Abstract
Aiming at the problems of the large storage, complex composition, low comprehensive utilization rate, and high environmental impact of coal gangue, this paper carried out experimental research on the preparation of iron oxide red from high-iron gangue by calcination activation, acid leaching, extraction, and the hydrothermal synthesis of coal gangue. The experimental results show that when the calcination temperature of coal gangue is 500 °C, the calcination time is 1.5 h, the optimal concentration of iron removal is 6 mol/L, the acid leaching temperature is 80 °C, the acid leaching time is 1 h, and the liquid——solid mass ratio is 4:1; the iron dissolution rate can reach 87.64%. A solvent extraction method (TBP-SK–hydrochloric acid system) was used to extract the leachate, and a solution with iron content up to 99.21% was obtained. By controlling the optimum hydrothermal conditions (pH = 9, temperature 170 °C, reaction time 5 h), high-purity iron oxide red product can be prepared; the yield is 80.07%. The red iron oxide was characterized by XRD, SEM-EDS, particle-size analysis, and ICP-OES. The results show that the red iron oxide peak has a cubic microstructure, an average particle size of 167.16 μm, and a purity of 99.16%. The quality of the prepared iron oxide red product meets the requirement of 98.5% of the “YHT4 Iron oxide Standard for ferrite”. It can be used as a raw material to produce high-performance soft magnetic ferrite. In summary, this experimental study on the preparation of iron oxide red from coal gangue is of great significance for the comprehensive utilization of coal gangue to realize the sustainable development of the environment and economy.
1. Introduction
Coal gangue is industrial waste generated during the mining and washing processes of coal, accounting for about 10–20% of coal production. Globally, coal gangue production is significant [1]. The large amount of coal gangue seriously harms the ecological environment and human health during transportation, stacking, and processing [2]. The annual output and growth rate of coal gangue in China from 2019 to 2023 are shown in Figure 1.The resource utilization of coal gangue in power generation [3], gas production [4], cement preparation [5], construction materials [6], and refractory materials [7] can reduce ecological environmental damage and increase industrial added value. However, it cannot achieve the high-value utilization of coal gangue. Therefore, exploring new pathways for preparing high-performance materials from coal gangue and improving its utilization efficiency is imperative [8].
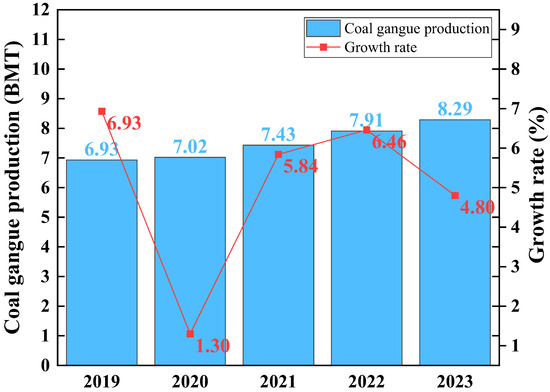
Figure 1.
China’s annual coal gangue production and growth rate from 2019 to 2023.
Coal gangue contains a large amount of Si, Al, and Fe elements, mainly in the form of minerals such as kaolinite, quartz, and hematite, making it a potential mineral resource [9]. According to the aluminum–silicon ratio (Al2O3:SiO2) in coal gangue, it can be divided into three categories: aluminum–silicon ratio ≥0.5, aluminum–silicon ratio between 0.3 and 0.5, and aluminum–silicon ratio ≤0.3. Regarding coal gangue with an aluminum–silicon ratio ≥0.5, it has a high aluminum content, relatively low silicon content, and characteristics such as a small particle size and good plasticity, making it widely used in the preparation of zeolite molecular sieves. Wang et al. [10] used coal gangue as a raw material and employed an impregnation–calcination method to prepare coal gangue molecular sieve Cu/Fe-X catalysts, which were then used for removing Rhodamine B from aqueous solutions. Under optimal conditions, the catalytic degradation rate reached 99.9%, with a high TOC removal rate of 98.5%. Zheng et al. [11] utilized coal gangue as a silicon and aluminum source to successfully synthesize ZSM-5 molecular sieves. The sample exhibited a microporous specific surface area of 169.6329 m2/g, an average pore size of 0.6285 nm, and a pore volume of 0.0988 cm3/g. For coal gangue with an aluminum–silicon ratio between 0.3 and 0.5, the moderate aluminum and silicon content allows for the extraction of Al and Si elements for the production of various products, such as Al2O3 [12], AlCl3 [13], Na2SiO3, and SiO2 [14,15,16]. Han et al. [17] activated coal gangue using alkaline supercritical water, revealing extraction rates of Al3+ and Si4+ as high as 78.9% and 69.2%, respectively, after activation. Al2O3 and SiO2 were prepared using an acid-base combination method, with purities of 99.3% and 96.2%, respectively. Wang et al. [18] used a process of hematite roasting–alkaline leaching–Bayer process to extract Al2O3 and SiO2 from high-alumina coal gangue. At an alkaline leaching temperature of 110 °C and a Bayer digestion temperature of 260 °C, they obtained 96% SiO2 and 97% Al2O3, respectively. For coal gangue with an aluminum–silicon ratio ≤0.3, the silicon content is much higher than the aluminum content. With a large particle size and poor plasticity, it can be used to produce cement, ceramic tiles, and split bricks [19].
Additionally, coal gangue can be classified into low-iron coal gangue (0.1–1.0 wt.%), medium-iron coal gangue (1.0–8.0 wt.%), high-iron coal gangue (8.0–18.0 wt.%), and ultra-high-iron coal gangue (>18.0 wt.%) based on its iron content. High-iron and ultra-high-iron coal gangue have a higher iron content, which can be utilized for the production of Fe2O3. Iron elements in coal gangue primarily exist in the form of siderite and hematite, with relatively low grades [20]. Conventional physical beneficiation techniques encounter challenges in separating and enriching iron minerals, resulting in low economic efficiency. Currently, iron extraction mainly relies on acid leaching, a process involving three stages: acid leaching, filtration, and purification [21]. Zheng et al. [22] employed a response surface experimental design to optimize the preparation process of iron oxide red pigment from coal gangue aluminum–iron separation liquid. The results revealed that under conditions of a Na2CO3 concentration of 107 g/L, reaction temperature of 43 °C, and Fe2+ concentration of 0.26 mol/L, the purity of the obtained iron oxide red product could reach 96.09%. Kong et al. [23] conducted research on extracting iron ions from coal gangue. When coal gangue was roasted at 675 °C for 1 h and leached with 6 mol/L hydrochloric acid at 93 °C for 4 h, the leaching rate of iron ions exceeded 90%. However, in the process of preparing iron oxide red in the aforementioned studies, iron ions were precipitated by adjusting the pH value, resulting in the precipitation of other metal elements (such as Al and Ca) from the leach solution. This method faces challenges in controlling the pH value and can lead to the precipitation of certain impurities, thereby affecting the purity of the iron oxide red. Solvent extraction, characterized by its high selectivity, good economic efficiency, and feasibility for continuous operation, has been proven to be highly effective in metal recovery from various solutions [24]. Zhang et al. [25] investigated the selective extraction of Fe3+ from high-acidity chloride leach solution using a mixed solvent extraction agent composed of tributyl phosphate (TBP) and methyl isobutyl ketone (MIBK). They found that TBP and MIBK exhibited a synergistic effect in extracting iron ions, achieving a Fe3+ extraction rate of 91.0% under the conditions of a 70% TBP volume fraction, 2 min of contact time, and 25 °C temperature. Duan et al. [26] added P2O4 to the traditional TBP-FeCl3–kerosene system to selectively extract lithium from high magnesium/lithium ratio brine, thereby solving the problem of Fe3+ loss and improving the stability of the system. The single-stage extraction rate of Li+ was 52.71%. Using the solvent extraction method (TBP-SK-HCl system), Fe ions in the leaching solution are extracted and back-extracted multiple times to obtain a high-purity FeCl3 solution. The main advantages of this process are as follows: (1) a short extraction time and high extraction efficiency; (2) the low cost of TBP and sulfonated kerosene (SK), recyclable with high selectivity [27]; and (3) no introduction of alkali or other precipitants throughout the entire process, greatly enhancing the purity of the ferric oxide red.
