Engineered Mesoporous Silica-Based Nanoparticles: Characterization of Surface Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
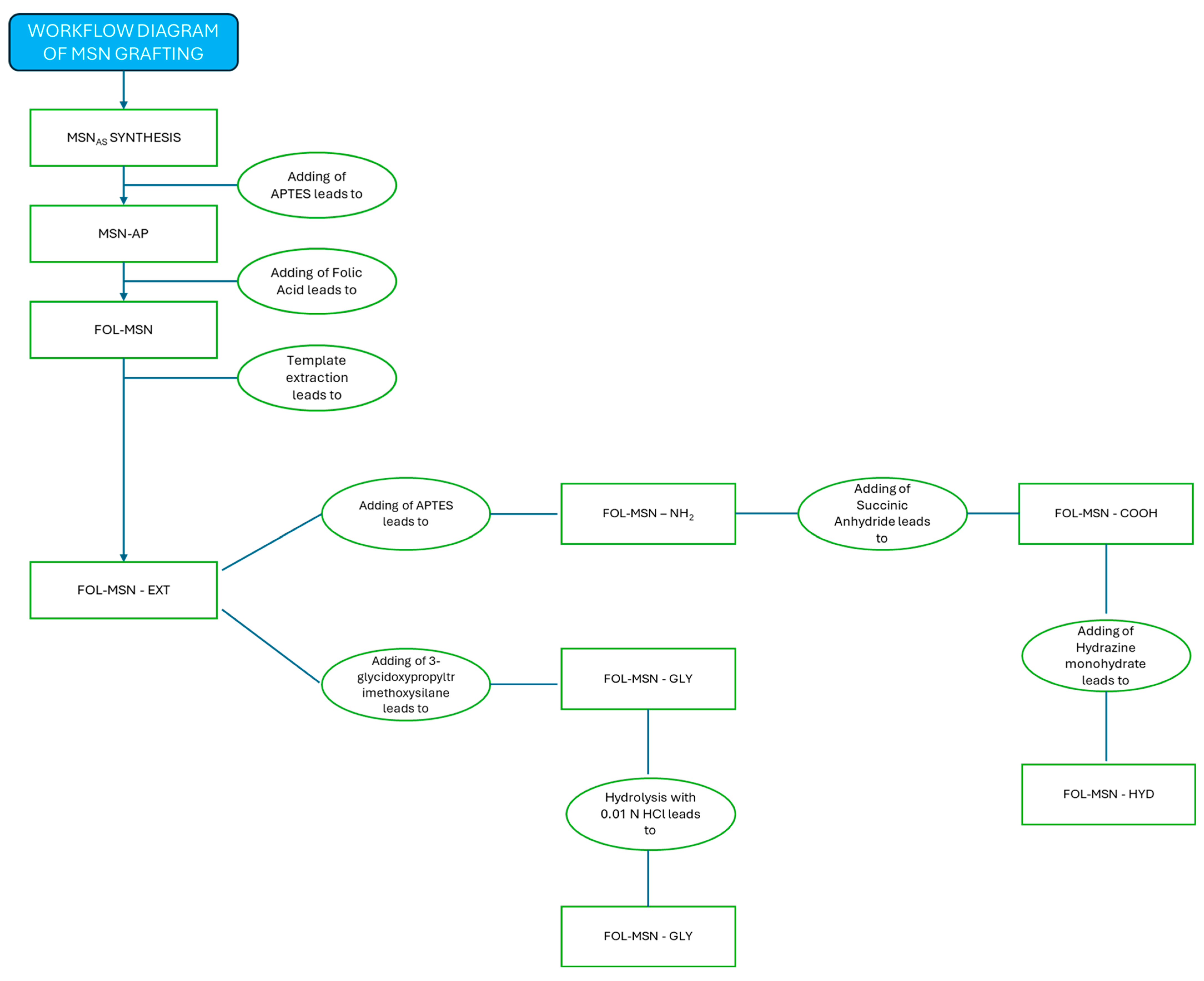
MSNAS Synthesis
2.2. Grafting Protocol
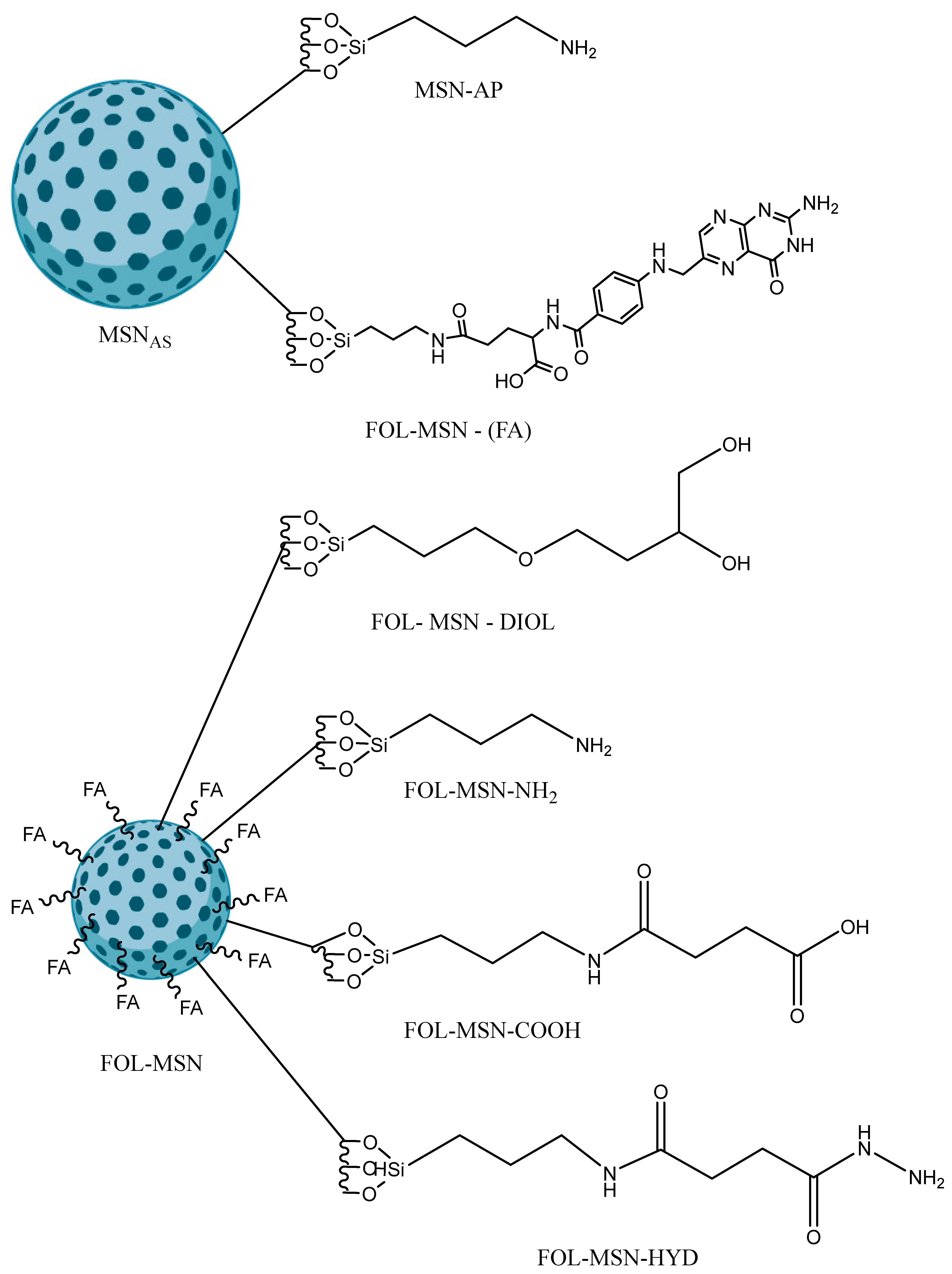
2.2.1. Synthesis of Amine-Functionalized MSNs
2.2.2. Synthesis of FOL-MSN
2.2.3. Template Extraction Protocol
2.2.4. Synthesis of FOL-MSN-DIOL
2.2.5. Synthesis of FOL-MSN-NH2
2.2.6. Synthesis of FOL-MSN-COOH
2.2.7. Synthesis of FOL-MSN-HYD
2.3. Instrumental Characterizations
3. Results
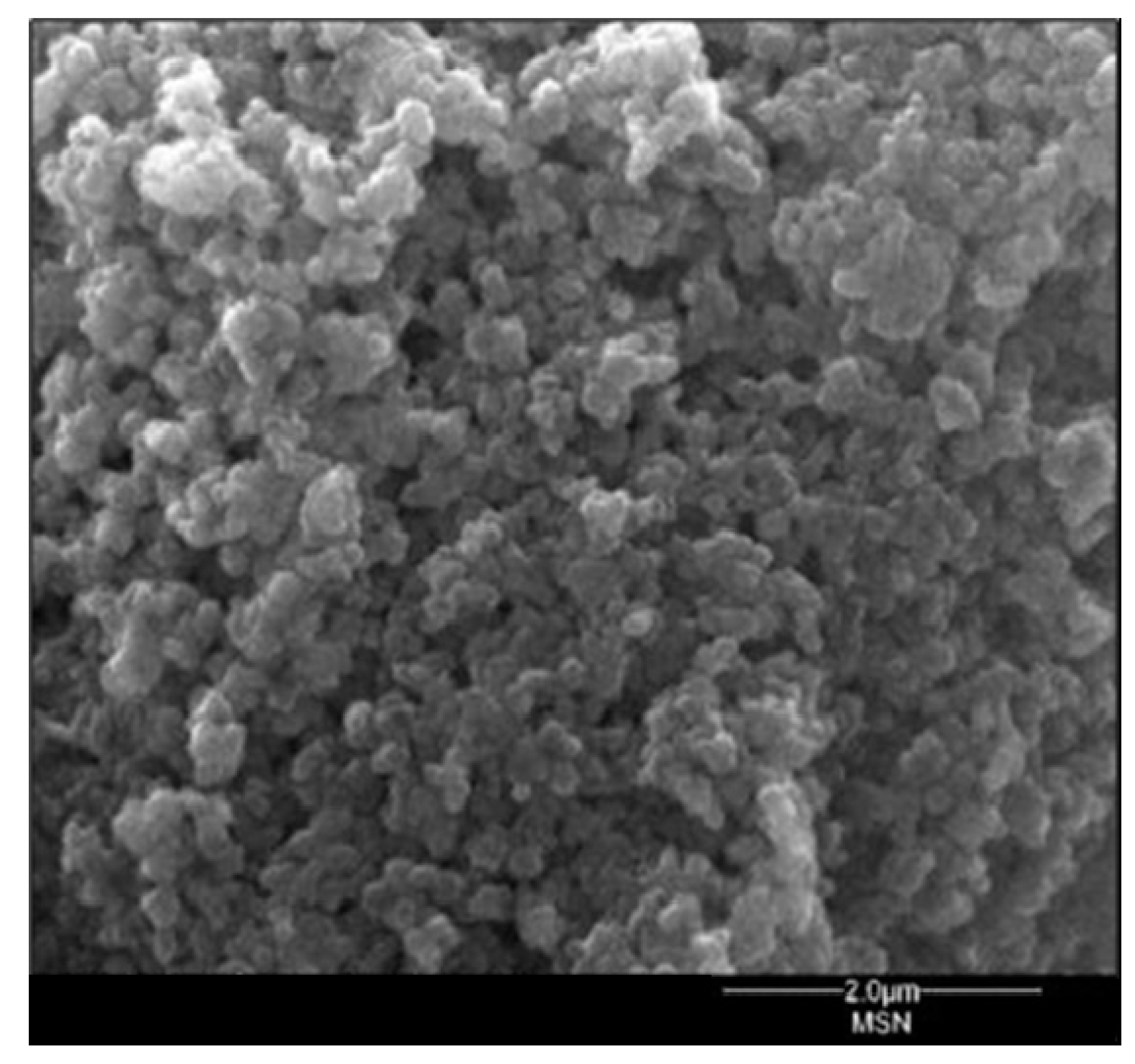
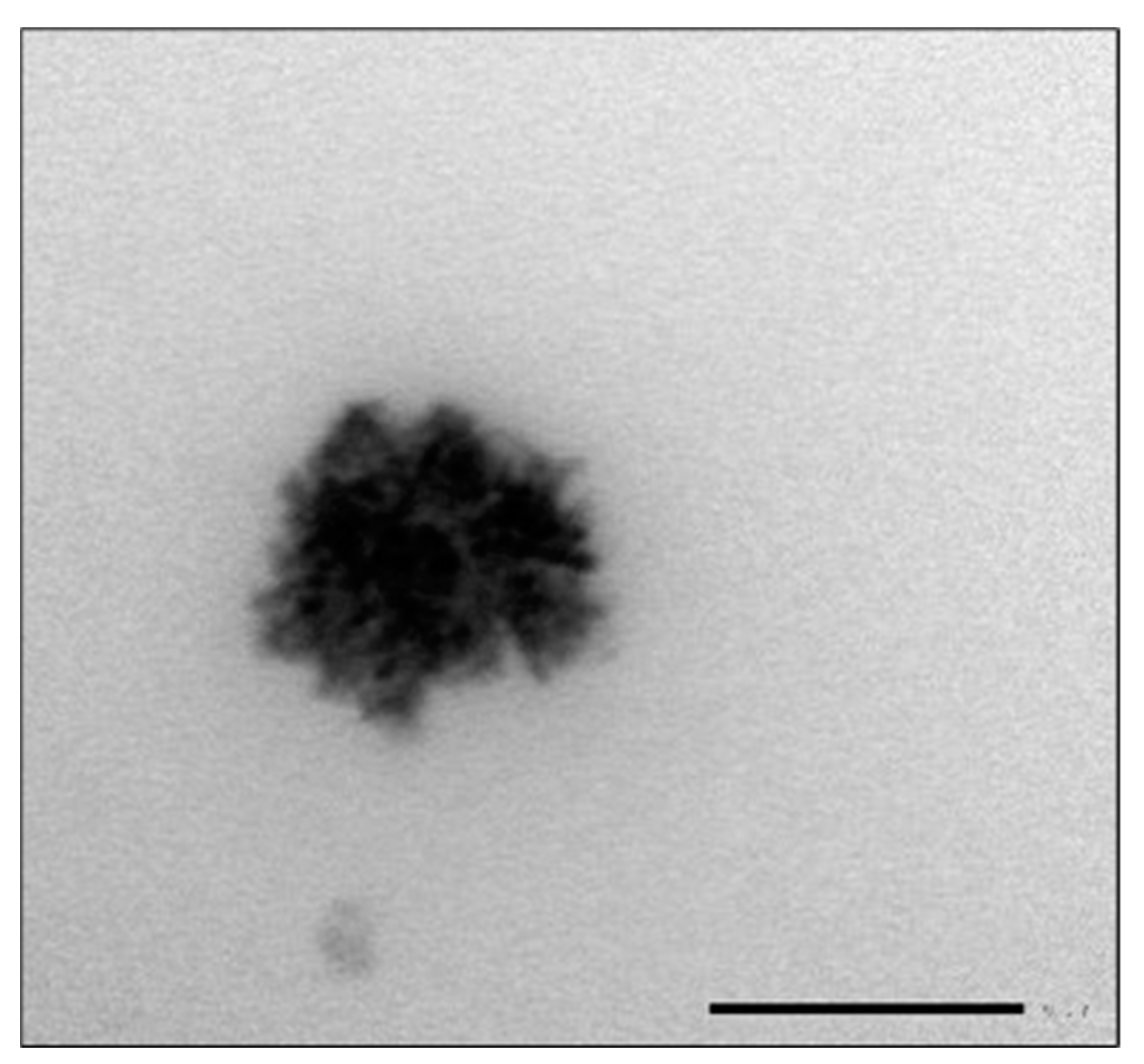
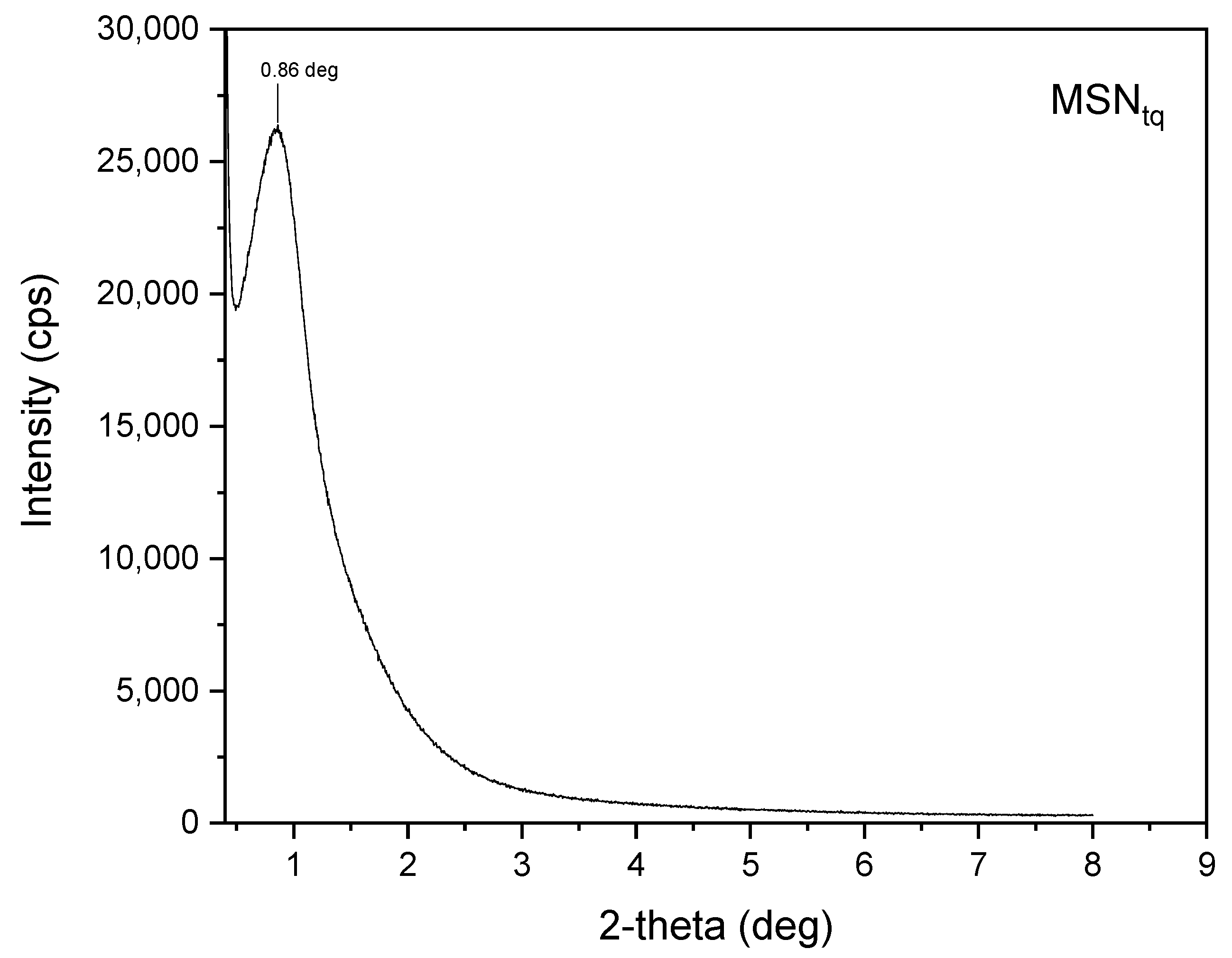
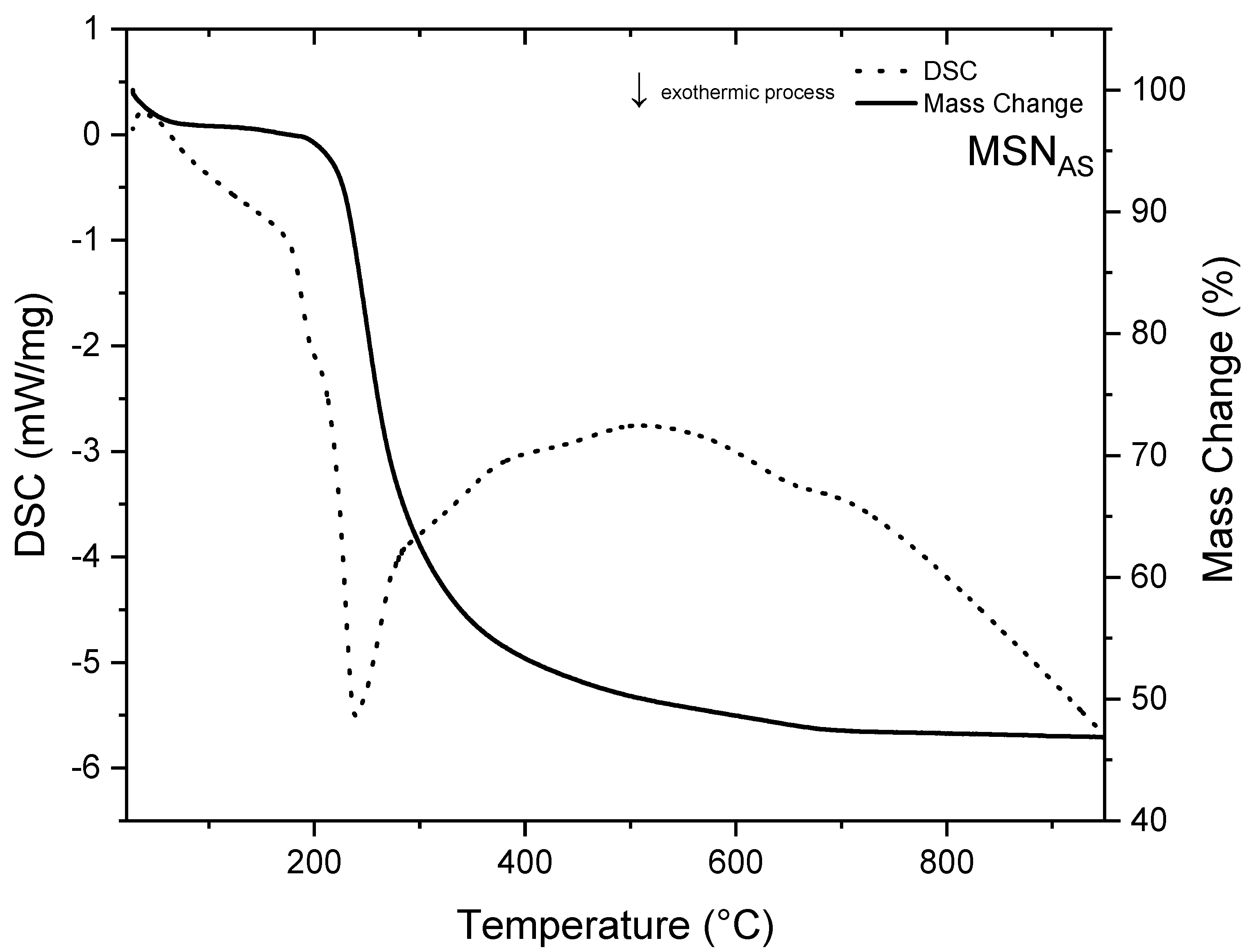
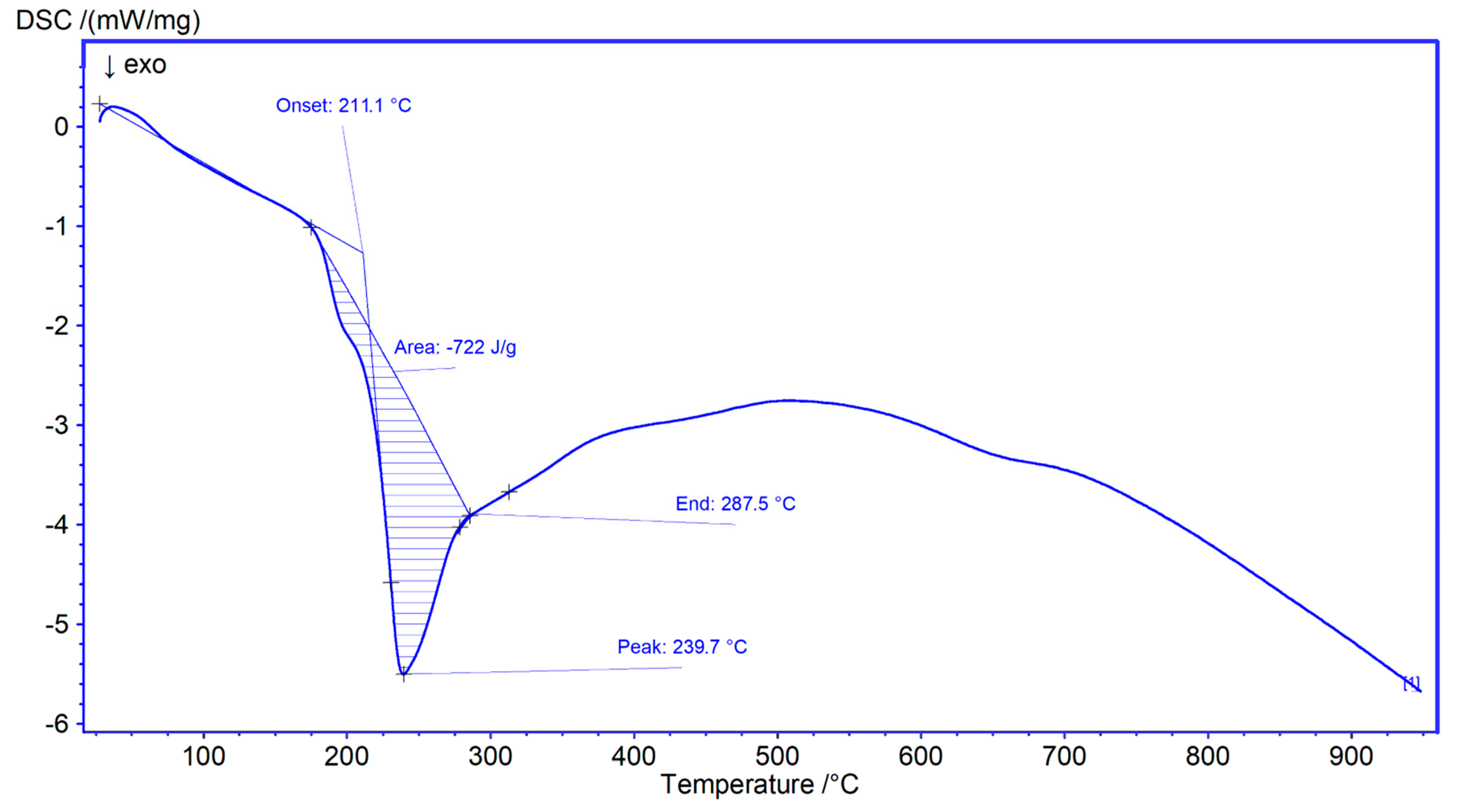
3.1. MSNAS Characterization
3.2. Functionalization of MSNAS Sample and Characterization of Obtained Materials
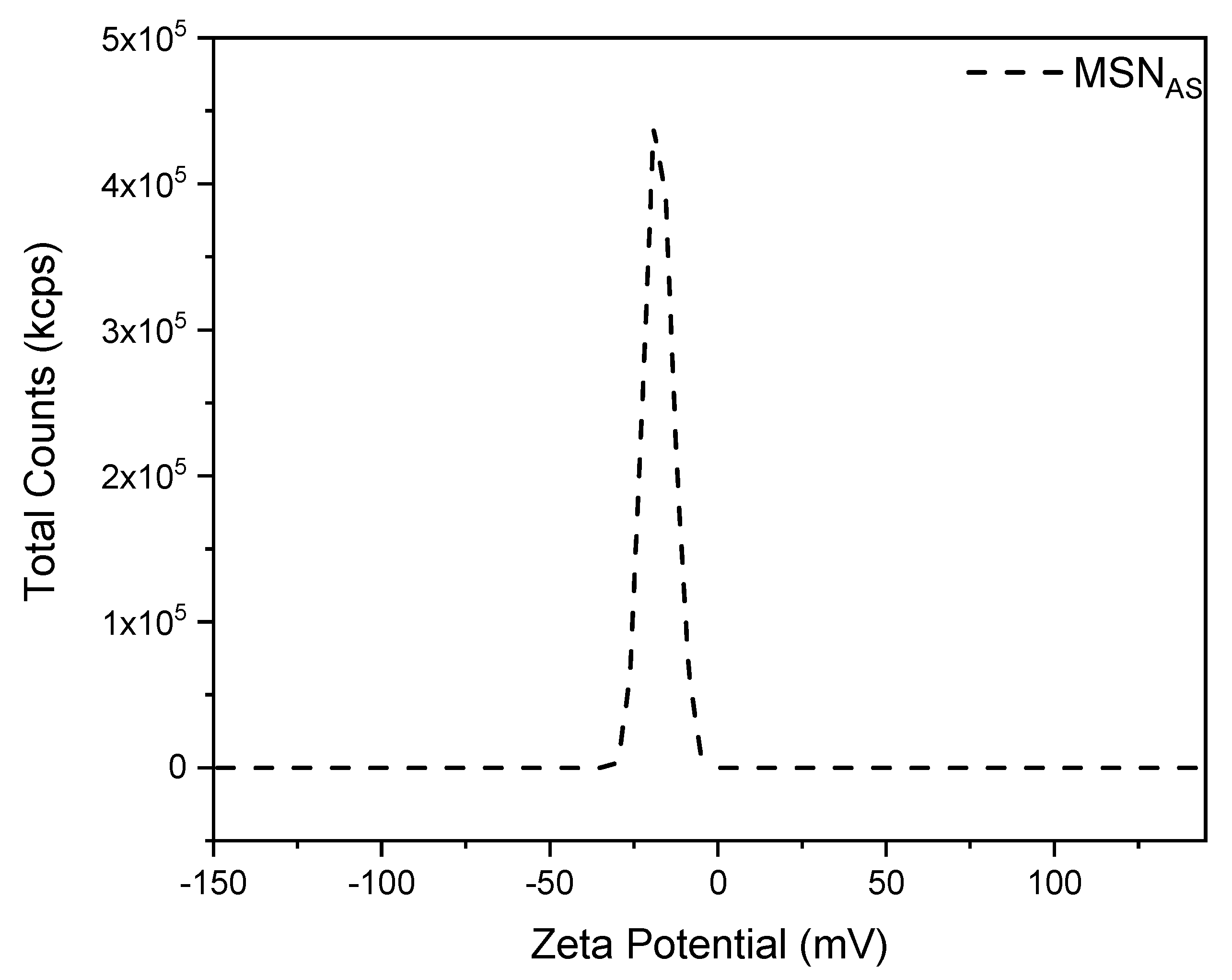
3.3. Z-Potential Characterization
3.4. FTIR Characterization
3.5. Nitrogen Adsorption Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vallet-Regí, M. Our contributions to applications of mesoporous silica nanoparticles. Acta Biomater. 2022, 137, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Liu, Z.; Ohsuna, T.; Sakamoto, K.; Terasaki, O.; Tatsumi, T. Synthesis and characterization of chiral mesoporous silica. Nature 2004, 429, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Preianò, M.; Pasqua, L.; Gallelli, L.; Galasso, O.; Gasparini, G.; Savino, R.; Terracciano, R. Simultaneous extraction and rapid visualization of peptidomic and lipidomic body fluids fingerprints using mesoporous aluminosilicate and MALDI-TOF MS. Proteomics 2012, 12, 3286–3294. [Google Scholar] [CrossRef]
