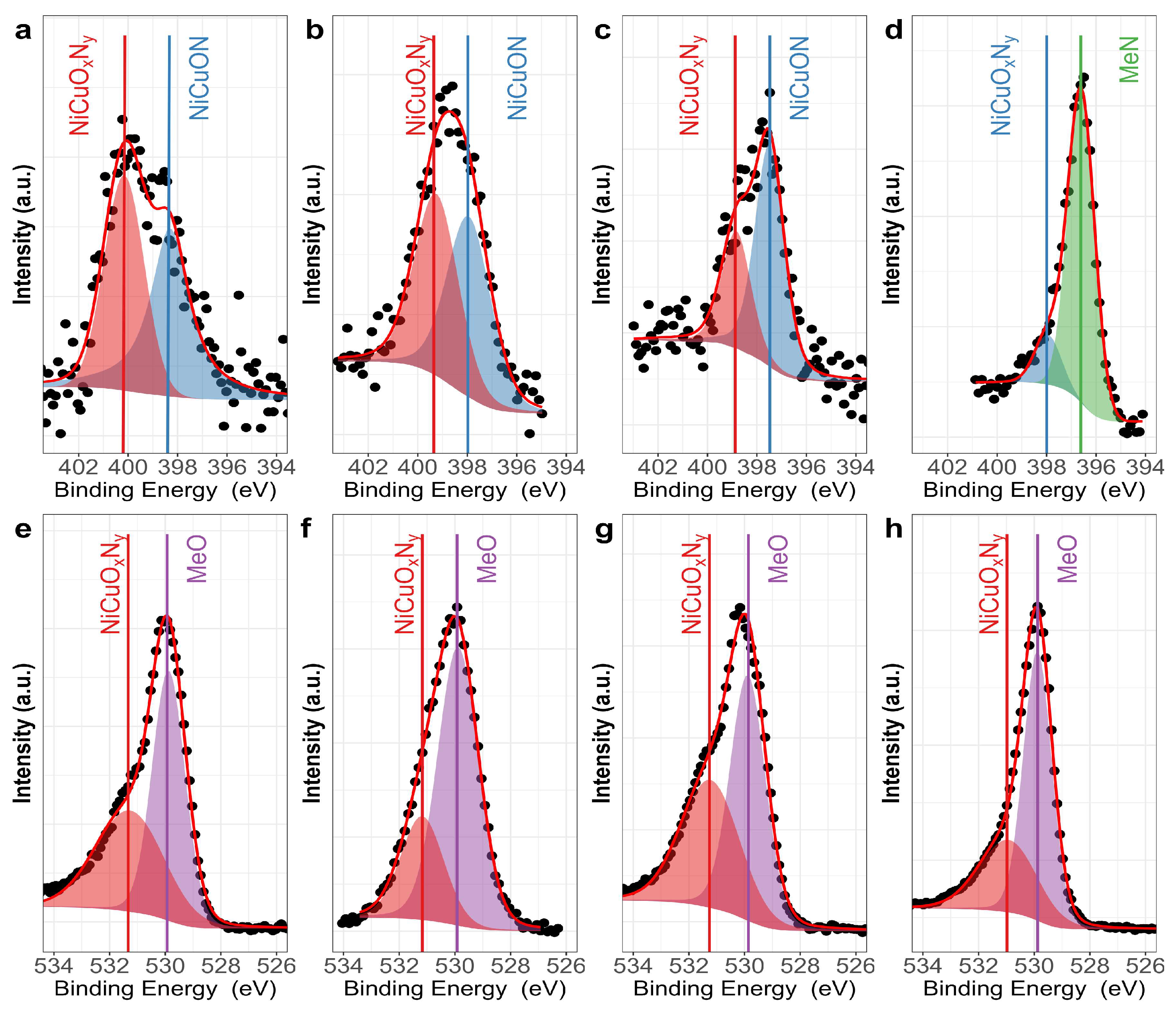
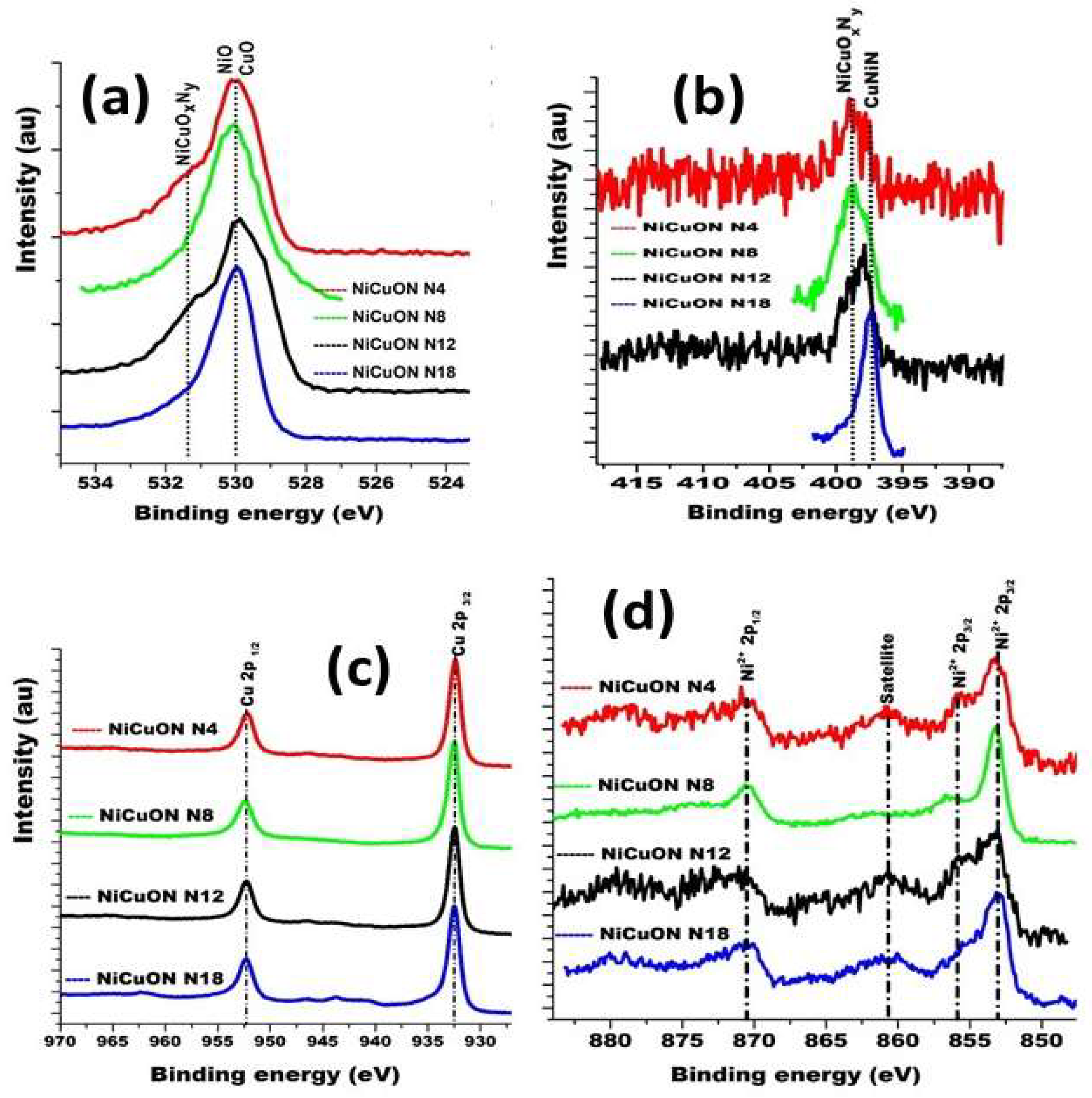
3.1. Chemical Composition
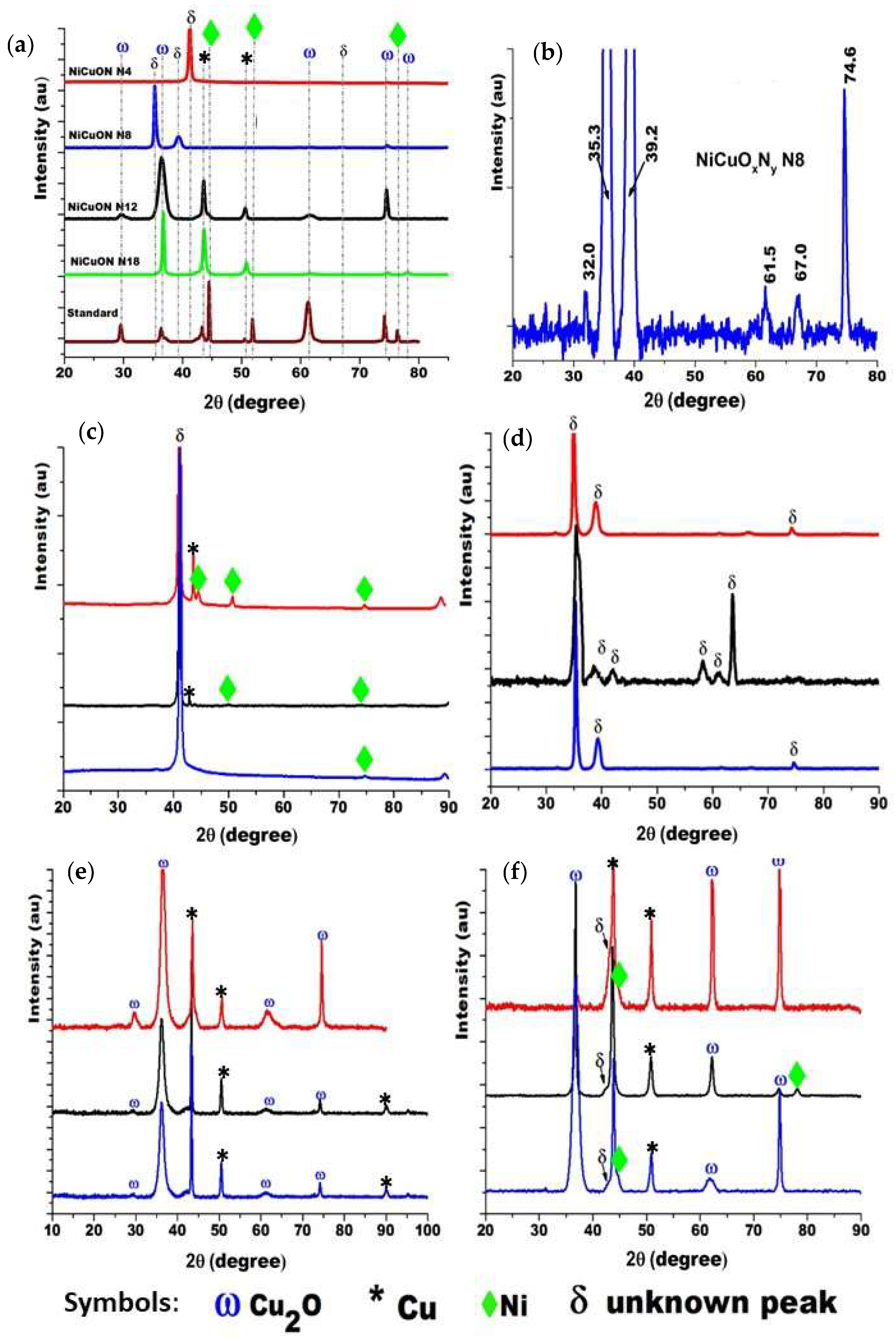
3.2. Crystal Structure
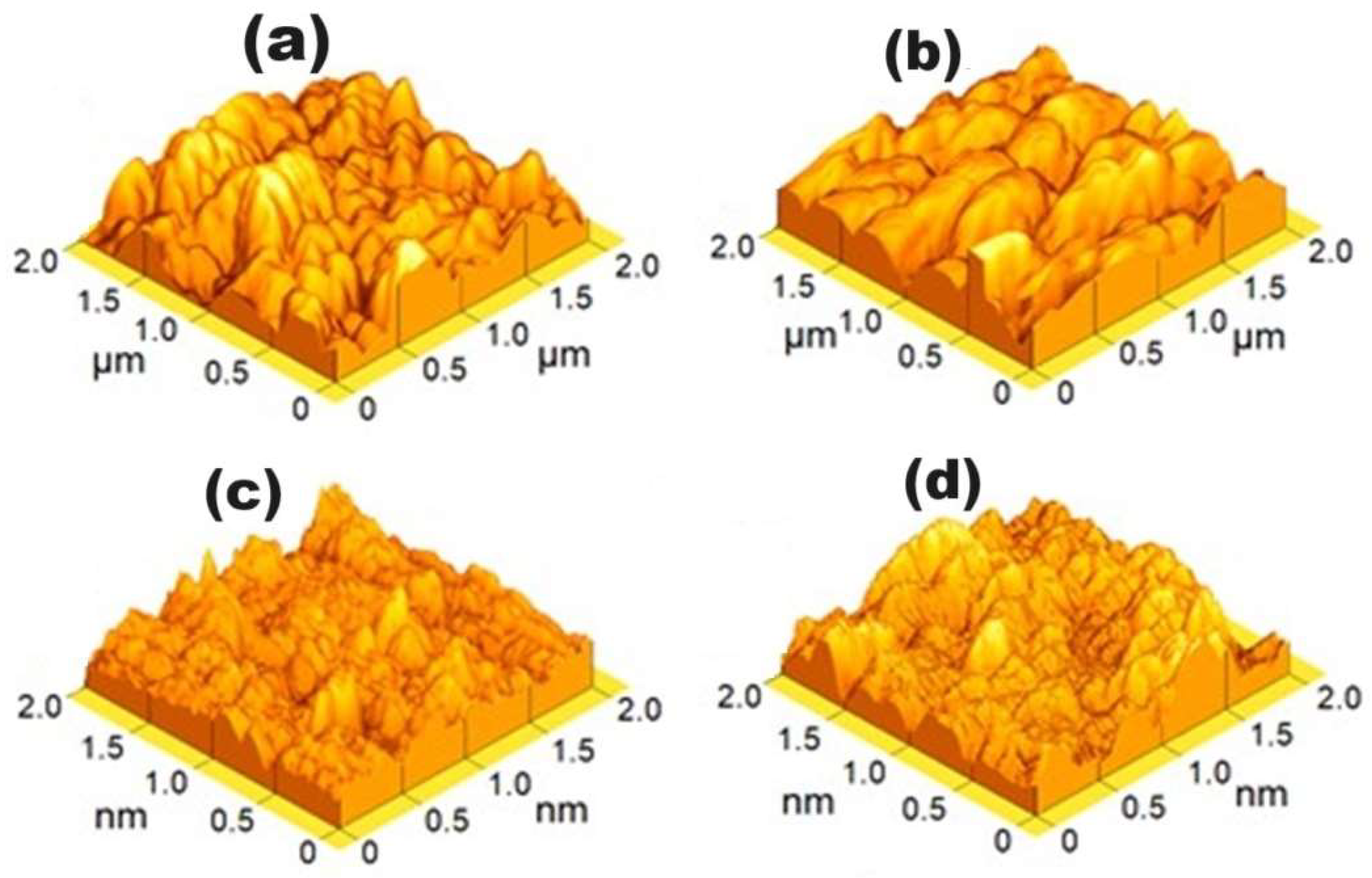
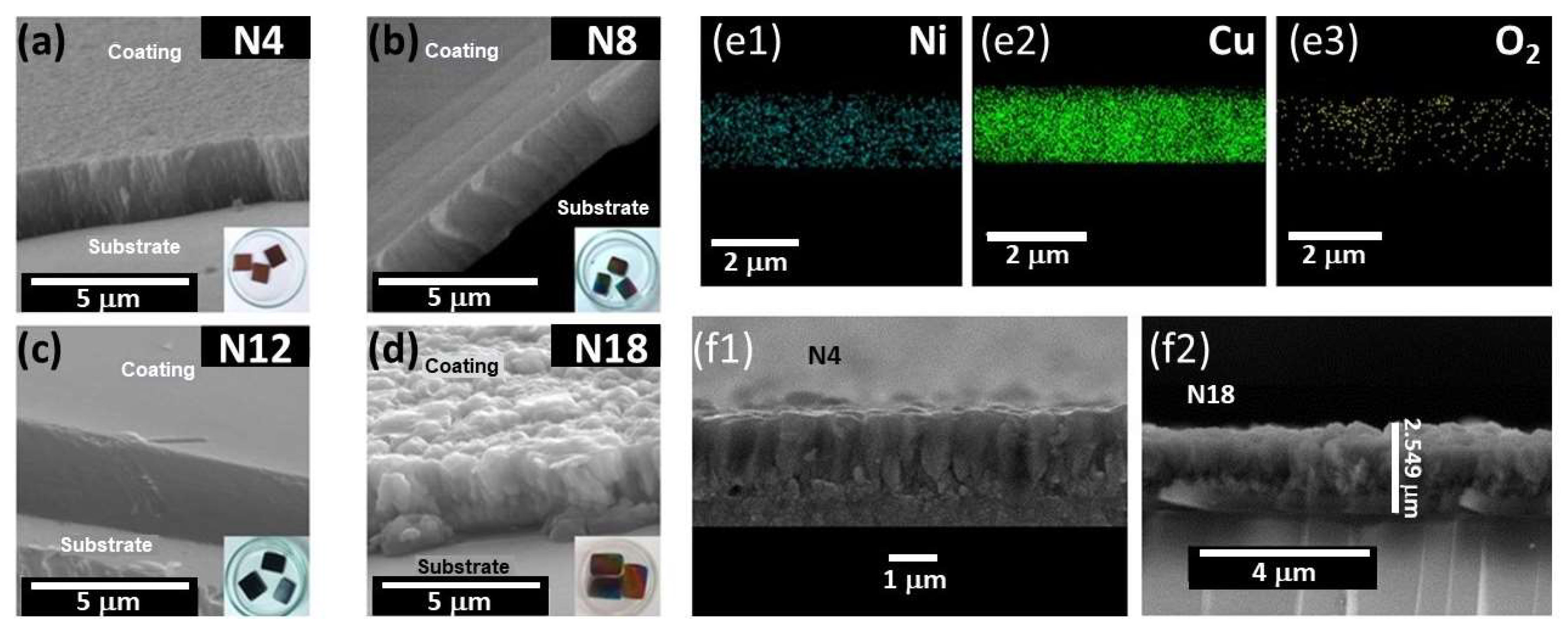
3.3. Morphology
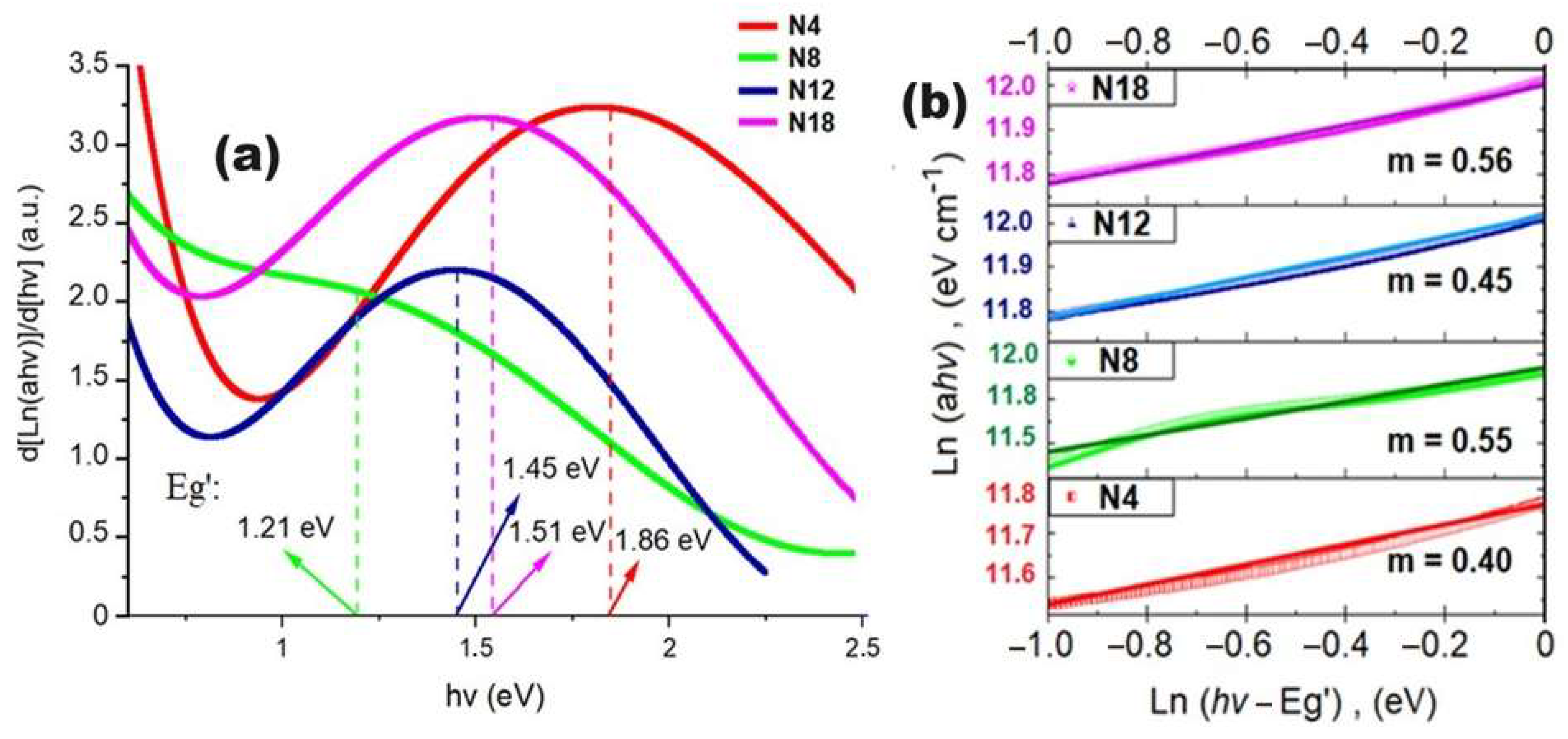
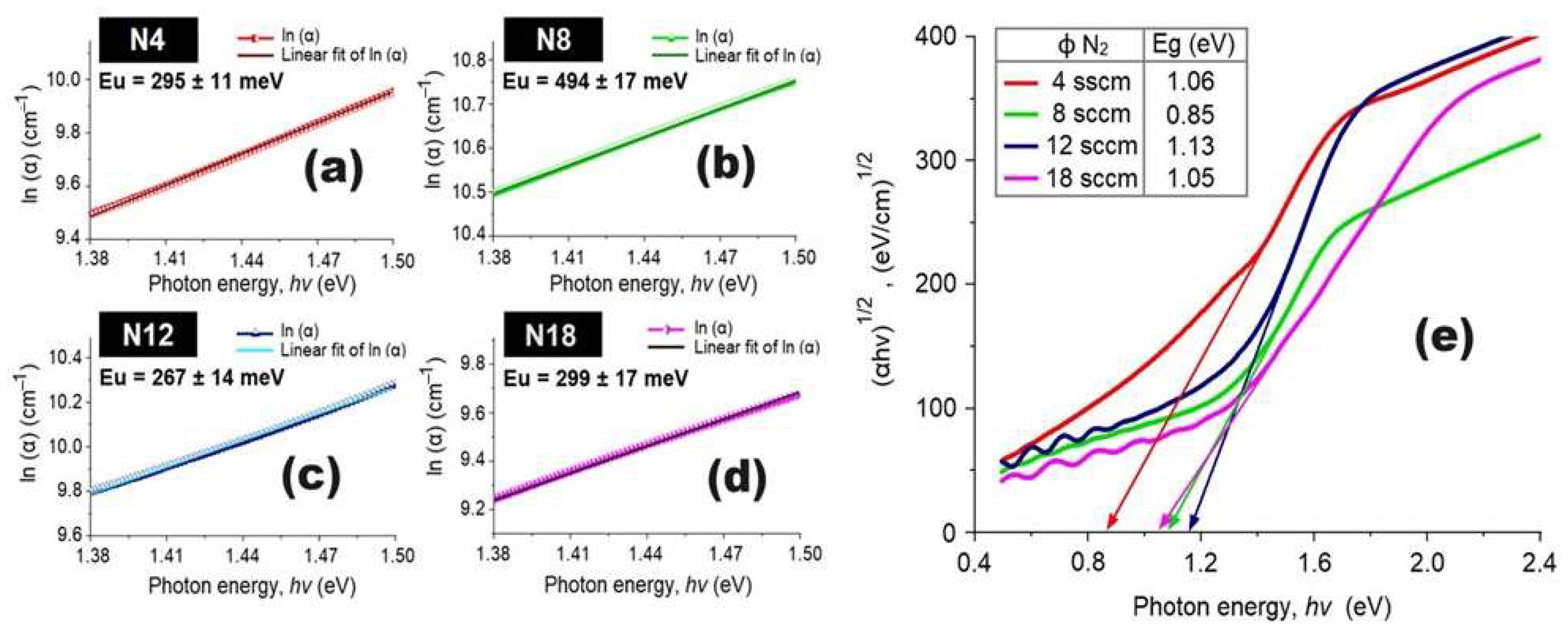
3.4. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, J.; Ni, J.; Irvine, T.S.J.; Ni, C. Transparent conductive oxide type materials as the anode of solid oxide fuel cells at a reduced temperature. J. Mater. Chem. A 2022, 10, 25249–25261. [Google Scholar] [CrossRef]
- Isherwood, P.J.M.; Walls, J.M. Cupric Oxide-based p-type Transparent Conductors. Energy Procedia 2014, 60, 129–134. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Murali, A. Plasma Synthesis of Advanced Metal Oxide Nanoparticles and Their Applications as Transparent Conducting Oxide Thin Films. Molecules 2021, 26, 1456. [Google Scholar] [CrossRef]
- Khemiri, N.; Chamekh, S.; Kanzaria, M. Properties of thermally evaporated CZTS thin films and numerical simulation of earth abundant and non-toxic CZTS/Zn(S,O) based solar cells. J. Sol. Energy 2020, 207, 496–502. [Google Scholar] [CrossRef]
- Mondon, A.; Jawaid, M.N.; Bartsch, J.; Glatthaar, M.; Glunz, S.W. Microstructure analysis of the interface situation and adhesion of thermally formed nickel silicide for plated nickel–copper contacts on silicon solar cells. Sol. Energy Mater. Sol. Cells 2013, 117, 209–213. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Yao, L.; Ren, D.; Sivula, K.; Grätzel, M.; Hagfeldt, A. Cu2O photocathodes with band-tail states assisted hole transport for standalone solar water splitting. Nat. Comm. 2020, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, J.; Lee, K.; Oh, S.K.; Kang, H.J.; Park, N.S. Electronic, Optical and Electrical Properties of Nickel Oxide Thin Films Grown by RF Magnetron Sputtering. Appl. Sci. Converg. Technol. 2015, 24, 72–76. [Google Scholar] [CrossRef]
