Electronic Properties and Mechanical Stability of Multi-Ion-Co-Intercalated Bilayered V2O5
Abstract
:1. Introduction
2. Methodology
Computational Details
3. Results and Discussion
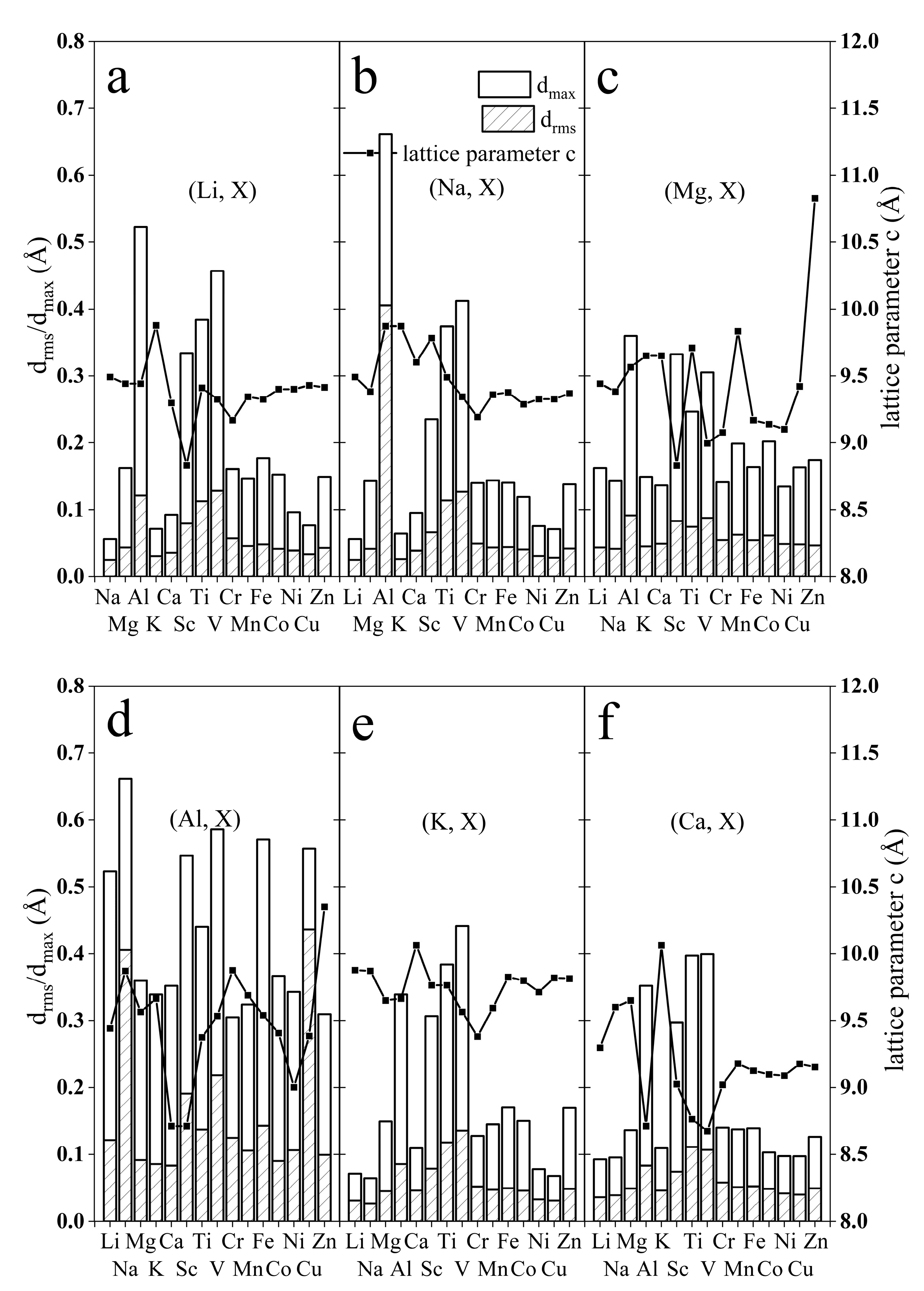
3.1. Structure Modification after Co-Intercalation
3.2. Stability and Mechanical Properties after Co-Intercalation
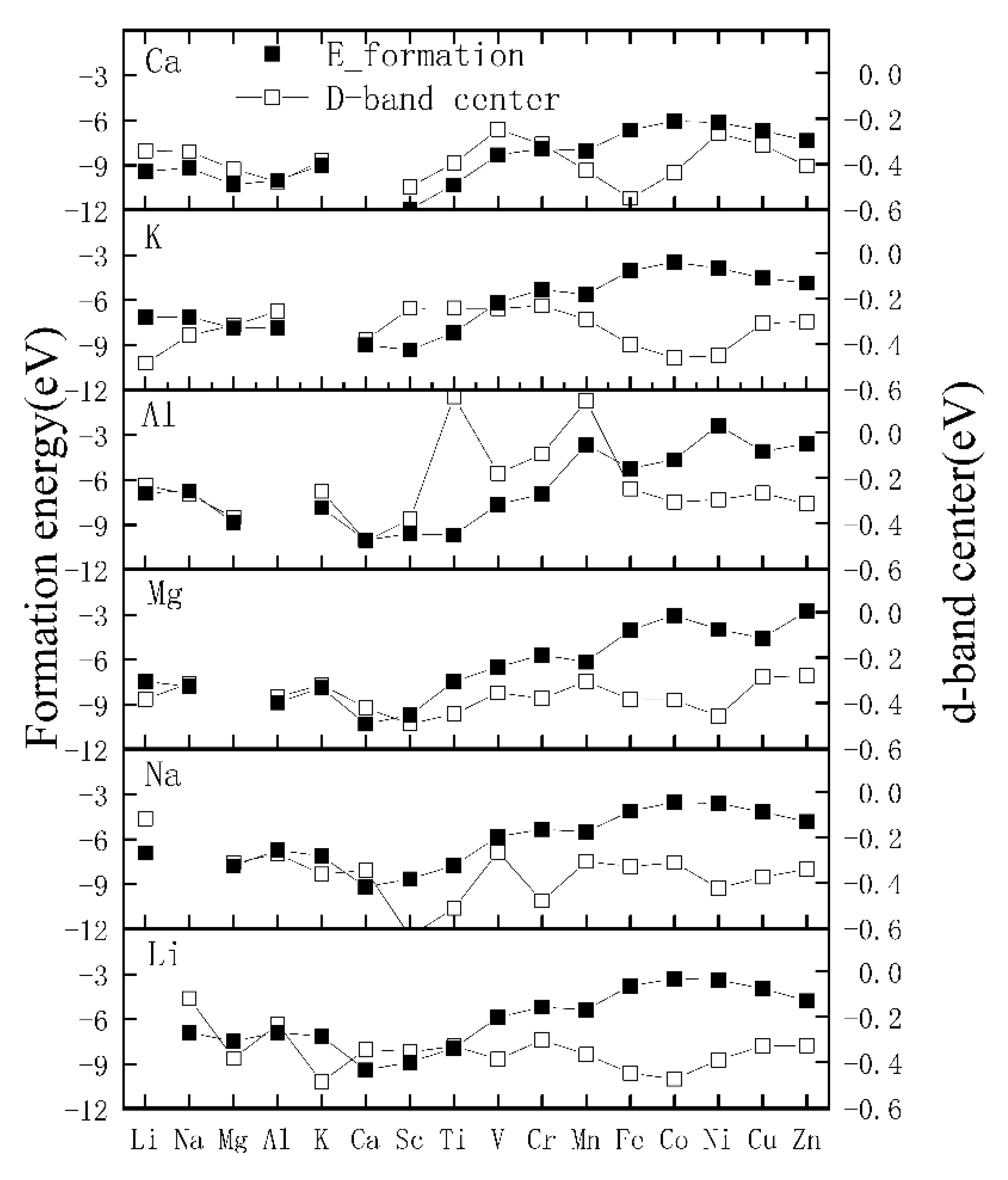
3.3. Electronic Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, F.; Qi, L. Recent Progress in Self-Supported Metal Oxide Nanoarray Electrodes for Advanced Lithium-Ion Batteries. Adv. Sci. 2016, 3, 1600049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Shi, J.-L.; Liang, J.-Y.; Yin, Y.-X.; Guo, Y.-G.; Wan, L.-J. Structurally modulated Li-rich cathode materials through cooperative cation doping and anion hybridization. Sci. China Chem. 2017, 60, 1554–1560. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Ji, S.; Pollet, B.G.; Wang, R. V2O5-SiO2 hybrid as anode material for aqueous rechargeable lithium batteries. Ionics 2016, 22, 1593–1601. [Google Scholar] [CrossRef]
- Champness, N.R. The future of metal-organic frameworks. Dalton Trans. 2011, 40, 10311–10315. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.T.; Liu, L.L.; Wu, Y.P.; Holze, R. Electrochemical behavior of V2O5·0.6H2O nanoribbons in neutral aqueous electrolyte solution. Electrochim. Acta 2013, 96, 8–12. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Lin, W.; Chen, F.; Chen, G.; Chen, D. Electrochemical properties and facile preparation of hollow porous V2O5 microspheres for lithium-ion batteries. J. Colloid Interface Sci. 2023, 638, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Reddy, I.N.; Akkinepally, B.; Manjunath, V.; Neelima, G.; Reddy, M.V.; Shim, J. SnO2 Quantum Dots Distributed along V2O5 Nanobelts for Utilization as a High-Capacity Storage Hybrid Material in Li-Ion Batteries. Molecules 2021, 26, 7262. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, C.; Zhang, F.; Dong, W.; Zhang, X.; Sang, Y.; Wang, J.-J.; Guo, Y.-G.; Liu, H.; Wang, S. Tunable Layered (Na, Mn)V8O20·nH2O Cathode Material for High-Performance Aqueous Zinc Ion Batteries. Adv. Sci. 2020, 7, 2000083. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, X.; Gao, T.; Sun, W.; Ma, Z.; Yang, C.; Han, F.; Xu, K.; Wang, C. High-Voltage Aqueous Magnesium Ion Batteries. ACS Cent. Sci. 2017, 3, 1121–1128. [Google Scholar] [CrossRef]