- Casadonte, F.; Pasqua, L.; Savino, R.; Terracciano, R. Smart trypsin adsorption into N-(2-aminoethyl)-3-aminopropyl-modified mesoporous silica for ultra-fast protein digestion. Chem. Eur. J. 2010, 30, 8998–9001. [Google Scholar] [CrossRef]
- Ceresa, C.; Nicolini, G.; Rigolio, R.; Bossi, M.; Pasqua, L.; Cavaletti, G. Functionalized mesoporous silica nanoparticles: A possible strategy to target cancer cells reducing peripheral nervous system uptake. Curr. Med. Chem. 2013, 20, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Han, Y.H.; Xia, H.Y.; Wang, S.B.; Chen, A.Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotech. 2022, 20, 126. [Google Scholar] [CrossRef]
- Costa, J.A.S.; de Jesus, R.A.; Santos, D.O.; Neris, J.B.; Figueiredo, R.T.; Paranhos, C.M. Synthesis, functionalization, and environmental application of silica-based mesoporous materials of the M41S and SBA-n families: A review. J. Environ. Chem. Eng. 2021, 9, 105259. [Google Scholar] [CrossRef]
- Roshan, A.; Kumar, M. Water end-use estimation can support the urban water crisis management: A critical review. J. Environ. Manag. 2020, 268, 110663. [Google Scholar] [CrossRef]
- Tevapitak, K.; Helmsing, A.B. The interaction between local governments and stakeholders in environmental management: The case of water pollution by SMEs in Thailand. J. Environ. Manag. 2019, 247, 840–848. [Google Scholar] [CrossRef]
- Sagbo, O.; Sun, Y.; Hao, A.; Gu, P. Effect of PAC addition on MBR process for drinking water treatment. Sep. Purif. Technol. 2008, 58, 320–327. [Google Scholar] [CrossRef]
- Samal, K.; Kar, S.; Trivedi, S. Ecological floating bed (EFB) for decontamination of polluted water bodies: Design, mechanism and performance. J. Environ. Manag. 2019, 251, 109550. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.A.; Muhammad, M.H. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Grisolia, A.; Dell’Olio, G.; Spadafora, A.; De Santo, M.; Morelli, C.; Leggio, A.; Pasqua, L. Hybrid polymer-silica nanostructured materials for environmental remediation. Molecules 2023, 28, 5105. [Google Scholar] [CrossRef] [PubMed]
- Youssef, H.M.; Abdullah, A.M.; Azzam, M.A.; Kenawy, I.M. Facile synthesis and characterization of folic acid-modified silica nanoparticles and its exploration for adsorptive removal of aluminum (III) from aqueous media. J. Dispers. Sci. Technol. 2023, 44, 1940–1952. [Google Scholar] [CrossRef]
- Almethen, A.A.; Alotaibi, K.M.; Alhumud, H.S.; Alswieleh, A.M. Highly efficient and rapid removal of methylene blue from aqueous solution using folic acid-conjugated dendritic mesoporous silica nanoparticles. Processes 2022, 10, 705. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Garud, H.B.; Thoravat, S.S.; Patil, V.S.; Shinde, P.S.; Burungale, S.H.; Patil, P.S. Synthesis and testing of functional mesoporous silica nanoparticles for removal of Cr (VI) ions from water. Biointerface Res. Appl. Chem. 2021, 11, 8599–8607. [Google Scholar]
- Cueto-Díaz, E.J.; Castro-Muñiz, A.; Suárez-García, F.; Gálvez-Martínez, S.; Torquemada-Vico, M.C.; Valles-González, M.P.; Mateo-Martí, E. Aptes-based silica nanoparticles as a potential modifier for the selective sequestration of CO2 gas molecules. Nanomaterials 2021, 11, 2893. [Google Scholar] [CrossRef] [PubMed]
- Cueto-Díaz, E.J.; Suárez-García, F.; Gálvez-Martínez, S.; Valles-González, M.P.; Mateo-Marti, E. CO2 adsorption capacities of amine-functionalized microporous silica nanoparticles. React. Funct. Polym. 2022, 170, 105100. [Google Scholar] [CrossRef]