- Visibile, A.; Wang, R.B.; Vertova, A.; Rondinini, S.; Minguzzi, A.; Ahlberg, E.; Busch, M. Influence of Strain on the Band Gap of Cu2O. Mater. Chem. 2019, 31, 4787–4792. [Google Scholar] [CrossRef]
- Omelchenko, S.T.; Tolstova, Y.; Atwater, H.A.; Lewis, N.S. Excitonic Effects in Emerging Photovoltaic Materials: A Case Study in Cu2O. ACS Energy Lett. 2017, 2, 2431–2437. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Q.; Zhang, Q.; Xiao, Y.; Zhong, X.; Dong, G.; Delpancke-Ogletree, M.P.; Terryn, H.; Baert, K.; Reiners, F. In situ electrochromic efficiency of a nickel oxide thin film: Origin of electrochemical process and electrochromic degradation. J. Mater. Chem. C 2018, 6, 646–653. [Google Scholar] [CrossRef]
- Wang, K.C.; Jeng, J.Y.; Shen, P.S.; Chang, Y.C.; Diau, E.W.G.; Tsai, C.H.; Chao, T.Y.; Hsu, H.C.; Lin, P.Y.; Chen, P.; et al. p-type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells. Sci. Rep. 2014, 4, 4756. [Google Scholar] [CrossRef] [PubMed]
- Mezher, S.J.; Dawood, M.O.; Abdulmunem, O.M.; Mejbel, M.K. Copper doped nickel oxide gas sensor. Vacuum 2020, 172, 109074. [Google Scholar] [CrossRef]
- Malvin, C.T.; Tsai, C.T.; Wang, Y.Y.; Chen, P.C.; Kao, C.S.Y. Improved hole-injection and external quantum efficiency of organic light-emitting diodes using an ultra-thin K-doped NiO buffer layer. J. Alloys Compd. 2019, 797, 159–165. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Hassan, D.A.; Hojjati-Najafabadi, A.; Li, W.; Liao, L.; Moshayedi, A.J.; Huang, X.; Khajavi, M.N. Quality enhancement of copper oxide thin film synthesized under elevated gravity acceleration by two-axis spin coating. Ceram. Int. 2020, 46, 7421–7429. [Google Scholar] [CrossRef]
