Grafting Carbon Fibers with Graphene via a One-Pot Aryl Diazonium Reaction to Refine the Interface Performance of T1100-Grade CF/BMI Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
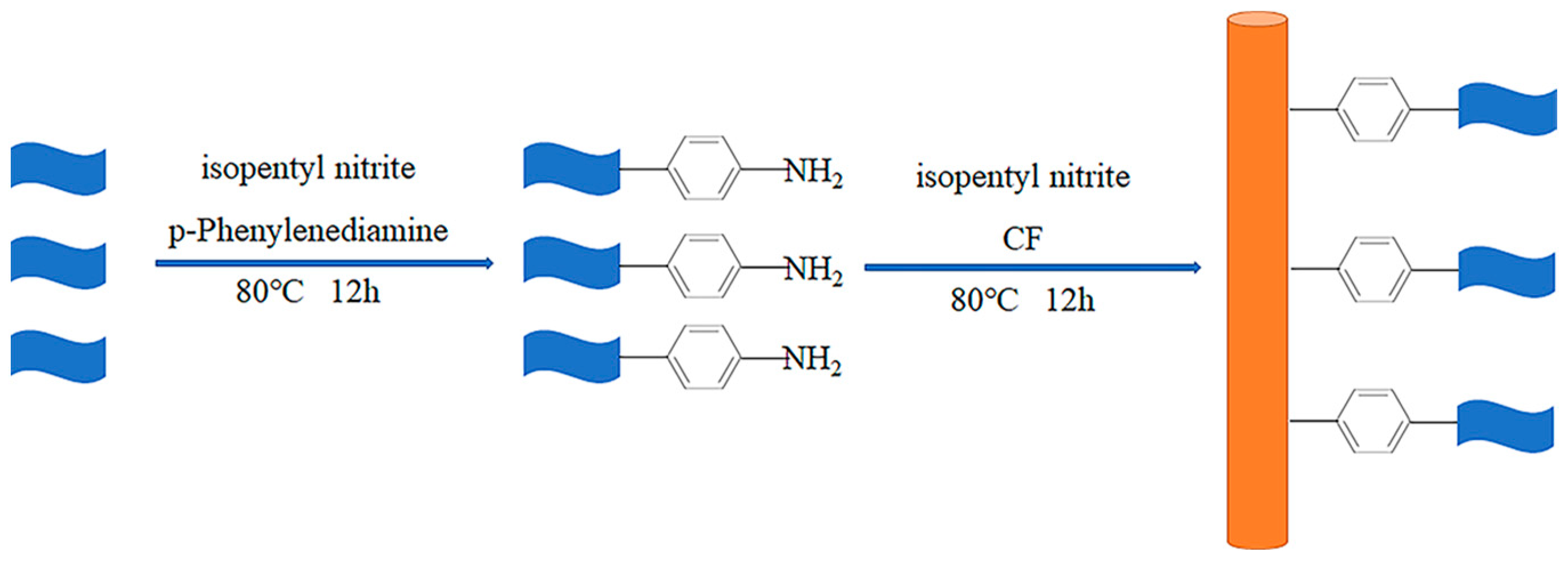
2.2. Preparation of CF-G
2.3. Characterization
3. Results and Discussion
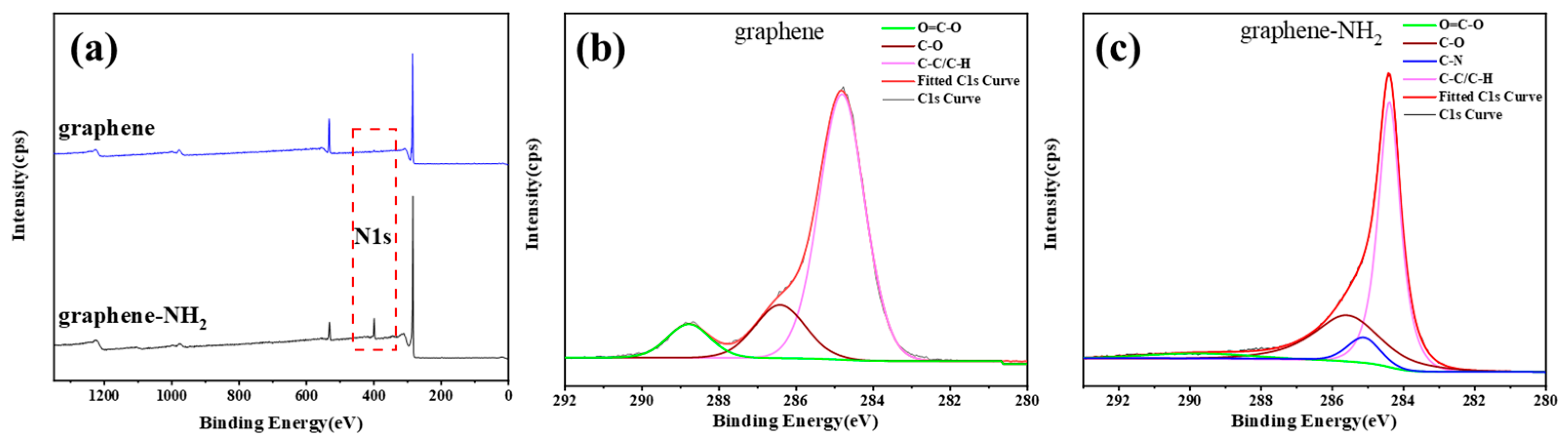
3.1. Characterization of Graphene-NH2
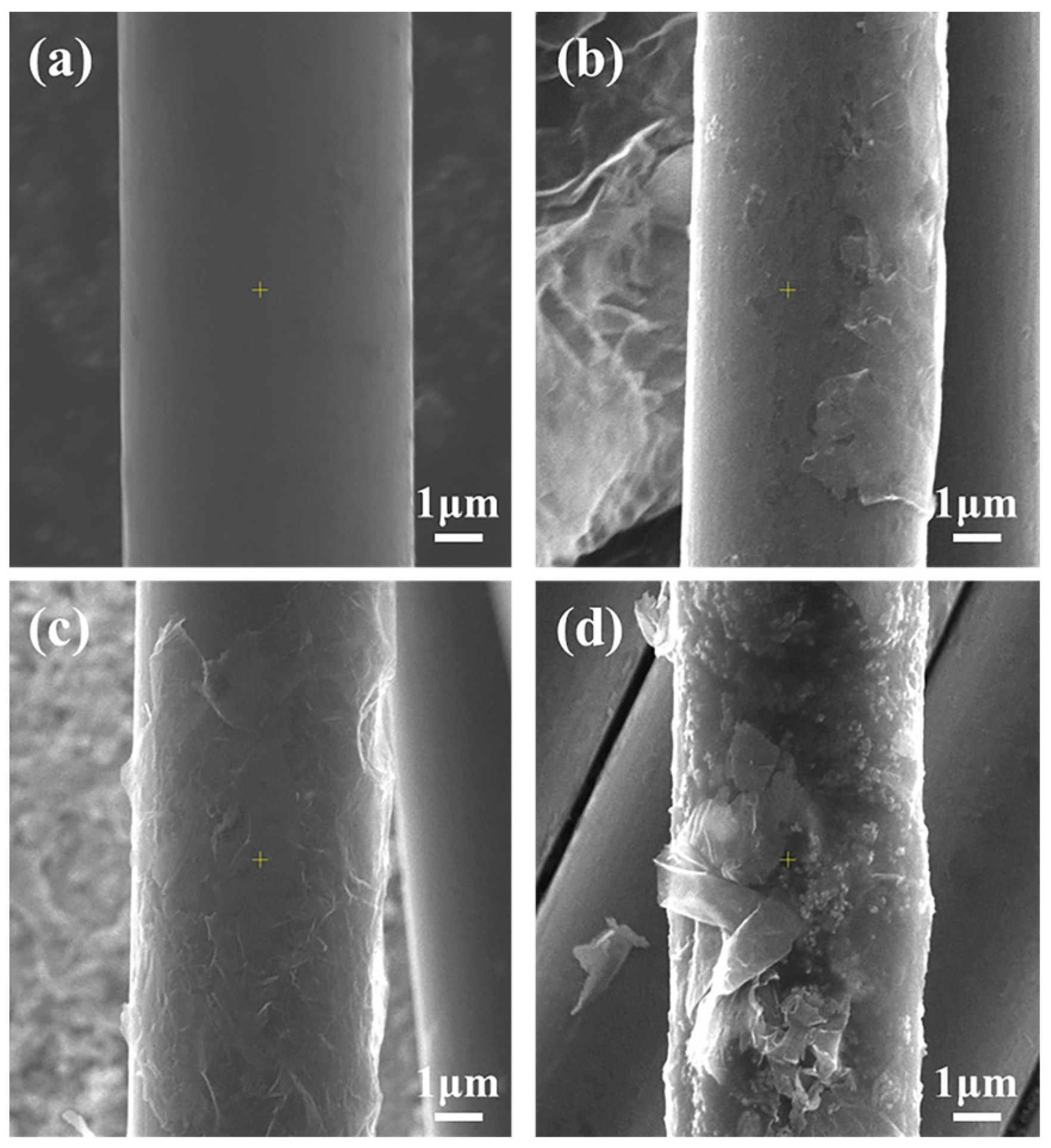
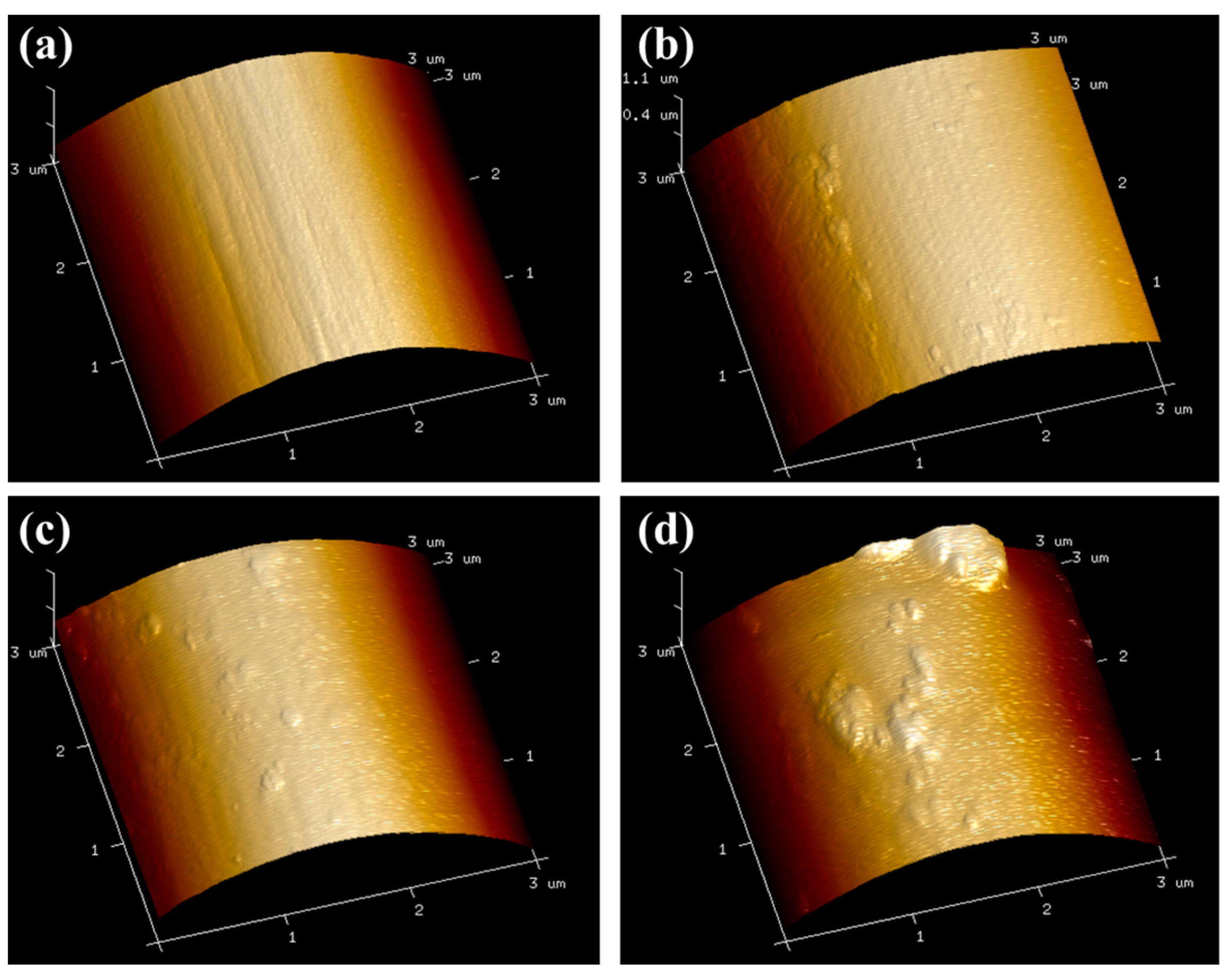
3.2. Surface Morphologies and Properties
3.3. Surface Chemical Characteristics of CF
3.4. Wettability of CF
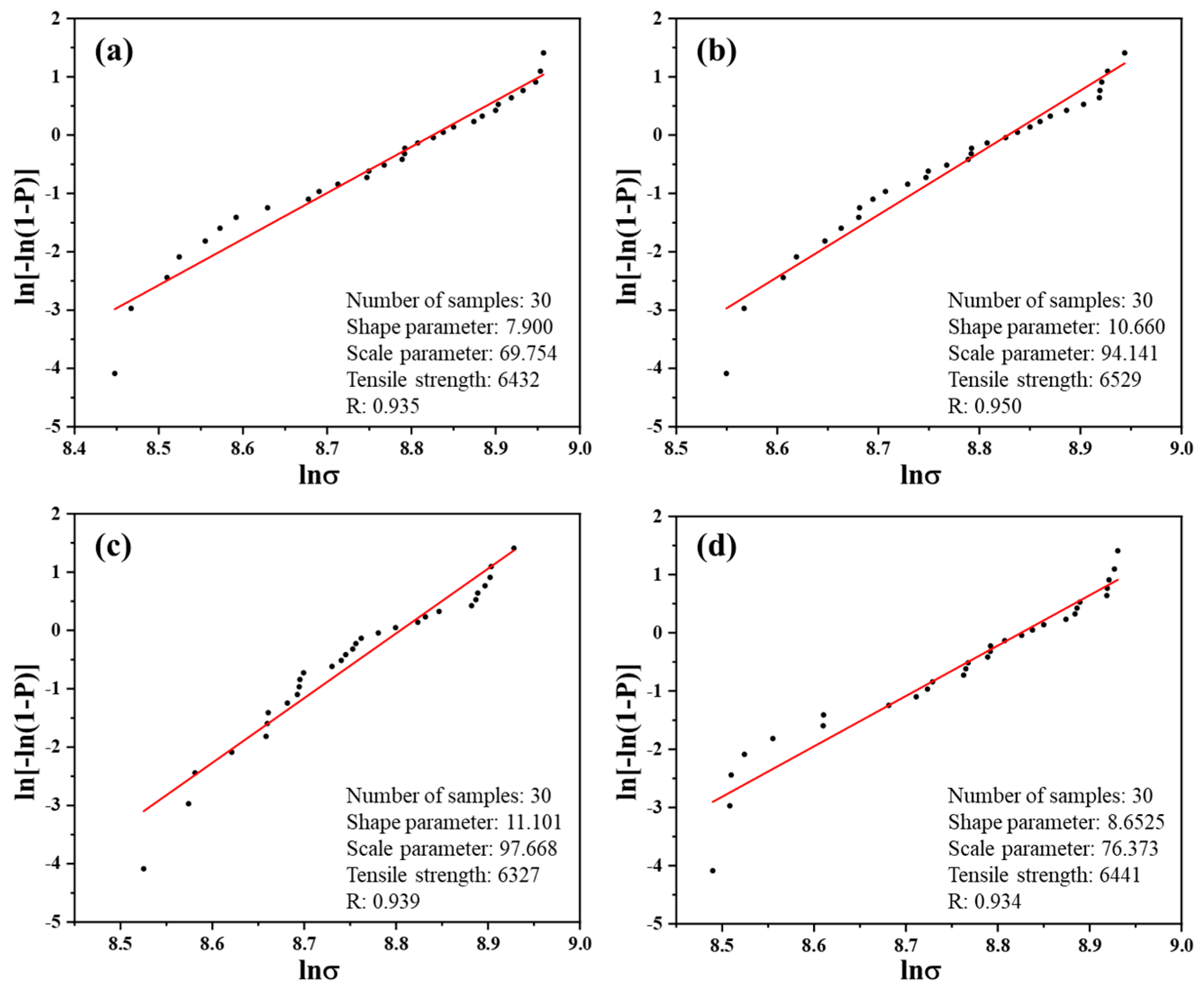
3.5. Tensile Properties of Monofilament
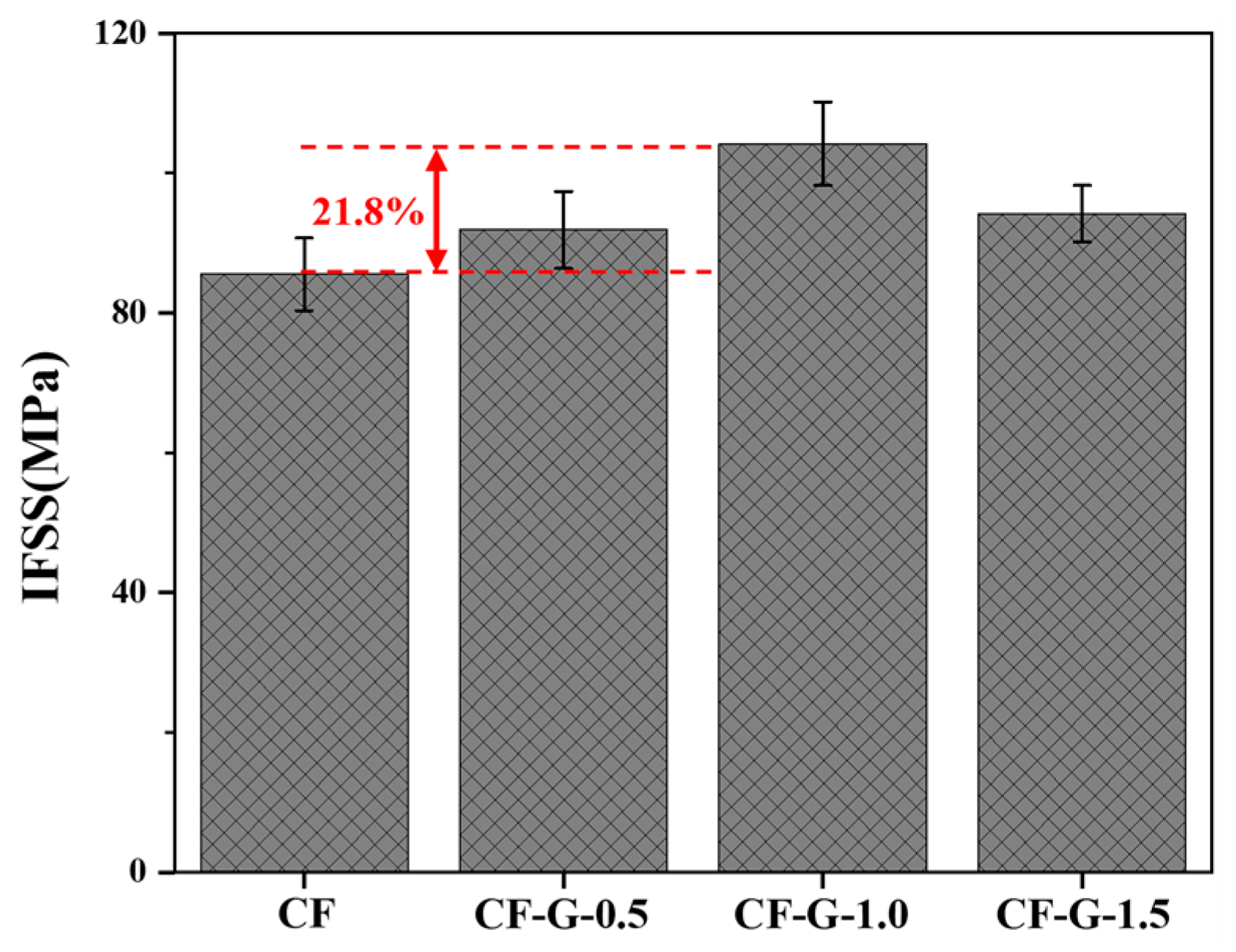
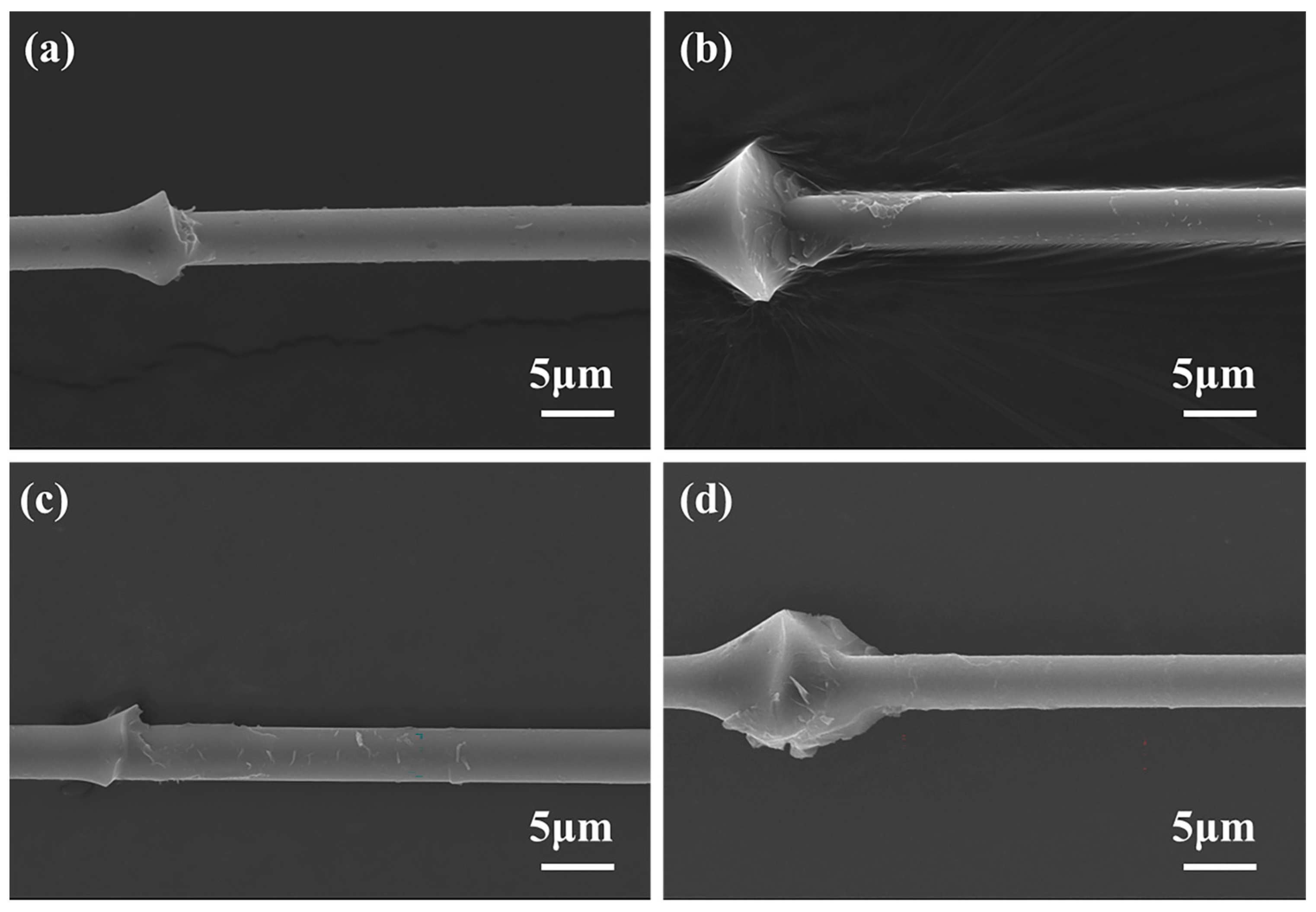
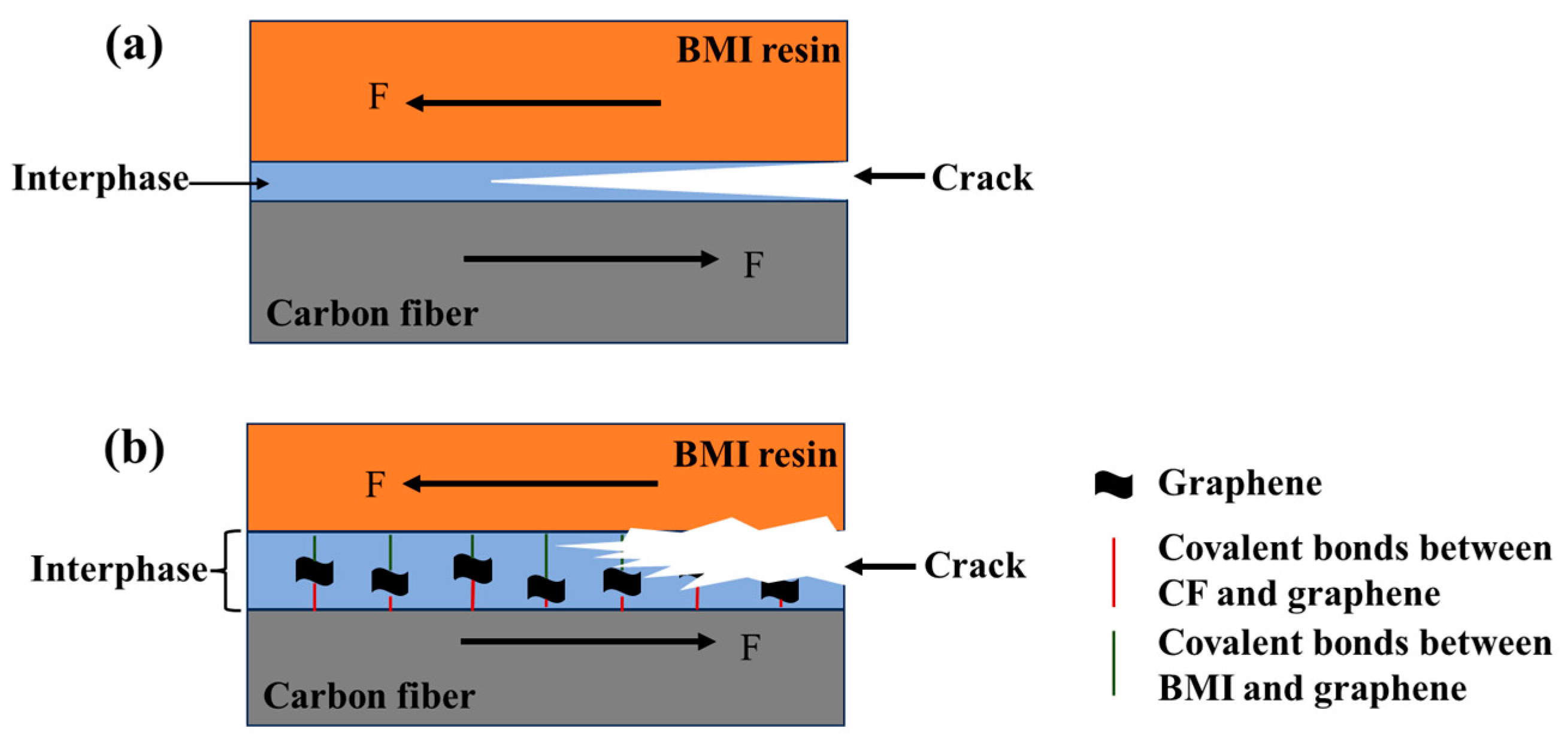
3.6. IFSS of CF/BMI Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hollaway, L.C. The evolution of and the way forward for advanced polymer composites in the civil infrastructure. Construct. Build. Mater. 2003, 17, 365–378. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Zhao, Z.; Wang, G.; Zhan, S.; Ding, J.; Liu, X.; Guo, Y.; Zhou, H.; Zhao, T. Improved toughness of phthalonitrile composites through synergistic toughening methods. Compos. Commun. 2021, 26, 100779. [Google Scholar] [CrossRef]