To achieve the comprehensive utilization of coal gangue, this study utilizes high-iron coal gangue as the raw material to conduct experimental research on the preparation of high-purity ferric oxide red through the processes of coal gangue calcination activation, acid leaching iron removal, extraction, and hydrothermal synthesis. The experiment investigates four process parameter conditions (calcination activation, acid leaching iron removal, extraction purification, and hydrothermal synthesis), to prepare high-purity ferric oxide red products. The properties of ferric oxide red are analyzed using characterization methods such as XRD, SEM-EDS, particle-size analysis, and ICP-OES.
2. Materials and Methods
2.1. Materials
The raw materials for the experiment were sourced from a coal-mining enterprise in Liupanshui, China. The elemental composition of the coal gangue was determined using a Japanese Rigaku Supermini 200 wavelength-dispersive X-ray fluorescence spectrometer (XRF, Rigaku Company, Tokyo, Japan), as shown in Table 1. The chemical composition of the coal gangue is quite complex, containing a large amount of Si, Fe, Al, Ca, and other elements. Specifically, the SiO2 content is 35.5%, Fe2O3 content is 25.1%, and Al2O3 content is 14.7%, indicating it as a typical high-iron–low-aluminum coal gangue. To further determine the Fe content in the coal gangue sample, an Agilent 5800 inductively coupled plasma emission spectrometer (ICP-OES, Agilent Technologies Inc, Santa Clara, CA, USA) was used for measurements, revealing an Fe element content of 12.93% in the coal gangue.

Table 1.
Chemical composition of coal gangue. Unit: %.
To ascertain the mineral composition and structural morphology of the coal gangue, a Japanese Rigaku Ultima IV X-ray diffractometer (XRD, Rigaku Company, Tokyo, Japan) and a German ZEISS Sigma 300 scanning electron microscope (FE-SEM, Carl Zeiss AG, Oberkochen, Germany) were used to conduct XRD, SEM, and SEM-EDS characterization analyses of the coal gangue samples. The XRD, SEM, and SEM-EDS spectra of the coal gangue samples are shown in Figure 2, Figure 3, Figure 4 and Figure 5, respectively. The XRD results indicate that the main mineral components of the coal gangue are quartz (SiO2) and kaolinite (Al4(Si4O10)(OH)6). The diffraction peaks of the quartz phase are sharp and clear, suggesting high crystallinity. Additionally, the coal gangue contains small amounts of clinoferrosilite (FeSiO3), siderite (FeCO3), and brookite (TiO2). The SEM results showed that the size distribution of coal gangue particles is uneven and shows an irregular morphology. The SEM-EDS point and area scan analyses of the coal gangue raw material are shown in Figure 4 and Figure 5, respectively. From Figure 4, it is evident that the coal gangue particles are formed by stacking lamellar crystals, exhibiting a typical layered structure. The Fe element is uniformly distributed within the coal gangue particles. The coal gangue is primarily composed of elements such as Si, Fe, Al, Ca, Mg, S, K, among others, which corresponds well with the analysis results in Table 1. From Figure 5, it can be observed that the combination and dispersion of O, Fe, Al, and Si are relatively uniform, consistent with the phase analysis results in Figure 2. The coal gangue is mainly composed of phases such as SiO2, Al4(Si4O10)(OH)6, and FeSiO3.
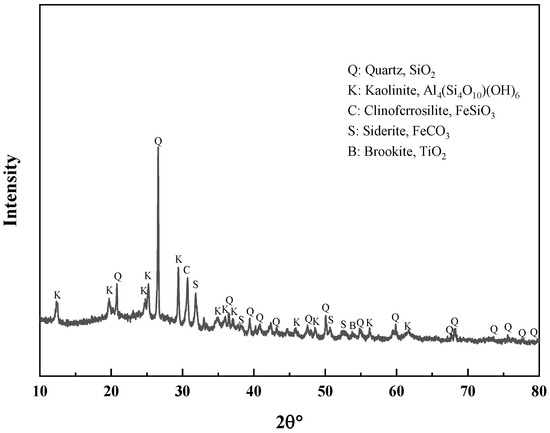
Figure 2.
XRD pattern of sample.
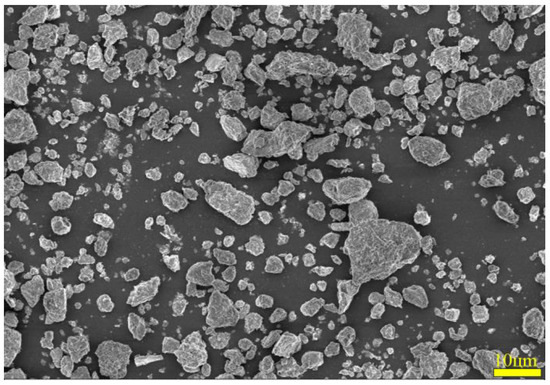
Figure 3.
SEM pattern of sample.
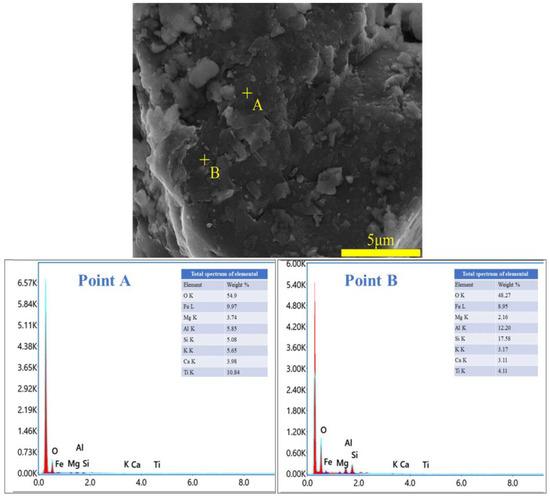
Figure 4.
SEM image and EDS point analysis of sample.
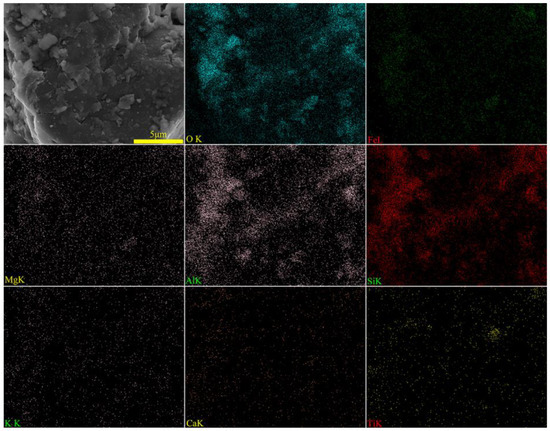
Figure 5.
SEM image and EDS mapping analysis of sample.
