Synthesis of Heterostructured TiO2 Nanopores/Nanotubes by Anodizing at High Voltages
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Anodizing Titanium with High Voltage
2.2. Surface Morphology Analysis and Electrochemical Characteristics
2.3. Biological Compatibility Evaluation
3. Results and Discussion
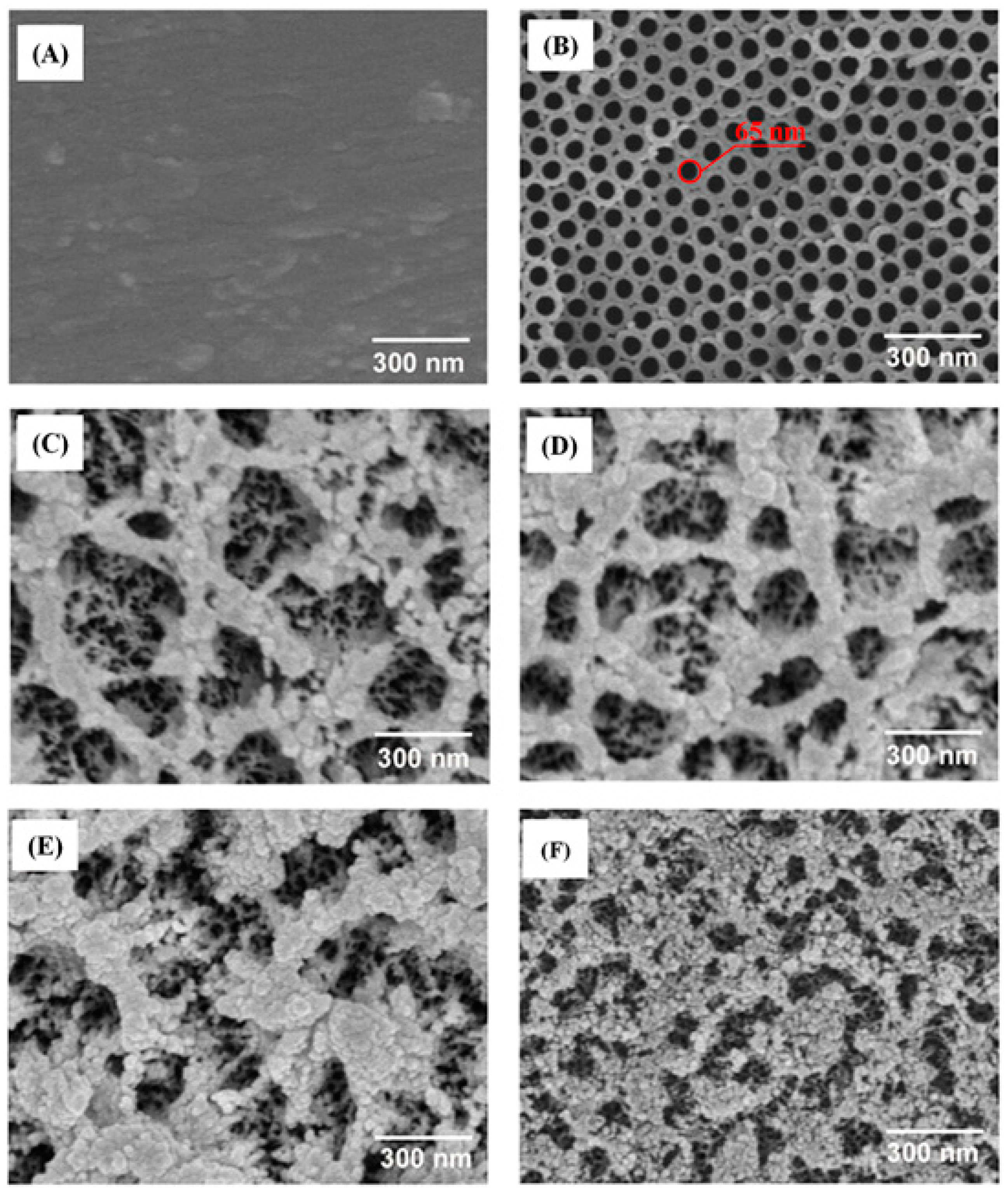
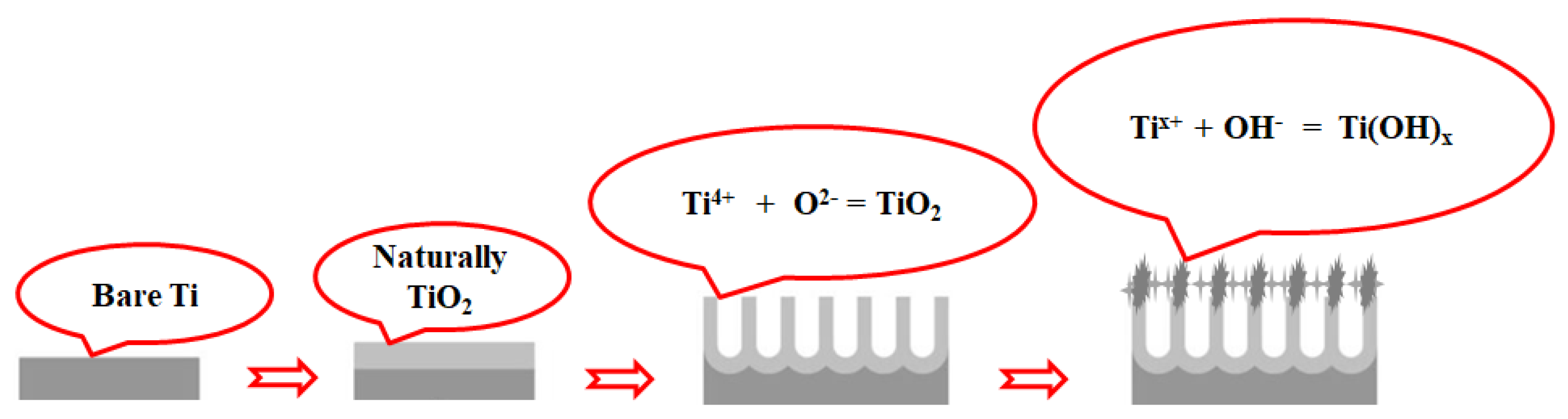
3.1. Surface Properties
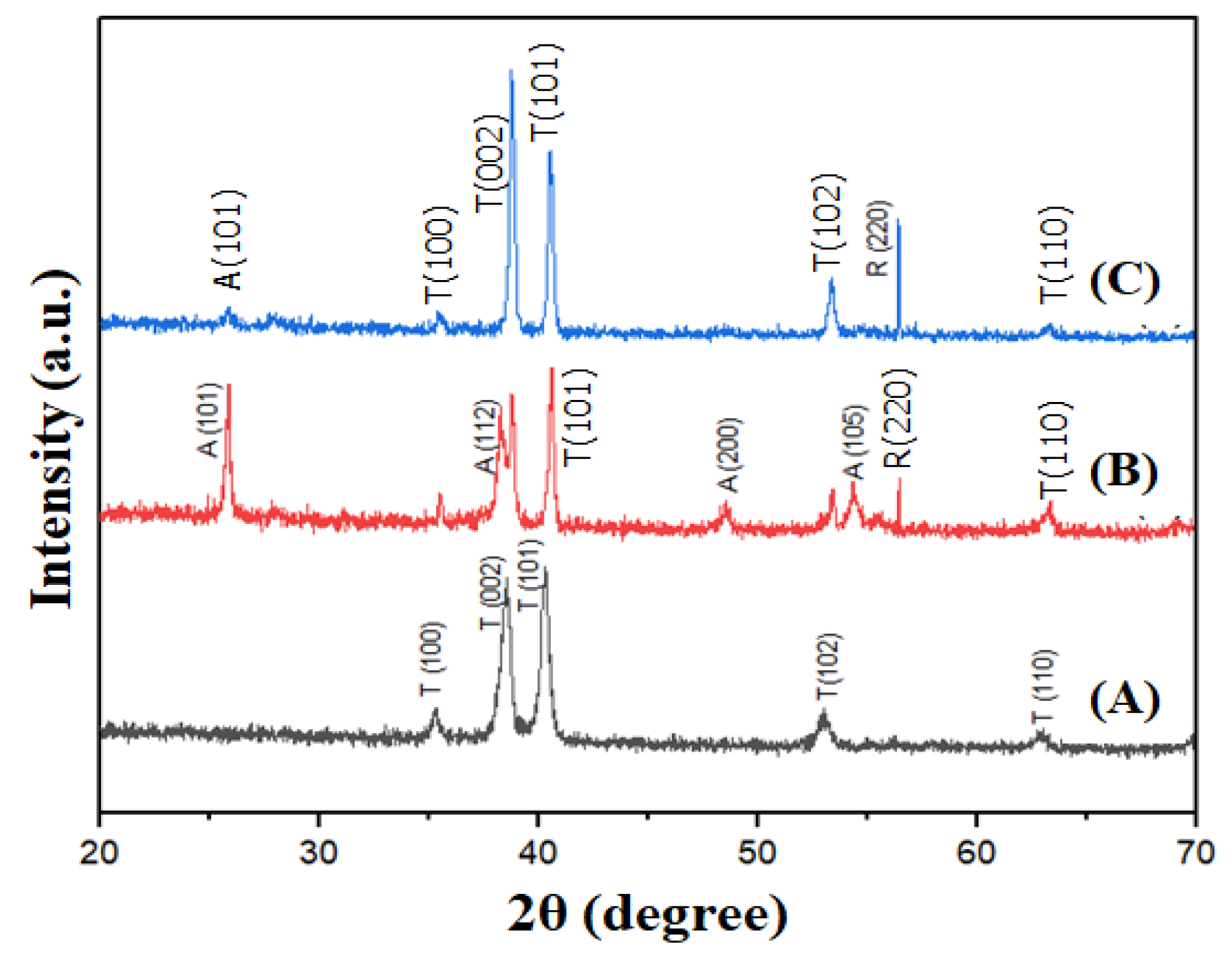
3.2. X-ray Diffraction Study
3.3. Electrochemical Properties
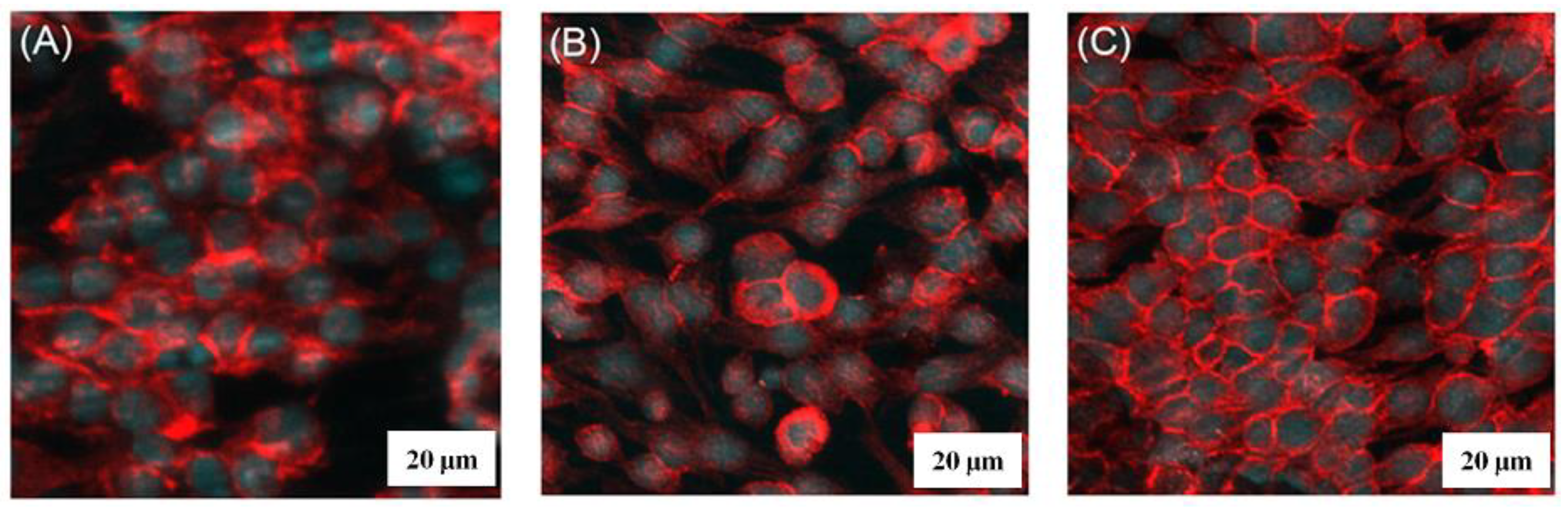
3.4. Cell Attachment Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manivasagam, V.K.; Popat, K.C. Hydrothermally treated titanium surfaces for enhanced osteogenic differentiation of adipose derived stem cells. Mater. Sci. Eng. C 2021, 128, 112315. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Komasa, S.; Morimoto, Y.; Sekino, T.; Kawazoe, T.; Okazaki, J. UV/ozone irradiation manipulates immune response for antibacterial activity and bone regeneration on titanium. Mater. Sci. Eng. C 2021, 129, 112377. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Desai, T.A. TiO2-Based Nanotopographical Cues Attenuate the Restenotic Phenotype in Primary Human Vascular Endothelial and Smooth Muscle Cells. ACS Biomater. Sci. Eng. 2020, 6, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, R.A.; Echeverry-Rendon, M.; Robledo, S.; Echeverría, F.E. Effect of TiO2 nanotubes size, heat treatment, and UV irradiation on osteoblast behavior. Mater. Chem. Phys. 2022, 275, 125137. [Google Scholar] [CrossRef]
