The Irradiation Effects in Ferritic, Ferritic–Martensitic and Austenitic Oxide Dispersion Strengthened Alloys: A Review
Abstract
:1. Introduction
2. Stainless Steels for Nuclear Applications
3. A Brief History of ODS Steels in the Context of Fission Reactors
4. Ferritic and FM ODS Alloys
5. Austenitic ODS Alloys
6. Conclusions
- Ferritic ODS steel:
- Advantages:
- -
- Bcc crystal structure of the matrix;
- -
- Higher operational temperature;
- -
- Low swelling;
- -
- High Cr content in bcc alloys increases the corrosion resistance;
- -
- Good oxidation resistance.
- Disadvantages:
- -
- Anisotropic mechanical properties;
- -
- The ferromagnetism;
- -
- Poor fracture toughness.
- Ferritic–martensitic (FM) ODS steel:
- Advantages:
- -
- Excellent creep resistance up to 800 °C as a consequence of a fine dispersion of insoluble oxide particles;
- -
- Large grain size and a high grain aspect ratio;
- -
- Nearly isotropic properties after heat treatment;
- -
- Scalable fabrication.
- Disadvantages:
- -
- Limited long-term applications at temperatures below 700 °C due to embrittlement associated with a precipitation of intermetallic phases;
- -
- The ferromagnetism;
- -
- Low oxidation resistance at high temperatures;
- -
- Poor fracture toughness.
- Austenitic ODS steel:
- Advantages:
- -
- Fcc crystal structure of the matrix;
- -
- Good ductility and toughness at high temperatures;
- -
- Better protection against corrosion;
- -
- Higher carbon solubility;
- -
- Non-magnetism;
- -
- High strength of austenitic ODS steels due to very small grain sizes and blocking of grain growth at high temperatures.
- Disadvantages:
- -
- The strength of the austenitic ODS steels is lower than ferritic counterparts at all temperatures;
- -
- Processing challenges associated with MA.
Author Contributions
Funding
Conflicts of Interest
References
- Hosemann, P.; Frazer, D.; Fratoni, M.; Bolind, A.; Ashby, M.F. Materials Selection for Nuclear Applications: Challenges and Opportunities. Scr. Mater. 2018, 143, 181–187. [Google Scholar] [CrossRef]
- Suri, A.K.; Krishnamurthy, N.; Batra, I.S. Materials Issues in Fusion Reactors. J. Phys. Conf. Ser. 2010, 208, 012001. [Google Scholar] [CrossRef]
- Odette, R.; Zinkle, S. (Eds.) Structural Alloys for Nuclear Energy Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-397046-6. [Google Scholar]
- Oka, H.; Watanabe, M.; Kinoshita, H.; Shibayama, T.; Hashimoto, N.; Ohnuki, S.; Yamashita, S.; Ohtsuka, S. In Situ Observation of Damage Structure in ODS Austenitic Steel during Electron Irradiation. J. Nucl. Mater. 2011, 417, 279–282. [Google Scholar] [CrossRef]
- Phaniraj, M.P.; Kim, D.I.; Shim, J.H.; Cho, Y.W. Microstructure Development in Mechanically Alloyed Yttria Dispersed Austenitic Steels. Acta Mater. 2009, 57, 1856–1864. [Google Scholar] [CrossRef]
- Seils, S.; Kauffmann, A.; Hinrichs, F.; Schliephake, D.; Boll, T.; Heilmaier, M. Temperature Dependent Strengthening Contributions in Austenitic and Ferritic ODS Steels. Mater. Sci. Eng. A 2020, 786, 139452. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Busby, J.T. Structural Materials for Fission & Fusion Energy. Mater. Today 2009, 12, 12–19. [Google Scholar] [CrossRef]
- Fave, L.; Pouchon, M.A.; Döbeli, M.; Schulte-Borchers, M.; Kimura, A. Helium Ion Irradiation Induced Swelling and Hardening in Commercial and Experimental ODS Steels. J. Nucl. Mater. 2014, 445, 235–240. [Google Scholar] [CrossRef]
- Garner, F.A.; Toloczko, M.B.; Sencer, B.H. Comparison of Swelling and Irradiation Creep Behavior of Fcc-Austenitic and Bcc-Ferritic/Martensitic Alloys at High Neutron Exposure. J. Nucl. Mater. 2000, 276, 123–142. [Google Scholar] [CrossRef]