2.2. Methods
2.2.1. Acid Leaching Experiments
The coal gangue is subjected to drying, crushing, and grinding treatments. A certain mass of coal gangue is placed in a muffle furnace and roasted at different temperatures (300 °C, 400 °C, 500 °C, 600 °C, 700 °C) and for different roasting times (0.5 h, 1.0 h, 1.5 h, 2.0 h, 2.5 h), then allowed to cool naturally before retrieval. Using deionized water to dilute the concentrated hydrochloric acid, we prepare hydrochloric acid solutions of different molar concentrations (1 mol/L, 3 mol/L, 4 mol/L, 6 mol/L, 8 mol/L). A total of 10 g of pre-roasted coal gangue samples is taken and placed in conical flasks with hydrochloric acid solutions of different molar concentrations. The heating, stirring, and leaching is performed under different reaction temperature (50 °C, 60 °C, 70 °C, 80 °C, 90 °C), reaction time (0.5 h, 1.0 h, 1.5 h, 2.0 h, 2.5 h), and liquid——solid mass ratio (2:1, 3:1, 4:1, 5:1, 6:1) conditions. After leaching, the mixture is filtered to obtain the leachate and filter residue. The Fe content in the leachate is determined by the ortho-phenanthroline colorimetric method using a TU-1901 UV–visible spectrophotometer (UV–Vis, Beijing Purkinje GENERAL Instrument Co., Ltd., Beijing, China), while the mass fraction of Fe in the coal gangue raw material is determined by ICP-OES. The leaching rate of Fe M% is calculated by the following formula:
where c (mg/L) represents the Fe concentration determined by UV–Vis spectrophotometer; n indicates the multiple of the dilution of the leachate; V (L) indicates the total volume of the leachate; m (g) indicates the mass of coal gangue; and ω (%) indicates the mass fraction of Fe in the coal gangue.
2.2.2. Extraction Experiments of Leachates
According to the optimal technological parameters, the coal gangue is calcined and leached, and multiple leaching solutions are collected and heated to concentrate them. The specific process is as follows: Five groups of leaching solutions are collected and put into beakers, heated to boiling on an electric stove, and concentrated to 500 mL. Then, the concentrated solution is used for subsequent extraction experiments. In the extraction process, Fe2+ in the percolate will be oxidized to Fe3+. After cooling, 12 mol/L HCl is added to increase the acid concentration of the solution to above 4 mol/L. Use tributyl phosphate as the extractant and sulfonated kerosene as the diluent. Extract Fe from the leachate under the conditions of 40% TBP concentration, O/A = 2:1 (organic phase/aqueous phase), an extraction temperature of 20 °C, and extraction time of 10 min. Strip the Fe using deionized water as the stripping agent under the conditions of O/A = 1:1, a stripping temperature of 20 °C, and stripping time of 10 min. Repeat the extraction–stripping process 2–3 times (TBP extract can be reused during the process). Finally, obtain the coal gangue-stripping solution containing high-purity Fe ions.
Use the Agilent 5800 inductively coupled plasma emission spectrometer to detect the ion concentrations of Fe, Al, Ca, and Mg in the leachate after extraction and in the back-extraction solution. The calculation formula is as follows:
C0: the concentration of the test solution elements, in mg/L; f: the dilution factor; V0: the volume after the sample is digested and made up to volume, in mL; m0: the mass of the coal gangue raw material taken, in grams; C1: the elemental concentration of the sample digestion solution, in mg/L; Cx: the concentration of the measured element, in mg/L.
2.2.3. Preparation of Iron Oxide Red
The extracted solution is transferred to a beaker, stirred at 60 °C, and then 2 mol/L NaOH solution is added to adjust the pH. The mixture is stirred for 1 h at 80 °C, then transferred to a hydrothermal reaction vessel. Under different hydrothermal temperature, time, and pH conditions, hydrothermal reactions are carried out. Finally, the solution is centrifuged, dried, and ground to obtain Fe2O3. The process flowchart for preparing ferric oxide from coal gangue is shown in Figure 6.
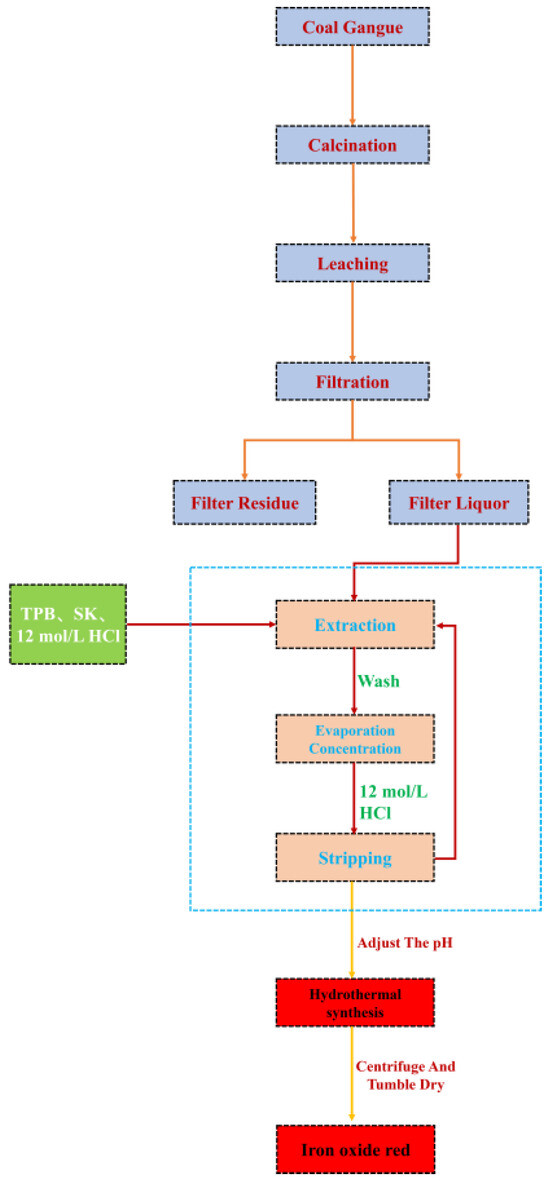
Figure 6.
Process flowchart of iron red prepared by iron liquid extraction by coal gangue acid leaching.
3. Results and Discussion
3.1. Experimental Study on Coal Gangue Roasting Process
The experiment was conducted under the conditions of an acid leaching temperature of 70 °C, leaching time of 2 h, liquid——solid mass ratio of 4:1, and acid concentration of 6 mol/L. The effects of different roasting temperatures and roasting times on the iron removal rate in coal gangue were investigated. The results are shown in Figure 7. Figure 7a illustrates that as the roasting temperature increases, the iron removal rate gradually increases. When the roasting temperature reaches 500 °C, the highest iron removal rate is 81.52%. At this temperature, the coal gangue exhibits the highest reaction activity and maximum specific surface area, which is conducive to the leaching of Fe2O3. However, a further increase in temperature leads to a decrease in the specific surface area of the coal gangue and a reduction in activity, resulting in a decrease in the iron removal rate [28]. Therefore, the optimal roasting temperature is determined to be 500 °C. Figure 7b shows that with an increase in roasting time, the iron removal rate gradually increases. After a roasting time exceeding 1.5 h, the change in the iron removal rate is insignificant. Therefore, the optimal roasting time is determined to be 1.5 h.
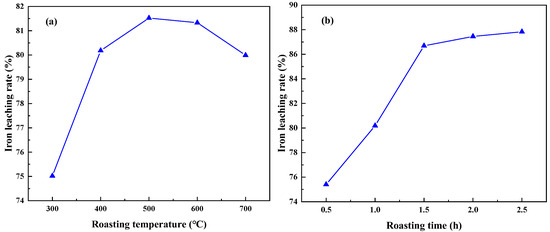
Figure 7.