- Motola, M.; Capek, J.; Zazpe, R.; Bacova, J.; Hromadko, L.; Bruckova, L.; Ng, S.; Handl, J.; Spotz, Z.; Knotek, P.; et al. Thin TiO2 Coatings by ALD Enhance the Cell Growth on TiO2 Nanotubular and Flat Substrates. ACS Appl. Bio Mater. 2020, 3, 6447–6456. [Google Scholar] [CrossRef] [PubMed]
- Demetrescu, I.; Pirvu, C.; Mitran, V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry 2010, 79, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.D.; Yook, S.W.; Kim, H.E.; Koh, Y.H. Fabrication of titanium scaffolds with porosity and pore size gradients by sequential freeze casting. Mater. Lett. 2009, 63, 1545–1547. [Google Scholar] [CrossRef]
- Pham, V.H.; Jang, T.S.; Jung, H.D.; Kim, H.E.; Koh, Y.H. Creation of nanoporous tantalum (Ta)-incorporated titanium (Ti) surface onto Ti implants by sputtering of Ta in Ar under extremely high negative substrate biases. J. Mater. Chem. 2012, 22, 24798–24804. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, H.; Zhao, L.; Sun, Y.; Guan, S.; Liu, B.; Kong, L. The effect of hierarchical micro/nanosurface titanium implant on osseointegration in ovariectomized sheep. Osteoporos. Int. 2011, 22, 1907–1913. