The Mineral Apposition Rate on Implants with Either a Sandblasted Acid-Etched Implant Surface (SLA) or a Nanostructured Calcium-Incorporated Surface (XPEED®): A Histological Split-Mouth, Randomized Case/Control Human Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgical Procedure
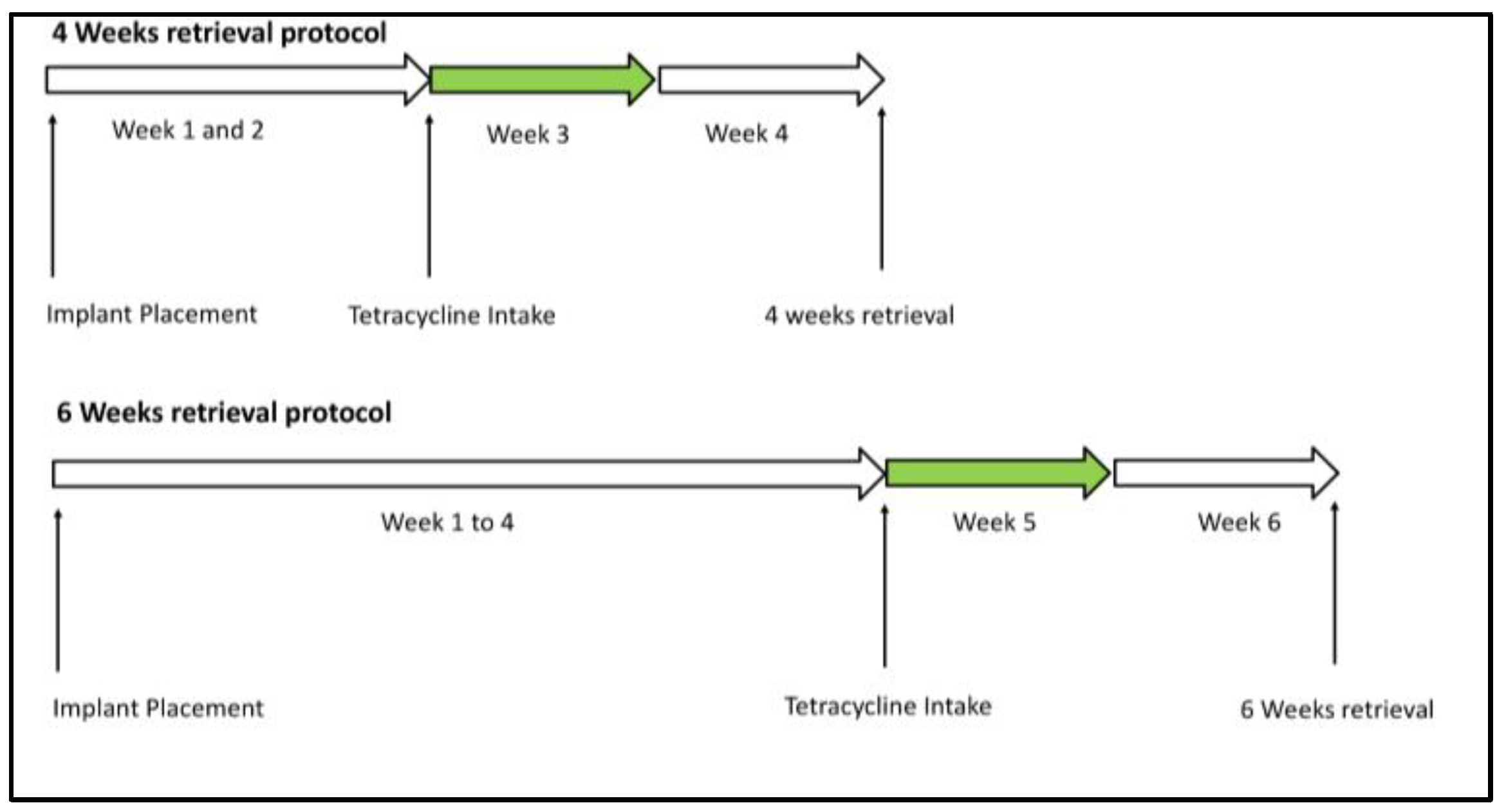
2.3. Working Mechanism and Fluorochrome Use
2.4. Histological Processing
2.5. Statistical Analysis
3. Results
3.1. Clinical Outcome
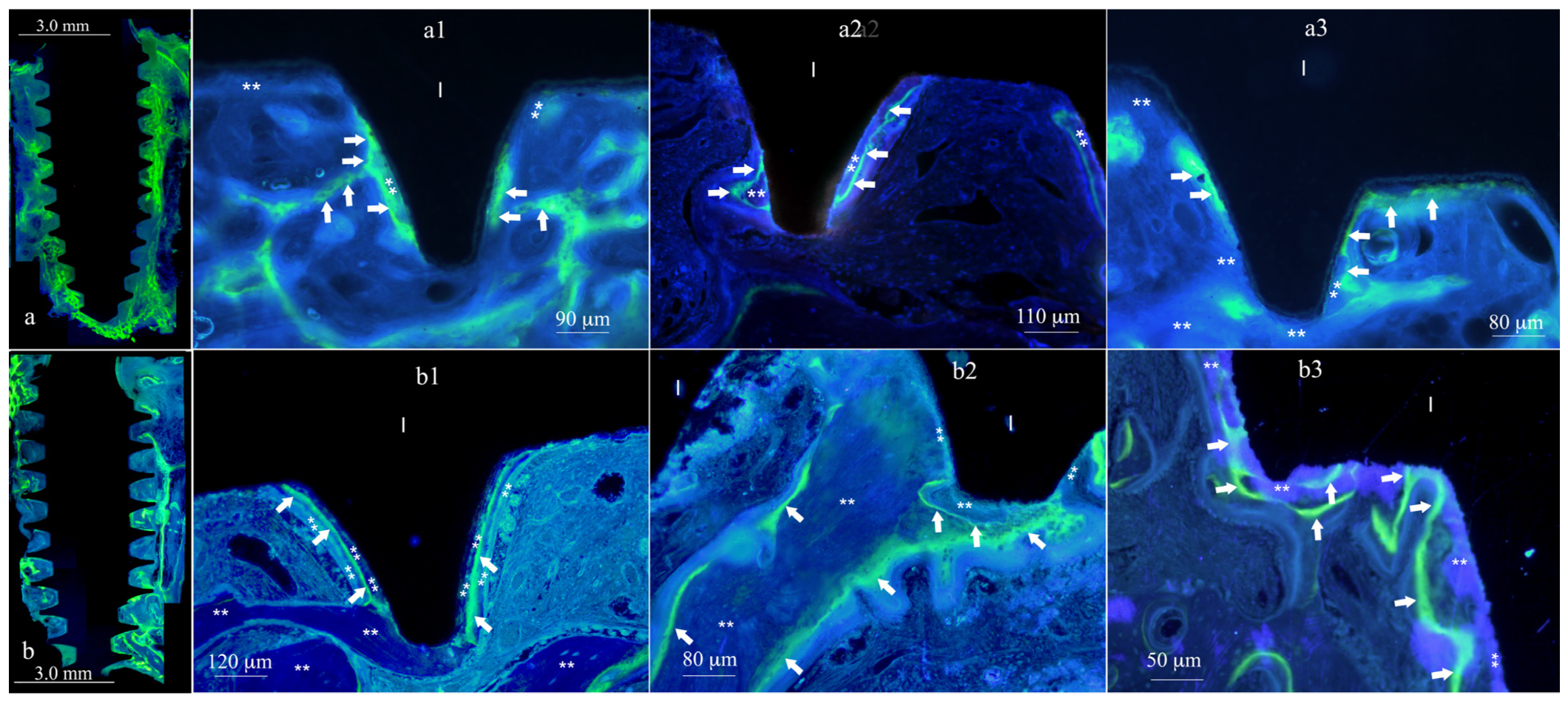
3.2. Histomorphometric
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fang, S.; Zhong, Q.; Qi, S. Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings 2022, 12, 1654. [Google Scholar] [CrossRef]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; Gil, J.; Jimenez-Guerra, A. Influence of the Titanium Implant Surface Treatment on the Surface Roughness and Chemical Composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.; Åström, M. The Influence of Implant Surface on Maintenance of Marginal Bone Levels for Three Premium Implant Brands: A Systematic Review and Meta-analysis. Int. J. Oral. Maxillofac. Implant. 2020, 35, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Makary, C.; Menhall, A.; Zammarie, C.; Lombardi, T.; Lee, S.Y.; Stacchi, C.; Park, K.B. Primary Stability Optimization by Using Fixtures with Different Thread Depth According To Bone Density: A Clinical Prospective Study on Early Loaded Implants. Materials 2019, 12, 2398. [Google Scholar] [CrossRef] [PubMed]
- Demiroglu, U.; Ocak, H.; Songur, T.; Çolpak, H.A. Which dental implant surface is more effective in osteointegration: RBM surface versus SLA surface. Ann. Clin. Anal. Med. 2021, 12, 736–739. [Google Scholar] [CrossRef]
- Şener-Yamaner, I.D.; Yamaner, G.; Sertgöz, A.; Çanakçi, C.F.; Özcan, M. Marginal Bone Loss Around Early-Loaded SLA and SLActive Implants: Radiological Follow-Up Evaluation Up to 6.5 Years. Implant. Dent. 2017, 26, 592–599. [Google Scholar] [CrossRef]
- Nicolau, P.; Guerra, F.; Reis, R.; Krafft, T.; Benz, K.; Jackowski, J. 10-year outcomes with immediate and early loaded implants with a chemically modified SLA surface. Quintessence Int. 2019, 50, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Makary, C.; Menhall, A.; Lahoud, P.; An, H.W.; Park, K.B.; Traini, T. Nanostructured Calcium-Incorporated Surface Compared to Machined and SLA Dental Implants—A Split-Mouth Randomized Case/Double-Control Histological Human Study. Nanomaterials 2023, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Harrison, J.E.; Reed, R.; Cruickshank, B. Bone