- Nalage, S.R.; Chougule, M.A.; Sen, S.; Joshi, P.B.; Patil, V.B. Sol-gel synthesis of nickel oxide thin films and their characterization. Thin Solid Film. 2012, 520, 4835–4840. [Google Scholar] [CrossRef]
- Khodair, Z.; Al-Jubbori, M.A.; Shano, A.M.; Sharrad, F.I. Study of Optical and Structural Properties of (NiO)1−x(CuO)x Nanostructures Thin Films. Chem. Data Coll. 2020, 28, 100414. [Google Scholar] [CrossRef]
- Sasaki, I.; Koutoulaki, A.; Kompitsas, M.; Charitidis, C. Structural electrical and mechanical properties of NiO thin films grown by pulsed laser deposition. Appl. Surf. Sci. 2010, 257, 429–433. [Google Scholar]
- Alfonso, J.E.; Olaya, J.J.; Pinzón, M.J.; Marco, J.F. Potentiodynamic Polarization Studies and Surface Chemical Composition of Bismuth Titanate (BixTiyOz) Films Produced through Radiofrequency Magnetron Sputtering. Materials 2013, 10, 4441–4449. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The same chemical state of carbon gives rise to two peaks in X-ray photoelectron spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Naslund, L.A.; Persson, I. XPS spectra curve fittings of Ti3C2Tx based on first principles thinking. Appl. Surf. Sci. 2022, 593, 153442. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water purification from chlorobenzenes using hetroatom-functionalized carbon nanofibers produced on self-organizing Ni-Pd catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Mishakov, I.V.; Bauman, Y.; Shubin, Y.V.; Maksimova, T.A.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Cristea, D.; Scărlătescu, A.I.; Croitoru, C.; Marin, A.; Velicu, I.L.; Tiron, V.; Martínez-Martínez, D.; da Silva Oliveira, C.I.; Cunha, L. Photocatalytical and corrosion behavior of sputtered zirconium oxynitride thin films doped with titanium. Surf. Interfaces 2023, 42, 103488. [Google Scholar] [CrossRef]
- Yazdi, S.T.; Shahri, R.P.; Shafei, S. First synthesis of In-doped vanadium pentoxide thin films and their structural, optical and electrical characterization. Mater. Sci. Eng. B 2021, 263, 114755. [Google Scholar] [CrossRef]
