Impact of Scattering Foil Composition on Electron Energy Distribution in a Clinical Linear Accelerator Modified for FLASH Radiotherapy: A Monte Carlo Study
Abstract
1. Introduction
2. Materials and Methods
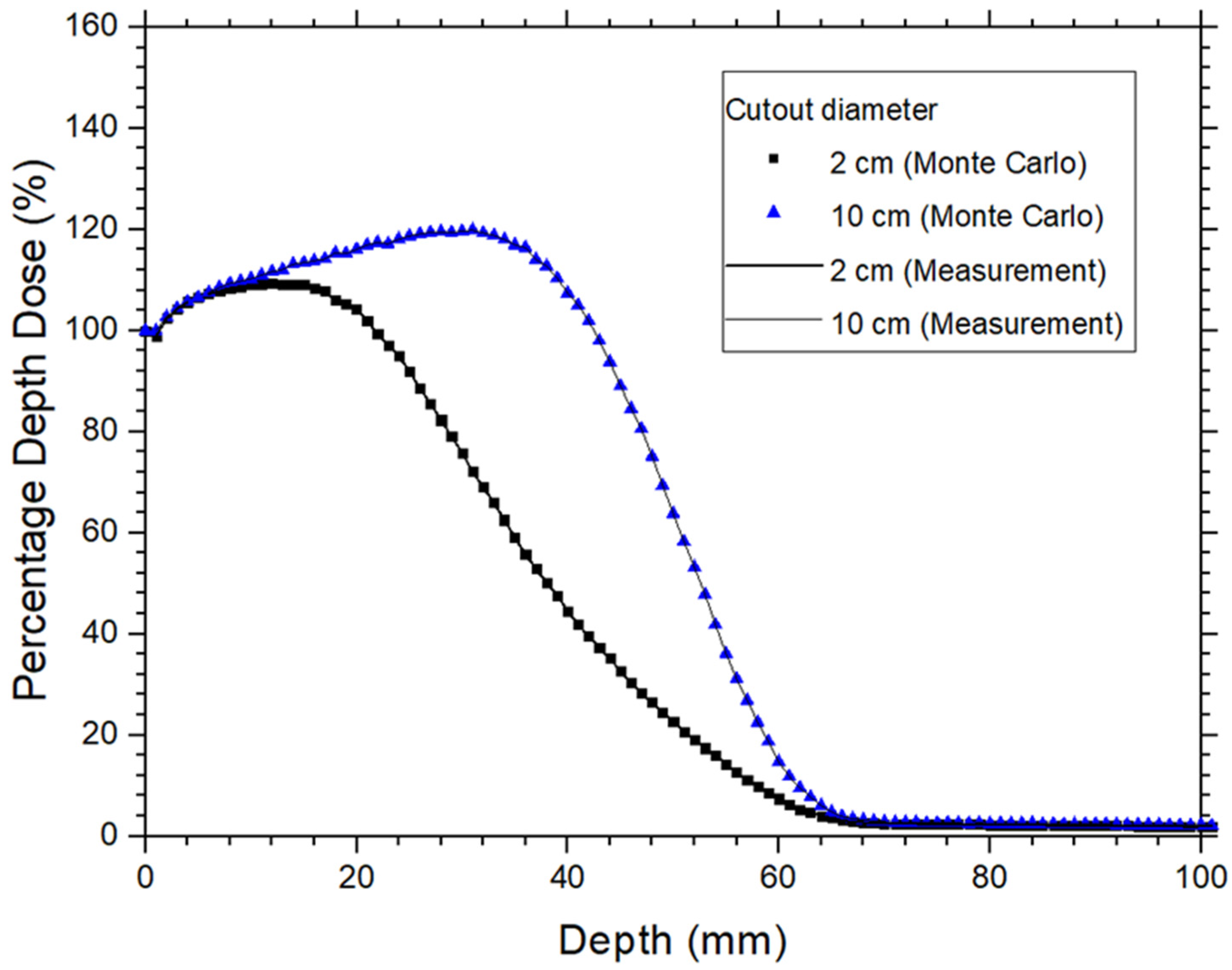
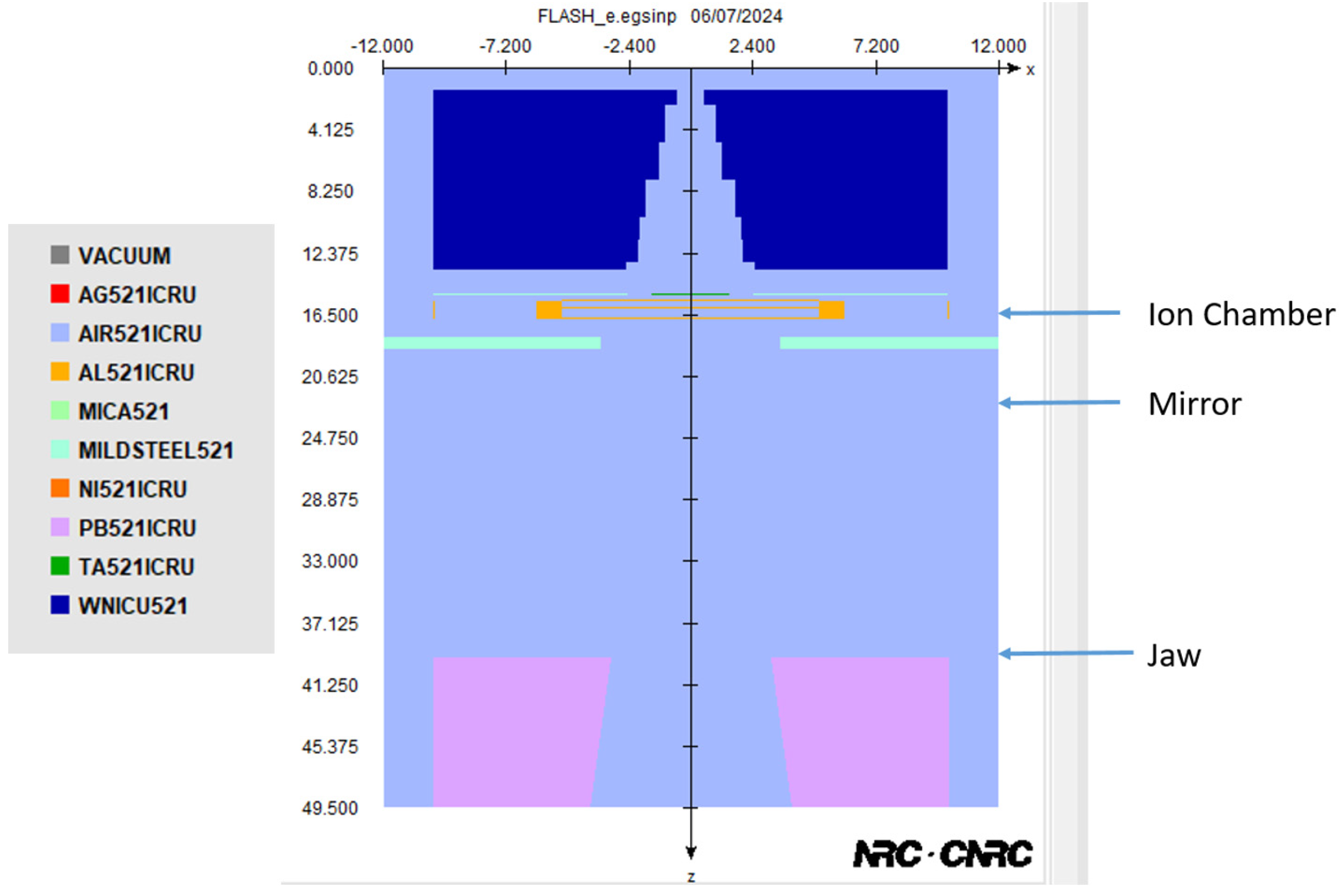
2.1. Monte Carlo Simulation, Phase Space File of Linac, and Model Verification
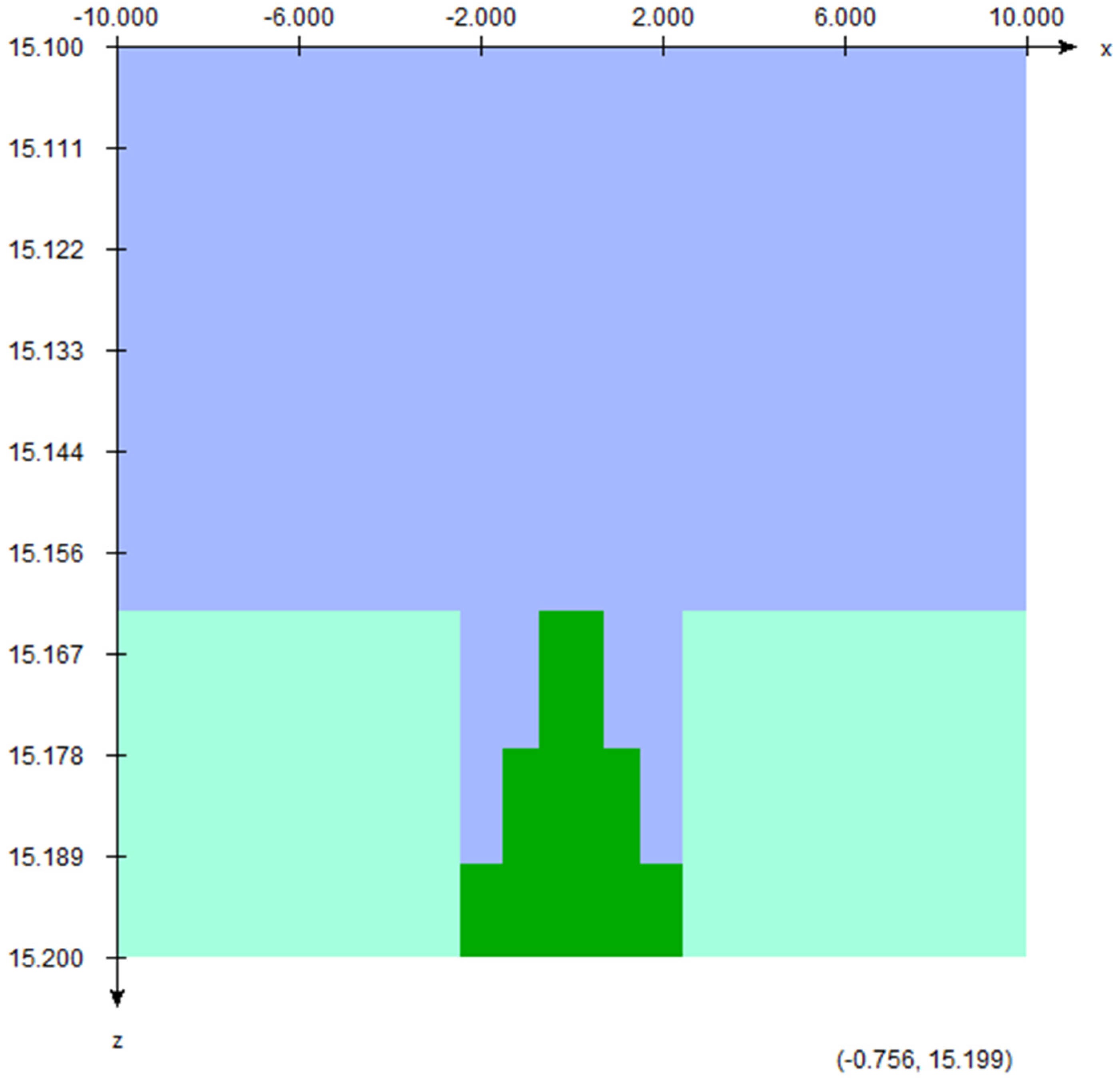
2.2. Scattering Foil Material, Position of Sampling Holder, and Calculation of the Electron Energy Spectra
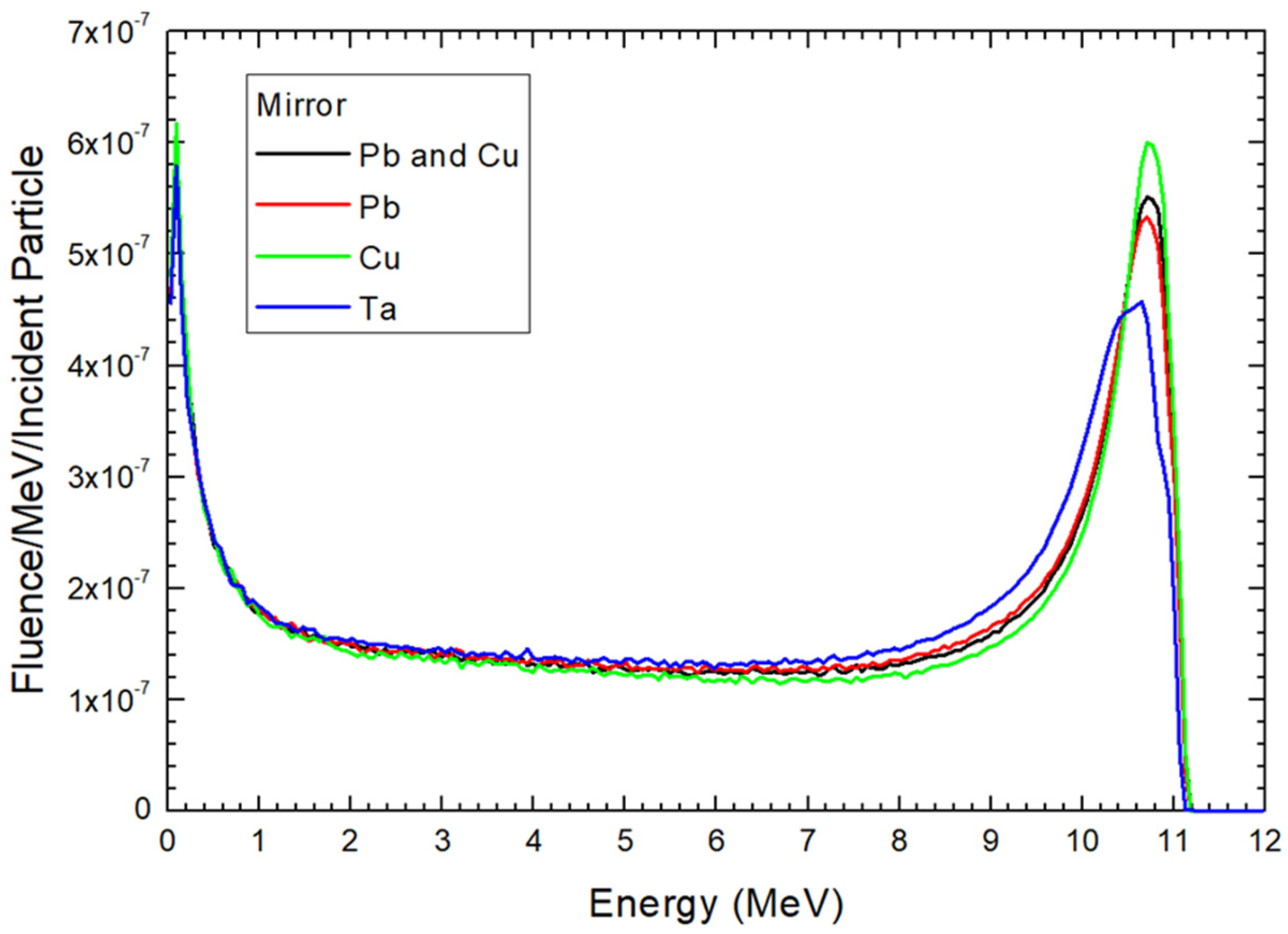
3. Results
4. Discussion
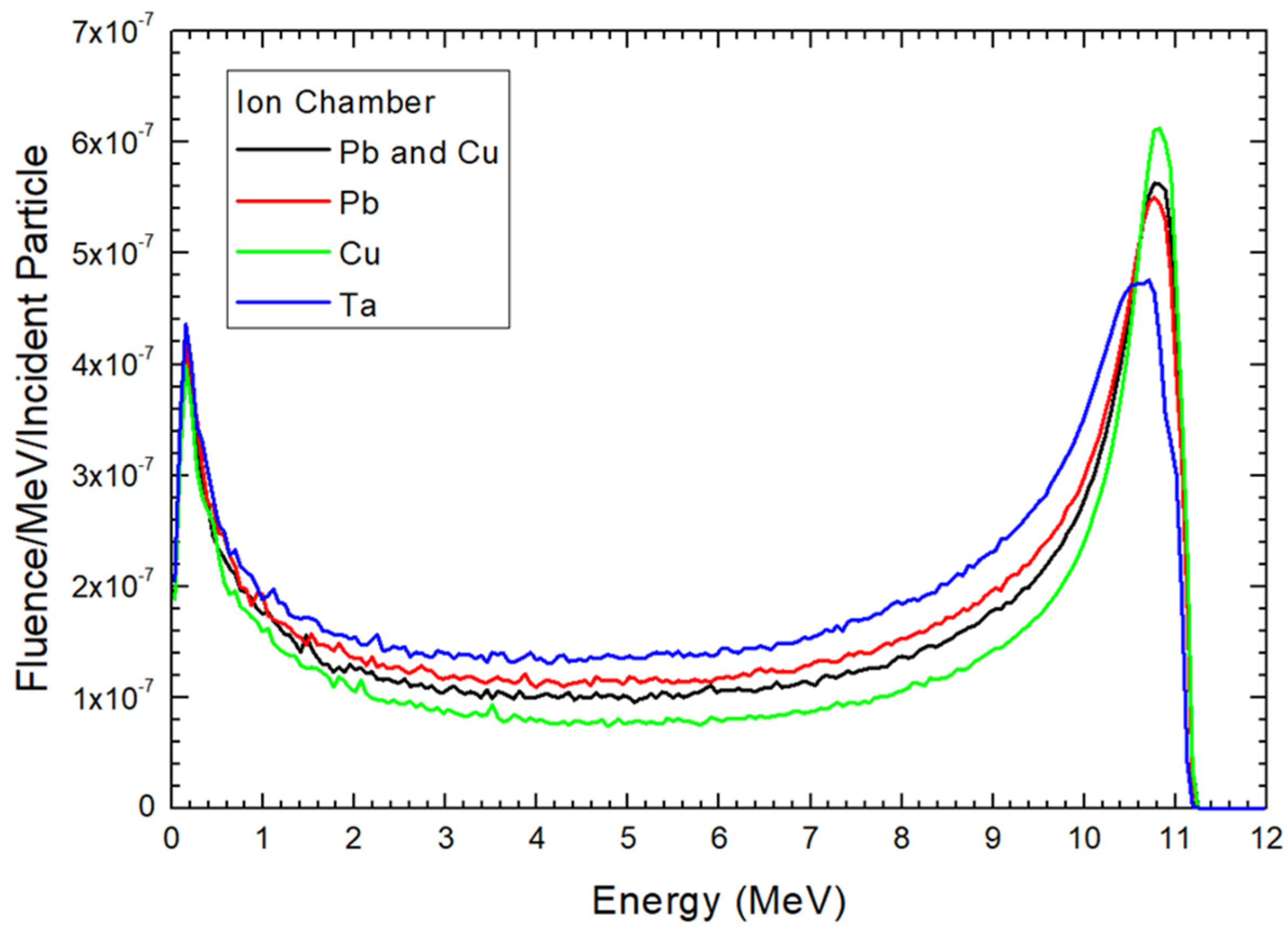
4.1. Dependences of Electron Energy Distribution on the Scattering Foil Material
4.2. Dependence of Electron Distribution on the Sampling Holder Position
4.3. Selection of Sampling Holder Location in a Modified Linac for FLASH Electron Radiotherapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, S.R.; Suhag, V.; Semwal, M.; Sharma, N. Radiotherapy: Basic concepts and recent advances. Med. J. Armed Forces India 2010, 66, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, D.I.; Tuohy, J.B. Back to the future: The history and development of the clinical linear accelerator. Phys. Med. Biol. 2006, 51, R343. [Google Scholar] [CrossRef] [PubMed]
- Do Huh, H.; Kim, S. History of radiation therapy technology. Prog. Med. Phys. 2020, 31, 124–134. [Google Scholar] [CrossRef]
- Biggs, P.J. Review of the improvements in the quality and applications of photon beams from linear accelerators in radiation therapy and a projection of future trends. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1987, 24, 1096–1099. [Google Scholar] [CrossRef]
- Chow, J.C.; Ruda, H.E. Flash radiotherapy: Innovative cancer treatment. Encyclopedia 2023, 3, 808–823. [Google Scholar] [CrossRef]
- Borghini, A.; Vecoli, C.; Labate, L.; Panetta, D.; Andreassi, M.G.; Gizzi, L.A. FLASH ultra-high dose rates in radiotherapy: Preclinical and radiobiological evidence. Int. J. Radiat. Biol. 2022, 98, 127–135. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Bourhis, J.; Montay-Gruel, P.; Jorge, P.G.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef]
- Lin, B.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. FLASH radiotherapy: History and future. Front. Oncol. 2021, 11, 644400. [Google Scholar] [CrossRef]
- Siddique, S.; Ruda, H.E.; Chow, J.C. FLASH radiotherapy and the use of radiation dosimeters. Cancers 2023, 15, 3883. [Google Scholar] [CrossRef]
- Chow, J.C.; Ruda, H.E. Mechanisms of Action in FLASH Radiotherapy: A Comprehensive Review of Physicochemical and Biological Processes on Cancerous and Normal Cells. Cells 2024, 13, 835. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Huang, D.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. Mechanisms of FLASH effect. Front. Oncol. 2022, 12, 995612. [Google Scholar] [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron neutron capture therapy: A review of clinical applications. Front. Oncol. 2021, 11, 601820. [Google Scholar] [CrossRef]