apposition rate as an index of bone metabolism. Metabolism 1978, 27, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Schopper, C.; Moser, D.; Spassova-Tzekova, E.; Russmueller, G.; Goriwoda, W.; Lagogiannis, G.; Ewers, R.; Redl, H. Mineral apposition rates provide significant information on long-term effects in BMP-induced bone regeneration. J. Biomed Mater. Res. A 2009, 89, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Kataoka, Y.; Sonobe, J.; Yamamoto, H.; Maruyama, H.; Yamamoto, T.; Bessho, K.; Ohura, K. Analysis of mineral apposition rates during alveolar bone regeneration over three weeks following transfer of BMP-2/7 gene via in vivo electroporation. Eur. J. Histochem. 2018, 62, 2947. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G. Bone mineral apposition rates at early implantation times around differently prepared titanium surfaces: A study in beagle dogs. J. Oral. Maxillofac. Implant. 2011, 26, 63–69. [Google Scholar]
- Ristow, O.; Nehrbass, D.; Zeiter, S.; Arens, D.; Moratin, J.; Pautke, C.; Hoffmann, J.; Freudlsperger, C.; Otto, S. Differences between auto-fluorescence and tetracycline-fluorescence in medication-related osteonecrosis of the jaw—A preclinical proof of concept study in the mini-pig. Clin. Oral. Investig. 2020, 24, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- van Gaalen, S.M.; Kruyt, M.C.; Geuze, R.E.; de Bruijn, J.D.; Alblas, J.; Dhert, W.J.A. Use of Fluorochrome Labels in In Vivo Bone Tissue Engineering Research. Tissue Eng. Part. B Rev. 2010, 16, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Erben, R.G. Bone-labeling techniques. In Handbook of Histology Methods for Bone and Cartilage; Springer: Berlin/Heidelberg, Germany, 2003; pp. 99–117. [Google Scholar]
- Júnior, B.K.; de Carvalho Lopes, C. Bone remodeling analysis after placement of dental implants using polyfluorochrome sequential labeling. Ann. Anat.-Anat. Anz. 2002, 184, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Zhao, B.; Jiang, S. Accelerated and enhanced osteointegration of MAO-treated implants: Histological and histomorphometric evaluation in a rabbit model. Int. J. Oral. Sci. 2018, 10, 11. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Solheim, T. Pluricolor fluorescent labeling of mineralizing tissue. Eur. J. Oral. Sci. 1974, 82, 19–27. [Google Scholar] [CrossRef]