Effect of different roasting temperature (a) and roasting time (b) on iron leaching rate (acid leaching temperature of 70 °C, leaching time of 2 h, liquid—solid mass ratio of 4:1, and acid concentration of 6 mol/L).
Under optimized roasting activation conditions, a comparative study was conducted to investigate the effects of unroasted and roasted activation on the iron removal rate of coal gangue. The results, as shown in Figure 8, indicate that the iron removal rate of unroasted coal gangue is 66.98%. Unroasted coal gangue exhibits a higher lattice structure and lower reaction activity. The iron removal rate of roasted activated coal gangue is 87.64%. Under high-temperature roasting, particles undergo intense thermal motion, causing ions such as Fe, Mg, and Ca to reselect filling positions and leading to the formation of short-chain, disordered, and unstable glassy and amorphous substances from silicon–oxygen tetrahedra and aluminum–oxygen octahedra. This greatly enhances the reaction activity [29]. Comparative experimental results demonstrate that the roasting activation of coal gangue contributes to an increased leaching rate of Fe.
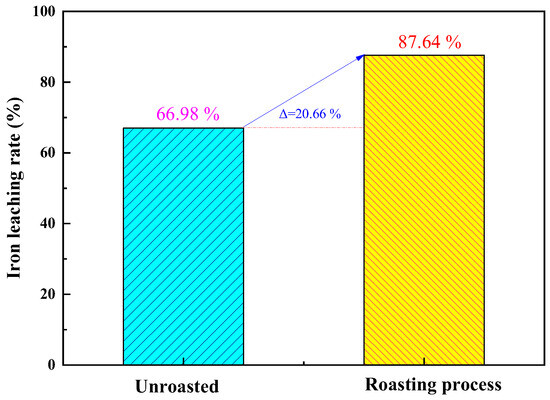
Figure 8.
Comparison of iron leaching rate between unroasted and roasting-activated treatments of coal gangue.
3.2. Experimental Study on Coal Gangue Roasting–Acid Leaching Iron Removal Process
3.2.1. The Influence of Different Leaching Conditions on Iron Leaching Rate
The experiment investigated the effects of different leaching temperatures, leaching times, liquid——solid mass ratios, and acid concentrations on the iron removal rate in coal gangue. The results are depicted in Figure 9. Figure 9a demonstrates that with an increase in leaching temperature, the iron removal rate gradually rises. Higher temperatures enhance the material activity within the coal gangue, promoting acid leaching reactions [30]. However, when the leaching temperature is increased from 80 °C to 90 °C, the iron removal rate remains almost unchanged. This mainly causes the evaporation of the solution water due to the high temperature, which accelerates the evaporation of the solution and increases the concentration of hydrochloric acid, which leads to a reduction in the diffusion of HCl and the surface of the gangue particles, thus hindering the leaching of Fe ions. Consequently, the optimal leaching temperature is determined to be 80 °C. The results in Figure 9b indicate that as the acid leaching time increases, the iron removal rate gradually rises. After 1.0 h of acid leaching, the iron removal rate increases slowly. Therefore, the optimal leaching time is determined to be 1.0 h. The results in Figure 9c demonstrate that at lower liquid——solid mass ratios, the iron removal rate increases with an increase in the liquid——solid mass ratio. This is because at lower liquid——solid mass ratios, the hydrochloric acid content is lower, resulting in an incomplete reaction between the iron oxide in the coal gangue and the hydrochloric acid, leading to a lower leaching rate of Fe ions. As the liquid——solid mass ratio gradually increases, the hydrochloric acid content increases, leading to a more complete reaction and promoting the leaching of Fe ions. When the liquid——solid mass ratio reaches 4:1, the reaction between the coal gangue and hydrochloric acid is sufficient, and the iron removal rate mainly depends on the temperature. As the liquid——solid mass ratio continues to increase, the iron removal rate remains relatively unchanged. Therefore, the optimal liquid——solid mass ratio is chosen to be 4:1. Figure 9d illustrates that as the hydrochloric acid concentration increases, the iron removal rate continues to rise. This is because, with the increase in hydrochloric acid concentration, the probability of H+ contacting iron bearing minerals increases, thereby enhancing the reaction rate. However, when the acid concentration exceeds 6 mol/L, the iron removal rate does not increase significantly. Moreover, an excessively high hydrochloric acid concentration exacerbates side reactions, which is detrimental to the subsequent wastewater treatment and deep processing of the coal gangue. Therefore, the optimal acid concentration was selected as 6 mol/L. In summary, the optimal conditions for each factor are a leaching temperature of 80 °C, an acid leaching time of 1 h, a liquid——solid mass ratio of 4:1, and an acid concentration of 6 mol/L, resulting in an iron removal rate of 87.64%.
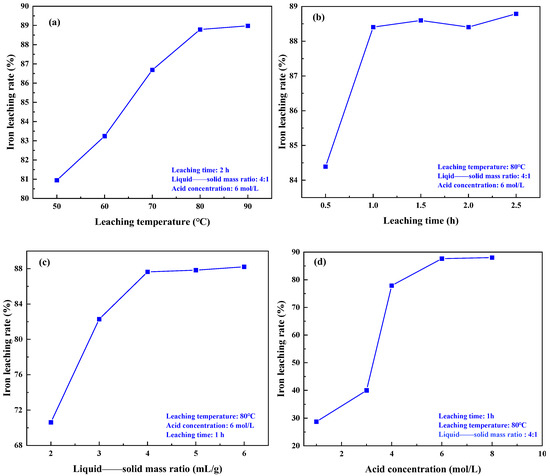
Figure 9.
Effect of different leaching conditions on iron leaching rate: (a) temperature, (b) time, (c) liquid——solid mass ratio, (d) acid concentration.
3.2.2. Coal Gangue Leaching Residue XRF, XRD, SEM-EDS, and Particle-Size Distribution Characterization Analysis
Table 2 shows the chemical composition of the coal gangue leaching slag, and by comparing it with Table 1, it is found that the Fe2O3 components in the coal gangue after acid leaching are basically removed and separated, and the contents of CaO and MgO are also greatly reduced, while the SiO2 and most of the Al2O3 components are retained, and the total mass of the two components reaches 78.2%. It can be seen that the grade of the acid leaching of the coal gangue has been effectively improved through heat activation.

Table 2.
Chemical composition of coal gangue leaching residue. Unit: %.