- Arba, Y.; Yamkane, Z.; Louafi, J.; Elouafi, A.; El Hachmi, A.; El Ouahbi, S.; Manoun, B.; Moubah, R.; Omari, L.H.; Lassri, H.; et al. Optical properties investigation of Sr2CaFe2WO9 triple perovskite for optoelectronic applications. Optik 2023, 288, 171153. [Google Scholar] [CrossRef]
- Lerch, M.; Krumeich, F.; Hock, R. Diffusion controlled formation of p type phases in the system ZrO2-Zr3N4. Solid State Ion. 1997, 95, 87–93. [Google Scholar] [CrossRef]
- Lerch, M.; Janek, J.; Becker, K.D.; Berendts, S.; Boysen, H.; Bredow, T.; Dronskowski, R.; Ebbinghaus, S.G.; Kilo, M.; Lumey, M.W.; et al. Oxide nitrides: From oxides to solids with mobile nitrogen ions. Prog. Solid State Chem. 2009, 37, 81–131. [Google Scholar] [CrossRef]
- Castro, J.D.; Lima, M.J.; Carvalho, I.; Henriques, M.; Carvalho, S. Cu oxidation mechanism on Cu-Zr(O)N coatings: Role on functional properties. Appl. Surf. Sci. 2021, 555, 149704. [Google Scholar] [CrossRef]
- Milosev, I.; Strehblow, H.; Gaberscek, M.; Navinsek, B. Comparison of TiN, ZrN and CrN hard nitride coatings: Electrochemical and thermal oxidation. Thin Solid Film. 1997, 303, 246–254. [Google Scholar] [CrossRef]
- Rizzo, A.; Signore, M.A.; Mirenghi, L.; Tapfer, L.; Piscopiello, E.; Salernitano, E. Sputtering deposition and characterization of zirconium nitride and oxynitride films. Thin Solid Film. 2012, 520, 3532–3538. [Google Scholar] [CrossRef]
- Bora, J.; Podder, S.; Gogoi, D.; Basumatary, B.; Pal, A.R. An all metal nitride nanostructure configuration: Study and exploitation in efficient photo-detection. J. Alloys Compd. 2021, 879, 160460. [Google Scholar] [CrossRef]
- Roman, D.; Bernardi, J.; De Amorim, C.; de Souza, F.; Spinelli, A.; Giacomelli, C.; Figueroa, C.; Baumvol, I.; Basso, R. Effect of deposition temperature on microstructure and corrosion resistance of ZrN thin films deposited by DC reactive magnetron sputtering. Mater. Chem. Phys. 2011, 130, 147–153. [Google Scholar] [CrossRef]