- Wang, H.; Bai, Y.; Chen, S.; Luo, X.; Wu, C.; Wu, F.; Lu, J.; Amine, K. Binder-free V2O5 cathode for greener rechargeable aluminum battery. ACS Appl. Mater. Interfaces 2015, 7, 80–84. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.; Zheng, J.; Jia, X.; Huang, J.; Yan, M.; Tian, M.; Wang, M.; Yang, J.; Cao, G. Expanded hydrated vanadate for high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 2273–2285. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Lei, Y.; Kandambeth, S.; Eddaoudi, M.; Alshareef, H.N. Layered MgxV2O5·nH2O as Cathode Material for High-Performance Aqueous Zinc Ion Batteries. ACS Energy Lett. 2018, 3, 2602–2609. [Google Scholar] [CrossRef]
- Sa, N.; Kinnibrugh, T.L.; Wang, H.; Sai Gautam, G.; Chapman, K.W.; Vaughey, J.T.; Key, B.; Fister, T.T.; Freeland, J.W.; Proffit, D.L.; et al. Structural Evolution of Reversible Mg Insertion into a Bilayer Structure of V2O5·nH2O Xerogel Material. Chem. Mater. 2016, 28, 2962–2969. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, Y.; Fang, G.; Shan, L.; Guo, J.; Zhang, W.; Wang, C.; Wang, L.; Zhou, J.; Liang, S. Li+ intercalated V2O5·nH2O with enlarged layer spacing and fast ion diffusion as an aqueous zinc-ion battery cathode. Energy Environ. Sci. 2018, 11, 3157–3162. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Mo, F.; Liu, Z.; Ma, L.; Li, X.; Fang, D.; Chen, S.; Zhang, S.; Zhi, C. Activating C-Coordinated Iron of Iron Hexacyanoferrate for Zn Hybrid-Ion Batteries with 10 000-Cycle Lifespan and Superior Rate Capability. Adv. Mater. 2019, 31, 1901521. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yang, Z.; Tan, L.; Zeng, L.; Zhu, Y.; Guo, L. Investigation of the Prussian Blue Analog Co3[Co(CN)6]2 as an Anode Material for Nonaqueous Potassium-Ion Batteries. Adv. Mater. 2018, 30, e1802510. [Google Scholar] [CrossRef]
- He, P.; Zhang, G.; Liao, X.; Yan, M.; Xu, X.; An, Q.; Liu, J.; Mai, L. Sodium Ion Stabilized Vanadium Oxide Nanowire Cathode for High-Performance Zinc-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702463. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Ed. Engl. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Pang, Q.; He, W.; Yu, X.; Yang, S.; Zhao, H.; Fu, Y.; Xing, M.; Tian, Y.; Luo, X.; Wei, Y. Aluminium pre-intercalated orthorhombic V2O5 as high-performance cathode material for aqueous zinc-ion batteries. Appl. Surf. Sci. 2021, 538, 148043. [Google Scholar] [CrossRef]
