Modeling of Concrete Deterioration under External Sulfate Attack and Drying–Wetting Cycles: A Review
Abstract
:1. Introduction
2. Moisture Transport Model
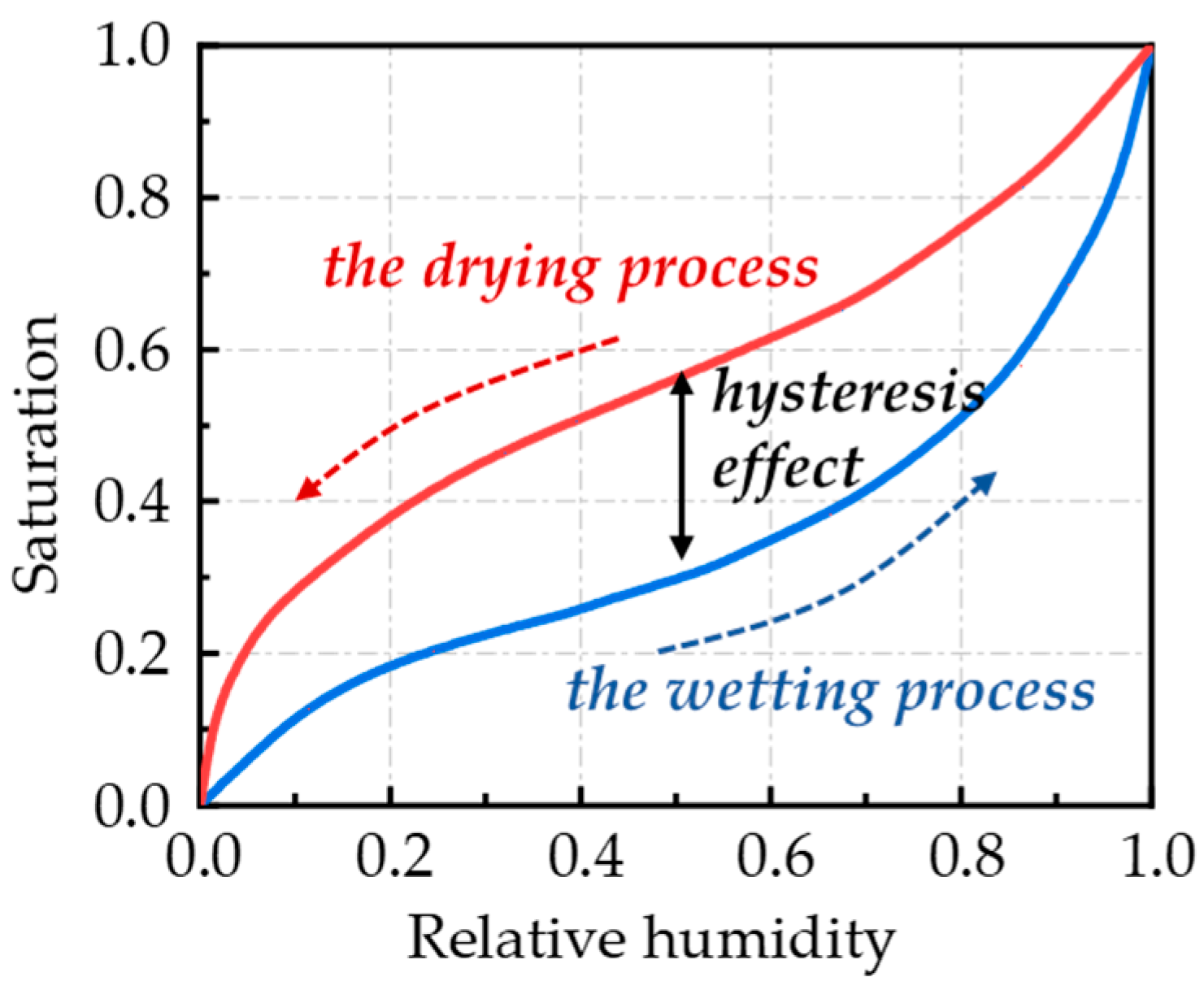
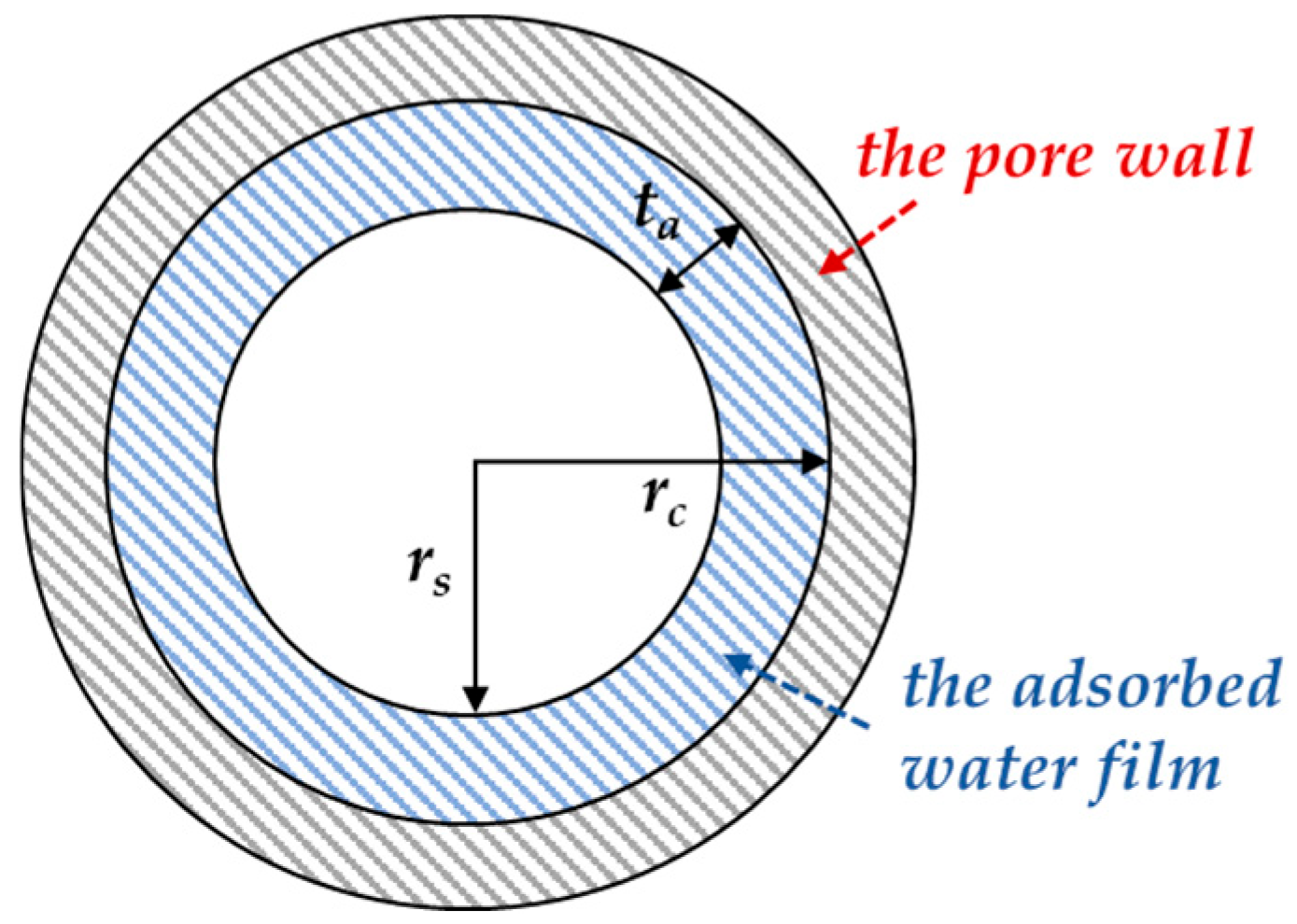
2.1. Theoretical Model
2.2. Empirical Model
3. Ion Transport Model
4. Expansion Damage Mechanism
4.1. Microscopic Expansion Mechanisms
4.2. Macroscopic Expansion Calculation
5. Future Work
6. Conclusions
Funding
Conflicts of Interest
References
- Elahi, M.M.A.; Shearer, C.R.; Reza, A.N.R.; Saha, A.K.; Khan, M.N.N.; Hossain, M.M.; Sarker, P.K. Improving the sulfate attack resistance of concrete by using supplementary cementitious materials (SCMs): A review. Constr. Build. Mater. 2021, 281, 122628. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Meheron, S.J. Deterioration of marine concrete exposed to wetting-drying action. J. Clean Prod. 2021, 278, 123383. [Google Scholar] [CrossRef]
- He, R.; Zheng, S.; Gan, V.J.; Wang, Z.; Fang, J.; Shao, Y. Damage mechanism and interfacial transition zone characteristics of concrete under sulfate erosion and Dry-Wet cycles. Constr. Build. Mater. 2020, 255, 119340. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 2. Proposed mechanisms. Cem. Concr. Res. 2003, 33, 341–346. [Google Scholar] [CrossRef]
- Ikumi, T.; Segura, I. Numerical assessment of external sulfate attack in concrete structures. A review. Cem. Concr. Res. 2019, 121, 91–105. [Google Scholar] [CrossRef]
- Chen, H.; Huang, H.; Qian, C. Study on the deterioration process of cement-based materials under sulfate attack and drying-wetting cycles. Struct. Concr. 2018, 19, 1225–1234. [Google Scholar] [CrossRef]
- Cefis, N.; Comi, C. Chemo-mechanical modelling of the external sulfate attack in concrete. Cem. Concr. Res. 2017, 93, 57–70. [Google Scholar] [CrossRef]
- Samson, E.; Marchand, J. Modeling the transport of ions in unsaturated cement-based materials. Comput. Struct. 2007, 85, 1740–1756. [Google Scholar] [CrossRef]
- Marchand, J.; Samson, E.; Maltais, Y.; Beaudoin, J.J. Theoretical analysis of the effect of weak sodium sulfate solutions on the durability of concrete. Cem. Concr. Compos. 2002, 24, 317–329. [Google Scholar] [CrossRef]
- Tixier, R.; Mobasher, B. Modeling of damage in cement-based materials subjected to external sulfate attack. I: Formulation. J. Mater. Civ. Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Cefis, N.; Comi, C. Degradation of Concrete Structures due to External Sulfate Attack. Key Eng. Mater. 2016, 711, 310–318. [Google Scholar] [CrossRef]