- Toloczko, M.B.; Garner, F.A.; Voyevodin, V.N.; Bryk, V.V.; Borodin, O.V.; Mel’nychenko, V.V.; Kalchenko, A.S. Ion-Induced Swelling of ODS Ferritic Alloy MA957 Tubing to 500 Dpa. J. Nucl. Mater. 2014, 453, 323–333. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Sugano, R.; Hasegawa, A.; Matsui, H. Annealing Behavior of Irradiation Hardening and Microstructure in Helium-Implanted Reduced Activation Martensitic Steel. J. Nucl. Mater. 2000, 283–287, 827–831. [Google Scholar] [CrossRef]
- Song, P.; Morrall, D.; Zhang, Z.; Yabuuchi, K.; Kimura, A. Radiation Response of ODS Ferritic Steels with Different Oxide Particles under Ion-Irradiation at 550 °C. J. Nucl. Mater. 2018, 502, 76–85. [Google Scholar] [CrossRef]
- Davis, J.W.; Michel, D.J. Ferritic Alloys for Use in Nuclear Energy Technologies; The Metallurgical Society, Inc.: Warrendale, PA, USA, 1984; Volume 27, ISBN 0-89520-458-4. [Google Scholar]
- Klueh, R.L.; Gelles, D.S.; Jitsukawa, S.; Kimura, A.; Odette, G.R.; Van der Schaaf, B.; Victoria, M. Ferritic/Martensitic Steels—Overview of Recent Results. J. Nucl. Mater. 2002, 307–311, 455–465. [Google Scholar] [CrossRef]
- Mann, F.M. Reduced Activation Calculations for the STARFIRE First Wall. J. Nucl. Mater. 1984, 123, 1053–1057. [Google Scholar] [CrossRef]
- Ehrlich, K.; Cierjacks, S.W.; Kelzenberg, S.; Möslang, A. The Development of Structural Materials for Reduced Long-Term Activation. ASTM Spec. Tech. Publ. 1996, 1270, 1109–1122. [Google Scholar] [CrossRef]
- Kohyama, A.; Kohno, Y.; Asakura, K.; Kayano, H. R&L D of Low Activation Ferritic Steels for Fusion in Japanese Universities. J. Nucl. Mater. 1994, 212, 684–689. [Google Scholar]
- Noda, T.; Abe, F.; Araki, H.; Okada, M. Development of Low Activation Ferritic Steels. J. Nucl. Mater. 1986, 141–143, 1102–1106. [Google Scholar] [CrossRef]
- Tupholme, K.W.; Dulieu, D.; Butterworth, G.J. The Development of Low-Activation Martensitic 9 and 11%Cr, W, V Stainless Steels for Fusion Reactor Applications. J. Nucl. Mater. 1988, 155–157, 650–655. [Google Scholar] [CrossRef]
- Ehrlich, K.; Kelzenberg, S.; Röhrig, H.D.; Schäfer, L.; Schirra, M. The Development of Ferritic-Martensitic Steels with Reduced Long-Term Activation. J. Nucl. Mater. 1994, 212–215, 678–683. [Google Scholar] [CrossRef]
- Cabet, C.; Dalle, F.; Gaganidze, E.; Henry, J.; Tanigawa, H. Ferritic-Martensitic Steels for Fission and Fusion Applications. J. Nucl. Mater. 2019, 523, 510–537. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Boutard, J.L.; Hoelzer, D.T.; Kimura, A.; Lindau, R.; Odette, G.R.; Rieth, M.; Tan, L.; Tanigawa, H. Development of next Generation Tempered and ODS Reduced Activation Ferritic/Martensitic Steels for Fusion Energy Applications. Nucl. Fusion 2017, 57, 092005. [Google Scholar] [CrossRef]
- Mazzone, G.; Aktaa, J.; Bachmann, C.; De Meis, D.; Frosi, P.; Gaganidze, E.; Gironimo, G.D.; Mariano, G.; Marzullo, D.; Porfiri, M.T.; et al. Choice of a Low Operating Temperature for the DEMO EUROFER97 Divertor Cassette. Fusion Eng. Des. 2017, 124, 655–658. [Google Scholar] [CrossRef]
- Furuya, K.; Wakai, E.; Ando, M.; Sawai, T.; Iwabuchi, A.; Nakamura, K.; Takeuchi, H. Tensile and Impact Properties of F82H Steel Applied to HIP-Bond Fusion Blanket Structures. Fusion Eng. Des. 2003, 69, 385–389. [Google Scholar] [CrossRef]
- Kim, S.W.; Kohyama, A.; Yoon, H.K. Fatigue Crack Growth Behavior and Microstructure of Reduced Activation Ferritic/Martensitic Steel (JLF-1). Fusion Eng. Des. 2006, 81, 1105–1110. [Google Scholar] [CrossRef]