- Salman, M.; Jahan, S.; Kanwal, S.; Mansoor, F. Recent advances in the application of silica nanostructures for highly improved water treatment: A review. Environ. Sci. Poll. Res. 2019, 26, 21065–21084. [Google Scholar] [CrossRef]
- Jayalath, S.; Larsen, S.C.; Grassian, V.H. Surface adsorption of Nordic aquatic fulvic acid on amine-functionalized and non-functionalized mesoporous silica nanoparticles. Environ. Sci. Nano 2018, 5, 2162–2171. [Google Scholar] [CrossRef]
- Qiao, M.; Liu, X.; Song, J.W.; Yang, T.; Chen, M.L.; Wang, J.H. Improving the adsorption capacity for ovalbumin by functional modification of aminated mesoporous silica nanoparticles with tryptophan. J. Mat. Chem. B 2018, 6, 7703–7709. [Google Scholar] [CrossRef] [PubMed]
- Tripaldi, L.; Callone, E.; D’Arienzo, M.; Dirè, S.; Giannini, L.; Mascotto, S.; Meyer, A.; Scotti, R.; Tadiello, L.; Di Credico, B. Silica hairy nanoparticles: A promising material for self-assembling processes. Soft Matter 2021, 17, 9434–9446. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Nigro, A.; Frattaruolo, L.; Fava, M.; De Napoli, I.; Greco, M.; Comandè, A.; Morelli, C. Bortezomib-loaded mesoporous silica nanoparticles selectively alter metabolism and induce death in multiple myeloma cells. Cancers 2020, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; De Santo, M.; Lombardo, D.; Leggio, A.; Pasqua, L. Mesoporous silicas in materials engineering: Nanodevices for bionanotechnologies. Mater. Today Bio 2022, 17, 100472. [Google Scholar] [CrossRef]
- De Santo, M.; Giovinazzo, A.; Fava, M.; Mazzotta, E.; De Napoli, I.E.; Greco, M.; Comandè, A.; Nigro, A.; Argurio, P.; Perrotta, I.; et al. Engineered mesoporous silica-based nanoparticles as smart chemotherapy nanodevice for bortezomib administration. Mater. Chem. Front. 2023, 7, 216–229. [Google Scholar] [CrossRef]