- Schmidt-Döhl, F.; Rostásy, F.S. A model for the calculation of combined chemical reactions and transport processes and its application to the corrosion of mineral-building materials Part II. Experimental verification. Cem. Concr. Res. 1999, 29, 1047–1053. [Google Scholar] [CrossRef]
- Sarkar, S.; Mahadevan, S.; Meeussen, J.C.L.; Van der Sloot, H.; Kosson, D.S. Numerical simulation of cementitious materials degradation under external sulfate attack. Cem. Concr. Compos. 2010, 32, 241–252. [Google Scholar] [CrossRef]
- Sarkar, S.; Mahadevan, S.; Meeussen, J.C.L.; van Der Sloot, H.; Kosson, D.S. Sensitivity analysis of damage in cement materials under sulfate attack and calcium leaching. J. Mater. Civ. Eng. 2012, 24, 430–440. [Google Scholar] [CrossRef]
- Idiart, A.E.; López, C.M.; Carol, I. Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: A meso-scale model. Cem. Concr. Compos. 2011, 33, 411–423. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Yang, J.; Zou, Y.; Wang, Z. Understanding of the deterioration characteristic of concrete exposed to external sulfate attack: Insight into mesoscopic pore structures. Constr. Build. Mater. 2020, 260, 119932. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Yang, J.; Zou, Y.; Wang, Z. Cracking characteristics and pore development in concrete due to physical attack. Mater. Struct. 2020, 53, 104. [Google Scholar] [CrossRef]
- Haufe, J.; Vollpracht, A. Tensile strength of concrete exposed to sulfate attack. Cem. Concr. Res. 2019, 116, 81–88. [Google Scholar] [CrossRef]
- Nehdi, M.L.; Suleiman, A.R.; Soliman, A.M. Investigation of concrete exposed to dual sulfate attack. Cem. Concr. Res. 2014, 64, 42–53. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, X.; Luo, W. Long-term behaviors of concrete under low-concentration sulfate attack subjected to natural variation of environmental climate conditions. Cem. Concr. Res. 2019, 116, 217–230. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Y.; Tan, Z.; Zhang, B. Investigating the failure process of concrete under the coupled actions between sulfate attack and drying–wetting cycles by using X-ray CT. Constr. Build. Mater. 2016, 108, 129–138. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Tang, L.; Li, X. Resistance of concrete against combined attack of chloride and sulfate under drying–wetting cycles. Constr. Build. Mater. 2016, 106, 650–658. [Google Scholar] [CrossRef]
- Gu, Y.; Martin, R.P.; Metalssi, O.O.; Fen-Chong, T.; Dangla, P. Pore size analyses of cement paste exposed to external sulfate attack and delayed ettringite formation. Cem. Concr. Res. 2019, 123, 105766. [Google Scholar] [CrossRef]