- Kuchena, S.F.; Wang, Y. V2O5 intercalated with polyaniline for improved kinetics in aqueous ammonium-ion batteries. Electrochim. Acta 2022, 425, 140751. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Hu, X.; Wang, T.; Parkin, I.P.; Wang, M.; Boruah, B.D. Polyaniline and water pre-intercalated V2O5 cathodes for high-performance planar zinc-ion micro-batteries. Chem. Eng. J. 2024, 487, 150384. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, Y.; Gao, Z.; Hu, D.; Jiang, H.; Hu, T.; Meng, C.; Zhang, Y. Construction interlayer structure of hydrated vanadium oxides with tunable P-band center of oxygen towards enhanced aqueous Zn-ion batteries. Adv. Powder Mater. 2024, 3, 100167. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Naveed, A.; Li, G.; Zhang, H.; Zhou, Y.; Dou, A.; Su, M.; Liu, Y.; Guo, R.; et al. Magnesium Ion Doping and Micro-Structural Engineering Assist NH4V4O10 as a High-Performance Aqueous Zinc Ion Battery Cathode. Adv. Funct. Mater. 2023, 33, 2306205. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Bréger, J.; Meng, Y.S.; Hinuma, Y.; Kumar, S.; Kang, K.; Shao-Horn, Y.; Ceder, G.; Grey, C.P. Effect of High Voltage on the Structure and Electrochemistry of LiNi0.5Mn0.5O2: A Joint Experimental and Theoretical Study. Chem. Mater. 2006, 18, 4768–4781. [Google Scholar] [CrossRef]
- Zhou, F.; Kang, K.; Maxisch, T.; Ceder, G.; Morgan, D. The electronic structure and band gap of LiFePO4 and LiMnPO4. Solid State Commun. 2004, 132, 181–186. [Google Scholar] [CrossRef]
- Kang, K.; Morgan, D.; Ceder, G. First principles study of Li diffusion in I-Li2NiO2 structure. Phys. Rev. B 2009, 79, 014305. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Sandagiripathira, K.; Moghaddasi, M.A.; Shepard, R.; Smeu, M. Investigating the role of structural water on the electrochemical properties of α-V2O5 through density functional theory. Phys. Chem. Chem. Phys. 2022, 24, 24271–24280. [Google Scholar] [CrossRef] [PubMed]