- Puzzo, M.; De Santo, M.; Morelli, C.; Leggio, A.; Pasqua, L. The Advent of Molecular Targeted Therapies Against Cancer. Toward Multi-Targeting Drugs Through Materials Engineering: A Possible Future Scenario. Small Sci. 2024, 2400113. [Google Scholar] [CrossRef]
- Morelli, C.; Maris, P.; Sisci, D.; Perrotta, E.; Brunelli, E.; Perrotta, I.; Panno, M.L.; Tagarelli, A.; Versace, C.; Casula, M.F.; et al. PEG-templated mesoporous silica nanoparticles exclusively target cancer cells. Nanoscale 2011, 3, 3198–3207. [Google Scholar] [CrossRef]
- Boissiere, C.; Larbot, A.; van Der Lee, A.; Kooyman, P.J.; Prouzet, E. A new synthesis of mesoporous MSU-X silica controlled by a two-step pathway. Chem. Mater. 2000, 12, 2902–2913. [Google Scholar] [CrossRef]
- He, Q.; Shi, J.; Chen, F.; Zhu, M.; Zhang, L. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials 2010, 31, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Quintanilla, D.; Sánchez, A.; del Hierro, I.; Fajardo, M.; Sierra, I. Synthesis and characterization of novel mesoporous silicas of the MSU-X family for environmental applications. J. Nanosci. Nanotechnol. 2009, 9, 4901–4909. [Google Scholar] [CrossRef] [PubMed]
- Rami, M.D.; Taghizadeh, M.; Akhoundzadeh, H. Synthesis and characterization of nano-sized hierarchical porous AuSAPO-34 catalyst for MTO reaction: Special insight on the influence of TX-100 as a cheap and green surfactant. Microporous Mesoporous Mater. 2019, 285, 259–270. [Google Scholar] [CrossRef]
- Breen, C.; Thompson, G.; Webb, M. Preparation, thermal stability and decomposition routes of clay/Triton-X100 composites. J. Mater. Chem. 1999, 9, 3159–3165. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, Netherlands, 2019; pp. 47–76. ISBN 9780128140314. [Google Scholar]