- Hossack, A.M.; Thomas, M.D. The effect of temperature on the rate of sulfate attack of Portland cement blended mortars in Na2SO4 solution. Cem. Concr. Res. 2015, 73, 136–142. [Google Scholar] [CrossRef]
- Yu, C.; Sun, W.; Scrivener, K. Mechanism of expansion of mortars immersed in sodium sulfate solutions. Cem. Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Ikumi, T.; Cavalaro, S.H.; Segura, I.; Aguado, A. Alternative methodology to consider damage and expansions in external sulfate attack modeling. Cem. Concr. Res. 2014, 63, 105–116. [Google Scholar] [CrossRef]
- Kunther, W.; Lothenbach, B.; Scrivener, K.L. On the relevance of volume increase for the length changes of mortar bars in sulfate solutions. Cem. Concr. Res. 2013, 46, 23–29. [Google Scholar] [CrossRef]
- Zhu, J.; Al-samawi, M.; Yang, Y.; Al-Shakhdha, N.A. An approach for simulating and evaluating the effect of sulfate attack on the durability of concrete bridges based on a three-dimensional cellular automata model. Eng. Struct. 2023, 291, 116451. [Google Scholar] [CrossRef]
- Ran, B.; Li, K.; Fen-Chong, T.; Omikrine-Metalssi, O.; Dangla, P. Spalling rate of concretes subject to combined leaching and external sulfate attack. Cem. Concr. Res. 2022, 162, 106951. [Google Scholar] [CrossRef]
- Melara, E.K.; Trentin, P.O.; Pereira, E.; Medeiros-Junior, R.A. Contribution to the service-life modeling of concrete exposed to sulfate attack by the inclusion of electrical resistivity data. Constr. Build. Mater. 2022, 322, 126490. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, M.; Zou, D.; Liu, T. A failure thickness prediction model for concrete exposed to external sulfate attack. Constr. Build. Mater. 2024, 416, 135202. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.X. Numerical modelling of mechanical deterioration of cement mortar under external sulfate attack. Constr. Build. Mater. 2018, 158, 490–502. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Sun, X.H.; Tang, Y.J. Macro-microscopically numerical analysis on expansion response of hardened cement paste under external sulfate attack. Constr. Build. Mater. 2019, 207, 600–615. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Tang, Y.J.; Ayinde, O.; Wang, J.L. Numerical simulation on time-dependent mechanical behavior of concrete under coupled axial loading and sulfate attack. Ocean Eng. 2017, 142, 115–124. [Google Scholar] [CrossRef]
- Sun, C.; Yuan, L.; Zhai, X.; Qu, F.; Li, Y.; Hou, B. Numerical and experimental study of moisture and chloride transport in unsaturated concrete. Constr. Build. Mater. 2018, 189, 1067–1075. [Google Scholar] [CrossRef]