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, K.; Choi, K.H.; Kim, S.H.; Kim, S.E.; Jeong, C.M.; Huh, J.B. Cell adhesion and in vivo osseointegration of sandblasted/acid etched/anodized dental implants. Int. J. Mol. Sci. 2015, 16, 10324–10336. [Google Scholar] [CrossRef]
- Wang, G.; Wan, Y.; Ren, B.; Liu, Z. Bioactivity of micropatterned TiO2 nanotubes fabricated by micro-milling and anodic oxidation. Mater. Sci. Eng. C 2019, 95, 114–121. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, T.; Wang, Z.; Jin, Y.; Liu, Z. Construction and characterization of micro/nano-topography on titanium alloy formed by micro-milling and anodic oxidation. Int. J. Adv. Manuf. Technol. 2018, 98, 29–35. [Google Scholar] [CrossRef]
- Fan, X.; Feng, B.; Liu, Z.; Tan, J.; Zhi, W.; Lu, X.; Wang, J.; Weng, J. Fabrication of TiO2 nanotubes on porous titanium scaffold and biocompatibility evaluation in vitro and in vivo. J. Biomed. Mater. Res. Part A 2012, 100A, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.E.; Shin, K.H.; Koh, Y.H. Improving the strength and biocompatibility of porous titanium scaffolds by creating elongated pores coated with a bioactive, nanoporous TiO2 layer. Mater. Lett. 2010, 64, 2526–2529. [Google Scholar] [CrossRef]
- Jeremiasz, K.; Jaroslaw, J. Effect of the High Voltage Anodic Oxidation on the Titanium Corrosion Resistance. Solid State Phenom. 2015, 227, 479–482. [Google Scholar] [CrossRef]