- Senocak, T.C.; Ezirmik, K.V.; Cengiz, S. The antibacterial properties and corrosion behavior of silver-doped niobium oxynitride coatings. Mater. Today Commun. 2022, 32, 103975. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Pelenovich, V.; Zeng, X.; Zeng, Z.; Hu, L.; Xu, T.; Zhan, X.; Lei, Y.; Chen, Y.; et al. Structure and mechanical properties of multi-principal AlCrNbSiTiON oxynitride coatings. Vacuum 2024, 227, 113366. [Google Scholar] [CrossRef]
- Muñoz, F.; Pascual, L.; Durán, A.; Rocherullé, J.; Marchand, R. Alkali and alkali–lead oxynitride phosphate glasses: A comparative structural study by NMR and XPS. Comptes Rendus Chim. 2002, 5, 731–738. [Google Scholar] [CrossRef]
- Liao, H.; Wang, Z. Adsorption removal of amaranth by nanoparticles-composed Cu2O Microspheres. J. Alloys Compd. 2018, 769, 1088–1095. [Google Scholar] [CrossRef]
- Wang, Y.; Lany, S.; Ghanbaja, J.; Fagot-Revurat, Y.; Chen, Y.P.; Soldera, F.; Horwat, D.; Mucklich, F.; Pierson, J.F. Electronic structures of Cu2O, Cu4O3, and CuO: A joint experimental and theoretical study. Phys. Rev. B 2016, 94, 245418. [Google Scholar] [CrossRef]
- Alajlani, Y.; Placido, F.; Barlow, A.; Chu, H.O.; Song, S.; Ur Rahman, S.; De Bold, R.; Gibson, D. Characterisation of Cu2O, Cu4O3, and CuO mixed phase thin films produced by microwave-activated reactive sputtering. Vacuum 2017, 144, 217–228. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.H.; Qia, M.Y.; Tanga, Z.R.; Xu, Y.J. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction. Appl. Catal. B Environ. 2020, 268, 118380. [Google Scholar] [CrossRef]
- Yang, G.; Qiua, P.; Xiong, J.; Zhua, X.; Cheng, G. Facilely anchoring Cu2O nanoparticles on mesoporous TiO2 nanorods for enhanced photocatalytic CO2 reduction through efficient charge transfer. Chin. Chem. Lett. 2022, 33, 3709–3712. [Google Scholar] [CrossRef]