- Whittaker, M.; Black, L. Current knowledge of external sulfate attack. Adv. Cem. Res. 2015, 27, 532–545. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, S.; Lyu, H.; Chen, C.; Zou, D.; Zhou, A.; Li, Y.; Liu, T. A transport-chemical-physical-mechanical model for concrete subjected to external sulfate attack and drying-wetting cycles. Eng. Fract. Mech. 2023, 293, 109726. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, M.; Qin, S.; Liu, T.; Tong, W.; Zhou, A.; Jivkov, A. Calcium leaching from cement hydrates exposed to sodium sulfate solutions. Constr. Build. Mater. 2022, 351, 128975. [Google Scholar] [CrossRef]
- Qin, S.; Zou, D.; Liu, T.; Jivkov, A. A chemo-transport-damage model for concrete under external sulfate attack. Cem. Concr. Res. 2020, 132, 106048. [Google Scholar] [CrossRef]
- Zou, D.; Qin, S.; Liu, T.; Jivkov, A. Experimental and numerical study of the effects of solution concentration and temperature on concrete under external sulfate attack. Cem. Concr. Res. 2021, 139, 106284. [Google Scholar] [CrossRef]
- Flatt, R.J.; Scherer, G.W. Thermodynamics of crystallization stresses in DEF. Cem. Concr. Res. 2008, 38, 325–336. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Kunther, W.; Lothenbach, B.; Scrivener, K. Influence of bicarbonate ions on the deterioration of mortar bars in sulfate solutions. Cem. Concr. Res. 2013, 44, 77–86. [Google Scholar] [CrossRef]
- Bary, B. Simplified coupled chemo-mechanical modeling of cement pastes behavior subjected to combined leaching and external sulfate attack. Int. J. Numer. Anal. Methods Geomech. 2008, 32, 1791–1816. [Google Scholar] [CrossRef]
- Bary, B.; Leterrier, N.; Deville, E.; Le Bescop, P. Coupled chemo-transport-mechanical modelling and numerical simulation of external sulfate attack in mortar. Cem. Concr. Compos. 2014, 49, 70–83. [Google Scholar] [CrossRef]
- Basista, M.; Weglewski, W. Micromechanical modeling of sulphate corrosion in concrete: Influence of ettringite forming reaction. Theor. Appl. Mech. 2008, 35, 29–52. [Google Scholar] [CrossRef]
- Pel, L. Moisture transport in porous building materials. Heron J. 1995, 41, 95–105. [Google Scholar]
- Zhang, Y. Mechanics of Chloride Ions Transport in Concrete. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2008. (In Chinese). [Google Scholar]
- Li, C. Study on Water and Ionic Transport Processes in Cover Concrete under Drying-wetting Cycles. Ph.D. Thesis, Tsinghua University, Beijing, China, 2009. (In Chinese). [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Chaube, R.; Kishi, T.; Maekawa, K. Modelling of Concrete Performance: Hydration, Microstructure and Mass Transport, 1st ed.; CRC Press: London, UK, 1999. [Google Scholar]