- Dobson, P.J.J.P.B. Physical Properties of Crystals—Their Representation by Tensors and Matrices. Phys. Bull. 1985, 36, 506. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.-X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Gao, X.P.; Jiang, Y.H.; Liu, Y.Z.; Zhou, R.; Feng, J. Stability and elastic properties of NbxCy compounds. Chin. Phys. B 2014, 23, 097704. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, H.; Cao, R.; Zhang, X.; Jiang, Z. Theoretical study on the initial stage of a magnesium battery based on a V2O5 cathode. Phys. Chem. Chem. Phys. 2014, 16, 18578–18585. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, H.H.; Metiu, H. Structure of V2O5·nH2O Xerogels. J. Phys. Chem. C 2016, 120, 3986–3992. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, H.; Qin, L.; Cao, X.; Zhou, J.; Pan, A.; Fang, G.; Liang, S. Interlayer Doping in Layered Vanadium Oxides for Low-cost Energy Storage: Sodium-ion Batteries and Aqueous Zinc-ion Batteries. ChemNanoMat 2020, 6, 1553–1566. [Google Scholar] [CrossRef]
- Guo, S.; Fang, G.; Liang, S.; Chen, M.; Wu, X.; Zhou, J. Structural perspective on revealing energy storage behaviors of silver vanadate cathodes in aqueous zinc-ion batteries. Acta Mater. 2019, 180, 51–59. [Google Scholar] [CrossRef]
- Shan, L.; Yang, Y.; Zhang, W.; Chen, H.; Fang, G.; Zhou, J.; Liang, S. Observation of combination displacement/intercalation reaction in aqueous zinc-ion battery. Energy Storage Mater. 2019, 18, 10–14. [Google Scholar] [CrossRef]
- Yu, X.; Hu, F.; Cui, F.; Zhao, J.; Guan, C.; Zhu, K. The displacement reaction mechanism of the CuV2O6 nanowire cathode for rechargeable aqueous zinc ion batteries. Dalton Trans. 2020, 49, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, C.; Tian, M.; Jia, X.; Jahrman, E.P.; Seidler, G.T.; Zhang, S.; Liu, Y.; Zhang, Y.; Meng, C.; et al. Fast and reversible zinc ion intercalation in Al-ion modified hydrated vanadate. Nano Energy 2020, 70, 104519. [Google Scholar] [CrossRef]
- Galy, J. Vanadium pentoxide and vanadium oxide bronzes—Structural chemistry of single (S) and double (D) layer MxV2O5 phases. J. Solid State Chem. 1992, 100, 229–245. [Google Scholar] [CrossRef]