- Klueh, R.L.; Alexander, D.J.; Sokolov, M.A. Effect of Rhenium and Osmium on Mechanical Properties of a 9Cr±2W±0.25V±0.07Ta±0.1C Steel. J. Nucl. Mater. 2000, 279, 91–99. [Google Scholar] [CrossRef]
- Chun, Y.B.; Kang, S.H.; Lee, D.W.; Cho, S.; Jeong, Y.H.; Żywczak, A.; Rhee, C.K. Development of Zr-Containing Advanced Reduced-Activation Alloy (ARAA) as Structural Material for Fusion Reactors. Fusion Eng. Des. 2016, 109–111, 629–633. [Google Scholar] [CrossRef]
- Huang, Q. Development Status of CLAM Steel for Fusion Application. J. Nucl. Mater. 2014, 455, 649–654. [Google Scholar] [CrossRef]
- Filacchioni, G.; Casagrande, E.; De Angelis, U.; De Santis, G.; Ferrara, D.; Pilloni, L. Tensile and Impact Behaviour of BATMAN II Steels, Ti-Bearing Reduced Activation Martensitic Alloys. J. Nucl. Mater. 1999, 271, 445–449. [Google Scholar] [CrossRef]
- Qiu, G.-X.; Dong-Ping, Z.; Cao, L.; Zhou-Hua, J. Review on Development of Reduced Activated Ferritic/Martensitic Steel for Fusion Reactor. J. Iron Steel Res. Int. 2022, 29, 1343–1356. [Google Scholar] [CrossRef]
- Jitsukawa, S.; Kimura, A.; Kohyama, A.; Klueh, R.L.; Tavassoli, A.A.; Van Der Schaaf, B.; Odette, G.R.; Rensman, J.W.; Victoria, M.; Petersen, C. Recent Results of the Reduced Activation Ferritic/Martensitic Steel Development. J. Nucl. Mater. 2004, 329–333, 39–46. [Google Scholar] [CrossRef]
- Sniegowski, J.J.; Wolfer, W.G. On the Physical Basis for the Swelling Resistance of Ferritic Steels; Metallurgical Society of AIME: Warrendale, PA, USA, 1984; ISBN 0-89520-458-4. [Google Scholar]
- Little, E.A.; Stow, D.A. Void-Swelling in Irons and Ferritic Steels: II. An Experimental Survey of Materials Irradiated in a Fast Reactor. J. Nucl. Mater. 1979, 87, 25–39. [Google Scholar] [CrossRef]
- Yan, W.; Wang, W.; Shan, Y.-Y.; Yang, K. Microstructural Stability of 9–12%Cr Ferrite/Martensite Heat-Resistant Steels. Front. Mater. Sci. 2013, 7, 1–27. [Google Scholar] [CrossRef]
- Zilnyk, K.D.; Oliveira, V.B.; Sandim, H.R.Z.; Möslang, A.; Raabe, D. Martensitic Transformation in Eurofer-97 and ODS-Eurofer Steels: A Comparative Study. J. Nucl. Mater. 2015, 462, 360–367. [Google Scholar] [CrossRef]
- Chun, Y.B.; Rhee, C.K.; Lee, D.W.; Park, Y.H. Enhanced High-Temperature Mechanical Properties of ARAA by Thermo-Mechanical Processing. Fusion Eng. Des. 2018, 136, 883–890. [Google Scholar] [CrossRef]
- Tan, L.; Byun, T.S.; Katoh, Y.; Snead, L.L. Stability of MX-Type Strengthening Nanoprecipitates in Ferritic Steels under Thermal Aging, Stress and Ion Irradiation. Acta Mater. 2014, 71, 11–19. [Google Scholar] [CrossRef]
- Gustafson, S. Coarsening of Precipitates in an Advanced Creep Resistant 9% Chromium Steel-Quantitative Microscopy and Simulations. Mater. Sci. Eng. 2002, 333, 279–286. [Google Scholar] [CrossRef]
- Du, J.; Jiang, S.; Cao, P.; Xu, C.; Wu, Y.; Chen, H.; Fu, E.; Lu, Z. Superior Radiation Tolerance via Reversible Disordering–Ordering Transition of Coherent Superlattices. Nat. Mater. 2023, 22, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Hishinuma, A.; Kohyama, A.; Klueh, R.L.; Gelles, D.S.; Dietz, W.; Ehrlich, K. Current Status and Future R&D for Reduced-Activation Ferritic/Martensitic Steels. J. Nucl. Mater. 1998, 258–263, 193–204. [Google Scholar] [CrossRef]
- Klueh, R.L.; Harries, D.R. High Chromium Ferritic and Martensitic Steels for Nuclear Applications; ASTM: West Conhohocken, PA, USA, 2001. [Google Scholar]
- Harries, D.R.; Dupouy, J.M.; Wu, C.H. Materials Research and Development for NET. J. Nucl. Mater. 1985, 133–134, 25–31. [Google Scholar] [CrossRef]
- Anderko, K.; Schäfer, L.; Materna-Morris, E. Effect of the δ-Ferrite Phase on the Impact Properties of Martensitic Chromium Steels. J. Nucl. Mater. 1991, 179–181, 492–495. [Google Scholar] [CrossRef]