- Blomquist, L.; Hanngren, Å. Fluorescence technique applied to whole body sections for distribution studies of tetracyclines. Biochem. Pharmacol. 1966, 15, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Ravidà, A.; Wang, H.L.; Helms, J.; Brunski, J. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral. Maxillofac. Implant. 2019, 34, S7–S23. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.P.; Cao, N.J.; Zhu, Y.H.; Wang, W. The osseointegration and stability of dental implants with different surface treatments in animal models: A network meta-analysis. Sci. Rep. 2021, 11, 13849. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Tovar, N.; Anchieta, R.; Machado, L.; Marin, C.; Teixeira, H.; Coelho, P. The combined effects of undersized drilling and implant macrogeometry on bone healing around dental implants: An experimental study. Int. J. Oral. Maxillofac. Surg. 2014, 43, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi, L.; Balzani, L.; Cucchi, A.; Ghensi, P.; Nocini, P. Primary and Secondary Stability of Implants in Postextraction and Healed Sites: A Randomized Controlled Clinical Trial. Int. J. Oral. Maxillofac. Implant. 2016, 31, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Açil, Y.; Sievers, J.; Gülses, A.; Ayna, M.; Wiltfang, J.; Terheyden, H. Correlation between resonance frequency, insertion torque and bone-implant contact in self-cutting threaded implants. Odontology 2017, 105, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Gasik, M.; Braem, A.; Chaudhari, A.; Duyck, J.; Vleugels, J. Titanium implants with modified surfaces: Meta-analysis of in vivo osteointegration. Mater. Sci. Eng. C 2015, 49, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials 2007, 28, 4748–4755. [Google Scholar] [CrossRef]
- Webster, T.J.; Ejiofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Ding, X.; Xu, B.; Wang, J.; Li, Y.; Chen, F.; Meng, F.; Song, W.; Zhang, Y. Nanoporous titanium implant surface promotes osteogenesis by suppressing osteoclastogenesis via integrin β1/FAKpY397/MAPK pathway. Bioact. Mater. 2022, 8, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Mangano, F.G.; Iezzi, G.; Shibli, J.A.; Pires, J.T.; Luongo, G.; Piattelli, A.; Mangano, C. Early bone formation around immediately loaded implants with nanostructured calcium-incorporated and machined surface: A randomized, controlled histologic and histomorphometric study in the human posterior maxilla. Clin. Oral. Investig. 2017, 21, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Milch, R.A.; Rall, D.P.; Tobie, J.E. Bone localization of the tetracyclines. J. Natl. Cancer Inst. 1957, 19, 87–93. [Google Scholar] [PubMed]