- Ramya, S.; Viruthagiri, G.; Gobi, R.; Shanmugam, N.; Kannadasan, N. Synthesis and characterization of Ni2+ ions incorporated CuO nanoparticles and its application in antibacterial activity. J. Mater. Sci. Mater. Electron. 2016, 27, 2701–2711. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serova, A.; Doan, H.; Danilovic, N.; Capuanob, C.B.; Sakamotoc, T.; Kishic, H.; Yamaguchic, S.; Mukerjeed, S.; Atanassova, P. Application of X-ray photoelectron spectroscopy to studies of electrodes in fuel cells and electrolyzers. J. Electron. Spectrosc. Relat. Phenom. 2019, 231, 127–139. [Google Scholar] [CrossRef]
- Domashevskaya, E.P.; Ryabtsev, S.V.; Terekhov, V.A.; Lenshin, A.S.; Chernyshov, F.M.; Kazakov, A.T.; Sidashov, A.V. XPS study of the oxidation of nanosize Ni/Si(100) films. J. Struct. Chem. 2011, 52, S115–S122. [Google Scholar] [CrossRef]
- Salunkhe, P.; Muhammed Ali, A.V.; Kekuda, D. Structural, spectroscopic and electrical properties of DC magnetron sputtered NiO thin films and an insight into different defect states. Appl. Phys. A 2021, 127, 390. [Google Scholar] [CrossRef]
- Park, S.H.; Cho, Y.H.; Choi, M.; Cho, H.; Kang, J.S.; Uma, J.H.; Choi, J.W.; Choe, H.; Sung, Y.E. Nickel-nitride-coated nickel foam as a counter electrode for dye-sensitized solar cells. Surf. Coat. Technol. 2014, 259, 560–569. [Google Scholar] [CrossRef]
- Bocîrnea, A.E.; Tanase, L.C.; Costescua, R.M.; Apostola, N.G.; Teodorcu, C.M. Band bending at magnetic Ni/Ge (001) interface investigated by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2017, 424, 269–274. [Google Scholar] [CrossRef]
- Suram, S.K.; Newhouse, P.F.; Gregoire, J.M. High Throughput Light Absorber Discovery, Part 1: An Algorithm for Automated Tauc Analysis. ACS Comb. Sci. 2016, 18, 673–681. [Google Scholar] [CrossRef]