- Xu, J.; Gui, J.; Song, S.; Li, F.; Zhou, H. Research on the change of prepreg tow width during automatic fiber placement process. Fiber Compos. 2022, 39, 120–124. [Google Scholar]
- Liu, H.; Sun, J.; Duan, Z.; Liu, Z.; Ma, X.; Lu, W.; Sun, T.; Li, Z.; Zhang, P.; Zhong, X.; et al. Study on properties influence of carbon fiber reinforced polyimide composites via surface modification using metal mesh. Polym. Compos. 2023, 44, 971–979. [Google Scholar] [CrossRef]
- Zou, M.; Zhao, W.; Wu, H.; Zhang, H.; Xu, W.; Yang, L. Single carbon fibers with a macroscopic-thickness, 3D highly porous carbon nanotube coating. Adv. Mater. 2018, 30, 1704419. [Google Scholar] [CrossRef]
- Liu, H.; Sun, J.; Zhang, L.; Liu, Z.; Huang, C.; Sun, M.; Duan, Z.; Wang, W.; Zhong, X.; Bao, J. Influence of the Second-Phase Resin Structure on the Interfacial Shear Strength of Carbon Fiber/Epoxy Resin. Materials 2024, 17, 1323. [Google Scholar] [CrossRef]
- Song, B.; Wang, T.; Wang, L.; Liu, H.; Mai, X.; Wang, X.; Wang, N.; Huang, Y.; Ma, Y.; Lu, Y.; et al. Interfacially reinforced carbon fiber/epoxy composite laminates via in-situ synthesized graphitic carbon nitride (g-C3N4). Compos. Part B Eng. 2019, 158, 259–268. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.; Cao, W. Enhancing the interfacial properties of high-modulus carbon fiber reinforced polymer matrix conposites via electrochemical surface oxidation and grafting. Compos. Part A 2020, 130, 105719. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Y.; He, X.; Lv, H.; Hu, P.; Shang, Y.; Wang, C.; Wang, R.; Sritharan, T.; Du, S. Interfacial enhancement of carbon fiber composites by poly (amido amine) functionalization. Compos. Sci. Technol. 2013, 74, 37–42. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Jiang, C.; Dai, Y.; Meng, C.; Luo, L.; Liu, X. Aramid fiber with excellent interfacial properties suitable for resin composite in a wide polarity range. Chem. Eng. J. 2018, 347, 483–492. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Arnold, C.L.; Randall, J.D.; Stojcevski, F.; Hendlmeier, A.; Stanfield, M.K.; Pinson, J.; Gengenbach, T.R.; Alexander, R.; Soulsby, L.C.; et al. Fiber with Butterfly Wings: Creating Colored Carbon Fibers with Increased Strength, Adhesion, and Reversible Malleability. ACS Appl. Mater. Interfaces 2019, 11, 41617–41625. [Google Scholar] [CrossRef]
- Li, J.; Sun, F.F. The Effect of Nitric Acid Oxidization Treatment on the Interface of Carbon Fiber-Reinforced Thermoplastic Polystyrene Composite. Polym.-Plast. Technol. Eng. 2009, 48, 711–715. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Y.; Zhang, C.; Liu, L.; Zhang, Y.; Wang, L. Effect of γ-ray irradiation grafting on the carbon fibers and interfacial adhesion of epoxy composites. Compos. Sci. Technol. 2007, 67, 3261–3270. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, F.; Yao, Y.; Jin, Z.; Liu, X.; Huang, Y. High efficient and continuous surface modification of carbon fibers with improved tensile strength and interfacial adhesion. Appl. Surf. Sci. 2017, 412, 424–435. [Google Scholar] [CrossRef]
- Oldham, T.; Simon, K.; Ferriell, D.R.; Belcher, M.A.; Rubin, A.; Thimsen, E. Highly Uniform Activation of Carbon Fiber Reinforced Thermoplastics by Low-Temperature Plasma. ACS Appl. Polym. Mater. 2019, 1, 2638–2648. [Google Scholar] [CrossRef]
- Chen, J.; Xu, H.; Liu, C.; Mi, L.; Shen, C. The effect of double grafted interface layer on the properties of carbon fiber reinforced polyamide 66 composites. Compos. Sci. Technol. 2018, 168, 20–27. [Google Scholar] [CrossRef]
- Qin, W.; Vautard, F.; Drzal, L.T.; Yu, J. Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber–matrix interphase. Compos. Part B Eng. 2015, 69, 335–341. [Google Scholar] [CrossRef]
- He, Y.; Yang, S.; Liu, H.; Shao, Q.; Chen, Q.; Lu, C.; Jiang, Y.; Liu, C.; Guo, Z. Reinforced carbon fiber laminates with oriented carbon nanotube epoxy nanocomposites: Magnetic field assisted alignment and cryogenic temperature mechanical properties. J. Colloid Interface Sci. 2018, 517, 40–51. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, C.; Su, X.; Wang, X. Effects of emulsion sizing with nano-SiO2 on interfacial properties of carbon fibers/epoxy composites. J. Mater. Sci. 2007, 42, 6347–6352. [Google Scholar] [CrossRef]
- Dong, J.; Jia, C.; Song, Y.; He, J.; Huang, Y. Improved interfacial properties of carbon fiber-reinforced epoxy composites with Fe2O3/graphene nanosheets using a magnetic field. J. Adhes. Sci. Technol. 2017, 32, 1018–1026. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, J.; Liu, F.; Fang, L.; Wang, J.; Li, D.; Wu, J. Influence of surface properties of graphene oxide/carbon fiber hybrid fiber by oxidative treatments combined with electrophoretic deposition. Surf. Interface Anal. 2016, 48, 212–217. [Google Scholar] [CrossRef]
- Zhang, R.L.; Gao, B.; Ma, Q.H.; Zhang, J.; Cui, H.Z.; Liu, L. Directly grafting graphene oxide onto carbon fiber and the effect on the mechanical properties of carbon fiber composites. Mater. Des. 2016, 93, 364–369. [Google Scholar] [CrossRef]
- He, M.; Xu, P.; Zhang, Y.; Liu, K.; Yang, X. Phthalocyanine nanowires@GO/carbon fiber composites with enhanced interfacial properties and electromagnetic interference shielding performance. Chem. Eng. J. 2020, 388, 124255. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, X.; Xu, C.; Su, Y.; Deng, C.; Liu, F.; Wu, J. Preparation of Graphene Oxide Coatings onto Carbon Fibers by Electrophoretic Deposition for Enhancing Interfacial Strength in Carbon Fiber Composites. J. Electrochem. Soc. 2016, 163, D133–D139. [Google Scholar] [CrossRef]
- Yan, F.; Yan, T.; Wang, G.; Li, G.; Dai, S.; Ao, Y.; Duan, J.; Liu, L. A novel thermoplastic water-soluble sizing agent for the interfacial enhancement of carbon fiber/polyether ether ketone composites. Compos. Part B 2024, 272, 111205. [Google Scholar] [CrossRef]
- Beggs, K.M.; Servinis, L.; Gengenbach, T.R.; Huson, M.G.; Fox, B.L.; Henderson, L.C. A systematic study of carbon fibre surface grafting via in situ diazonium generation for improved interfacial shear strength in epoxy matrix composites. Compos. Sci. Technol. 2015, 118, 31–38. [Google Scholar] [CrossRef]
- Cougnon, C.; Nguyen, N.H.; Dabos-Seignon, S.; Mauzeroll, J.; Bélanger, D. Carbon surface derivatization by electrochemical reduction of a diazonium salt in situ produced from the nitro precursor. J. Electroanal. Chem. 2011, 661, 13–19. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, L.; Fan, L.; Wu, G.; Ma, L.; Huang, Y. Preparation and properties of carbon nanotube/carbon fiber hybrid reinforcement by a two-step aryl diazonium reaction. RSC Adv. 2015, 5, 44492–44498. [Google Scholar] [CrossRef]