- Li, Q.; Rui, X.; Chen, D.; Feng, Y.; Xiao, N.; Gan, L.; Zhang, Q.; Yu, Y.; Huang, S. A High-Capacity Ammonium Vanadate Cathode for Zinc-Ion Battery. Nano-Micro Lett. 2020, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Z.; Wang, X.; Meng, J.; Liu, X.; Wu, B.; Han, C.; Mai, L. Comprehensive understanding of the roles of water molecules in aqueous Zn-ion batteries: From electrolytes to electrode materials. Energy Environ. Sci. 2021, 14, 3796–3839. [Google Scholar] [CrossRef]
- Wu, T.; Zhu, K.; Qin, C.; Huang, K. Unraveling the role of structural water in bilayer V2O5 during Zn2+-intercalation: Insights from DFT calculations. J. Mater. Chem. A 2019, 7, 5612–5620. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- McColl, K.; Corà, F. Phase stability of intercalated V2O5 battery cathodes elucidated through the Goldschmidt tolerance factor. Phys. Chem. Chem. Phys. 2019, 21, 7732–7744. [Google Scholar] [CrossRef]
- Tang, B.; Fang, G.; Zhou, J.; Wang, L.; Lei, Y.; Wang, C.; Lin, T.; Tang, Y.; Liang, S. Potassium vanadates with stable structure and fast ion diffusion channel as cathode for rechargeable aqueous zinc-ion batteries. Nano Energy 2018, 51, 579–587. [Google Scholar] [CrossRef]
- De, P.; Halder, J.; Priya, S.; Srivastava, A.K.; Chandra, A. Two-Dimensional V2O5 Nanosheets as an Advanced Cathode Material for Realizing Low-Cost Aqueous Aluminum-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 753–762. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Feng, J.; Zhou, R. Elasticity, electronic properties and hardness of MoC investigated by first principles calculations. Phys. B Condens. Matter 2013, 419, 45–50. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Ding Ying-Chun, X.B. Electronic Structure, Mechanical Properties and Intrinsic Hardness of a New Superhard Material BeP2N4. Acta Phys. Chim. Sin. 2011, 27, 1621–1632. [Google Scholar]
- Vaitheeswaran, G.; Kanchana, V.; Svane, A.; Delin, A. Elastic properties of MgCNi3—a superconducting perovskite. J. Phys. Condens. Matter 2007, 19, 326214. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Long, C.; Dong, B.; Fang, D.; Liu, Z.; Zhao, Y.; Li, X.; Fan, J.; Chen, S.; et al. Achieving Both High Voltage and High Capacity in Aqueous Zinc-Ion Battery for Record High Energy Density. Adv. Funct. Mater. 2019, 29, 1906142. [Google Scholar] [CrossRef]
