Effect of Solution-to-Binder Ratio and Molarity on Volume Changes in Slag Binder Activated by Sodium Hydroxide at Early Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Characterization
2.2. Pastes Design
3. Methods and Devices
3.1. Workability
3.2. Compressive Strength
3.3. Setting Times
3.4. Cumulative Heat, Heat Flow, and Activation Energy
3.5. Internal Relative Humidity
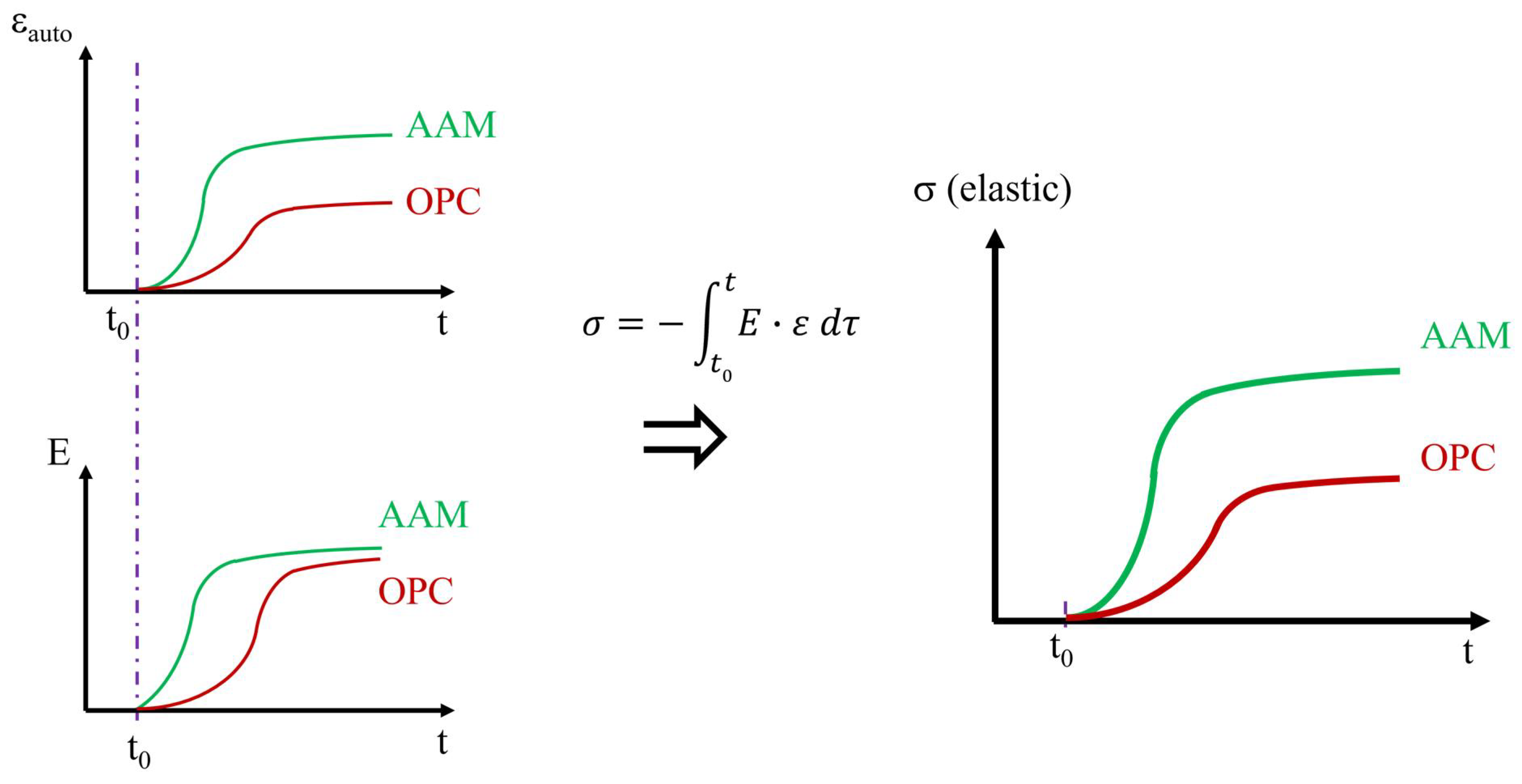
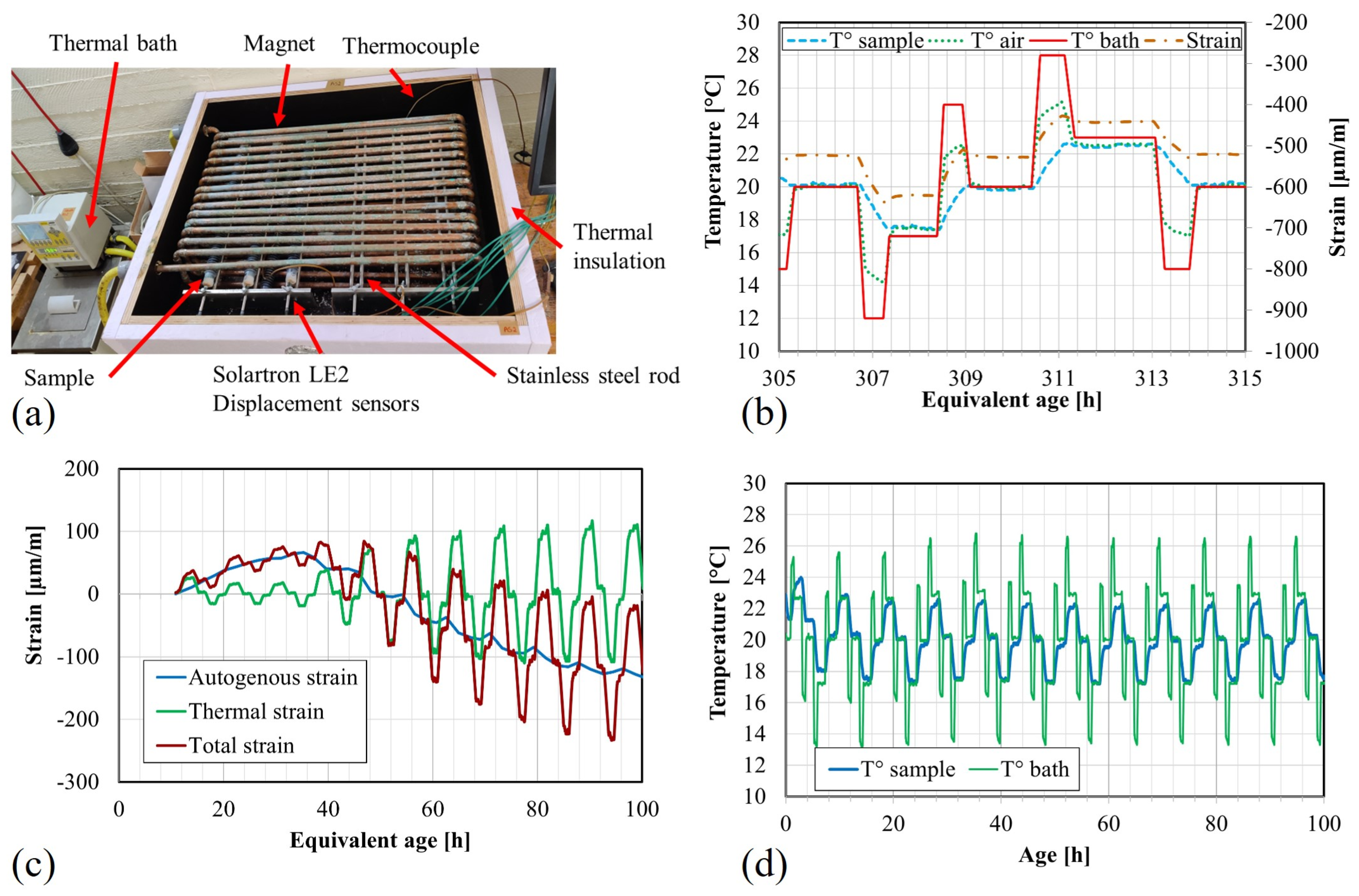
3.6. Autogenous Strain and Coefficient of Thermal Expansion
4. Results and Discussion
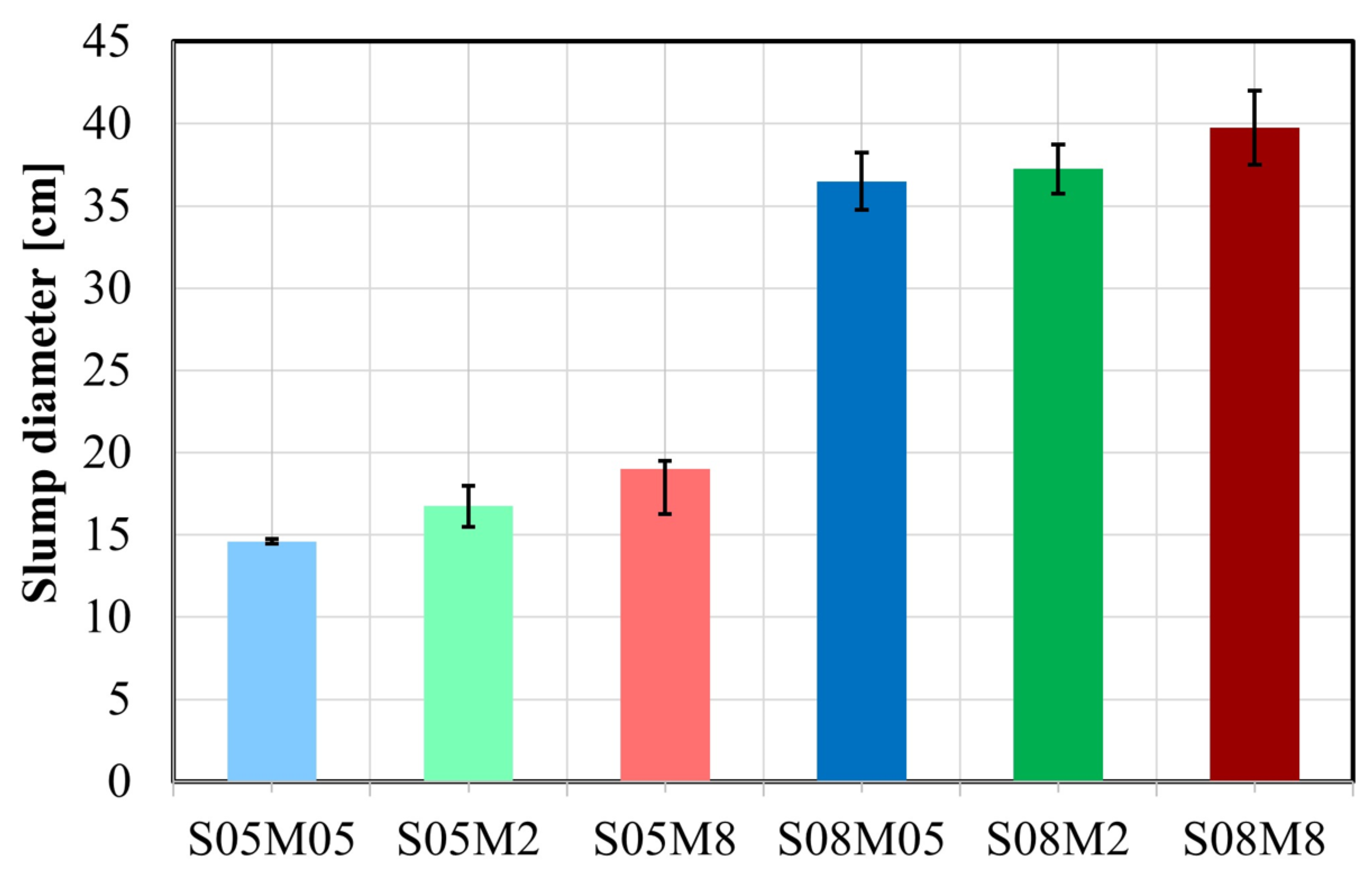
4.1. Workability
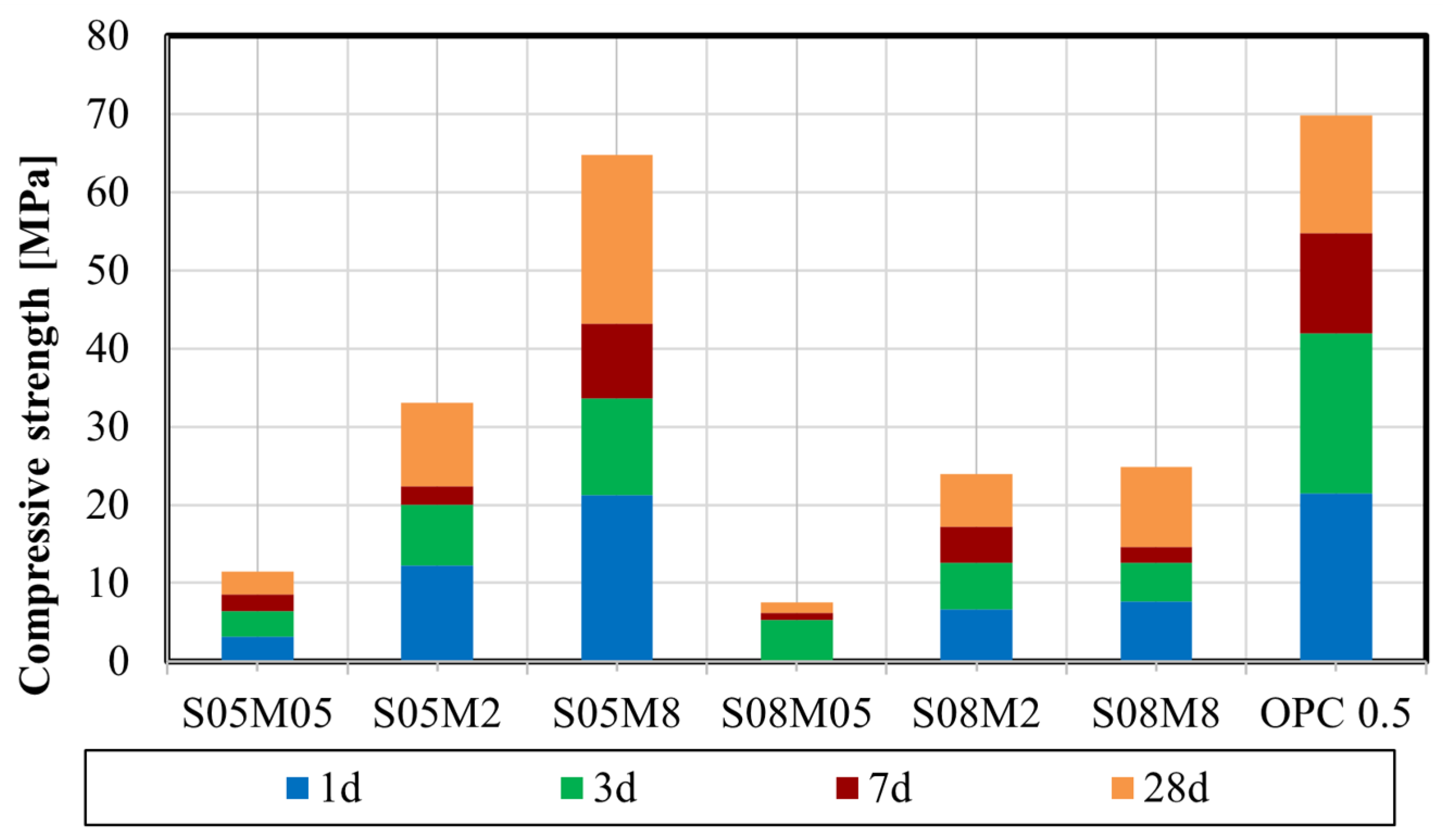
4.2. Compressive Strength