- Asumpinwong, W.; Kanokwan, S.; Viritpon, S. Different constant voltages of anodization on the corrosion behavior of Ti-6Al-4V alloy. Chiang Mai J. Sci. 2015, 42, 239–248. [Google Scholar]
- Il, S.P.; Tae, G.W.; Min, H.L.; Seung, G.A. Effects of anodizing voltage on the anodized and hydrothermally treated titanium surface. Met. Mater. Int. 2006, 12, 505–511. [Google Scholar] [CrossRef]
- Chayanis, V.; Tachakorn, K.; Pimduen, R.; Pisaisit, C. Color Formation on Titanium Surface Treated by Anodization and the Surface Characteristics: A Review. CM Dent. J. 2023, 44, 13–21. [Google Scholar] [CrossRef]
- Lucas, A.G.; Andrea, G.S.; Emilio, J.P.; Wido, H.S.; Silvia, M.C.; Josefina, B. Chemical and mechanical properties of anodized cp-titanium in NH4H2PO4/NH4F media for biomedical applications. Surf. Coat. Technol. 2012, 206, 4791–4798. [Google Scholar] [CrossRef]
- Giuseppe, N.; Marco, P.; Tiziana, V.; Andrea, D.S. Colouring titanium alloys by anodic oxidation. Metalurgija 2018, 57, 111. [Google Scholar]
- Bradford, J.P.; Hernandez-Moreno, G.; Pillai, R.R.; Hernandez-Nichols, A.L.; Thomas, V. Low-Temperature Plasmas Improving Chemical and Cellular Properties of Poly (Ether Ether Ketone) Biomaterial for Biomineralization. Materials 2024, 17, 171. [Google Scholar] [CrossRef]