- Liu, Y.T.; Wu, G.P.; Lu, C.X. Grafting of carbon nanotubes onto carbon fiber surfaces by step-wise reduction of in-situ generated diazonium salts for enhancing carbon/epoxy interfaces. Mater. Lett. 2014, 134, 75–79. [Google Scholar] [CrossRef]
- Sun, M.; Li, X.; Liu, H.; Huang, C.; Wang, K.; Zhao, Y. Effect of Electrochemical Aryl Diazonium Salt Modification on Interfacial Properties of CF/PEEK Composites. Materials 2024, 17, 2899. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Sun, M.; Liu, Z.; Liu, H.; Xiong, S.; Li, S.; Song, J.; Wang, K. Functionalized carbon fibers with MXene via electrochemistry aryl diazonium salt reaction to improve the interfacial properties of carbon fiber/epoxy composites. J. Mater. Res. Technol. 2022, 19, 3699–3712. [Google Scholar] [CrossRef]
- Wu, G.; Liu, L.; Huang, Y. Grafting of active carbon nanotubes onto carbon fiber using one-pot aryl diazonium reaction for superior interfacial strength in silicone resin composites. Compos. Commun. 2019, 13, 103–106. [Google Scholar] [CrossRef]
- ASTM D 4018; Standard Test MethodS for Properties of Continuous Filament Carbon and Graphite Fiber Tows. C28.07. ASTM: West Conshohocken, PA, USA, 2023.