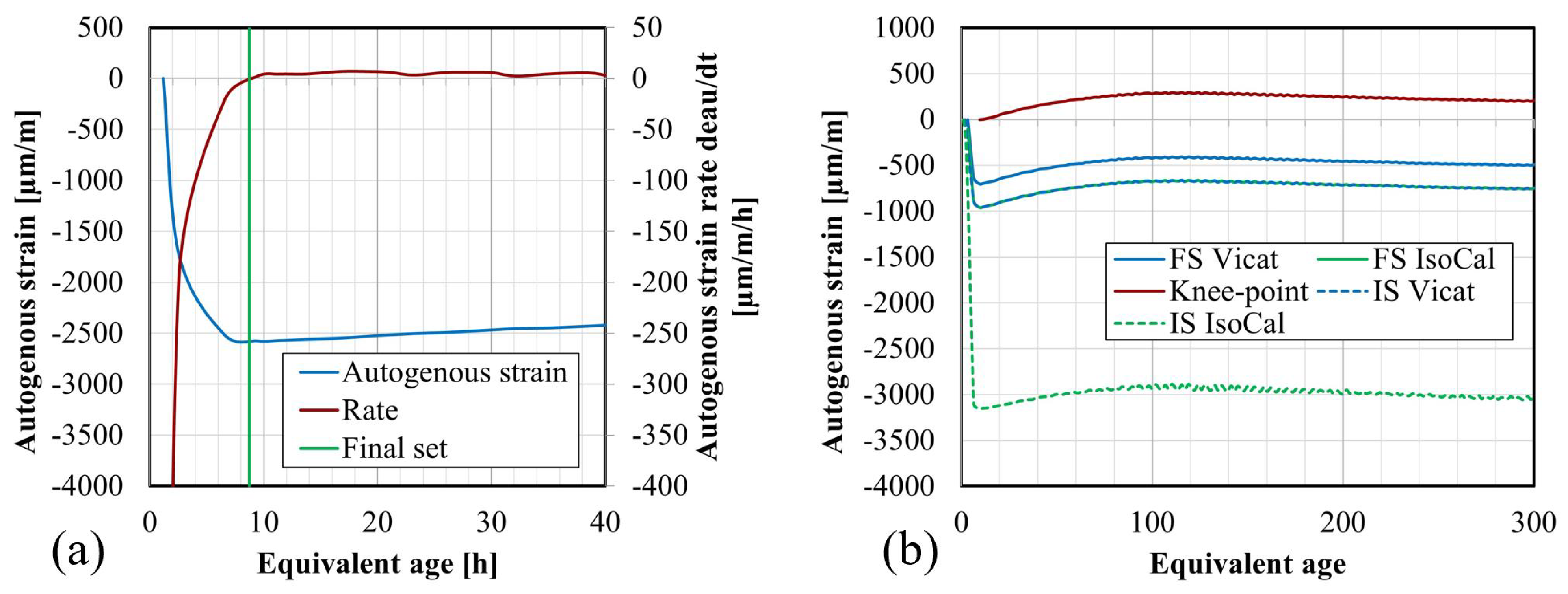
4.3. Setting Times
4.4. Isothermal Calorimetry
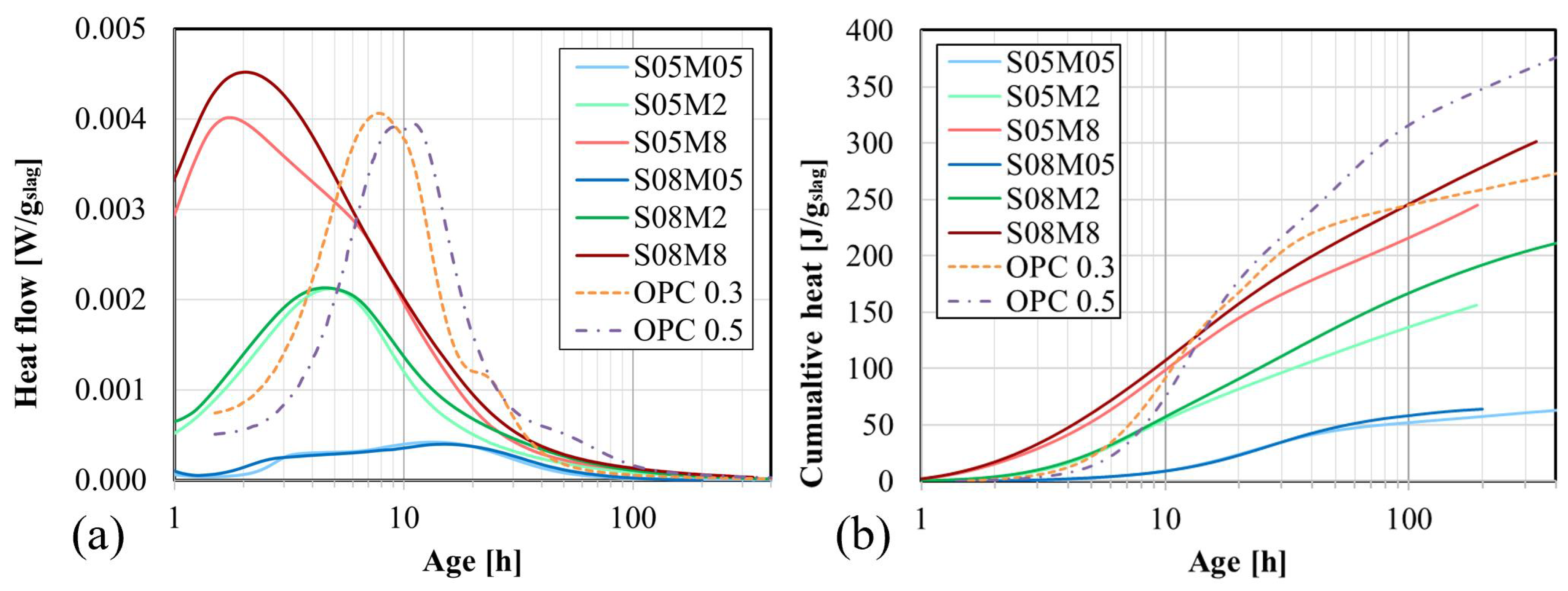
4.4.1. Heat Flow and Cumulative Heat
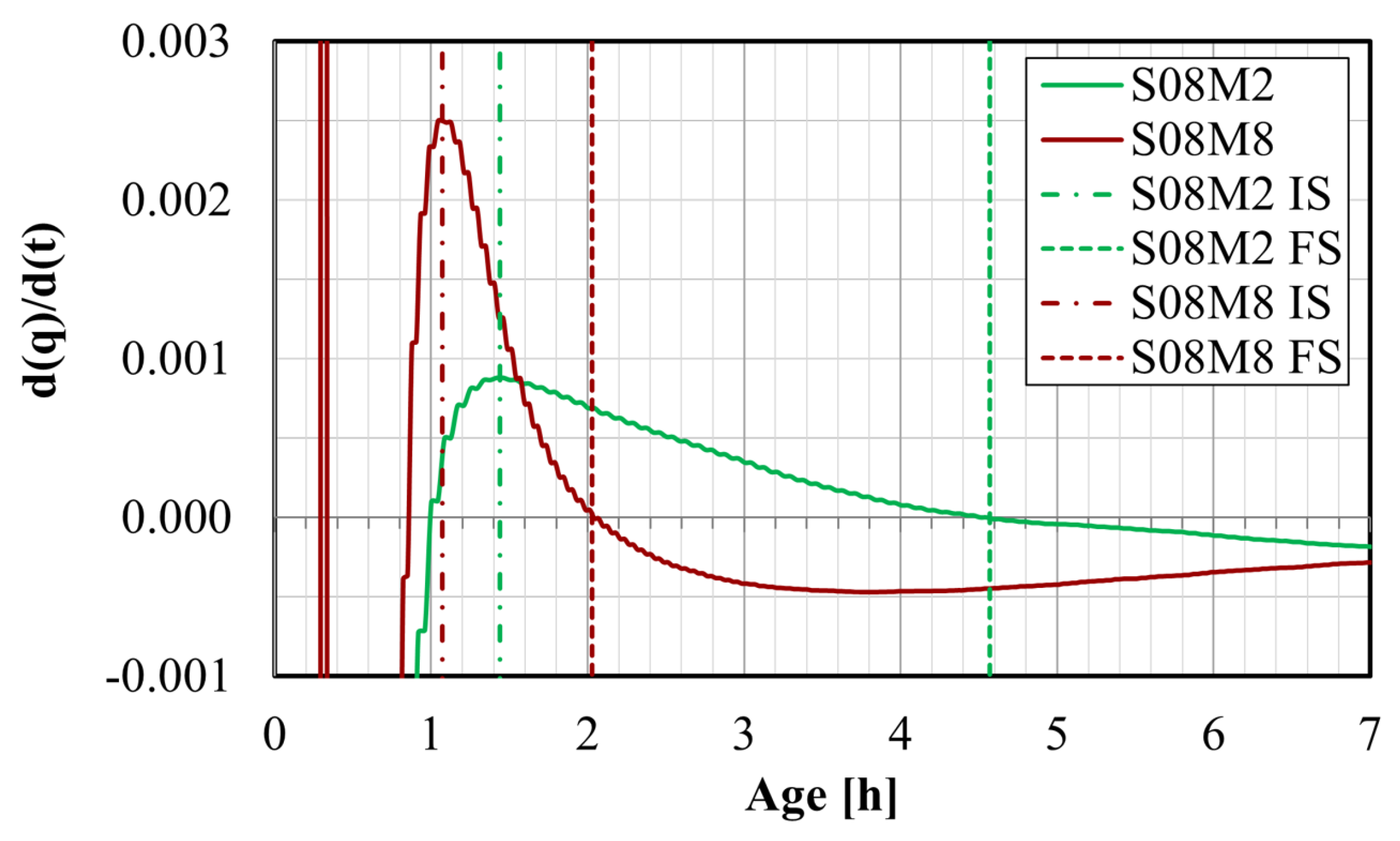
4.4.2. Setting Times Defined from Isothermal Calorimetry
4.4.3. Apparent Activation Energy
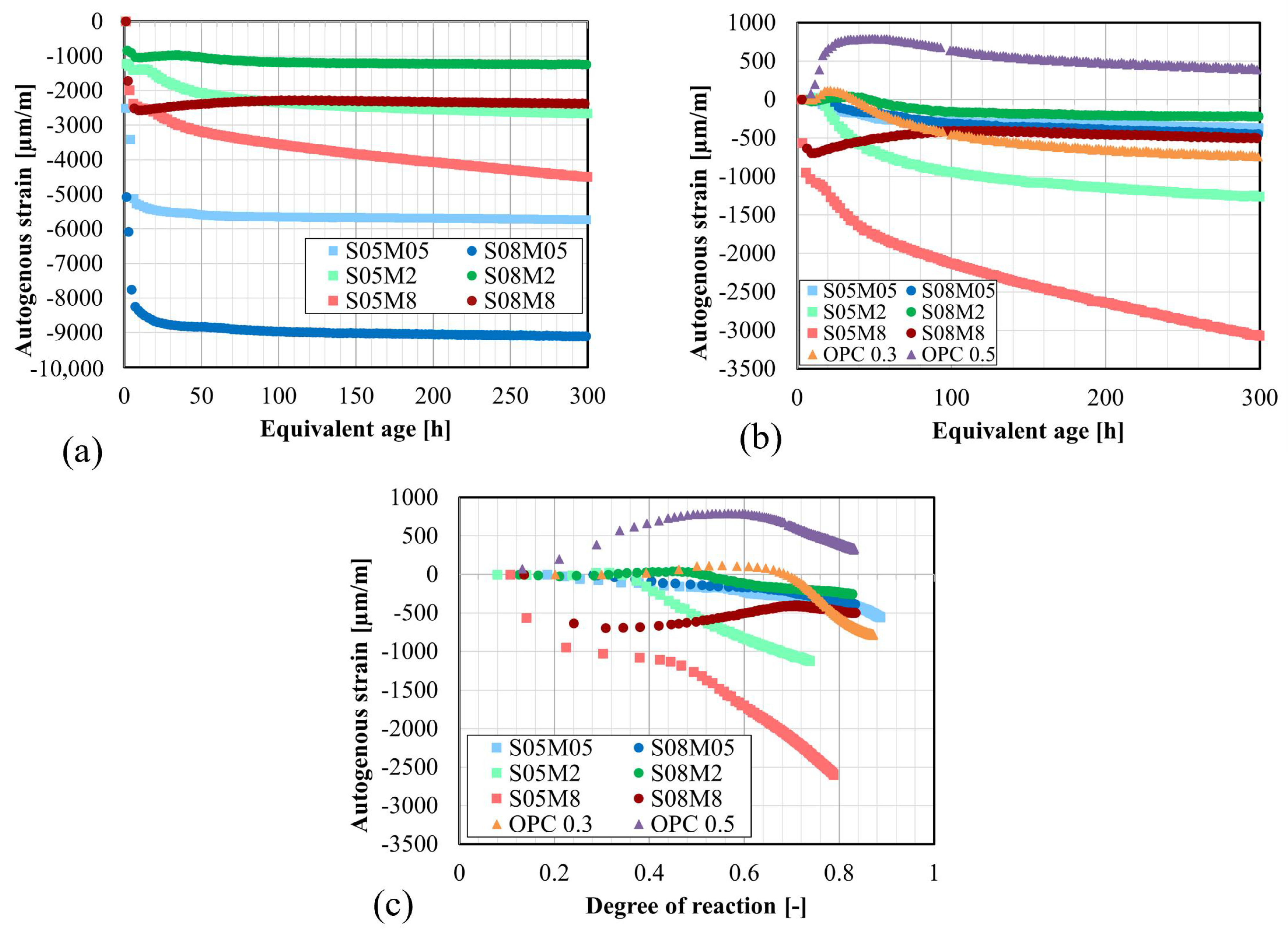