- Delgado, Á.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef] [PubMed]
- Xu, R. Progress in nanoparticles characterization: Sizing and zeta potential measurement. Particuology 2008, 6, 112–115. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS Pharmscitech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Sikora, A.; Shard, A.G.; Minelli, C. Size and ζ-potential measurement of silica nanoparticles in serum using tunable resistive pulse sensing. Langmuir 2016, 32, 2216–2224. [Google Scholar] [CrossRef]
- Talavera-Pech, W.A.; Esparza-Ruiz, A.; Quintana-Owen, P.; Vilchis-Nestor, A.R.; Carrera-Figueiras, C.; Ávila-Ortega, A. Effects of different amounts of APTES on physicochemical and structural properties of amino-functionalized MCM-41-MSNs. J. Sol-Gel Sci. Technol. 2016, 80, 697–708. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wang, J.; Yang, Y.; Li, Y.; Yuan, Y.; Liu, C. Charge-reversal APTES-modified mesoporous silica nanoparticles with high drug loading and release controllability. ACS Appl. Mater. Interfaces 2016, 8, 17166–17175. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Villegas, M.R.; Rodríguez, V.; Villaverde, G.; Lozano, D.; Baeza, A.; Vallet-Regí, M. Janus mesoporous silica nanoparticles for dual targeting of tumor cells and mitochondria. ACS Appl. Mater. Interfaces 2017, 9, 26697–26706. [Google Scholar] [CrossRef] [PubMed]
- Poovarodom, S.; Berg, J.C. Effect of particle and surfactant acid–base properties on charging of colloids in apolar media. J. Colloid Interface Sci. 2010, 346, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, X.; Wang, L.; Cheng, B.; Wu, Y.; Zhu, W. Preparation of modified SiO2 colloidal spheres with succinic acid and the assembly of colloidal crystals. Chin. Sci. Bull. 2007, 52, 461–466. [Google Scholar] [CrossRef]