- Dubuisson, P.; Gilbon, D.; Séran, J.L. Microstructural Evolution of Ferritic-Martensitic Steels Irradiated in the Fast Breeder Reactor Phénix. J. Nucl. Mater. 1993, 205, 178–189. [Google Scholar] [CrossRef]
- Shen, T.L.; Wang, Z.G.; Yao, C.F.; Sun, J.R.; Li, Y.F.; Wei, K.F.; Zhu, Y.B.; Pang, L.L.; Cui, M.H.; Wang, J.; et al. The Sink Effect of the Second-Phase Particle on the Cavity Swelling in RAFM Steel under Ar-Ion Irradiation at 773 K. Nucl. Instrum. Methods Phys. Res. Sect. B 2013, 307, 512–515. [Google Scholar] [CrossRef]
- Tan, L.; Snead, L.L.; Katoh, Y. Development of New Generation Reduced Activation Ferritic-Martensitic Steels for Advanced Fusion Reactors. J. Nucl. Mater. 2016, 478, 42–49. [Google Scholar] [CrossRef]
- Tan, L.; Katoh, Y.; Snead, L.L. Stability of the Strengthening Nanoprecipitates in Reduced Activation Ferritic Steels under Fe2+ Ion Irradiation. J. Nucl. Mater. 2014, 445, 104–110. [Google Scholar] [CrossRef]
- Terentyev, D.; Puype, A.; Kachko, O.; Van Renterghem, W.; Henry, J. Development of RAFM Steel for Nuclear Applications with Reduced Manganese, Silicon and Carbon Content. Nucl. Mater. Energy 2021, 29, 101070. [Google Scholar] [CrossRef]
- Spätig, P.; Chen, J.-C.; Odette, G.R. Ferritic and Tempered Martensitic Steels. In Structural Alloys for Nuclear Energy Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 485–527. [Google Scholar]
- Xie, Z.; Guo, L.; Chen, Y.; Long, Y.; Lv, P.; Li, F.; Luo, H.; Lin, W.; Yin, Z.; Cao, J.; et al. Deterioration of Irradiation Resistance of ODS-F/M Steel under High Concentration of Helium. J. Nucl. Mater. 2023, 577, 154293. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Zinkle, S.J.; Henry, J.; Levine, S.M.; Edmondson, P.D.; Gilbert, M.R.; Tanigawa, H.; Kessel, C.E. Irradiation Damage Concurrent Challenges with RAFM and ODS Steels for Fusion Reactor First-Wall/Blanket: A Review. J. Phys. Energy 2022, 4, 034003. [Google Scholar] [CrossRef]
- Jordan, M.S.L.; Ramsay, P.; Verrall, K.E.; van Staveren, T.O.; Brown, M.; Davies, B.; Tzelepi, A.; Metcalfe, M.P. Determining the Electrical and Thermal Resistivities of Radiolytically-Oxidised Nuclear Graphite by Small Sample Characterisation. J. Nucl. Mater. 2018, 507, 68–77. [Google Scholar] [CrossRef]
- Beck, P.W. Nuclear Energy in the Twenty-First Century: Examination of a Contentious Subject. Annu. Rev. Energy Environ. 1999, 24, 113–137. [Google Scholar] [CrossRef]
- Brook, B.W.; Bradshaw, C.J.A. Key Role for Nuclear Energy in Global Biodiversity Conservation. Conserv. Biol. 2015, 29, 702–712. [Google Scholar] [CrossRef]
- Murty, K.L.; Charit, I. Structural Materials for Gen-IV Nuclear Reactors: Challenges and Opportunities. J. Nucl. Mater. 2008, 383, 189–195. [Google Scholar] [CrossRef]
- Cawthorne, C.; Fulton, E.J. Voids in Irradiated Stainless Steel. Nature 1967, 216, 575–576. [Google Scholar] [CrossRef]
- De Bremaecker, A. Past Research and Fabrication Conducted at SCK•CEN on Ferritic ODS Alloys Used as Cladding for FBR’s Fuel Pins. J. Nucl. Mater. 2012, 428, 13–30. [Google Scholar] [CrossRef]
- Huet, J.J.; Massaux, H.; De Wilde, L.; Noels, J. Fabrication et Propriétés d’aciers Ferritiques Renforcés Par Dispersion. Rev. Métallurgie 1968, 65, 863–869. [Google Scholar] [CrossRef]
- Huet, J.J.; Leroy, V. Dispersion Strengthened Ferritic Steels as Fast Reactor Structural Materials. Nucl. Technol. 1974, 24, 216–224. [Google Scholar] [CrossRef]
- Sands, R.L.; Phelps, L.A.; Morgan, W.R. Dispersion-Strengthened Stainless Steel. Powder Metall. 1962, 5, 158–170. [Google Scholar] [CrossRef]