The XRD spectra of the gangue feedstock, calcined samples, and leaching residue are shown in Figure 10, which shows that the diffraction peak of siderite disappears after calcination, and siderite is oxidized at high temperatures to form Fe2O3 [31]. The XRD pattern of the leaching residue lacks the diffraction peaks associated with siderite and orthopyroxene phases, indicating their reaction with hydrochloric acid to produce Fe ions and SiO2. Additionally, the diffraction pattern of the leaching residue is similar to that before acid leaching, but with different intensities. This is primarily because quartz and brookite do not react with hydrochloric acid. The dissolution of Fe and Al ions after acid leaching increases the relative content of the remaining substances, resulting in an enhanced diffraction intensity. The SEM micrographs of the calcined sample and the leaching residue of the coal gangue are shown in Figure 11. From the images, it can be observed that the calcined sample contains many large particles, ranging from 15 to 20 μm or even larger. However, after acid leaching, the particle size decreases, with most particles having diameters smaller than 5 μm [32]. This reduction in particle size is attributed to the leaching of Fe and Al ions, which leads to the loosening of residual particles, resulting in the presence of numerous fragments. The SEM-EDS spectrum of the calcined sample is shown in Figure 12. Compared to the raw material, the surface of the calcined sample particles becomes porous and loose. This is mainly attributed to the reaction of certain components of the coal gangue (such as kaolinite and siderite) at high temperatures, leading to the formation of numerous pores within the coal gangue, thus resulting in a porous and loose structure. This structure provides favorable conditions for subsequent acid leaching processes, enhancing the leaching of Fe ions. The iron (Fe) content in the calcined sample is 9.81 wt%, with the elemental content ranking as follows: O (47.36 wt%) > Si (17.35 wt%) > Fe (9.81 wt%) > Al (9.67 wt%) > Ca (8.37 wt%) > Mg (4.11 wt%). The SEM-EDS spectrum of the leaching residue is shown in Figure 13. Compared to the calcined sample, the surface of the leaching residue exhibits obvious signs of erosion, indicating that hydrochloric acid has dissolved some elements from the coal gangue and entered the leachate. Additionally, the iron (Fe) content in the leaching residue is only 1.91 wt%, with the elemental content ranking as follows: O (45.16 wt%) > Si (31.35 wt%) > Al (12.14 wt%) > Fe (1.91 wt%) > Mg (1.57 wt%) > Ca (0.32 wt%). This suggests that most of the iron in the coal gangue has been leached out through the acid leaching process, which is consistent with the XRD analysis results. The particle size analysis of the calcined sample and leaching residue was conducted using a Winner2005A laser particle size analyzer (LPS, JINAN WINNER PARTICLE INSTRUMENT STOCK CO., LTD., Jinan, China), with the results shown in Figure 14. The particle-size distribution of the calcined samples is shown in Figure 14a and ranges from 0.01 to 780 μm, with an average particle size of 61.64 μm. The cumulative volume of particles smaller than 100 μm is 84.19%, and for particles smaller than 500 μm, the cumulative volume is 96.30%. The volume-specific surface area is 0.557 m2/cm3. The particle-size distribution of the leaching residue is shown in Figure 14b and ranges from 0.01 to 780 μm, with an average particle size of 3.045 μm. The cumulative volume of particles smaller than 100 μm is 59.90%, and for particles smaller than 500 μm, the cumulative volume is 91.92%. The volume-specific surface area is 41.33 m2/cm3. After acid leaching, the overall particle size of the coal gangue decreased significantly, which was consistent with the characterization results of the SEM. This phenomenon may be caused by the destruction and dissolution of the activated components in the coal gangue.
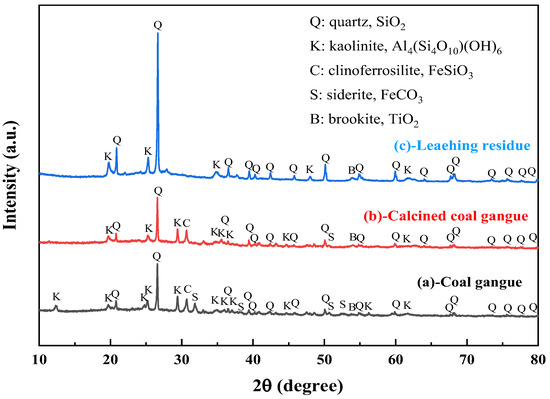
Figure 10.
XRD patterns of coal gangue raw materials (a), calcined coal gangue (b) and leaching residue (c).
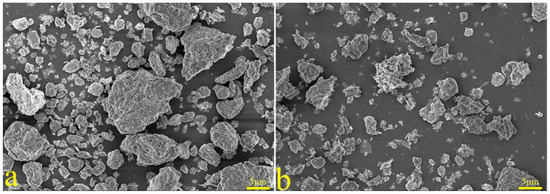
Figure 11.
SEM patterns of calcined coal gangue (a) and leaching residue (b).
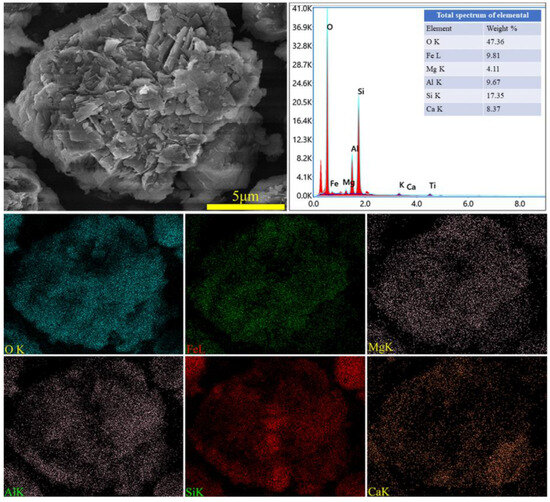
Figure 12.
SEM image and EDS mapping analysis of calcined coal gangue.
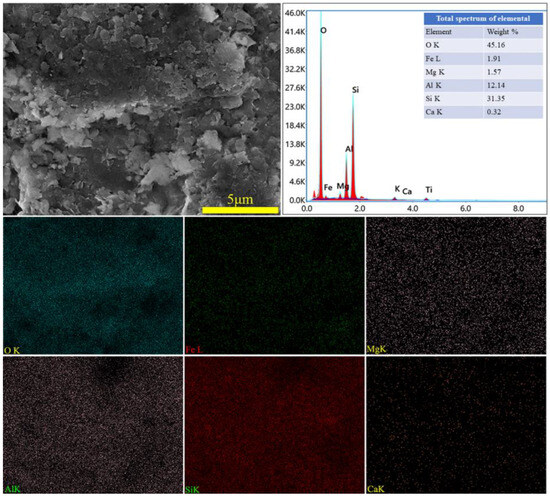
Figure 13.
SEM image and EDS mapping analysis of leaching residue.
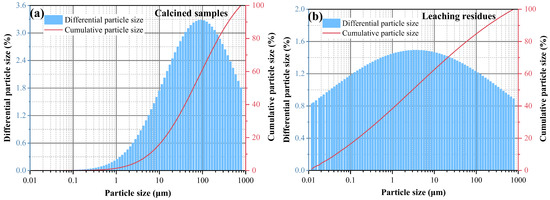
Figure 14.
Particle-size distribution diagrams of coal gangue (a) calcined samples and (b) leaching residues.
3.3. Experimental Study on Preparation of Iron Oxide Red from Iron-Bearing Leachate of Coal Gangue
The leachate obtained by filtering the acid-leached samples of coal gangue after roasting activation was subjected to an extraction experiment, and the ion contents in the leachate and the extraction solution were calculated, as shown in Table 3. An analysis of the results presented in Table 3 shows that the iron ion content in the leachate from coal gangue was 47.33%, whereas after extraction, the iron ion content in the solution increased dramatically to 99.21%. This indicates that the extraction process effectively separates iron ions and other impurity components from the leachate. Additionally, further experiments were conducted using the high-iron content back-extraction solution to study the process of preparing iron oxide red.

Table 3.
The concentration of each ion in the leaching solution and the back-extraction solution. Unit: mg/L.
3.3.1. The Influence of pH on the Properties of Iron Oxide Red
The effect of different pH values (pH = 3, 5, 9, 11) on the crystallinity of iron oxide red was studied at 150 °C and 5 h. The XRD spectra of iron oxide red under different pH conditions are shown in Figure 15. It can be observed from the graph that when the pH is 11, the diffraction peaks of Fe2O3 are sharper compared to other conditions, indicating that the crystallinity of Fe2O3 is highest at pH 11. In the hydrothermal synthesis of Fe2O3, pH is an important influencing factor. A higher pH leads to a faster hydrolysis of Fe3+ ions and a higher degree of hydrolysis, resulting in the higher purity of the prepared Fe2O3.