- Tian, G.; Dong, L.; Wei, C.; Huang, J.; He, H.; Shao, J. Investigation on microstructure and optical properties of titanium dioxide coatings annealed at various temperature. Opt. Mater. 2006, 28, 1058–1063. [Google Scholar] [CrossRef]
- Mohan, L.; Dennis, C.; Padmapriya, N.; Anandan, C.; Rajendran, N. Effect of Electrolyte Temperature and Anodization Time on Formation of TiO2 Nanotubes for Biomedical Applications. Mater. Today Commun. 2020, 23, 101103. [Google Scholar] [CrossRef]
- Ali, G.; Kim, H.J.; Kim, J.J.; Cho, S.O. Formation of self-organized Zircaloy-4 oxide nanotubes in organic viscous electrolyte via anodization. Nanoscale Res. Lett. 2014, 9, 553. [Google Scholar] [CrossRef]
- Guan, D.; Wang, Y. Synthesis and growth mechanism of multilayer TiO2 nanotube arrays. Nanoscale 2012, 4, 2968–2977. [Google Scholar] [CrossRef]
- Shokuhfar, T.; Sinha-Ray, S.; Sukotjo, C.; Yarin, A.L. Intercalation of anti-inflammatory drug molecules within TiO2 nanotubes. RSC Adv. 2013, 3, 17380. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Xue, Q.G.; Annabella, S. Reactivity of Anatase TiO2 Nanoparticles: The Role of the Minority (001) Surface. J. Phys. Chem. B 2005, 109, 19560–19562. [Google Scholar] [CrossRef]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in Biomedical Applications-Properties and Fabrication: A Review. J. Biomater. Tissue Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Hu, R.; Shi, B.; Zhang, L.; Huang, Q.; Yang, Y.; Tang, P.; Lin, C. Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation. Bioact. Mater. 2023, 27, 15–57. [Google Scholar] [CrossRef]