- Ukai, S.; Harada, M.; Okada, H.; Inoue, M.; Nomura, S.; Shikakura, S.; Asabe, K.; Nishida, T.; Fujiwara, M. Alloying Design of Oxide Dispersion Strengthened Ferritic Steel for Long Life FBRs Core Materials. J. Nucl. Mater. 1993, 204, 65–73. [Google Scholar] [CrossRef]
- Ukai, S.; Nishida, T.; Okada, H.; Okuda, T.; Fujiwara, M.; Asabe, K. Development of Oxide Dispersion Strengthened Ferritic Steels for Fbr Core Application. J. Nucl. Sci. Technol. 1997, 34, 256–263. [Google Scholar] [CrossRef]
- Ukai, S.; Nishida, T.; Okuda, T.; Yoshitake, T. Development of Oxide Dispersion Strengthened Steels for FBR Core Application, (II): Morphology Improvement by Martensite Transformation. J. Nucl. Sci. Technol. 1998, 35, 294–300. [Google Scholar] [CrossRef]
- Okuda, T.; Fujiwara, M. Dispersion Behaviour of Oxide Particles in Mechanically Alloyed ODS Steel. J. Mater. Sci. Lett. 1995, 14, 1600–1603. [Google Scholar] [CrossRef]
- Hoelzer, D.T. History and Outlook of ODS/NFA Ferritic Alloys for Nuclear Applications. In Proceedings of the Transactions of the American Nuclear Society, Philadelphia, PA, USA, 17–21 June 2018; Volume 118, p. 1587. [Google Scholar]
- Larson, D.J.; Maziasz, P.J.; Kim, I.S.; Miyahara, K. Three-Dimensional Atom Probe Observation of Nanoscale Titanium-Oxygen Clustering in an Oxide-Dispersion-Strengthened Fe-12Cr-3W-0.4Ti + Y2O3 Ferritic Alloy. Scr. Mater. 2001, 44, 359–364. [Google Scholar] [CrossRef]
- Miller, M.K.; Hoelzer, D.T.; Kenik, E.A.; Russell, K.F. Nanometer Scale Precipitation in Ferritic MA/ODS Alloy MA957. J. Nucl. Mater. 2004, 329–333, 338–341. [Google Scholar] [CrossRef]
- Hoelzer, D.T.; Unocic, K.A.; Sokolov, M.A.; Byun, T.S. Influence of Processing on the Microstructure and Mechanical Properties of 14YWT. J. Nucl. Mater. 2016, 471, 251–265. [Google Scholar] [CrossRef]
- Byun, T.S.; Hoelzer, D.T.; Kim, J.H.; Maloy, S.A. A Comparative Assessment of the Fracture Toughness Behavior of Ferritic-Martensitic Steels and Nanostructured Ferritic Alloys. J. Nucl. Mater. 2017, 484, 157–167. [Google Scholar] [CrossRef]
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. On the Role of Alloy Composition and Processing Parameters in Nanocluster Formation and Dispersion Strengthening in Nanostuctured Ferritic Alloys. Acta Mater. 2009, 57, 392–406. [Google Scholar] [CrossRef]
- Hoelzer, D.T.; Bentley, J.; Sokolov, M.A.; Miller, M.K.; Odette, G.R.; Alinger, M.J. Influence of Particle Dispersions on the High-Temperature Strength of Ferritic Alloys. J. Nucl. Mater. 2007, 367–370, 166–172. [Google Scholar] [CrossRef]
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent Developments in Irradiation-Resistant Steels. Annu. Rev. Mater. Res. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Odette, G.R. Recent Progress in Developing and Qualifying Nanostructured Ferritic Alloys for Advanced Fission and Fusion Applications. JOM 2014, 66, 2427–2441. [Google Scholar] [CrossRef]
- Klueh, R.L.; Maziasz, P.J.; Kim, I.S.; Heatherly, L.; Hoelzer, D.T.; Hashimoto, N.; Kenik, E.A.; Miyahara, K. Tensile and Creep Properties of an Oxide Dispersion-Strengthened Ferritic Steel. J. Nucl. Mater. 2002, 307–311, 773–777. [Google Scholar] [CrossRef]
- Tuček, K.; Tsige-Tamirat, H.; Ammirabile, L.; Lázaro, A.; Grah, A.; Carlsson, J.; Döderlein, C.; Oettingen, M.; Fütterer, M.A.; D’Agata, E.; et al. Generation IV Reactor Safety and Materials Research by the Institute for Energy and Transport at the European Commission’s Joint Research Centre. Nucl. Eng. Des. 2013, 265, 1181–1193. [Google Scholar] [CrossRef]