- Mohammadi, H.; Heidari, R.; Niknezhad, S.V.; Jamshidzadeh, A.; Farjadian, F. In vitro and in vivo evaluation of succinic acid-substituted mesoporous silica for ammonia adsorption: Potential application in the management of hepatic encephalopathy. Int. J. Nanomed. 2020, 15, 10085–10098. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.; Kumar, K.V.; Ramaswamy, A.V.; Halligudi, S.B. Au–Pt nanoparticles in amine functionalized MCM-41: Catalytic evaluation in hydrogenation reactions. Catal. Commun. 2007, 8, 629–634. [Google Scholar] [CrossRef]
- Freitas, L.B.O.; Bravo, I.J.G.; Macedo, W.A.D.A.; de Sousa, E.M.B. Mesoporous silica materials functionalized with folic acid: Preparation, characterization and release profile study with methotrexate. J. Sol-Gel Sci. Technol. 2016, 77, 186–204. [Google Scholar] [CrossRef]
- Smith, B. The Infrared Spectra of Polymers V: Epoxies. Spectroscopy 2022, 37, 17–19. [Google Scholar] [CrossRef]
- Selvakumar, R.; Geib, S.J.; Premkumar, T.; Govindarajan, S. Hydrolysis of hydrazido-oxalic acid leading to the formation of hydrazinium cadmium hydrazido-oxalate/uranium oxalate species with a polymeric structure. Polyhedron 2015, 87, 321–328. [Google Scholar] [CrossRef]
- Shi, M.; Huang, R.; Qi, W.; Su, R.; He, Z. Synthesis of superhydrophobic and high stable Zr-MOFs for oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125102. [Google Scholar] [CrossRef]
- Pasqua, L.; Testa, F.; Aiello, R.; Cundari, S.; Nagy, J.B. Preparation of bifunctional hybrid mesoporous silica potentially useful for drug targeting. Microporous Mesoporous Mater. 2007, 103, 166–173. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Bao, L.; Chen, C. Engineering Nano–Bio Interfaces from Nanomaterials to Nanomedicines. Acc. Mater. Res. 2022, 3, 812–829. [Google Scholar] [CrossRef]