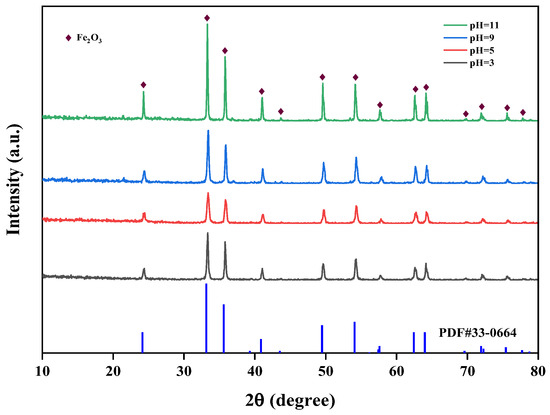
Figure 15.
Effect of different hydrothermal pH on iron-leaching rate.
3.3.2. The Impact of Temperature on the Properties of Iron Oxide Red
The effects of different temperatures (110 °C, 140 °C, 170 °C, 200 °C, 230 °C) on the crystallinity of iron oxide red were studied at pH 11 and a time of 5 h, and the XRD spectra of Fe2O3 at different hydrothermal temperatures are shown in Figure 16. From the figure, it is evident that at hydrothermal temperatures of 110 °C and 140 °C, the Fe(OH)3 phase appears. This is primarily due to the low temperature, where some of the Fe(OH)3 phase in the solution has not yet converted to the Fe2O3 phase. However, at 230 °C, the Fe(OH)3 phase reappears, possibly because the high temperature leads to rapid hydrolysis, causing Fe(OH)3 to enter the lattice without sufficient conversion. When the hydrothermal temperature is 170 °C and 200 °C, no Fe(OH)3 phase is observed, indicating that these temperature conditions are suitable for the transformation from the Fe(OH)3 phase to the Fe2O3 phase. From the perspective of energy conservation, 170 °C was chosen as the optimal hydrothermal temperature for subsequent experiments.
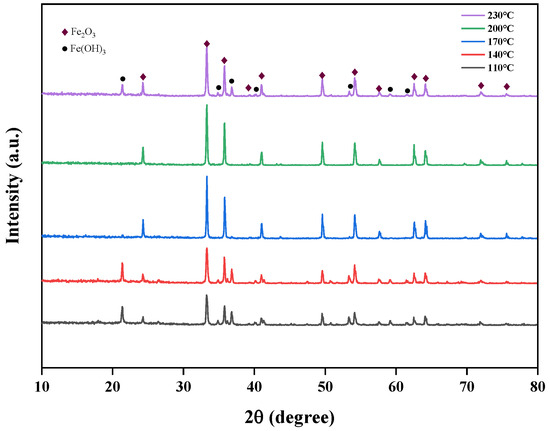
Figure 16.
Effect of different hydrothermal temperatures on iron-leaching rate.
3.3.3. The Effect of Time on the Properties of Iron Oxide Red and the Preparation of Iron Oxide Red under Optimized Process Conditions
At an optimal temperature of 170 °C and pH value of 11, the effect of different durations (1 h, 3 h, 5 h, 7 h, 9 h) on the crystallinity of iron oxide red was investigated. The XRD spectra of iron oxide red under different hydrothermal durations are shown in Figure 17. It can be observed that with an increasing reaction time, both the Fe2O3 and Fe(OH)3 phases are present in the samples. However, as the reaction time extends, the intensity of the Fe(OH)3 phase noticeably decreases and its relative content gradually diminishes, indicating that a prolonged reaction time is conducive to purity enhancement. Nevertheless, when the hydrothermal time exceeds 5 h, the peak intensity of the Fe2O3 phase weakens, and concurrently, the appearance of the Fe(OH)3 phase begins. Therefore, the optimal hydrothermal duration for Fe2O3 phase generation is 5 h.
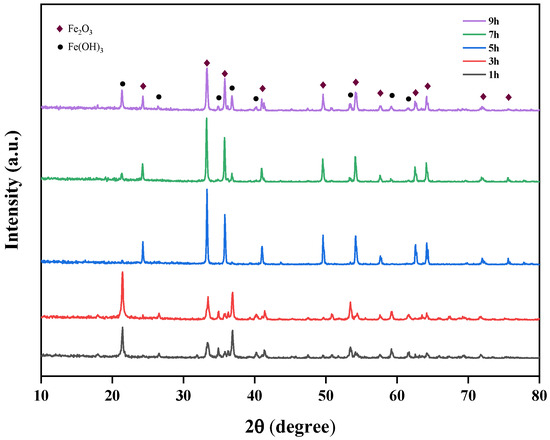
Figure 17.
Effect of different hydrothermal times on iron-leaching rate.
The preparation experiment for iron oxide red was carried out under the conditions of optimal reaction pH = 11, reaction temperature 170 °C, and a reaction time of 5 h, and the yield of iron oxide red was 80.07%. The XRD spectrum of the prepared iron oxide is shown in Figure 18. All the diffraction peaks of the sample correspond to the standard card of Fe2O3 and exhibit sharp peaks [33], indicating a high degree of crystallinity. Additionally, there are no other excess peaks observed in the diffraction peaks, indicating the high purity of the prepared Fe2O3. The particle-size distribution of iron oxide red is shown in Figure 19, ranging from 25 to 780 μm, with an average particle size of 167.16 μm. The cumulative volume of particles less than 200 μm is 61.95%, and that of particles less than 500 μm is 98.78%. ICP-OES was used for the elemental analysis of the iron oxide red samples. The analysis results are shown in Table 4. The purity of the iron oxide red prepared using coal gangue reached 99.16%, exceeding the purity standard (98.5%) required by the “Iron Oxide for Ferrite YHT4 Standard” (GB/T 24244-2009) [34], making it suitable as a raw material for producing high-performance soft magnetic ferrites.
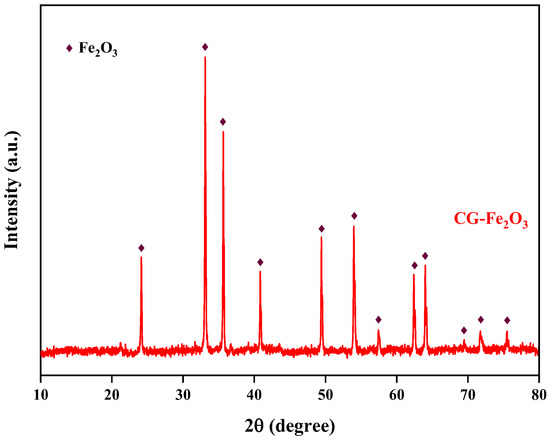
Figure 18.
XRD pattern of iron oxide red sample.
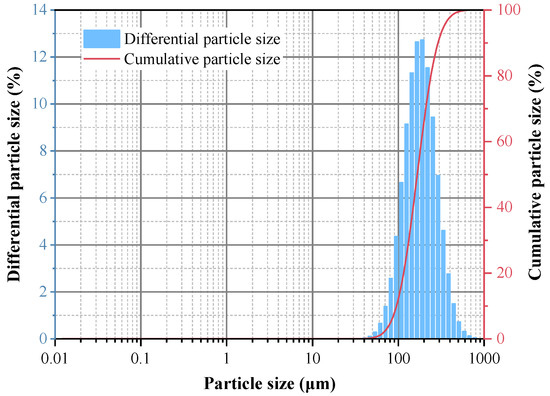
Figure 19.
Particle-size distribution of iron oxide red.