- Wu, J. Modelling and Simulation of Chloride Ions Transmission in Concrete. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2012. (In Chinese). [Google Scholar]
- Li, C.; Li, K.; Chen, Z. Numerical analysis of moisture influential depth in concrete during drying-wetting cycles. Tsinghua Sci. Technol. 2008, 13, 696–701. [Google Scholar] [CrossRef]
- Guan, B.W.; Wu, J.Y.; Yang, T.; Xu, A.H.; Sheng, Y.P.; Chen, H.X. Developing a model for chloride ions transport in cement concrete under dynamic flexural loading and dry-wet cycles. Math. Probl. Eng. 2017, 2017, 5760512. [Google Scholar] [CrossRef]
- Gummerson, R.J.; Hall, C.; Hoff, W.D.; Hawkes, R.; Holland, G.N.; Moore, W.S. Unsaturated water flow within porous materials observed by NMR imaging. Nature 1979, 281, 56–57. [Google Scholar] [CrossRef]
- Yin, G.J.; Li, L.B.; Wen, X.D.; Miao, L.; Wang, S.S.; Zuo, X.B. Numerical modelling on moisture and sulfate ion transport in unsaturated concrete slab under dry-wet cycles. J. Build. Eng. 2024, 89, 109296. [Google Scholar] [CrossRef]
- Wong, S.F.; Wee, T.H.; Swaddiwudhipong, S.; Lee, S.L. Study of water movement in concrete. Mag. Concr. Res. 2001, 53, 205–220. [Google Scholar] [CrossRef]
- Liu, P. Study on the Moisture Transport Law of Concrete under Dry-wet Cycles and Sulfate Attack. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2013. (In Chinese). [Google Scholar]
- Zheng, S.; He, R.; Chen, H.; Wang, Z.; Huang, X.; Liu, S. Three-dimensional reconstruction and sulfate ions transportation of interfacial transition zone in concrete under dry-wet cycles. Constr. Build. Mater. 2021, 291, 123370. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, M.; Hou, D.; Li, Z. External sulfate attack to reinforced concrete under drying-wetting cycles and loading condition: Numerical simulation and experimental validation by ultrasonic array method. Constr. Build. Mater. 2017, 139, 365–373. [Google Scholar] [CrossRef]
- Li, J.; Xie, F.; Zhao, G.; Li, L. Experimental and numerical investigation of cast-in-situ concrete under external sulfate attack and drying-wetting cycles. Constr. Build. Mater. 2020, 249, 118789. [Google Scholar] [CrossRef]