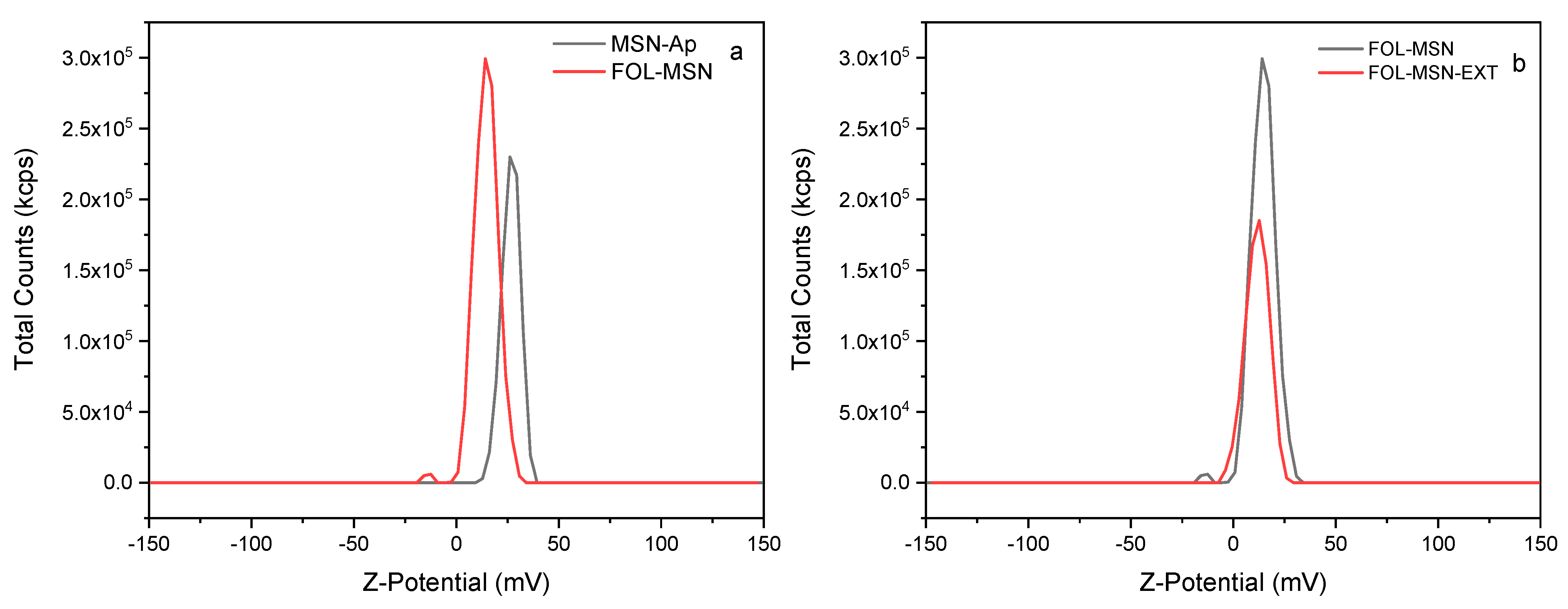


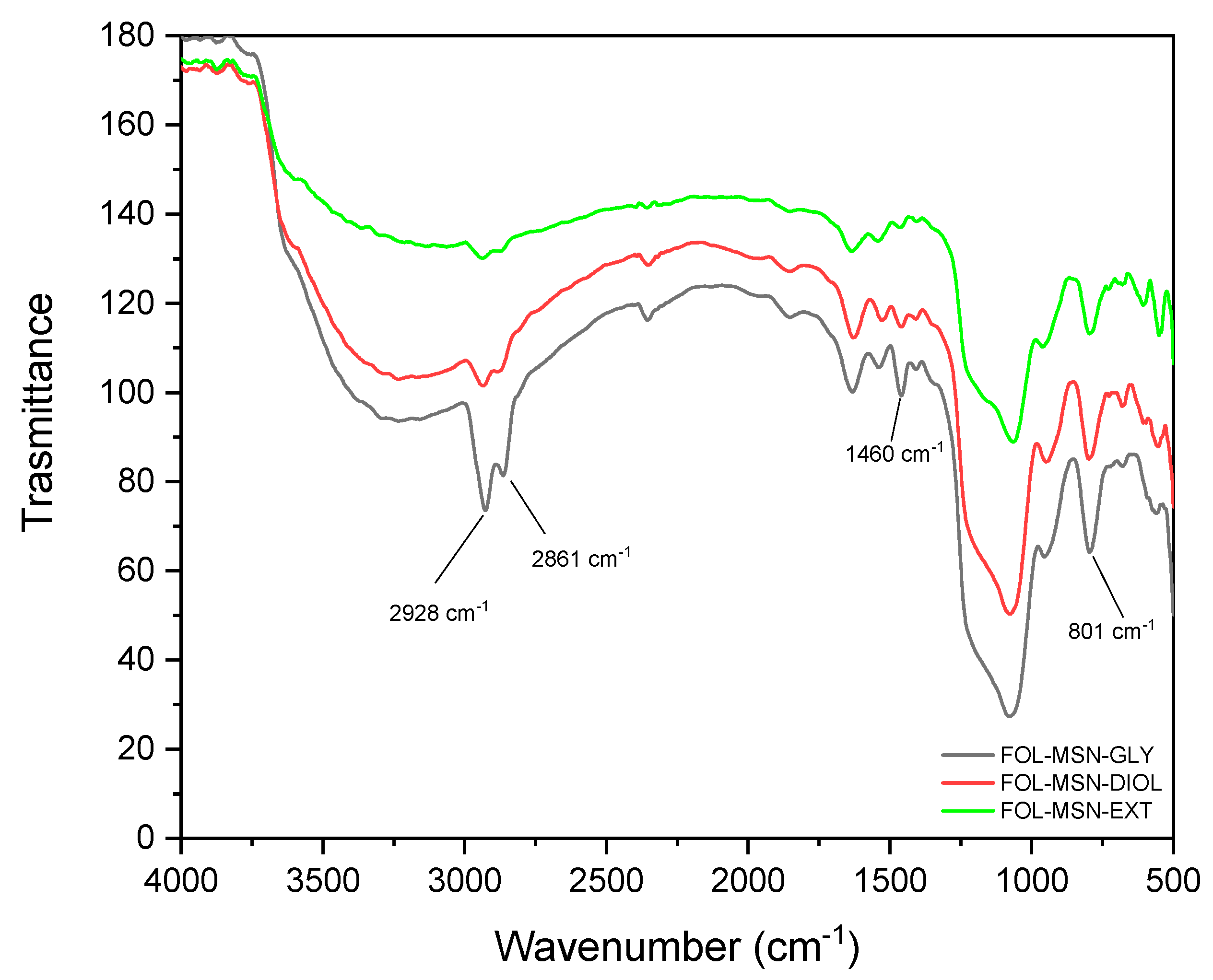



| Sample | Z-Potential Value (mV) | Std. Dev. (mV) |
|---|---|---|
| MSNAS | −18.0 | ±0.71 |
| MSN-AP | +27.7 | ±5.19 |
| FOL-MSN | +10.5 | ±5.74 |
| FOL-MSN-EXT | +9.91 | ±4.81 |
| FOL-MSN-DIOL | +25.50 | ±6.18 |
| FOL-MSN-NH2 | +18.40 | ±3.80 |
| FOL-MSN-COOH | −19.10 | ±3.40 |
| FOL-MSN-HYD | +16.90 | ±3.59 |
| Sample | BET Surface Area (m2∙g−1) | Pore Volume (cm3/g) At P/P0 = 0.96 | Average Pore Width Desorption Branch (BJH) (nm) |
|---|---|---|---|
| MSN-AP | 250.45 | 0.615 | 11.55 |
| FOL-MSN | 207.45 | 0.361 | 11.04 |
| FOL-MSN-EXT | 423.38 | 1.512 | 16.67 |
| FOL-MSN-DIOL | 349.02 | 0.820 | 12.33 |
| FOL-MSN-NH2 | 295.09 | 1.187 | 15.83 |
| FOL-MSN-COOH | 224.47 | 0.675 | 12.40 |
| FOL-MSN-HYD | 226.52 | 0.572 | 16.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grisolia, A.; De Santo, M.; Curcio, M.; Cavallaro, P.A.; Morelli, C.; Leggio, A.; Pasqua, L. Engineered Mesoporous Silica-Based Nanoparticles: Characterization of Surface Properties. Materials 2024, 17, 3352. https://doi.org/10.3390/ma17133352
Grisolia A, De Santo M, Curcio M, Cavallaro PA, Morelli C, Leggio A, Pasqua L. Engineered Mesoporous Silica-Based Nanoparticles: Characterization of Surface Properties. Materials. 2024; 17(13):3352. https://doi.org/10.3390/ma17133352
Chicago/Turabian StyleGrisolia, Antonio, Marzia De Santo, Manuela Curcio, Palmira Alessia Cavallaro, Catia Morelli, Antonella Leggio, and Luigi Pasqua. 2024. "Engineered Mesoporous Silica-Based Nanoparticles: Characterization of Surface Properties" Materials 17, no. 13: 3352. https://doi.org/10.3390/ma17133352