- GB/T 31290; Standard Test Method for Single-Filament Tensile Strength for Carbon Fiber. Toggle Navigation: Beijing, China, 2014.
- Miller, B.; Muri, P.; Rebenfeld, L. A microbond method for determination of the shear strength of a fiber/resin interface. Compos. Sci. Technol. 1987, 28, 17–32. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.; Wang, K.; Zhong, X.; Bao, J. Preparation and properties of aminated MXene cross-linked polyimide aerogels. High Perform. Polym. 2023, 35, 923–935. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Randall, J.D.; Stojcevski, F.; Sarlin, E.; Henderson, L.C. Examining interfacial interactions in a range of polymers using poly(ethylene oxide) functionalized carbon fibers. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106053. [Google Scholar] [CrossRef]


| Properties | Tensile Strength (MPa) | Elastic Modulus (GPa) | Elongation (%) |
|---|---|---|---|
| Value | 6663 | 342 | 1.93 |
| Samples | CF | CF-G-0.5 | CF-G-1.0 | CF-G-1.5 |
|---|---|---|---|---|
| Rq/nm | 5.7 ± 1.4 | 10.2 ± 1.4 | 16.7 ± 2.3 | 19.7 ± 1.5 |
| Ra/nm | 4.4 ± 1.0 | 7.28 ± 0.8 | 13.7 ± 1.8 | 15.7 ± 2.5 |
| Sample | Relative Content of Atom (%) | O/C | N/C | ||
|---|---|---|---|---|---|
| C1s | O1s | N1s | |||
| CF | 85.82 | 11.73 | 2.45 | 0.137 | 0.028 |
| CF-G-0.5 | 84.39 | 12.82 | 2.79 | 0.152 | 0.033 |
| CF-G-1.0 | 83.36 | 13.59 | 3.05 | 0.163 | 0.037 |
| CF-G-1.5 | 83.46 | 12.87 | 3.67 | 0.154 | 0.044 |
| Samples | -C-C-/-C-H | -C-O- | -O-C=O | -C-N- | Active Functional Groups Ratio (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| B.E. (eV) | P.C. (%) | B.E. (eV) | P.C. (%) | B.E. (eV) | P.C. (%) | B.E. (eV) | P.C. (%) | ||
| CF | 284.8 | 72.34 | 286.4 | 18.55 | 288.7 | 6.96 | 285.7 | 2.15 | 27.66 |
| CF-G-0.5 | 284.8 | 69.93 | 286.5 | 17.31 | 288.7 | 7.88 | 285.7 | 4.88 | 30.07 |
| CF-G-1.0 | 284.8 | 67.91 | 286.4 | 17.53 | 288.7 | 8.33 | 285.7 | 6.23 | 32.09 |
| CF-G-1.5 | 284.6 | 67.06 | 286.3 | 20.77 | 288.7 | 4.41 | 285.6 | 7.76 | 32.94 |
| Carbon Fiber | Number of Samples | Shape Parameter | Scale Parameter | Tensile Strength/MPa | R |
|---|---|---|---|---|---|
| CF | 30 | 7.900 | 69.754 | 6432 | 0.935 |
| CF-G-0.5 | 30 | 10.660 | 94.141 | 6529 | 0.950 |
| CF-G-1.0 | 30 | 11.101 | 97.668 | 6327 | 0.939 |
| CF-G-1.5 | 30 | 8.653 | 76.373 | 6441 | 0.934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Duan, Z.; Sun, M.; Shen, P.; Yang, H.; Zhong, X.; Zhang, Y.; Hu, X.; Bao, J. Grafting Carbon Fibers with Graphene via a One-Pot Aryl Diazonium Reaction to Refine the Interface Performance of T1100-Grade CF/BMI Composites. Materials 2024, 17, 3288. https://doi.org/10.3390/ma17133288
Li W, Duan Z, Sun M, Shen P, Yang H, Zhong X, Zhang Y, Hu X, Bao J. Grafting Carbon Fibers with Graphene via a One-Pot Aryl Diazonium Reaction to Refine the Interface Performance of T1100-Grade CF/BMI Composites. Materials. 2024; 17(13):3288. https://doi.org/10.3390/ma17133288
Chicago/Turabian StyleLi, Weidong, Ziqi Duan, Mingchen Sun, Pengfei Shen, Huanzhi Yang, Xiangyu Zhong, Yang Zhang, Xiaolan Hu, and Jianwen Bao. 2024. "Grafting Carbon Fibers with Graphene via a One-Pot Aryl Diazonium Reaction to Refine the Interface Performance of T1100-Grade CF/BMI Composites" Materials 17, no. 13: 3288. https://doi.org/10.3390/ma17133288
APA StyleLi, W., Duan, Z., Sun, M., Shen, P., Yang, H., Zhong, X., Zhang, Y., Hu, X., & Bao, J. (2024). Grafting Carbon Fibers with Graphene via a One-Pot Aryl Diazonium Reaction to Refine the Interface Performance of T1100-Grade CF/BMI Composites. Materials, 17(13), 3288. https://doi.org/10.3390/ma17133288