- Shan, Z.Q.; Yin, G.J.; Wen, X.D.; Miao, L.; Wang, S.S.; Zuo, X.B. Numerical simulation on transport-crystallization-mechanical behavior in concrete structure under external sulfate attack and wetting–drying cycles. Mater. Des. 2024, 241, 112908. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Lothenbach, B.; Bary, B.; Le Bescop, P.; Schmidt, T.; Leterrier, N. Sulfate ingress in Portland cement. Cem. Concr. Res. 2010, 40, 1211–1225. [Google Scholar] [CrossRef]
- Scherer, G.W. Factors affecting crystallization pressure. In Proceedings of the International RILEM Workshop on International Sulfate Attack and Delayed Ettringite Formation, Villars, Switzerland, 4–6 September 2002. [Google Scholar]
- Scrivener, K.L.; Taylor, H.F.W. Delayed ettringite formation: A microstructural and microanalytical study. Adv. Cem. Res. 1993, 5, 139–146. [Google Scholar] [CrossRef]
- Chen, H. Strengths Prediction of Concrete in Marine Environments Based on Dry-Wet Cycles and Salt Crystallization Translated into Stress. Ph.D. Thesis, Southeast University, Nanjing, China, 2018. (In Chinese). [Google Scholar]
- Yang, F. Damage Modeling of Concrete subjected to Coupled Action of Crystallization of Sulfate and Drying-Wetting Cycles. Master’s Thesis, Southeast University, Nanjing, China, 2012. (In Chinese). [Google Scholar]
- Ren, J.; Lai, Y.; Bai, R.; Qin, Y. The damage mechanism and failure prediction of concrete under wetting–drying cycles with sodium sulfate solution. Constr. Build. Mater. 2020, 264, 120525. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Sun, X.H.; Tang, Y.J. Numerical investigation of the external sulfate attack induced expansion response of cement paste by using crystallization pressure. Model. Simul. Mater. Sci. Eng. 2019, 27, 025006. [Google Scholar] [CrossRef]
- De Maio, U.; Greco, F.; Lonetti, P.; Pranno, A. A combined ALE-cohesive fracture approach for the arbitrary crack growth analysis. Eng. Fract. Mech. 2024, 301, 109996. [Google Scholar] [CrossRef]
- Rimkus, A.; Cervenka, V.; Gribniak, V.; Cervenka, J. Uncertainty of the smeared crack model applied to RC beams. Eng. Fract. Mech. 2020, 233, 107088. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, S.; Chen, C.; Zhang, M. Modeling of Concrete Deterioration under External Sulfate Attack and Drying–Wetting Cycles: A Review. Materials 2024, 17, 3334. https://doi.org/10.3390/ma17133334
Qin S, Chen C, Zhang M. Modeling of Concrete Deterioration under External Sulfate Attack and Drying–Wetting Cycles: A Review. Materials. 2024; 17(13):3334. https://doi.org/10.3390/ma17133334
Chicago/Turabian StyleQin, Shanshan, Chuyu Chen, and Ming Zhang. 2024. "Modeling of Concrete Deterioration under External Sulfate Attack and Drying–Wetting Cycles: A Review" Materials 17, no. 13: 3334. https://doi.org/10.3390/ma17133334