- Zhu, Q.; Huang, W.; Huang, C.; Gao, L.; Su, Y.; Qiao, L. The d band center as an indicator for the hydrogen solution and diffusion behaviors in transition metals. Int. J. Hydrog. Energy 2022, 47, 38445–38454. [Google Scholar] [CrossRef]

| D-V2O5 | a (Å) | b (Å) | c (Å) |
|---|---|---|---|
| PBE (this work) | 11.69 | 3.61 | 9.85 |
| DFT-PBE-D3 [48] | 11.36 | 3.56 | 9.37 |
| PBE + U [46] | 11.58 | 3.65 | 8.59 |
| C11 | C22 | C33 | B (GPa) | E (GPa) | G (GPa) | v | ||
|---|---|---|---|---|---|---|---|---|
| Li, Na | 175.26 | 220.71 | 41.20 | 86.30 | 84.67 | 31.68 | 0.34 | metal |
| Li, Mg | 176.67 | 223.58 | 39.78 | 92.33 | 75.35 | 27.62 | 0.36 | metal |
| Li, Al * | 137.08 | 229.34 | 44.59 | 72.64 | 63.91 | 23.61 | 0.35 | metal |
| Li, K | 167.36 | 214.76 | 34.34 | 81.77 | 86.38 | 32.62 | 0.32 | metal |
| Li, Ca | 191.37 | 242.23 | 43.84 | 99.24 | 96.45 | 36.04 | 0.34 | metal |
| LI, Sc | 172.38 | 225.90 | 89.33 | 99.16 | 90.72 | 33.66 | 0.35 | metal |
| Li, Ti | 107.21 | 235.99 | 96.20 | 89.31 | 82.64 | 30.71 | 0.35 | metal |
| Li, V | 117.16 | 240.22 | 100.75 | 91.08 | 88.82 | 33.20 | 0.34 | metal |
| Li, Cr | 181.39 | 238.00 | 73.39 | 102.71 | 97.23 | 36.22 | 0.34 | metal |
| Li, Mn | 181.03 | 242.48 | 64.85 | 100.64 | 90.84 | 33.65 | 0.35 | metal |
| Li, Fe | 174.04 | 230.18 | 48.86 | 95.77 | 76.12 | 27.83 | 0.37 | metal |
| Li, Co | 175.50 | 227.10 | 27.49 | 84.06 | 82.85 | 31.01 | 0.34 | metal |
| Li, Ni | 180.49 | 225.17 | 35.08 | 92.08 | 88.26 | 32.93 | 0.34 | metal |
| Li, Cu | 174.10 | 229.84 | 39.47 | 89.79 | 84.08 | 31.28 | 0.34 | metal |
| Li, Zn | 183.26 | 227.57 | 45.33 | 92.43 | 86.73 | 32.28 | 0.34 | metal |
| Na, Mg | 192.11 | 229.62 | 52.22 | 95.88 | 94.63 | 35.43 | 0.34 | metal |
| Na, Al * | 134.19 | 213.54 | 27.69 | 67.29 | 70.94 | 26.78 | 0.32 | metal |
| Na, K | 163.82 | 210.07 | 35.39 | 80.41 | 84.65 | 31.96 | 0.32 | metal |
| Na, Ca | 176.04 | 236.56 | 45.93 | 93.15 | 92.19 | 34.53 | 0.34 | metal |
| Na, Sc * | 168.74 | 227.96 | 42.56 | 86.82 | 76.12 | 28.11 | 0.35 | metal |
| Na, Ti | 132.90 | 236.19 | 64.50 | 85.31 | 83.13 | 31.07 | 0.34 | metal |
| Na, V | 139.34 | 224.61 | 81.76 | 81.30 | 91.10 | 34.69 | 0.31 | metal |
| Na, Cr | 188.66 | 231.72 | 67.35 | 99.89 | 98.81 | 37.01 | 0.34 | metal |
| Na, Mn | 192.04 | 228.80 | 51.41 | 95.43 | 94.42 | 35.36 | 0.34 | metal |