- Bates, J.F.; Powell, R.W. Irradiation-Induced Swelling in Commercial Alloys. J. Nucl. Mater. 1981, 102, 200–213. [Google Scholar] [CrossRef]
- Pint, B.A.; Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T. Development of ODS FeCrAl for Compatibility in Fusion and Fission Energy Applications. JOM 2014, 66, 2458–2466. [Google Scholar] [CrossRef]
- Terrani, K.A.; Zinkle, S.J.; Snead, L.L. Advanced Oxidation-Resistant Iron-Based Alloys for LWR Fuel Cladding. J. Nucl. Mater. 2014, 448, 420–435. [Google Scholar] [CrossRef]
- Pint, B.A.; Terrani, K.A.; Brady, M.P.; Cheng, T.; Keiser, J.R. High Temperature Oxidation of Fuel Cladding Candidate Materials in Steam–Hydrogen Environments. J. Nucl. Mater. 2013, 440, 420–427. [Google Scholar] [CrossRef]
- Allen, T.R.; Gan, J.; Cole, J.I.; Miller, M.K.; Busby, J.T.; Shutthanandan, S.; Thevuthasan, S. Radiation Response of a 9 Chromium Oxide Dispersion Strengthened Steel to Heavy Ion Irradiation. J. Nucl. Mater. 2008, 375, 26–37. [Google Scholar] [CrossRef]
- Certain, A.G.; Field, K.G.; Allen, T.R.; Miller, M.K.; Bentley, J.; Busby, J.T. Response of Nanoclusters in a 9Cr ODS Steel to 1 Dpa, 525 °C Proton Irradiation. J. Nucl. Mater. 2010, 407, 2–9. [Google Scholar] [CrossRef]
- Schäublin, R.; Ramar, A.; Baluc, N.; de Castro, V.; Monge, M.A.; Leguey, T.; Schmid, N.; Bonjour, C. Microstructural Development under Irradiation in European ODS Ferritic/Martensitic Steels. J. Nucl. Mater. 2006, 351, 247–260. [Google Scholar] [CrossRef]
- Klimiankou, M.; Lindau, R.; Möslang, A. HRTEM Study of Yttrium Oxide Particles in ODS Steels for Fusion Reactor Application. J. Cryst. Growth 2003, 249, 381–387. [Google Scholar] [CrossRef]
- London, A.J.; Lozano-Perez, S.; Moody, M.P.; Amirthapandian, S.; Panigrahi, B.K.; Sundar, C.S.; Grovenor, C.R.M. Quantification of Oxide Particle Composition in Model Oxide Dispersion Strengthened Steel Alloys. Ultramicroscopy 2015, 159, 360–367. [Google Scholar] [CrossRef]
- Ribis, J.; Lozano-Perez, S. Nano-Cluster Stability Following Neutron Irradiation in MA957 Oxide Dispersion Strengthened Material. J. Nucl. Mater. 2014, 444, 314–322. [Google Scholar] [CrossRef]
- Hsiung, S.; Ritz, J.; Jones, R.; Eiland, J. Design and Evaluation of a Microcontroller Training System for Hands-on Distance and Campus-Based Classes. J. Ind. Technol. 2010, 26, 1–10. [Google Scholar]
- Okita, T.; Wolfer, W.G.; Garner, F.A.; Sekimura, N. Effects of Titanium Additions to Austenitic Ternary Alloys on Microstructural Evolution and Void Swelling. Philos. Mag. 2005, 85, 2033–2048. [Google Scholar] [CrossRef]
- Ukai, S.; Ohtsuka, S.; Kaito, T.; de Carlan, Y.; Ribis, J.; Malaplate, J. Oxide Dispersion-Strengthened/Ferrite-Martensite Steels as Core Materials for Generation IV Nuclear Reactors. In Structural Materials for Generation IV Nuclear Reactors; Woodhead Publishing: Sawston, UK, 2017; pp. 357–414. [Google Scholar] [CrossRef]
- McKimpson, M.G.; O’Donnell, D. Joining ODS Materials for High-Temperature Applications. JOM 1994, 46, 49–51. [Google Scholar] [CrossRef]
- Gessinger, G.H. Joining Techniques for P/M Superalloys. In Powder Metallurgy of Superalloys; Butterworth-Heinemann: Oxford, UK, 1984; pp. 295–327. [Google Scholar] [CrossRef]
- Doyen, O.; Gloannec, B.L.; Deschamps, A.; De Geuser, F.; Pouvreau, C.; Poulon-Quintin, A. Ferritic and Martensitic ODS Steel Resistance Upset Welding of Fuel Claddings: Weldability Assessment and Metallurgical Effects. J. Nucl. Mater. 2019, 518, 326–333. [Google Scholar] [CrossRef]
- Dubuisson, P.; Carlan, Y.D.; Garat, V.; Blat, M. ODS Ferritic/Martensitic Alloys for Sodium Fast Reactor Fuel Pin Cladding. J. Nucl. Mater. 2012, 428, 6–12. [Google Scholar] [CrossRef]