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med. Phys. 2022, 49, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Radiotherapy using high-intensity pulsed radiation beams (FLASH): A radiation-chemical perspective. Radiat. Res. 2020, 194, 607–617. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A.; Schuemann, J.; Dokic, I.; Lundh, O.; Zhao, T.; Ramos-Méndez, J.; Dong, L.; Petersson, K. Development of ultra-high dose-rate (FLASH) particle therapy. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 6, 252–262. [Google Scholar] [CrossRef]
- Rahman, M.; Trigilio, A.; Franciosini, G.; Moeckli, R.; Zhang, R.; Böhlen, T.T. FLASH radiotherapy treatment planning and models for electron beams. Radiother. Oncol. 2022, 175, 210–221. [Google Scholar] [CrossRef]
- Vozenin, M.C.; Bourhis, J.; Durante, M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 791–803. [Google Scholar] [CrossRef] [PubMed]
- No, H.J.; Wu, Y.F.; Dworkin, M.L.; Manjappa, R.; Skinner, L.; Ashraf, M.R.; Lau, B.; Melemenidis, S.; Viswanathan, V.; Yu, A.S.; et al. Clinical linear accelerator-based electron FLASH: Pathway for practical translation to FLASH clinical trials. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 482–492. [Google Scholar] [CrossRef]
- Schüler, E.; Trovati, S.; King, G.; Lartey, F.; Rafat, M.; Villegas, M.; Praxel, A.J.; Loo, B.W., Jr.; Maxim, P.G. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 195–203. [Google Scholar] [CrossRef]
- No, H.J.; Wu, Y.F.; Manjappa, R.; Skinner, L.; Lau, B.; Melemenidis, S.; Yu, S.J.; Surucu, M.; Schueler, E.; Bush, K.; et al. Feasibility of clinically practical ultra-high dose rate (FLASH) radiation delivery by a reversible configuration of a standard clinical-use linear accelerator. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, S32. [Google Scholar] [CrossRef]
- Okumura, Y.; Kitagawa, T.; Kitabatake, T. Scattering foil device for high-energy electron-beam therapy. Radiology 1969, 93, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Bieda, M.R.; Antolak, J.A.; Hogstrom, K.R. The effect of scattering foil parameters on electron-beam Monte Carlo calculations. Med. Phys. 2001, 28, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Zabihzadeh, M.; Sedaghat, Z.; Shahbazian, H. Determination the dosimetric properties of scattering foil and scattering foil free electron beams in clinical linear accelerator. Int. J. Radiat. Res. 2023, 21, 217–226. [Google Scholar]
- Andreo, P. Monte Carlo simulations in radiotherapy dosimetry. Radiat. Oncol. 2018, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.W.; Kawrakow, I.; Seuntjens, J.P.; Walters, B.R.; Mainegra-Hing, E. NRC User Codes for EGSnrc; NRCC Report PIRS-702 (Rev. B); National Research Council of Canada: Ottawa, ON, Canada, 2003.