- Milch, R.A.; Rall, D.P.; Tobie, J.E. Fluorescence of Tetracycline Antibiotics in Bone. JBJS 1958, 40, 897–910. [Google Scholar] [CrossRef]
- Frost, H.M. measurement of human bone formation by means of tetracycline labelling. Can. J. Biochem. Physiol. 1963, 41, 31–42. [Google Scholar] [CrossRef]
- Duewelhenke, N.; Krut, O.; Eysel, P. Influence on Mitochondria and Cytotoxicity of Different Antibiotics Administered in High Concentrations on Primary Human Osteoblasts and Cell Lines. Antimicrob. Agents Chemother. 2007, 51, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Fernandes, M.H. Effect of therapeutic levels of doxycycline and minocycline in the proliferation and differentiation of human bone marrow osteoblastic cells. Arch. Oral. Biol. 2007, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Rauch, F. Watching bone cells at work: What we can see from bone biopsies. Pediatr. Nephrol. 2006, 21, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Krasnoshtein, F.; Hryhorenko, L.; Sindrey, D. Quantification of Bone Formation on Calcium Phosphate Ceramic Thin Film, in Vitro by Tetracycline Labelling. Microsc. Microanal. 1998, 4 (Suppl. S2), 936–937. [Google Scholar] [CrossRef]
- Frost, H.M. Tetracycline-based histological analysis of bone remodeling. Calcif. Tissue Res. 1969, 3, 211–237. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Simpo, S.; Lee, M.; Oikawa, T.; Yoshii, T.; Noda, K.; Kuwahara, Y.; Kawasaki, K. Histology and tetracycline labeling of a single section of alveolar bone of first molars in the rat. Biotech. Histochem. 2000, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lum, L.B.; Ross Beirne, O. Viability of the retained bone core in the core-vent dental implant. J. Oral. Maxillofac. Surg. 1986, 44, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Haider, R.; Watzek, G.; Plenk, H. Effects of Drill Cooling and Bone Structure on IMZ Implant Fixation. Int. J. Oral. Maxillofac. Implant. 1993, 8, 83–91. [Google Scholar]
- Degidi, M.; Scarano, A.; Piattelli, M.; Perrotti, V.; Piattelli, A. Bone Remodeling in Immediately Loaded and Unloaded Titanium Dental Implants: A Histologic and Histomorphometric Study in Humans. J. Oral. Implantol. 2005, 31, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans: Early osseointegration on implant surfaces. Clin. Oral. Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J. The Effect of Implant Thread Design on Implant Stability in the Early Post-operative Period. Clin. Oral. Implant. Res. 2017, 28, 1218–1226. [Google Scholar] [CrossRef]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Height of the residual bone crest in the programmed implant site ≥ 9 mm and thickness ≥ 7 mm. | Myocardial infarction within the past 6 months |