- Monnet, I. Stabilité Sous Irradiation de Particules d’oxydes Finement Dispersées Dans Des Alliages Ferritiques. Doctoral Dissertation, Ecole Centrale de Paris, Châtenay-Malabry, France, 1999. [Google Scholar]
- Barnes, R.S. Embrittlement of Stainless Steels and Nickel-Based Alloys at High Temperature Induced by Neutron Radiation. Nature 1965, 206, 1307–1310. [Google Scholar] [CrossRef]
- Ullmaier, H. Introductory Remarks—Helium in Metals. Radiat. Eff. 1983, 78, 1–10. [Google Scholar] [CrossRef]
- Ongena, J.; Ogawa, Y. Nuclear Fusion: Status Report and Future Prospects. Energy Policy 2016, 96, 770–778. [Google Scholar] [CrossRef]
- Ukai, S.; Kaito, T.; Ohtsuka, S.; Narita, T.; Fujiwara, M.; Kobayashi, T. Production and Properties of Nano-Scale Oxide Dispersion Strengthened (ODS) 9Cr Martensitic Steel Claddings. ISIJ Int. 2003, 43, 2038–2045. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Kaito, T.; Tanno, T.; Yano, Y.; Koyama, S.; Tanaka, K. Microstructure and High-Temperature Strength of High Cr ODS Tempered Martensitic Steels. J. Nucl. Mater. 2013, 442, S89–S94. [Google Scholar] [CrossRef]
- Henry, J.; Averty, X.; Alamo, A. Tensile and Impact Properties of 9Cr Tempered Martensitic Steels and ODS-FeCr Alloys Irradiated in a Fast Reactor at 325 °C up to 78 Dpa. J. Nucl. Mater. 2011, 417, 99–103. [Google Scholar] [CrossRef]
- Zhang, C.H.; Kimura, A.; Kasada, R.; Jang, J.; Kishimoto, H.; Yang, Y.T. Characterization of the Oxide Particles in Al-Added High-Cr ODS Ferritic Steels. J. Nucl. Mater. 2011, 417, 221–224. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al Added High-Cr ODS Steels for Fuel Cladding of next Generation Nuclear Systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Stratil, L.; Horník, V.; Dymáček, P.; Roupcová, P.; Svoboda, J. The Influence of Aluminum Content on Oxidation Resistance of New-Generation ODS Alloy at 1200 °C. Metals 2020, 10, 1478. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wang, C.; Shen, H. Effect of Al Content on the Oxidation Behavior of Y2Ti2O7-Dispersed Fe-14Cr Ferritic Alloys. Corros. Sci. 2016, 104, 17–25. [Google Scholar] [CrossRef]
- Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T.; Massey, C.P.; Pint, B.A. Development of Low-Cr ODS FeCrAl Alloys for Accident-Tolerant Fuel Cladding. J. Nucl. Mater. 2018, 501, 59–71. [Google Scholar] [CrossRef]
- Lee, J.H. Development of Oxide Dispersion Strengthened Ferritic Steels with and without Aluminum. Front. Energy 2012, 6, 29–34. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Z.; Mo, K.; Miao, Y.; Li, S.; Liu, X.; Wang, M.; Park, J.S.; Almer, J.; Stubbins, J.F. The Comparison of Microstructures and Mechanical Properties between 14Cr-Al and 14Cr-Ti Ferritic ODS Alloys. Mater. Des. 2016, 98, 61–67. [Google Scholar] [CrossRef]
- Kim, I.S.; Hunn, J.D.; Hashimoto, N.; Larson, D.L.; Maziasz, P.J.; Miyahara, K.; Lee, E.H. Defect and Void Evolution in Oxide Dispersion Strengthened Ferritic Steels under 3.2 MeV Fe+ Ion Irradiation with Simultaneous Helium Injection. J. Nucl. Mater. 2000, 280, 264–274. [Google Scholar] [CrossRef]
- de Carlan, Y.; Bechade, J.-L.; Dubuisson, P.; Seran, J.-L.; Billot, P.; Bougault, A.; Cozzika, T.; Doriot, S.; Hamon, D.; Henry, J.; et al. CEA Developments of New Ferritic ODS Alloys for Nuclear Applications. J. Nucl. Mater. 2009, 386–388, 430–432. [Google Scholar] [CrossRef]
- Wasilkowska, A.; Bartsch, M.; Messerschmidt, U.; Herzog, R.; Czyrska-Filemonowicz, A. Creep Mechanisms of Ferritic Oxide Dispersion Strengthened Alloys. J. Mater. Process Technol. 2003, 133, 218–224. [Google Scholar] [CrossRef]