| Na, Fe | 184.22 | 220.59 | 41.70 | 95.18 | 86.56 | 32.10 | 0.35 | metal |
| Na, Co * | 182.06 | 235.02 | 27.00 | 78.03 | 81.14 | 30.58 | 0.33 | metal |
| Na, Ni * | 171.86 | 229.76 | 62.91 | 79.50 | 90.02 | 34.32 | 0.31 | metal |
| Na, Cu | 173.65 | 223.93 | 47.30 | 89.93 | 80.87 | 29.95 | 0.35 | metal |
| Na, Zn | 191.81 | 225.46 | 48.41 | 95.09 | 93.25 | 34.89 | 0.34 | metal |
| Mg, Al | 181.36 | 257.38 | 62.95 | 99.18 | 103.06 | 38.84 | 0.33 | metal |
| Mg, K | 179.63 | 225.05 | 39.73 | 90.08 | 100.97 | 38.45 | 0.31 | metal |
| Mg, Ca | 207.43 | 248.33 | 68.19 | 108.05 | 111.40 | 41.94 | 0.33 | 0.16 |
| Mg, Sc | 190.59 | 202.86 | 101.40 | 93.06 | 95.58 | 35.97 | 0.33 | 0.19 |
| Mg, Ti * | 178.62 | 224.56 | 16.78 | 84.77 | 69.08 | 25.32 | 0.36 | metal |
| Mg, V | 215.85 | 255.24 | 89.67 | 116.66 | 100.84 | 37.20 | 0.36 | metal |
| Mg, Cr | 216.94 | 252.68 | 63.09 | 113.40 | 97.13 | 35.78 | 0.36 | 0.15 |
| Mg, Mn | 175.28 | 252.18 | 59.21 | 99.06 | 90.10 | 33.41 | 0.35 | metal |
| Mg, Fe * | 192.59 | 240.38 | 39.68 | 101.32 | 70.01 | 25.28 | 0.38 | metal |
| Mg, Co | 155.68 | 216.09 | 36.41 | 72.94 | 77.57 | 29.32 | 0.32 | metal |
| Mg, Ni | 178.05 | 243.94 | 63.53 | 101.67 | 88.47 | 32.65 | 0.35 | metal |
| Mg, Cu | 176.35 | 228.65 | 47.06 | 95.33 | 81.21 | 29.90 | 0.36 | metal |
| Mg, Zn | 138.83 | 181.10 | 25.19 | 63.24 | 68.06 | 25.77 | 0.32 | metal |
| Al, K | 144.54 | 226.89 | 30.74 | 74.99 | 94.67 | 36.71 | 0.29 | metal |
| Al, Ca | 190.85 | 248.94 | 110.96 | 113.22 | 112.94 | 42.34 | 0.33 | metal |
| Al, Sc * | 177.47 | 220.54 | 86.65 | 95.23 | 77.35 | 28.34 | 0.36 | metal |
| Al, Ti | 190.12 | 278.23 | 75.31 | 102.18 | 122.25 | 47.00 | 0.30 | metal |
| Al, V | 174.82 | 265.06 | 63.13 | 99.00 | 112.96 | 43.12 | 0.31 | metal |
| Al, Cr | 203.13 | 305.86 | 144.41 | 133.42 | 155.83 | 59.69 | 0.31 | metal |
| Al, Mn | 140.36 | 219.45 | 43.54 | 71.93 | 78.79 | 29.90 | 0.32 | metal |
| Al, Fe | 169.46 | 249.93 | 56.82 | 98.69 | 97.10 | 36.34 | 0.34 | metal |
| Al, Co | 182.68 | 261.93 | 66.51 | 101.06 | 108.13 | 40.91 | 0.32 | metal |
| Al, Ni | 154.21 | 253.78 | 43.35 | 83.72 | 85.90 | 32.32 | 0.33 | metal |
| Al, Cu * | 143.62 | 219.17 | 31.41 | 70.33 | 53.84 | 19.61 | 0.37 | metal |