Table 4.
Analysis results of iron oxide products composition. Unit: %.
3.3.4. SEM-EDS Analysis of Iron Oxide Red
A SEM image of the iron oxide red sample is shown in Figure 20, depicting particles with a cubic structure and a certain degree of dispersion. The SEM-EDS spectrum of the iron oxide red sample is illustrated in Figure 21, indicating that the particles contain only Fe and O elements. This suggests that the prepared Fe2O3 has a high purity without any other impurities. Specifically, the Fe element accounts for 75.23% of the total sample mass, while the O element accounts for 24.77%.
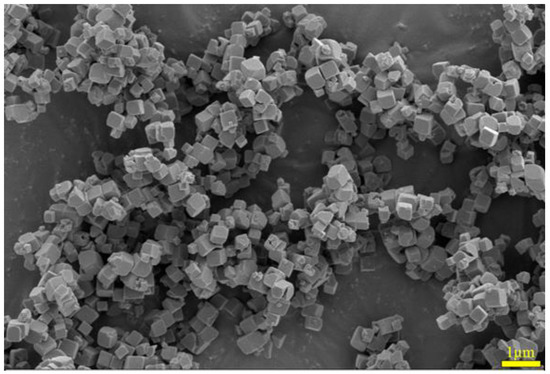
Figure 20.
SEM image of iron oxide red sample.
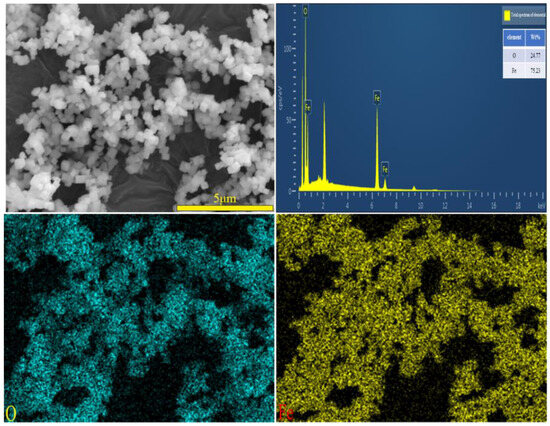
Figure 21.
SEM image and EDS mapping analysis of iron oxide red sample.
3.4. Mechanism of Coal Gangue Roasting Activation–Acidic Iron Removal-Extraction–Hydrothermal Preparation of Iron Oxide Red
The schematic diagram of the process for coal gangue roasting activation, acid leaching iron removal, extraction purification, and the hydrothermal synthesis of Fe2O3 is shown in Figure 22. During the coal gangue roasting activation stage, some kaolinite and siderite are destroyed [35], and the following reactions occur:
Al2Si2O5(OH)4→Al2O3·2SiO2 + 2H2O↑
FeCO3 + O2→Fe2O3 + CO2↑
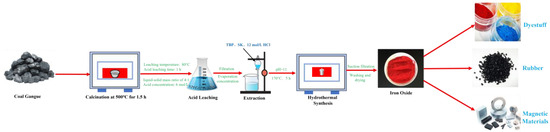
Figure 22.
A schematic diagram of the mechanism of coal gangue roasting and activation–acid iron removal–extraction–hydrothermal synthesis of iron oxide red.
During the acid leaching iron removal stage, the extraction and separation of Fe from impurity ions such as Al, Mg, and Ca take place, with the specific reactions as follows:
Al2O3·2SiO2 + 6HCl→2AlCl3 + 2SiO2 + 3H2O
Fe2O3 + 6HCl→2FeCl3 + 3H2O
MgO + 2HCl→MgCl2 + H2O
CaO + 2HCl→CaCl2 + H2O
During the extraction purification–hydrothermal synthesis of Fe2O3 stage, Fe ions are enriched and Al, Mg, and Ca ions are removed. Subsequently, NaOH solution is added, and the mixture is placed into a hydrothermal reactor for synthesis. The reaction equation is as follows:
FeCl3 + 3NaOH→Fe(OH)3↓+3NaCl
Fe(OH)3→Fe2O3 + 3H2O
In summary, the coal gangue undergoes the stages of roasting activation pretreatment, acidic iron removal, extraction purification, and hydrothermal synthesis, leading to the production of high-purity iron oxide red products. This further facilitates the utilization of coal gangue as a solid waste resource.
4. Conclusions
- (1)
- The roasting temperature of coal gangue was controlled at 500 °C for 1.5 h, and the iron-leaching rate was 87.64% under the conditions of an optimal iron removal acid concentration of 6 mol/L, an acid leaching temperature of 80 °C, an acid leaching time of 1 h, and a liquid——solid mass ratio of 4:1, which was increased by 20.66% compared with unroasted activation.
- (2)
- A solvent extraction method (TBP-SK-hydrochloric acid system) was employed to extract the leachate, resulting in an iron ion content as high as 99.21% in the extracted solution. Additionally, the extraction agent is economically viable, environmentally friendly, and can be reused.
- (3)
- Iron oxide red products with a yield of 80.07% and a purity of 99.16% were prepared by keeping to an optimal hydrothermal pH = 9, temperature 170 °C and time of 5 h. The characterization analysis using XRD, SEM-EDS, particle-size analysis, and ICP-OES showed that the synthesized iron oxide red showed sharp peaks, the microstructure was similar to the cubic morphology, the average particle size was 167.16 μm, and the purity was 99.16%, which exceeded the purity standard (98.5%) specified in the “Iron Oxide for Ferrite-YHT4” standard (GB/T 24244-2009). This material can serve as a raw material for producing high-performance soft ferrite materials.
- (4)
- The preparation of high-purity iron oxide red through the processes of coal gangue roasting activation pretreatment, acid leaching for iron removal, solvent extraction purification, and hydrothermal synthesis demonstrates significant importance in promoting the high-value utilization of coal gangue resources and alleviating environmental pressure.
Author Contributions
X.Y.: Writing—Original Draft, Conceptualization, and Methodology, Investigation, Validation; A.M.: Investigation, Validation, Funding Acquisition, Supervision, Writing—Review and Editing; M.C.: Supervision; J.D.: Methodology, Investigation; X.Z.: Visualization, Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Discipline Team of Liupanshui Normal University (LPSSY2023XKTD07), the Guizhou Provincial First-class course (GZSylkc202222), the Guizhou Provincial First-class professional (GZSylzy202103), the Key Cultivation Disciplines of Liupanshui Normal University (LPSSYZDXK202001), and the Jointly Trains Postgraduate Special Scientific Research Project of Liupanshui Normal University (LPSSYLPY202329).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to technical or time limitations.