| Availability, in each sector, of sufficient mesiodistal space allowing for the placement of 2 standard-sized implants and at least 2 mini-implants (3.5 × 8.5 mm) for retrieval | Poorly controlled diabetes (HBA1c > 7.5%) |
| Healed bone crest (≥3 months elapsed after extraction or tooth loss). | Coagulation disorders |
| Age > 18 years | Radiotherapy to the head/neck area within the past two years |
| Ability to examine and fully understand the study protocol | Present or past treatment with intravenous bisphosphonates |
| Immunocompromised patients | |
| Psychological or psychiatric problems | |
| Alcohol or drug abuse | |
| Poor oral hygiene and motivation (full mouth plaque score > 30% and/or full mouth bleeding score > 20%) | |
| Uncontrolled periodontal disease |
| Dose Taken | Dose at Half-Life Time (12 h) | Time Lapse (hours) |
|---|---|---|
| 500 mg | 250 mg | 12 |
| 500 mg | 325 mg | 24 |
| 500 mg | 412.5 mg | 36 |
| 500 mg | 456.25 mg | 48 |
| - | 228 mg | 52 |
| - | 114 mg | 64 |
| - | 57 mg | 76 |
| - | 28.5 mg | 88 |
| - | 14.25 mg | 100 |
| - | 7.12 mg | 112 |
| - | 3.56 mg | 124 |
| - | 1.78 mg | 136 |
| - | 0.89 mg | 148 |
| - | 0.445 mg | 160 |
| Column | Size | Missing | Mean | Std Dev | Std. Error | C.I. of Mean |
|---|---|---|---|---|---|---|
| SLA MAR3w | 3 | 0 | 1.570 | 0.106 | 0.0611 | 0.263 |
| XPEED MAR3w | 3 | 0 | 2.043 | 0.180 | 0.104 | 0.447 |
| SLA MAR5w | 5 | 0 | 1.152 | 0.129 | 0.0576 | 0.160 |
| XPEED MAR5w | 6 | 0 | 1.292 | 0.233 | 0.0951 | 0.244 |
| Column | Range | Max | Min | Median | 25% | 75% |
| SLA MAR3w | 0.200 | 1.690 | 1.490 | 1.530 | 1.500 | 1.650 |
| XPEED MAR3w | 0.330 | 2.250 | 1.920 | 1.960 | 1.930 | 2.178 |
| SLA MAR5w | 0.310 | 1.280 | 0.970 | 1.140 | 1.068 | 1.273 |
| XPEED MAR5w | 0.670 | 1.650 | 0.980 | 1.280 | 1.130 | 1.430 |
| Column | Skewness | Kurtosis | K-S Dist. | K-S Prob. | Sum | Sum of Squares |
| SLA MAR3w | 1.458 | -- | 0.314 | 0.268 | 4.710 | 7.417 |
| XPEED MAR3w | 1.636 | -- | 0.345 | 0.175 | 6.130 | 12.590 |
| SLA MAR5w | −0.475 | −0.867 | 0.220 | 0.499 | 5.760 | 6.702 |
| XPEED MAR5w | 0.336 | 0.164 | 0.170 | 0.690 | 7.750 | 10.282 |
| Group Name | N | Missing | Mean | Std Dev | SEM |
|---|---|---|---|---|---|
| SLA MAR3w | 3 | 0 | 1.570 | 0.106 | 0.0612 |
| XPEED MAR3w | 3 | 0 | 2.043 | 0.180 | 0.104 |