| Al, Zn * | 118.89 | 204.58 | 33.62 | 66.12 | 66.29 | 24.87 | 0.33 | metal |
| K, Ca * | 156.52 | 219.61 | 54.43 | 87.04 | 62.61 | 22.68 | 0.38 | metal |
| K, Sc | 154.01 | 223.96 | 46.59 | 81.08 | 85.07 | 32.10 | 0.33 | metal |
| K, Ti | 116.71 | 221.52 | 89.44 | 77.60 | 94.77 | 36.55 | 0.30 | metal |
| K, V | 155.35 | 231.92 | 101.99 | 92.75 | 104.50 | 39.82 | 0.31 | metal |
| K, Cr | 186.73 | 227.42 | 54.76 | 96.52 | 106.68 | 40.54 | 0.32 | metal |
| K, Mn | 187.15 | 223.47 | 45.57 | 92.77 | 101.68 | 38.59 | 0.32 | metal |
| K, Fe | 166.40 | 209.33 | 34.01 | 81.24 | 87.45 | 33.11 | 0.32 | metal |
| K, Co | 164.14 | 220.29 | 40.81 | 83.72 | 91.57 | 34.75 | 0.32 | metal |
| K, Ni | 167.36 | 217.93 | 37.09 | 86.53 | 87.23 | 32.74 | 0.33 | metal |
| K, Cu | 176.80 | 225.94 | 38.47 | 89.41 | 84.04 | 31.28 | 0.34 | metal |
| K, Zn | 170.14 | 218.06 | 36.94 | 83.59 | 89.45 | 33.84 | 0.32 | metal |
| Ca, Sc | 190.92 | 249.69 | 66.62 | 106.57 | 108.02 | 40.58 | 0.33 | metal |
| Ca, Ti | 191.04 | 240.23 | 132.97 | 112.17 | 109.79 | 41.06 | 0.34 | metal |
| Ca, V | 194.87 | 244.95 | 130.29 | 114.00 | 116.08 | 43.63 | 0.33 | metal |
| Ca, Cr | 201.92 | 251.90 | 94.50 | 113.01 | 117.09 | 44.11 | 0.33 | 0.17 † |
| Ca, Mn | 205.68 | 248.31 | 66.70 | 107.45 | 110.49 | 41.58 | 0.33 | 0.13 |
| Ca, Fe | 205.88 | 252.55 | 75.81 | 110.53 | 111.69 | 41.94 | 0.33 | metal |
| Ca, Co * | 178.11 | 251.16 | 68.70 | 103.27 | 105.75 | 39.78 | 0.33 | metal |
| Ca, Ni * | 183.65 | 244.71 | 83.99 | 105.95 | 90.26 | 33.23 | 0.36 | metal |
| Ca, Cu | 177.89 | 246.19 | 68.00 | 99.96 | 94.01 | 34.99 | 0.34 | metal |
| Ca, Zn | 208.13 | 247.74 | 70.06 | 108.41 | 110.38 | 41.49 | 0.33 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Zhou, B. Electronic Properties and Mechanical Stability of Multi-Ion-Co-Intercalated Bilayered V2O5. Materials 2024, 17, 3364. https://doi.org/10.3390/ma17133364
Ma C, Zhou B. Electronic Properties and Mechanical Stability of Multi-Ion-Co-Intercalated Bilayered V2O5. Materials. 2024; 17(13):3364. https://doi.org/10.3390/ma17133364
Chicago/Turabian StyleMa, Chunhui, and Bo Zhou. 2024. "Electronic Properties and Mechanical Stability of Multi-Ion-Co-Intercalated Bilayered V2O5" Materials 17, no. 13: 3364. https://doi.org/10.3390/ma17133364