- Nadjia, L.; Abdelkader, E.; Naceur, B.; Ahmed, B. CeO2 nanoscale particles: Synthesis, characterization and photocatalytic activity under UVA light irradiation. J. Rare Earth 2018, 36, 575–587. [Google Scholar] [CrossRef]
- Lin, K.S.; Chowdhury, S. Synthesis, Characterization, and Application of 1-D Cerium Oxide Nanomaterials: A Review. Int. J. Mol. Sci. 2010, 11, 3226–3251. [Google Scholar] [CrossRef]
- Patel, M.; Ray, A. Magnetron sputtered Cu doped SnS thin films for improved photoelectrochemical and heterojunction solar cells. RSC Adv. 2014, 74, 39343–39350. [Google Scholar] [CrossRef]
- Jamal, M.S.; Shahahmadi, S.A.; Chelvanathan, P.; Alharbi, H.F.; Karim, M.R.; Dar, M.A.; Luqman, M.; Alharthi, N.H.; Al-Harthi, Y.S.; Aminuzzaman, M.; et al. Effects of growth temperature on the photovoltaic properties of RF sputtered undoped NiO thin films. Results Phys. 2019, 14, 102360. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Natarajan, K.; Saraf, M.; Mobin, S.M. Visible-Light-Induced Water Splitting Based on a Novel α-Fe2O3/CdS Heterostructure. ACS Omega 2017, 7, 3447–3456. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, I.; Ranjith Kumar, E.; Suriyanarayanan, N.; Mohanraj, K.; Srinivas Ch Mehar, M.V.K. Effect of Al doping concentration on the structural, optical, morphological, and electrical properties of V2O5 nanostructures. New J. Chem. 2018, 42, 4278–4288. [Google Scholar] [CrossRef]




| Sample | Sputtering Deposition Parameters | |||
|---|---|---|---|---|
| ϕN2 (sccm) | Power (W) | T (K) | Cu/Ni Ratio in Target | |
| NiCuOxNy N4 | 4.00 | 250 | 433 | 6.14 |
| NiCuOxNy N8 | 8.00 | |||
| NiCuOxNy N12 | 12.00 | |||
| NiCuOxNy N18 | 18.00 | |||
| Coating Sample | ϕN2 (sccm) | BE N1s (eV) | Chemical Formula |
|---|---|---|---|
| NiCuOxNy N4 | 4.00 | 400 | CuNi0.2O0.93N0.09 |
| 397 | CuNi0.2O0.93N0.12 | ||
| NiCuOxNy N8 | 8.00 | 400 | CuNi0.4O0.8N0.8 |
| 397 | CuNi0.4O0.9N0.7 | ||