- Rogers, D.W.; Walters, B.R.; Kawrakow, I. BEAMnrc Users Manual; NRC Report PIRS-0509; National Research Council of Canada: Ottawa, ON, Canada, 2009; p. 12.
- Walters, B.R.; Kawrakow, I.; Rogers, D.W. DOSXYZnrc Users Manual; NRC Report PIRS-794; National Research Council of Canada: Ottawa, ON, Canada, 2005; pp. 57–58.
- Rogers, D.W.; Bielajew, A.F. Differences in electron depth-dose curves calculated with EGS and ETRAN and improved energy-range relationships. Med. Phys. 1986, 13, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C. Calculation of lateral buildup ratio using Monte Carlo simulation for electron radiotherapy. Med. Phys. 2007, 34, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bielajew, A.F.; Rogers, D.W. PRESTA: The parameter reduced electron-step transport algorithm for electron Monte Carlo transport. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1986, 18, 165–181. [Google Scholar] [CrossRef]
- Chow, J.C.; Grigorov, G.N. Effect of electron beam obliquity on lateral buildup ratio: A Monte Carlo dosimetry evaluation. Phys. Med. Biol. 2007, 52, 3965. [Google Scholar] [CrossRef]
- Chow, J.C. Depth dose enhancement on flattening-filter-free photon beam: A Monte Carlo study in nanoparticle-enhanced radiotherapy. Appl. Sci. 2020, 10, 7052. [Google Scholar] [CrossRef]
- Ahmed, M.M.; El Bardouni, T.; Boukhal, H.; Azahra, M.; Chakir, E. Implementation of the EGSnrc/BEAMnrc Monte Carlo code-Application to medical accelerator SATURNE43. Int. J. Innov. Appl. Stud. 2014, 6, 635. [Google Scholar]
- Chow, J.C.; Owrangi, A.M. Mucosal dosimetry on unflattened photon beams: A Monte Carlo phantom study. Biomed. Phys. Eng. Express 2018, 5, 015007. [Google Scholar] [CrossRef]
- Favaudon, V.; Labarbe, R.; Limoli, C.L. Model studies of the role of oxygen in the FLASH effect. Med. Phys. 2022, 49, 2068–2081. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, J.C.L.; Ruda, H.E. Impact of Scattering Foil Composition on Electron Energy Distribution in a Clinical Linear Accelerator Modified for FLASH Radiotherapy: A Monte Carlo Study. Materials 2024, 17, 3355. https://doi.org/10.3390/ma17133355
Chow JCL, Ruda HE. Impact of Scattering Foil Composition on Electron Energy Distribution in a Clinical Linear Accelerator Modified for FLASH Radiotherapy: A Monte Carlo Study. Materials. 2024; 17(13):3355. https://doi.org/10.3390/ma17133355
Chicago/Turabian StyleChow, James C. L., and Harry E. Ruda. 2024. "Impact of Scattering Foil Composition on Electron Energy Distribution in a Clinical Linear Accelerator Modified for FLASH Radiotherapy: A Monte Carlo Study" Materials 17, no. 13: 3355. https://doi.org/10.3390/ma17133355
APA StyleChow, J. C. L., & Ruda, H. E. (2024). Impact of Scattering Foil Composition on Electron Energy Distribution in a Clinical Linear Accelerator Modified for FLASH Radiotherapy: A Monte Carlo Study. Materials, 17(13), 3355. https://doi.org/10.3390/ma17133355