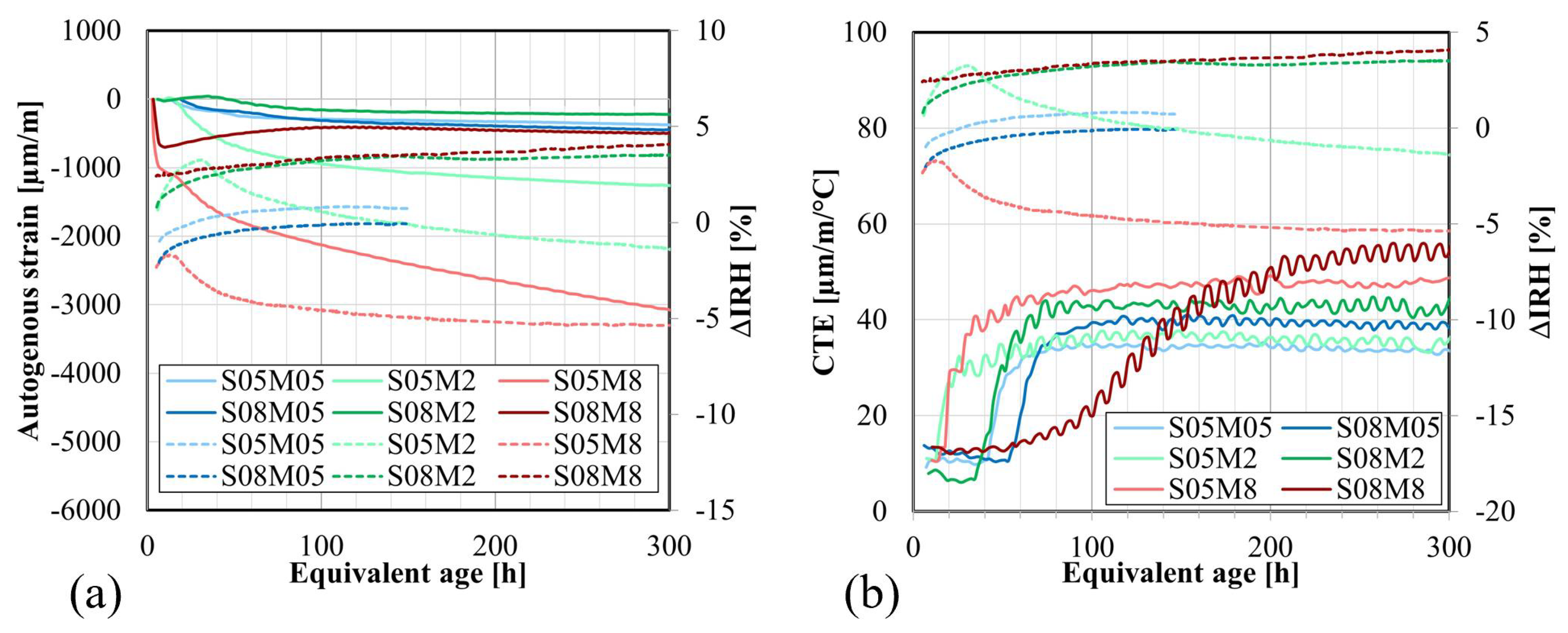
4.5. Autogenous Strain
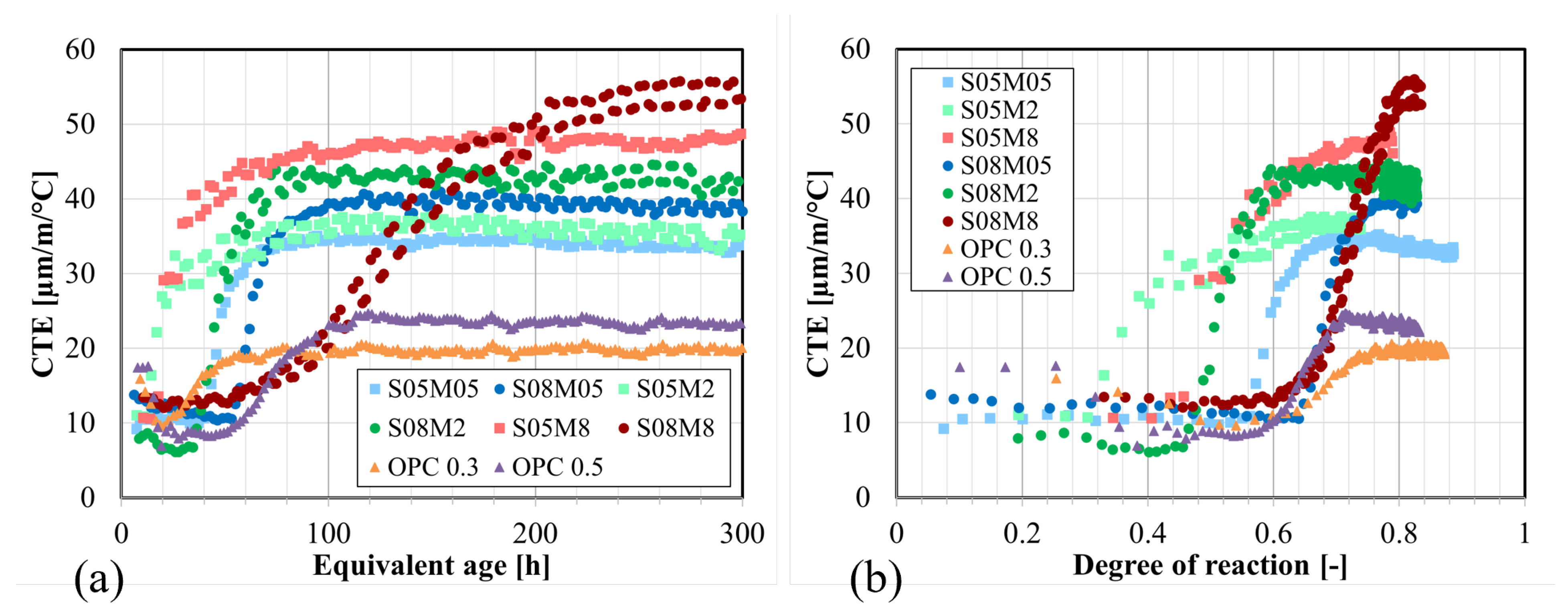
4.6. Coefficient of Thermal Expansion
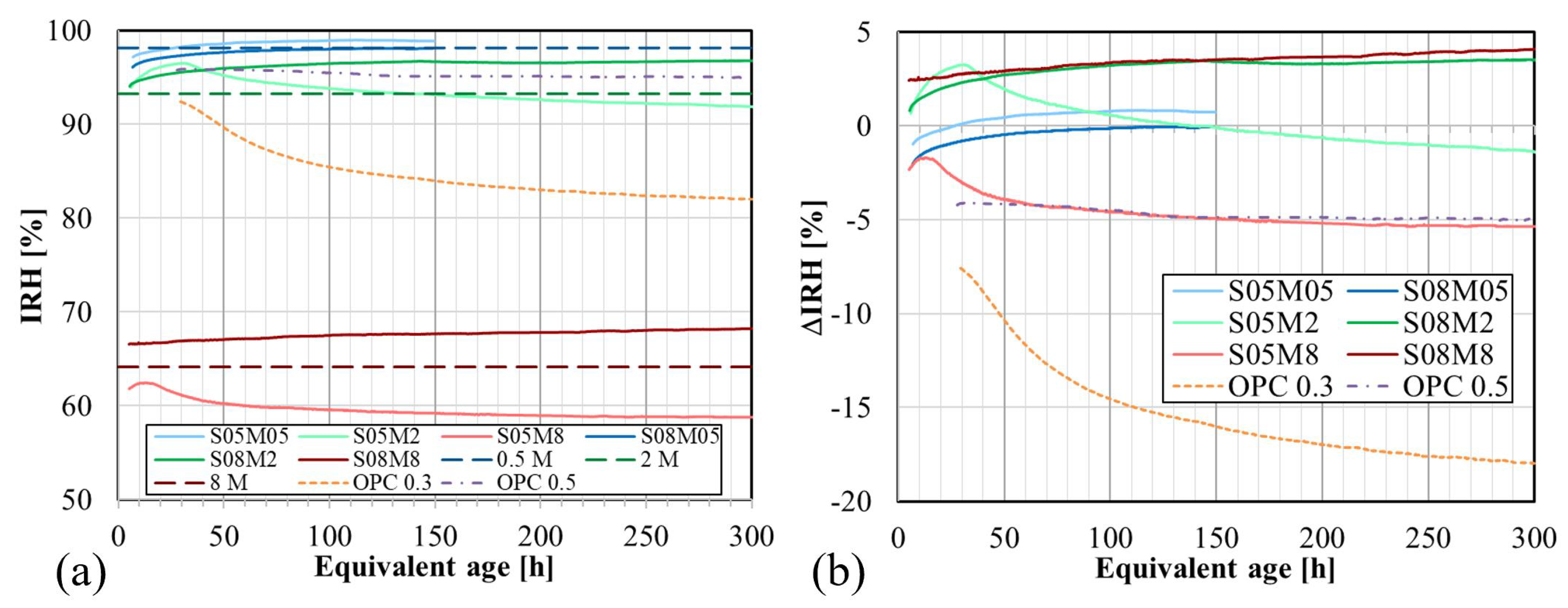
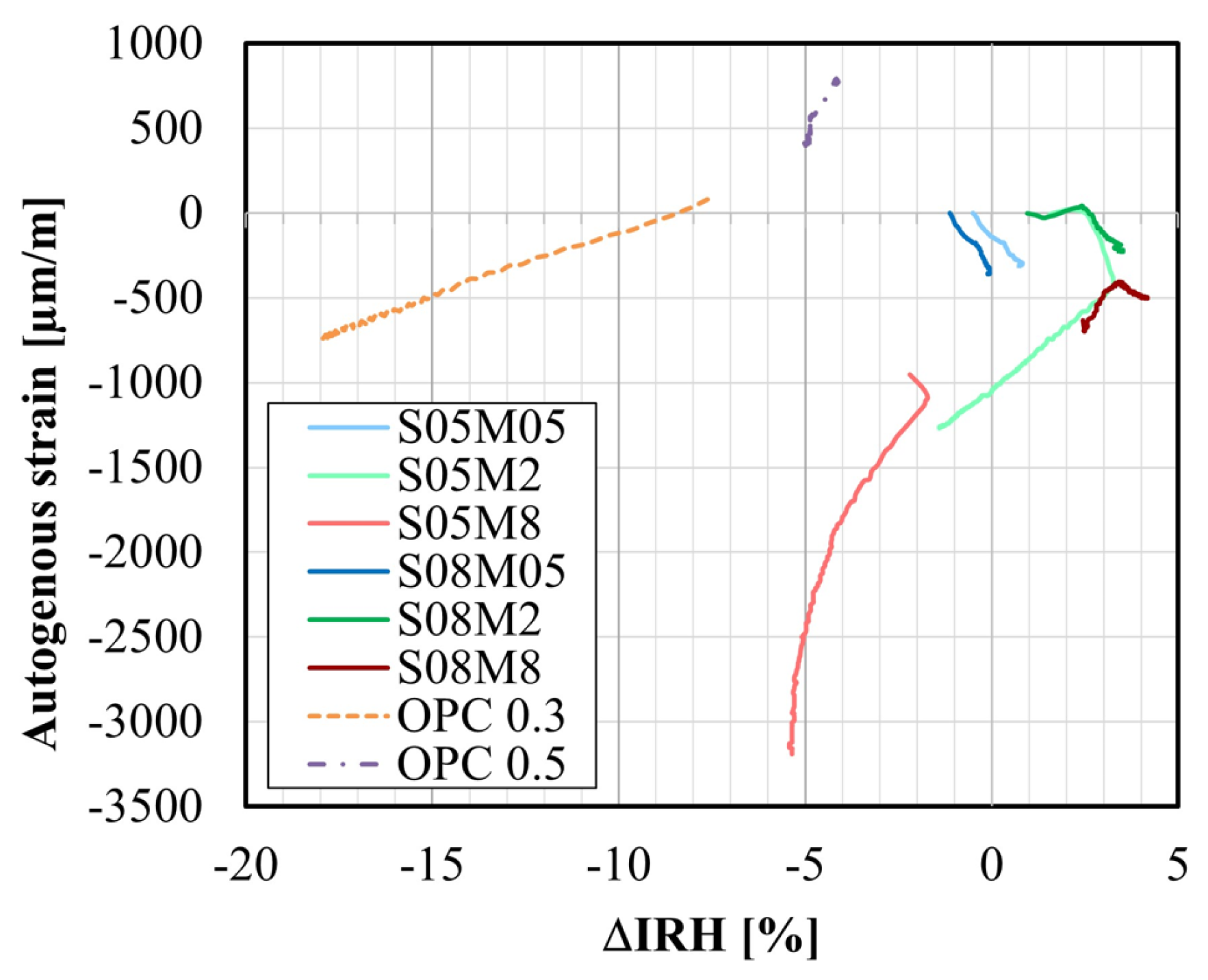
4.7. Internal Relative Humidity
- p′: pore pressure of fluid;
- IRH: internal relative humidity;
- R: universal gas constant;