Acknowledgments
The authors acknowledge financial support to carry out this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Lv, B.; Zhao, Z.; Deng, X.; Fang, C.; Dong, B.; Zhang, B. Sustainable and clean utilization of coal gangue: Activation and preparation of silicon fertilizer. J. Mater. Cycles Waste Manag. 2022, 24, 1579–1590. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhu, M.; Cheng, F.; Zhang, D. Interactions of coal gangue and pine sawdust during combustion of their blends studied using differential thermogravimetric analysis. Bioresour. Technol. 2016, 214, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, X.; Qiao, X. Thermogravimetric experimental study on the co-combustion of coal gangue and polypropylene. Thermochim. Acta 2024, 732, 179660. [Google Scholar] [CrossRef]
- Wu, J.; Wong, H.S.; Zhang, H.; Yin, Q.; Jing, H.; Ma, D. Improvement of cemented rockfill by premixing low-alkalinity activator and fly ash for recycling gangue and partially replacing cement. Cem. Concr. Compos. 2024, 145, 105345. [Google Scholar] [CrossRef]
- Guan, H.; Yu, J.; Umuhuza Kibugenza, A.S.; Sun, Q. Preparation of coal gangue ceramsite high-strength concrete and investigation of its physico-mechanical properties. Sci. Rep. 2022, 12, 16369. [Google Scholar] [CrossRef]
- Chen, F.; Sang, S.; Ma, Y.; Li, Y. Preparation of microporous mullite refractories with high strength based on low-grade bauxite and coal gangue. Appl. Ceram. Technol. 2024, 21, 1648–1657. [Google Scholar] [CrossRef]
- Xiao, J.; Li, F.; Zhong, Q.; Bao, H.; Wang, B.; Huang, J.; Zhang, Y. Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 2015, 155, 118–124. [Google Scholar] [CrossRef]
- Zhou, M.; Dou, Y.; Zhang, Y.; Zhang, Y.; Zhang, B. Effects of the variety and content of coal gangue coarse aggregate on the mechanical properties of concrete. Constr. Build. Mater. 2019, 220, 386–395. [Google Scholar] [CrossRef]
- Lixiang, W.; Xuelu, L.; Rui, Z.; Xiaoli, W.; Miaosen, Z. Synthesis of Zeolite-Based Cu/Fe–X from Coal Gangue for Fenton-Like Catalytic Degradation of Rhodamine B. J. Inorg. Organomet. Polym. Mater. 2024, 34, 722–734. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, J.; Ma, Z.; Weng, X.; Cheng, L.; Tang, G. Preparation of a High-Silicon ZSM-5 Molecular Sieve Using Only Coal Gangue as the Silicon and Aluminum Sources. Materials 2023, 16, 4338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, H.; Zhang, H.; Zhang, P.; Bei, R. Aluminum extraction from activated coal gangue with carbide slag. J. Anal. Appl. Pyrolysis 2022, 163, 105504. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, H.; Yang, X.; Cheng, F. AlCl3·6H2O recovery from the acid leaching liquor of coal gangue by using concentrated hydrochloric inpouring. Sep. Purif. Technol. 2015, 151, 177–183. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, H.; Zhang, H.; Zhang, P. Preparation of monodisperse silica powder from acid residue after extracting alumina from coal gangue. Chem. Eng. 2023, 18, e2845. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, P.; Sun, H. Ambient-Dried Silica Aerogel Powders Derived from Coal Gangue by Using One-Pot Method. Materials 2022, 15, 1454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhao, C.; Hu, X.; Tong, Z.; Yun, J.; Wei, N.; Wang, K.; Liu, C.; Zou, Y.; Chen, Z. Utilization of coal gangue for preparing high-silica porous materials with excellent ad/desorption performance on VOCs. Chem. Technol. Biotechnol. 2022, 97, 3498–3510. [Google Scholar] [CrossRef]
- Han, L.; Ren, W.; Wang, B.; He, X.; Ma, L.; Huo, Q.; Wang, J.; Bao, W.; Chang, L. Extraction of SiO2 and Al2O3 from coal gangue activated by supercritical water. Fuel 2019, 253, 1184–1192. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Liu, C.; Shen, L. Comprehensive Extraction of Silica and Alumina from High-Alumina Coal Gangue (HACG): Hematite Involved Roasting − Alkaline Leaching − Bayer Digestion Process. J. Sustain. Metall. 2021, 7, 1686–1698. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, H.; Lv, S.; Wang, X.; Li, S.; Zhang, J. Preparation of Sintered Brick with Aluminum Dross and Optimization of Process Parameters. Coating 2021, 11, 1039. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Guo, X.; Yan, K.; Guo, Y. Valuable element recovery of Fe and Al by reduction roasting of red mud and coal gangue. Chin. J. Environ. Eng. 2021, 15, 3306–3315. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, Z.; Song, S.; Jiang, R. Acid Leaching Extraction Mechanism of Aluminum and Iron Ions from Coal Gangue Based on CaF2 Assistance and Process Optimization. Materials 2023, 16, 499. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, J.; Xia, J.; Luo, Z.; Geng, R. Preparation Iron Oxide Red Based on Acid Soluble Substances of Coal Gangue Using Response Surface Methodology. Chin. J. Process Eng. 2017, 17, 327–332. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, Z.; Jiang, R.; Song, S.; Feng, S.; Lian, M. Extraction of Aluminum and Iron Ions from Coal Gangue by Acid Leaching and Kinetic Analyses. Materials 2022, 12, 215. [Google Scholar] [CrossRef]
- Niu, Y.; Peng, X.; Li, J.; Zhang, Y.; Song, F.; Shi, D.; Li, L. Recovery of Li2CO3 and FePO4 from spent LiFePO4 by coupling technics of isomorphic substitution leaching and solvent extraction. Chin. J. Chem. Eng. 2023, 54, 306–315. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Wei, G.; Zhao, H.; Wang, L.; Qi, T.; Meng, F.; Meng, L. Extraction of iron (III) from chloride leaching liquor with high acidity using tri-n-butyl phosphate and synergistic extraction combined with methyl isobutyl ketone. Sep. Purif. Technol. 2015, 150, 132–138. [Google Scholar] [CrossRef]
- Duan, W.; Wang, Y.; Li, R.; Ren, Z.; Zhou, Z. Selective extraction of lithium from high magnesium/lithium ratio brines with a TBP–FeCl3–P2O4–kerosene extraction system. Sep. Purif. Technol. 2024, 328, 125066. [Google Scholar] [CrossRef]
- Yu, X.; Fan, X.; Guo, Y.; Deng, T. Recovery of lithium from underground brine by multistage centrifugal extraction using tri-isobutyl phosphate. Sep. Purif. Technol. 2019, 211, 790–798. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal treatment of kaolin: The effect of mineralogy on the pozzolanic activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Seetharaman, S.; Liu, L.; Wang, X.; Zhang, Z. Effects of chemistry and mineral on structural evolution and chemical reactivity of coal gangue during calcination: Towards efficient utilization. Mater. Struct. 2015, 48, 2779–2793. [Google Scholar] [CrossRef]
- Yang, P.; Li, D.; Zhang, W.; Wang, N.; Yang, Z.; Wang, D.; Ma, T. Flocculation-dewatering behavior of waste activated sludge particles under chemical conditioning with inorganic polymer flocculant: Effects of typical sludge properties. Chemosphere 2019, 218, 930–940. [Google Scholar] [CrossRef]
- Niu, X.; Guo, S.; Gao, L.; Cao, Y.; Wei, X.-X. Mercury Release during Thermal Treatment of Two Coal Gangues and Two Coal Slimes under N2 and in Air. Energy Fuels 2017, 31, 8648–8654. [Google Scholar] [CrossRef]
- Kong, D.; Jiang, R. Preparation of NaA Zeolite from High Iron and Quartz Contents Coal Gangue by Acid Leaching—Alkali Melting Activation and Hydrothermal Synthesis. Materials 2021, 11, 1198. [Google Scholar] [CrossRef]
- Almeida, T.P.; Fay, M.; Zhu, Y.; Brown, P.D. Process Map for the Hydrothermal Synthesis of α-Fe2O3 Nanorods. J. Phys. Chem. C 2009, 113, 18689–18698. [Google Scholar] [CrossRef]
- GB/T 24244—2009; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2009.
- Hu, Y.; Han, X.; Sun, Z.; Jin, P.; Li, K.; Wang, F.; Gong, J. Study on the Reactivity Activation of Coal Gangue for Efficient Utilization. Materials 2023, 16, 6321. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).