- Mutalik, C.; Hsiao, Y.-C.; Chang, Y.-H.; Krisnawati, D.I.; Alimansur, M.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Wang, D.-Y.; Kuo, T.-R. High UV-Vis-NIR Light-Induced Antibacterial Activity by Heterostructured TiO2-FeS2 Nanocomposites. Int. J. Nanomed. 2020, 15, 8911–8920. [Google Scholar] [CrossRef] [PubMed]
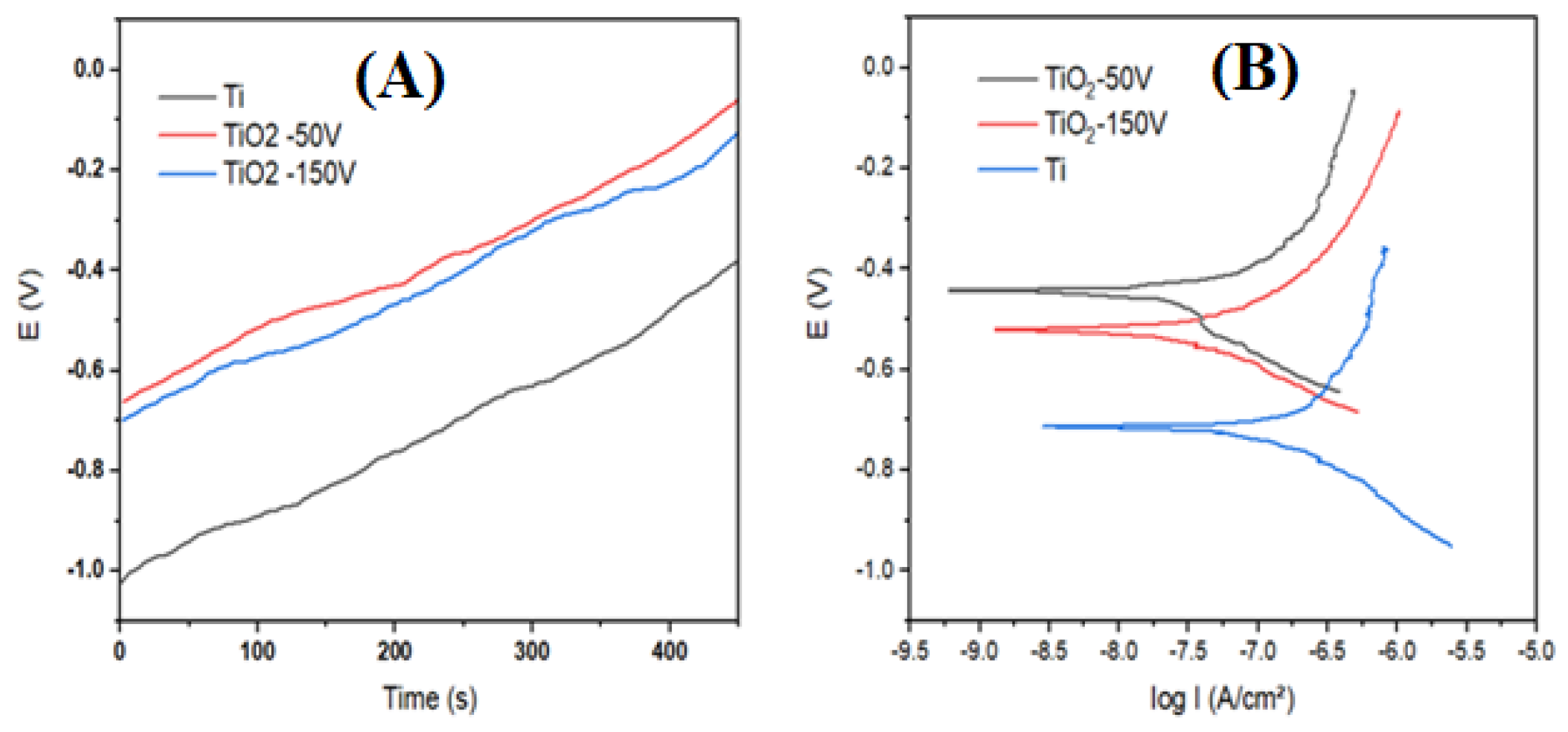

| Samples | Ecorr (V) | icorr (10−7 A/cm2) | Rcorr (kΩcm2) | Corrosion Rate (mg/m2h) | CP Efficient H (%) |
|---|---|---|---|---|---|
| Bare Ti | −0.717 | 1.280 | 332 | 5.73 | 0% |
| TiO2 50 V | −0.442 | 0.157 | 1059 | 0.70 | 87.78% |
| TiO2 150 V | −0.515 | 0.305 | 945 | 1.36 | 76.26% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuan, T.Q.; Toan, L.V.; Pham, V.-H. Synthesis of Heterostructured TiO2 Nanopores/Nanotubes by Anodizing at High Voltages. Materials 2024, 17, 3347. https://doi.org/10.3390/ma17133347
Tuan TQ, Toan LV, Pham V-H. Synthesis of Heterostructured TiO2 Nanopores/Nanotubes by Anodizing at High Voltages. Materials. 2024; 17(13):3347. https://doi.org/10.3390/ma17133347
Chicago/Turabian StyleTuan, Ta Quoc, Le Van Toan, and Vuong-Hung Pham. 2024. "Synthesis of Heterostructured TiO2 Nanopores/Nanotubes by Anodizing at High Voltages" Materials 17, no. 13: 3347. https://doi.org/10.3390/ma17133347