- T: absolute temperature;
- v′: molar volume of solution.
5. Conclusions
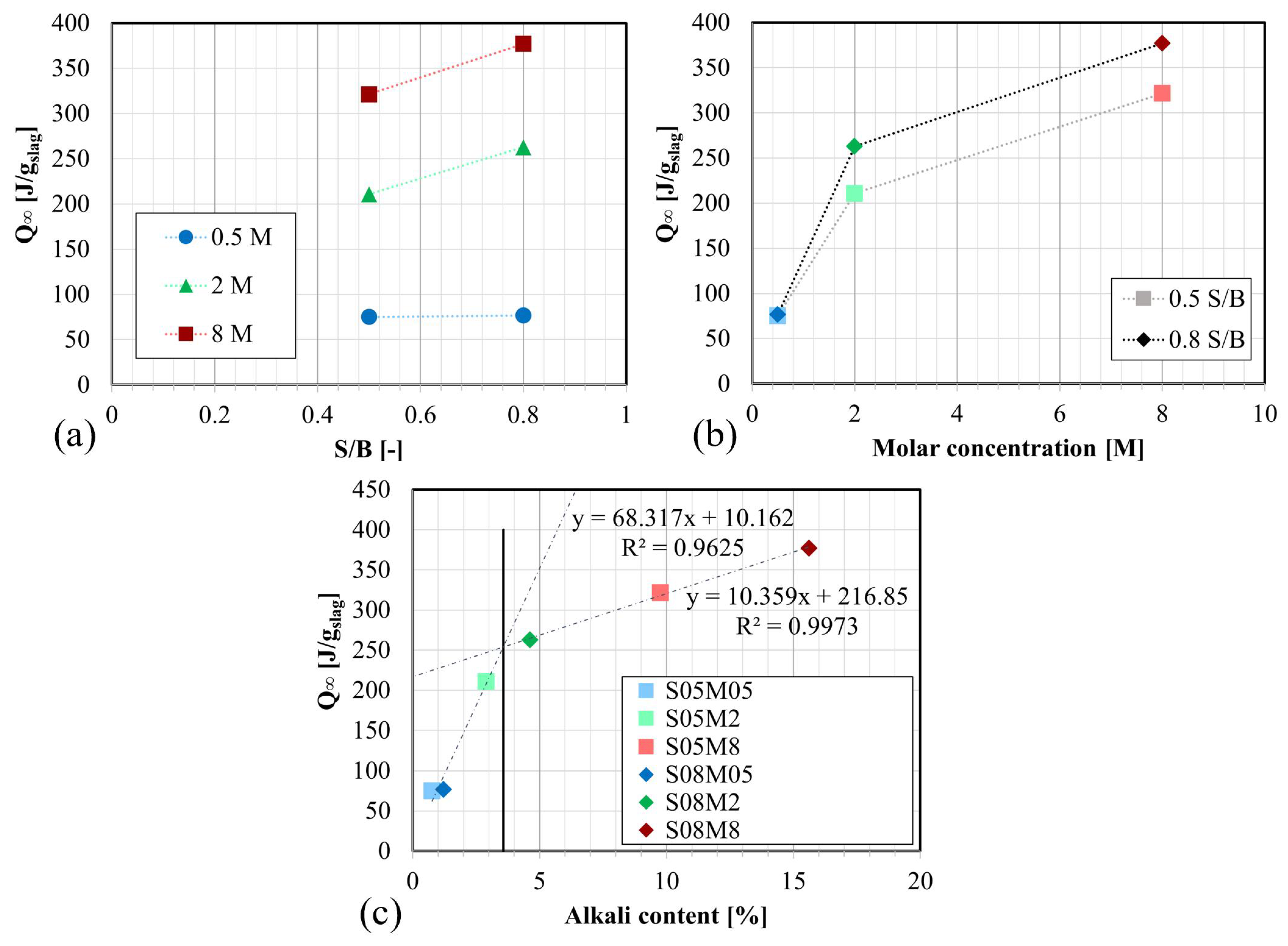
- The autogenous swelling is powered by the increase in the S/B ratio and the increase in the molarity, while the self-desiccation shrinkage is decreased by increasing S/B or decreasing the concentration of the alkaline solution.