| Difference −0.473 | |||||
| t = −3.922 with 4 degrees of freedom. (p = 0.017) | |||||
| 95 percent confidence interval for difference of means: −0.808 to −0.138 | |||||
| The difference in mean values of the two groups is greater than the values expected by chance; there is a statistically significant difference between the input groups (p = 0.017). | |||||
| Group Name | N | Missing | Mean | Std Dev | SEM |
| SLA MAR5w | 5 | 0 | 1.150 | 0.1000 | 0.0447 |
| XPEED MAR5w | 6 | 0 | 1.290 | 0.1000 | 0.0408 |
| Difference −0.140 | |||||
| t = −2.312 with 9 degrees of freedom. (p = 0.046) | |||||
| 95 percent confidence interval for difference of means: −0.277 to −0.00302 | |||||
| The difference in the mean values of the two groups is greater than the values expected by chance; there is a statistically significant difference between the input groups (p = 0.046). | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menhall, A.; Lahoud, P.; Yang, K.R.; Park, K.B.; Razukevicius, D.; Traini, T.; Makary, C. The Mineral Apposition Rate on Implants with Either a Sandblasted Acid-Etched Implant Surface (SLA) or a Nanostructured Calcium-Incorporated Surface (XPEED®): A Histological Split-Mouth, Randomized Case/Control Human Study. Materials 2024, 17, 3341. https://doi.org/10.3390/ma17133341
Menhall A, Lahoud P, Yang KR, Park KB, Razukevicius D, Traini T, Makary C. The Mineral Apposition Rate on Implants with Either a Sandblasted Acid-Etched Implant Surface (SLA) or a Nanostructured Calcium-Incorporated Surface (XPEED®): A Histological Split-Mouth, Randomized Case/Control Human Study. Materials. 2024; 17(13):3341. https://doi.org/10.3390/ma17133341
Chicago/Turabian StyleMenhall, Abdallah, Pierre Lahoud, Kyung Ran Yang, Kwang Bum Park, Dainius Razukevicius, Tonino Traini, and Christian Makary. 2024. "The Mineral Apposition Rate on Implants with Either a Sandblasted Acid-Etched Implant Surface (SLA) or a Nanostructured Calcium-Incorporated Surface (XPEED®): A Histological Split-Mouth, Randomized Case/Control Human Study" Materials 17, no. 13: 3341. https://doi.org/10.3390/ma17133341
APA StyleMenhall, A., Lahoud, P., Yang, K. R., Park, K. B., Razukevicius, D., Traini, T., & Makary, C. (2024). The Mineral Apposition Rate on Implants with Either a Sandblasted Acid-Etched Implant Surface (SLA) or a Nanostructured Calcium-Incorporated Surface (XPEED®): A Histological Split-Mouth, Randomized Case/Control Human Study. Materials, 17(13), 3341. https://doi.org/10.3390/ma17133341