| NiCuOxNy N12 | 12.0 | 400 | CuNi0.2O0.5N0.03 |
| 397 | CuNi0.2O0.4N0.03 | ||
| NiCuOxNy N18 | 18.0 | 400 | CuNi0.2O0.7N0.04 |
| 397 | CuNi0.2O0.7N0.04 |
| Coating Sample | Average Roughness Parameters (Mean ± SD) | Thickness | |
|---|---|---|---|
| RMS (nm) | Gran Size (nm) | μm | |
| NiCuOxNy N4 | 38.20 ± 0.86 | 131.00 ± 9 | 2.43 |
| NiCuOxNy N8 | 64.70 ± 0.95 | 252.00 ± 14 | 2.50 |
| NiCuOxNy N12 | 1.93 ± 0.07 | 9.00 ± 1 | 4.30 |
| NiCuOxNy N18 | 3.55 ± 0.03 | 14.00 ± 1 | 2.86 |
| Coating Sample | Element wt (%) | |||
|---|---|---|---|---|
| Ni | Cu | O2 | N2 | |
| NiCuOxNy N4 | 6.27 | 90.3 | 3.45 | ND |
| NiCuOxNy N8 | 5.42 | 92.3 | 2.26 | ND |
| NiCuOxNy N12 | 5.35 | 85.3 | 4.13 | 5.18 |
| NiCuOxNy N18 | 6.51 | 81.0 | 3.93 | 8.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-Méndez, K.L.; Alfonso, J.E.; Bethencourt, M.; Cifredo, G.; Cubillos, G.I. Characterization of NiCuOxNy Coatings Obtained via RF Sputtering: Structure, Morphology, and Optical Properties. Materials 2024, 17, 3264. https://doi.org/10.3390/ma17133264
Velásquez-Méndez KL, Alfonso JE, Bethencourt M, Cifredo G, Cubillos GI. Characterization of NiCuOxNy Coatings Obtained via RF Sputtering: Structure, Morphology, and Optical Properties. Materials. 2024; 17(13):3264. https://doi.org/10.3390/ma17133264
Chicago/Turabian StyleVelásquez-Méndez, Karen Lizzette, José Edgar Alfonso, Manuel Bethencourt, Gustavo Cifredo, and Gloria Ivonne Cubillos. 2024. "Characterization of NiCuOxNy Coatings Obtained via RF Sputtering: Structure, Morphology, and Optical Properties" Materials 17, no. 13: 3264. https://doi.org/10.3390/ma17133264
APA StyleVelásquez-Méndez, K. L., Alfonso, J. E., Bethencourt, M., Cifredo, G., & Cubillos, G. I. (2024). Characterization of NiCuOxNy Coatings Obtained via RF Sputtering: Structure, Morphology, and Optical Properties. Materials, 17(13), 3264. https://doi.org/10.3390/ma17133264