- The CTE of AAS (33–55 µm/m/°C) is notably higher than that of OPC (20–25 µm/m/°C) due to the elevated CTE of the solution (2.27 to 2.45 times higher than the CTE of water), implying that the thermal strains in AAS will be higher than in OPC. A higher S/B leads to a higher CTE due to the addition of solution, which has a higher CTE than solids. It also slows down the evolution of the CTE. Similarly, an increase in the concentration enhances the CTE and accelerates its evolution.
- The IRH primarily depends on the concentration of the alkaline solution. Increasing the concentration leads to a decreased IRH, amounting to about 64% when using an 8 M solution. In compositions with S/B = 0.5, IRH initially increases because of the decrease in the total ion concentration before decreasing due to self-desiccation. Conversely, at S/B = 0.8, only an increase in IRH is observed, correlating with higher swelling and reduced self-desiccation observed in the autogenous strain. The opposite trend established between the IRH and the CTE for OPC was observed when examining the effect of the concentration. However, this was not fully consistent with the effect of S/B.
- Vicat and isothermal calorimetry methods demonstrate good agreement for the determination of the setting times, for lower S/B and higher concentrations. However, the knee-point method presents challenges for compositions lacking apparent swelling. Generally, increasing S/B prolongs setting times due to the addition of water to the paste, while higher concentration accelerates setting times by promoting polymerization.
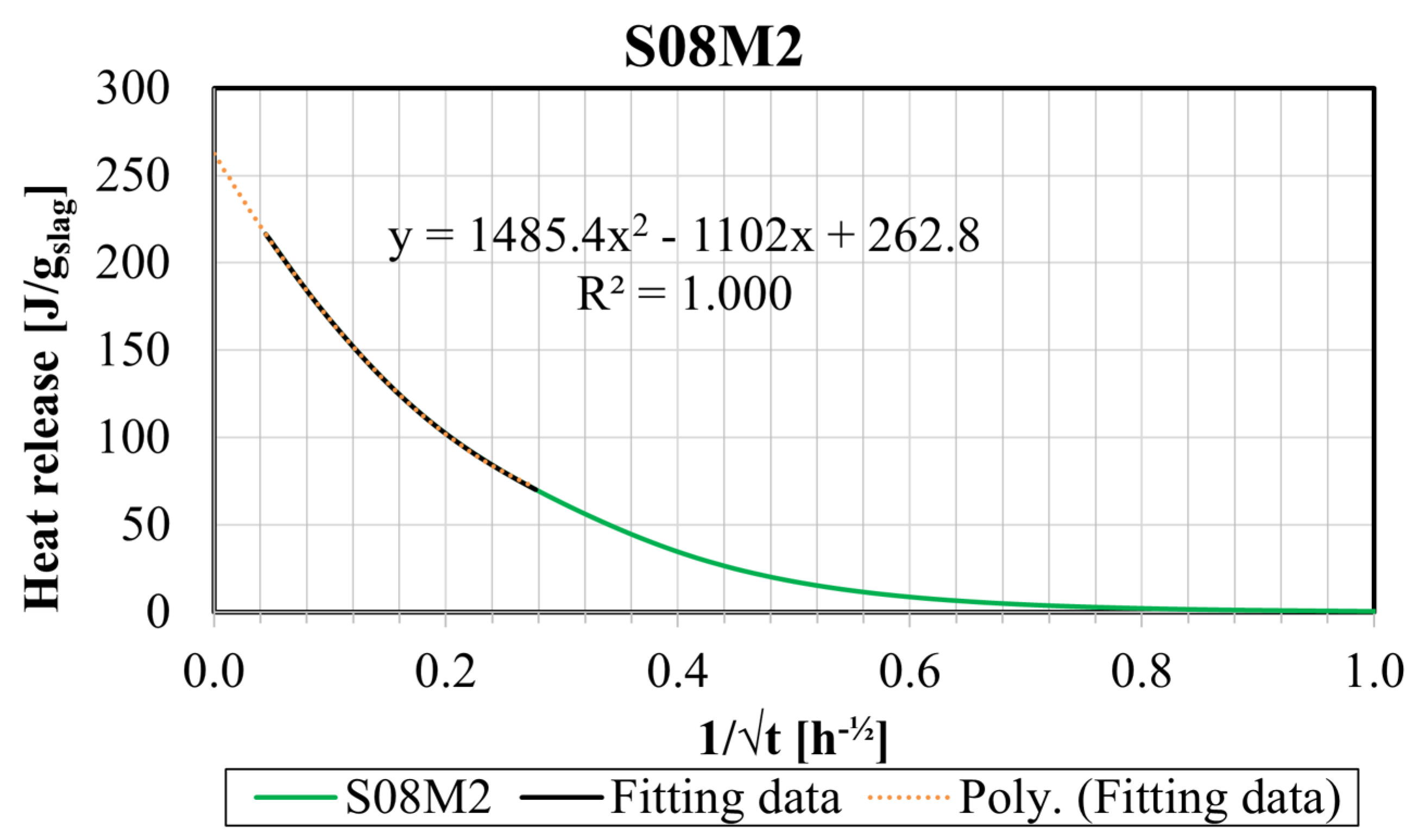
- The heat flow and the cumulative heat are predominantly influenced by the concentration of the alkaline solution due to the higher availability of OH−. Over time, a higher S/B results in increased heat release. Extrapolation revealed an optimum ultimate heat for an alkali content of 3.57%. Increasing S/B and the concentration generally enhanced the ultimate heat. The average apparent activation energy of 75.5 kJ/mol was computed.
- Enhanced workability is observed with increasing S/B and concentration due to the addition of solution and higher particle dispersion. The compressive strength increases with the increase in the concentration and the decrease in S/B, attributed to the formation of the strength-giving gel and the reduction in the pores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAM | Alkali-activated materials |
| AAS | Alkali-activated slag |
| CTE | Coefficient of thermal expansion |
| IRH | Internal relative humidity |
| M | Mol/l |
| OPC | Ordinary Portland cement |
| RH | Relative humidity |
| S/B | Solution-to-binder mass ratio |
| W/C | Water-to-cement mass ratio |
References
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Damtoft, J.; Lukasik, J.; Herfort, D.; Sorrentino, D.; Gartner, E. Sustainable development and climate change initiatives. Cem. Concr. Res. 2008, 38, 115–127. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Munteam, M.; Peters, J. Trends in global CO2 Emissions: 2015 Report; Technical Report; Ispra: European Commission, Joint Research Centre; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2015. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. (Eds.) Alkali Activated Materials; RILEM State-of-the-Art Reports; Springer: Dordrecht, The Netherlands, 2014; Volume 13. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Alkali activation of Australian slag cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Karahan, O.; Yakupoğlu, A. Resistance of alkali-activated slag mortar to abrasion and fire. Adv. Cem. Res. 2011, 23, 289–297. [Google Scholar] [CrossRef]
- Ye, H.; Chen, Z.; Huang, L. Mechanism of sulfate attack on alkali-activated slag: The role of activator composition. Cem. Concr. Res. 2019, 125, 105868. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; van Breugel, K. Modelling of autogenous shrinkage of hardening cement paste. Constr. Build. Mater. 2020, 264, 120708. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, C.; Zhang, Z. Recent progress in understanding setting and hardening of alkali-activated slag (AAS) materials. Cem. Concr. Compos. 2022, 134, 104795. [Google Scholar] [CrossRef]
- Serdar, M.; Gabrijel, I.; Schlicke, D.; Staquet, S.; Azenha, M. (Eds.) Advanced Techniques for Testing of Cement-Based Materials; Springer Tracts in Civil Engineering; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Bendimerad, A.; Delsaute, B.; Rozière, E.; Staquet, S.; Loukili, A. Advanced techniques for the study of shrinkage-induced cracking of concrete with recycled aggregates at early age. Constr. Build. Mater. 2020, 233, 117340. [Google Scholar] [CrossRef]
- Turcry, P.; Loukili, A.; Barcelo, L.; Casabonne, J.M. Can the maturity concept be used to separate the autogenous shrinkage and thermal deformation of a cement paste at early age? Cem. Concr. Res. 2002, 32, 1443–1450. [Google Scholar] [CrossRef]
- Maruyama, I.; Lura, P. Properties of early-age concrete relevant to cracking in massive concrete. Cem. Concr. Res. 2019, 123, 105770. [Google Scholar] [CrossRef]
- Li, L.; Dao, V.; Lura, P. Autogenous deformation and coefficient of thermal expansion of early-age concrete: Initial outcomes of a study using a newly-developed Temperature Stress Testing Machine. Cem. Concr. Compos. 2021, 119, 103997. [Google Scholar] [CrossRef]
- Sirotti, M.; Delsaute, B.; Staquet, S. New Experimental Evidence for Drying Shrinkage of Alkali-Activated Slag with Sodium Hydroxide. Materials 2023, 16, 5659. [Google Scholar] [CrossRef]
- Sirotti, M.; Carette, J.; Staquet, S. New Insights into Pore Structure and Hydraulic Conductivity of Sodium Hydroxide Alkali-Activated Slag through Advanced Modelling. Materials 2024, 17, 363. [Google Scholar] [CrossRef]
- Li, L. Delayed Deformations of Alkali Sulphates Activated Materials under Autogenous Condition. Ph.D. Thesis, Institut Nationnal des Sciences Appliquées, Rennes, France, 2023. [Google Scholar]
- Le Chatelier, H. Sur les changements de volume qui accompagnent le durcissement des ciments. Bull. Soc. L’Encourag. L’Ind. Natl. 1900, 5, 54–57. [Google Scholar]
- Fang, G.; Bahrami, H.; Zhang, M. Mechanisms of autogenous shrinkage of alkali-activated fly ash-slag pastes cured at ambient temperature within 24 h. Constr. Build. Mater. 2018, 171, 377–387. [Google Scholar] [CrossRef]
- Carette, J.; Joseph, S.; Cizer, O.; Staquet, S. Decoupling the autogenous swelling from the self-desiccation deformation in early age concrete with mineral additions: Micro-macro observations and unified modelling. Cem. Concr. Compos. 2018, 85, 122–132. [Google Scholar] [CrossRef]
- Mehta, P. Mechanism of expansion associated with ettringite formation. Cem. Concr. Res. 1973, 3, 1–6. [Google Scholar] [CrossRef]
- Stefan, L.; Boulay, C.; Torrenti, J.M.; Bissonnette, B.; Benboudjema, F. Influential factors in volume change measurements for cementitious materials at early ages and in isothermal conditions. Cem. Concr. Compos. 2018, 85, 105–121. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Provis, J.L.; Cizer, O.; Ye, G. Autogenous shrinkage of alkali-activated slag: A critical review. Cem. Concr. Res. 2023, 172, 107244. [Google Scholar] [CrossRef]
- Uppalapati, S. Early-Age Structural Development and Autogenous Shrinkage of Alkali-Activated Slag/Fly Ash Cements. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2020. [Google Scholar]
- Li, Y.; Li, J. Capillary tension theory for prediction of early autogenous shrinkage of self-consolidating concrete. Constr. Build. Mater. 2014, 53, 511–516. [Google Scholar] [CrossRef]
- Tang, S.; Huang, D.; He, Z. A review of autogenous shrinkage models of concrete. J. Build. Eng. 2021, 44, 103412. [Google Scholar] [CrossRef]
- Wittmann, F. Surface tension skrinkage and strength of hardened cement paste. Matér. Constr. 1968, 1, 547–552. [Google Scholar] [CrossRef]
- Powers, T. Mechanisms of shrinkage and reversible creep of hardened cement paste. In The Structure of Concrete and Its Behaviour under Load: Proceedings of an International Conference, International Conference on the Structure of Concrete, London, UK; Brooks, A.E., Newman, K., Eds.; Cement and Concrete Association: London, UK, 1965; pp. 319–344. [Google Scholar]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Wang, J.; Thom, N. Effects of alkali dosage and silicate modulus on autogenous shrinkage of alkali-activated slag cement paste. Cem. Concr. Res. 2021, 141, 106322. [Google Scholar] [CrossRef]
- Ballekere Kumarappa, D.; Peethamparan, S.; Ngami, M. Autogenous shrinkage of alkali activated slag mortars: Basic mechanisms and mitigation methods. Cem. Concr. Res. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Sellevold, E.J.; Bjontegaard, O. Coefficient of thermal expansion of cement paste and concrete: Mechanisms of moisture interaction. Mater. Struct. 2006, 39, 809–815. [Google Scholar] [CrossRef]
- Maruyama, I.; Teramoto, A. Impact of time-dependant thermal expansion coefficient on the early-age volume changes in cement pastes. Cem. Concr. Res. 2011, 41, 380–391. [Google Scholar] [CrossRef]
- Ma, J.; Dehn, F. Investigations on the coefficient of thermal expansion of a low-calcium fly ash-based geopolymer concrete. Struct. Concr. 2017, 18, 781–791. [Google Scholar] [CrossRef]
- Delsaute, B.; Staquet, S. Impact of recycled sand and gravels in concrete on volume change. Constr. Build. Mater. 2020, 232, 117279. [Google Scholar] [CrossRef]
- Fairbairn, E.M.; Azenha, M. (Eds.) Thermal Cracking of Massive Concrete Structures; RILEM State-of-the-Art Reports; Springer International Publishing: Cham, Switzerland, 2019; Volume 27. [Google Scholar] [CrossRef]
- Lacante, M.; Delsaute, B.; Gambacorta, J.; Königsberger, M.; Staquet, S. Development of early age autogenous and thermal strains of alkali-activated slag-fly ash pastes. Front. Built Environ. 2022, 8, 1–16. [Google Scholar] [CrossRef]
- Srinivasamurthy, L.; Chevali, V.S.; Zhang, Z.; Wang, H. Effect of fly ash to slag ratio and Na2O content on leaching behaviour of fly Ash/Slag based alkali activated materials. Constr. Build. Mater. 2023, 383, 131234. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; De Schutter, G. Early age reaction, rheological properties and pore solution chemistry of NaOH-activated slag mixtures. Cem. Concr. Compos. 2022, 133, 104715. [Google Scholar] [CrossRef]
- Naqi, A.; Delsaute, B.; Königsberger, M.; Staquet, S. Effect of Solution-to-Binder Ratio and Alkalinity on Setting and Early-Age Properties of Alkali-Activated Slag-Fly Ash Binders. Materials 2022, 16, 373. [Google Scholar] [CrossRef]
- Baroghel-Bouny, V.; Mounanga, P.; Khelidj, A.; Loukili, A.; Rafaï, N. Autogenous deformations of cement pastes. Cem. Concr. Res. 2006, 36, 123–136. [Google Scholar] [CrossRef]
- Nedeljković, M.; Li, Z.; Ye, G. Setting, Strength, and Autogenous Shrinkage of Alkali-Activated Fly Ash and Slag Pastes: Effect of Slag Content. Materials 2018, 11, 2121. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- Delsaute, B. New Approach for Monitoring and Modelling of the Creep and Shrinkage Behaviour of Cement Pastes, Mortars and Concretes since Setting Time. Ph.D. Thesis, Université libre de Bruxelles, BATir, Belgium and Université Paris-Est, Ifsttar, France, 2016. [Google Scholar]
- ASTM C230/C230M-08; Standard Specification for Flow Table for Use in Tests of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM C109/C109M-16a; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2016.
- EN 196-3:2016; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- Zhang, M.H.; Sisomphon, K.; Ng, T.S.; Sun, D.J. Effect of superplasticizers on workability retention and initial setting time of cement pastes. Constr. Build. Mater. 2010, 24, 1700–1707. [Google Scholar] [CrossRef]
- Uppalapati, S.; Vandewalle, L.; Cizer, O. Monitoring the setting process of alkali-activated slag-fly ash cements with ultrasonic P-wave velocity. Constr. Build. Mater. 2021, 271, 121592. [Google Scholar] [CrossRef]
- Tenório Filho, J.R.; Pereira Gomes de Araújo, M.A.; Snoeck, D.; De Belie, N. Discussing Different Approaches for the Time-Zero as Start for Autogenous Shrinkage in Cement Pastes Containing Superabsorbent Polymers. Materials 2019, 12, 2962. [Google Scholar] [CrossRef]
- Wadso, L. The Study of Cement Hydration by Isothermal Calorimetry; Technical Report; Lund University: Lund, Sweden; TA Instruments: New Castle, DE, USA. Available online: https://www.tainstruments.com/pdf/literature/M100.pdf (accessed on 4 January 2023).
- TA Instruments. TAM Air Calorimeter Operator’s Manual; Technical Report; TA Instruments: New Castle, DE, USA, 2006. [Google Scholar]
- TA Instruments. Tam Air Isothermal Calorimetry; TA Instruments: New Castle, DE, USA, 2013. [Google Scholar]
- Germann. Auto-Shrink Installation Manual Automatic Logging System; Germann: Copenhagen, Denmark, 2015. [Google Scholar]
- Mejlhede Jensen, O.; Freiesleben Hansen, P. A dilatometer for measuring autogenous deformation in hardening portland cement paste. Mater. Struct. 1995, 28, 406–409. [Google Scholar] [CrossRef]
- Delsaute, B.; Staquet, S. Decoupling Thermal and Autogenous Strain of Concretes with Different Water/Cement Ratios During the Hardening Process. Adv. Civ. Eng. Mater. 2017, 6, 20160063. [Google Scholar] [CrossRef]
- Siddique, R.; Khan, M.I. Supplementary Cementing Materials; Engineering Materials; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2011; Volume 37. [Google Scholar] [CrossRef]
- Sun, B.; Sun, Y.; Ye, G.; De Schutter, G. A mix design methodology of slag and fly ash-based alkali-activated paste. Cem. Concr. Compos. 2022, 126, 104368. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Mehrotra, S.P. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Saloma; Saggaff, A.; Hanafiah; Mawarni, A. Geopolymer Mortar with Fly Ash. MATEC Web Conf. 2016, 78, 01026. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Hossain, M.M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A review on alkali-activated binders: Materials composition and fresh properties of concrete. Constr. Build. Mater. 2020, 260, 119788. [Google Scholar] [CrossRef]
- Gijbels, K.; Pontikes, Y.; Samyn, P.; Schreurs, S.; Schroeyers, W. Effect of NaOH content on hydration, mineralogy, porosity and strength in alkali/sulfate-activated binders from ground granulated blast furnace slag and phosphogypsum. Cem. Concr. Res. 2020, 132, 106054. [Google Scholar] [CrossRef]
- Mohamed, R.; Abd Razak, R.; Abdullah, M.M.A.B.; Abd Rahim, S.Z.A.; Yuan-Li, L.; Subaer; Sandu, A.V.; Wysłocki, J.J. Heat evolution of alkali-activated materials: A review on influence factors. Constr. Build. Mater. 2022, 314, 125651. [Google Scholar] [CrossRef]
- Gargouri, A.; Daoud, A.; Loulizi, A.; Kallel, A. Laboratory Investigation of Self-Consolidating Waste Tire Rubberized Concrete. ACI Mater. J. 2016, 113, 661–668. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Joseph, S.; Uppalapati, S.; Cizer, O. Instantaneous activation energy of alkali activated materials. RILEM Tech. Lett. 2019, 3, 121–123. [Google Scholar] [CrossRef]
- Broda, M. Conception of an isothermal calorimeter for concrete—Determination of the apparent activation energy. Mater. Struct. 2002, 35, 389–394. [Google Scholar] [CrossRef]
- Laboratoire Central des Ponts et Chaussées. Résistance du Béton dans L’ouvrage: La Maturométrie; Laboratoire Central des Ponts et Chaussées: Paris, France, 2003. [Google Scholar]
- Königsberger, M.; Delsaute, B.; Staquet, S. Thermo-poro-micromechanics of cementitious materials: Hydration-induced evolution of thermal. In Proceedings of the 18th International Conference on Experimental Mechanics, ICEM18, Brussels, Belgium, 1–5 July 2018. [Google Scholar]
- Naqi, A.; Delsaute, B.; Königsberger, M.; Staquet, S. Monitoring early age elastic and viscoelastic properties of alkali-activated slag mortar by means of repeated minute-long loadings. Dev. Built Environ. 2023, 16, 100275. [Google Scholar] [CrossRef]
- Canciam, C.A. Estudo do coefficiente de expansão térmica de soluções aquosas de soda cáustica. Rev. Eng. Tecnol. 2014, 6, 72–79. [Google Scholar]
- Yeon, J.H.; Choi, S.; Won, M.C. Effect of Relative Humidity on Coefficient of Thermal Expansion of Hardened Cement Paste and Concrete. Transp. Res. Rec. J. Transp. Res. Board 2009, 2113, 83–91. [Google Scholar] [CrossRef]
- Hu, Z.; Wyrzykowski, M.; Lura, P. Estimation of reaction kinetics of geopolymers at early ages. Cem. Concr. Res. 2020, 129, 105971. [Google Scholar] [CrossRef]
- Yssorche-Cubaynes, M.P.; Ollivier, J.P. La microfissuration d’autodessiccation et la durabilité des BHP et BTHP. Mater. Struct. 1999, 32, 14–21. [Google Scholar] [CrossRef]
- Wang, H.; Mang, H.; Yuan, Y.; Pichler, B.L.A. Multiscale Thermoelastic Analysis of the Thermal Expansion Coefficient and of Microscopic Thermal Stresses of Mature Concrete. Materials 2019, 12, 2689. [Google Scholar] [CrossRef]



| SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | TiO2 | SO3 | Na2O | BaO | MnO |
|---|---|---|---|---|---|---|---|---|---|---|
| 33.30 | 12.30 | 0.39 | 40.80 | 0.67 | 7.84 | 1.29 | 2.30 | 0.44 | 0.31 | 0.36 |
| Paste | S/B [-] | BFS [g] | NaOH 0.5 M [g] | NaOH 2 M [g] | NaOH 8 M [g] |
|---|---|---|---|---|---|
| S05M05 | 0.5 | 100 | 50 | 0 | 0 |
| S05M2 | 0.5 | 100 | 0 | 50 | 0 |
| S05M8 | 0.5 | 100 | 0 | 0 | 50 |
| S08M05 | 0.8 | 100 | 80 | 0 | 0 |
| S08M2 | 0.8 | 100 | 0 | 80 | 0 |
| S08M8 | 0.8 | 100 | 0 | 0 | 80 |
| Composition | Q∞ [J/g] | R2 [-] | Extrapolation Interval [h] |
|---|---|---|---|
| S05M05 | 75.07 | 0.9920 | 15.5–800.0 |
| S05M2 | 210.67 | 0.9993 | 13.0–190.0 |
| S05M8 | 321.37 | 0.9972 | 8.0–190.0 |
| S08M05 | 76.72 | 0.9985 | 18.0–200.0 |
| S08M2 | 262.80 | 1.0000 | 18.0–500.0 |
| S08M8 | 376.97 | 0.9982 | 18.0–333.0 |
| Composition | IS (Vicat) [h] | FS (Vicat) [h] | IS (IsoCal) [h] | FS (IsoCal) [h] | Diff IS [h] | Diff FS [h] |
|---|---|---|---|---|---|---|
| S05M05 | 11.68 | 13.08 | 2.76 | 13.14 | 8.92 | 0.06 |
| S05M2 | 3.08 | 4.05 | 1.31 | 4.78 | 1.78 | 0.73 |
| S05M8 | 0.90 | 2.72 | 1.06 | 1.74 | 0.16 | 0.98 |
| S08M05 | 15.40 | 19.10 | 2.04 | 14.24 | 13.36 | 4.86 |
| S08M2 | 4.48 | 5.85 | 1.44 | 4.57 | 3.04 | 1.28 |
| S08M8 | 2.03 | 3.33 | 1.07 | 2.03 | 0.96 | 1.30 |
| Composition | S05M05 | S05M2 | S05M8 | S08M05 | S08M2 | S08M8 | Average |
|---|---|---|---|---|---|---|---|
| Ea | 74.18 | 71.99 | 83.63 | 69.08 | 73.08 | 81.05 | 75.50 |
| Composition | FS (Vicat) [h] | FS (IsoCal) [h] | FS Knee-Point Method [h] |
|---|---|---|---|
| S05M05 | 13.08 | 13.14 | 35.58 |
| S05M2 | 4.05 | 4.78 | 5.85 |
| S05M8 | 2.72 | 1.74 | 12.28 |
| S08M05 | 19.10 | 14.24 | 49.03 |
| S08M2 | 5.85 | 4.57 | 8.75 |
| S08M8 | 3.33 | 2.03 | 9.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacante, M.; Delsaute, B.; Staquet, S. Effect of Solution-to-Binder Ratio and Molarity on Volume Changes in Slag Binder Activated by Sodium Hydroxide at Early Age. Materials 2024, 17, 3308. https://doi.org/10.3390/ma17133308
Lacante M, Delsaute B, Staquet S. Effect of Solution-to-Binder Ratio and Molarity on Volume Changes in Slag Binder Activated by Sodium Hydroxide at Early Age. Materials. 2024; 17(13):3308. https://doi.org/10.3390/ma17133308
Chicago/Turabian StyleLacante, Maïté, Brice Delsaute, and Stéphanie Staquet. 2024. "Effect of Solution-to-Binder Ratio and Molarity on Volume Changes in Slag Binder Activated by Sodium Hydroxide at Early Age" Materials 17, no. 13: 3308. https://doi.org/10.3390/ma17133308