- Lim, J.; Nam, H.O.; Hwang, I.S.; Kim, J.H. A Study of Early Corrosion Behaviors of FeCrAl Alloys in Liquid Lead–Bismuth Eutectic Environments. J. Nucl. Mater. 2010, 407, 205–210. [Google Scholar] [CrossRef]
- Lee, S.K.; Garrison, B.E.; Capps, N.A.; Pastore, G.; Massey, C.P.; Linton, K.D.; Brown, N.R. BISON Validation of FeCrAl Cladding Mechanical Failure during Simulated Reactivity-Initiated Accident Conditions. J. Nucl. Mater. 2022, 564, 153676. [Google Scholar] [CrossRef]
- Aghamiri, S.M.S.; Sugawara, T.; Ukai, S.; Oono, N.; Sakamoto, K.; Yamashita, S. Orientation Dependence of Yield Strength in a New Single Crystal-like FeCrAl Oxide Dispersion Strengthened Alloy. Mater. Charact. 2021, 176, 111043. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, R.; Long, D.; Sun, Y.; Zhou, Z. Effect of Zirconium Content on Mechanical Properties of ODS FeCrAl Alloy. Mater. Sci. Eng. A 2022, 839, 142824. [Google Scholar] [CrossRef]
- Ukai, S.; Fujiwara, M. Perspective of ODS Alloys Application in Nuclear Environments. J. Nucl. Mater. 2002, 307–311, 749–757. [Google Scholar] [CrossRef]
- Pereira, V.S.M.; Wang, S.; Morgan, T.; Schut, H.; Sietsma, J. Microstructural Evolution and Behavior of Deuterium in a Ferritic ODS 12 Cr Steel Annealed at Different Temperatures. Metall. Mater. Trans. A 2022, 53, 874–892. [Google Scholar] [CrossRef]
- Meza, A.; Macía, E.; García-Junceda, A.; Díaz, L.A.; Chekhonin, P.; Altstadt, E.; Serrano, M.; Rabanal, M.E.; Campos, M. Development of New 14 Cr ODS Steels by Using New Oxides Formers and B as an Inhibitor of the Grain Growth. Metals 2020, 10, 1344. [Google Scholar] [CrossRef]
- Dash, M.K.; Mythili, R.; Dasgupta, A.; Saroja, S. Optimization of Consolidation Parameters of 18Cr-ODS Ferritic Steel through Microstructural and Microtexture Characterization. AIP Conf. Proc. 2018, 1942, 140063. [Google Scholar] [CrossRef]
- Paúl, A.; Alves, E.; Alves, L.C.; Marques, C.; Lindau, R.; Odriozola, J.A. Microstructural Characterization of Eurofer-ODS RAFM Steel in the Normalized and Tempered Condition and after Thermal Aging in Simulated Fusion Conditions. Fusion Eng. Des. 2005, 75–79, 1061–1065. [Google Scholar] [CrossRef]
- Sittiho, A.; Tungala, V.; Charit, I.; Mishra, R.S. Microstructure, Mechanical Properties and Strengthening Mechanisms of Friction Stir Welded Kanthal APMT Steel. J. Nucl. Mater. 2018, 509, 435–444. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kane, K.; Pint, B.; Trofimov, A.; Wang, H. Report on Exploration of New FeCrAl Heat Variants with Improved Properties; Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 2019. [Google Scholar]
- Stan, T.; Wu, Y.; Ciston, J.; Yamamoto, T.; Odette, G.R. Characterization of Polyhedral Nano-Oxides and Helium Bubbles in an Annealed Nanostructured Ferritic Alloy. Acta Mater. 2020, 183, 484–492. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Snead, L.L. Designing Radiation Resistance in Materials for Fusion Energy. Annu. Rev. Mater. Res. 2014, 44, 241–267. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. Polymorphic and Coherency Transition of Y–Al Complex Oxide Particles with Extrusion Temperature in an Al-Alloyed High-Cr Oxide Dispersion Strengthened Ferritic Steel. Acta Mater. 2011, 59, 992–1002. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Yao, L.; Wang, X.L.; Miller, M.K. Vacancy-Controlled Ultrastable Nanoclusters in Nanostructured Ferritic Alloys. Sci. Rep. 2015, 5, 10600. [Google Scholar] [CrossRef] [PubMed]
- Oono, N.; Ukai, S. Precipitation of Oxide Particles in Oxide Dispersion Strengthened (ODS) Ferritic Steels. Mater. Trans. 2018, 59, 1651–1658. [Google Scholar] [CrossRef]
- Ardell, A.J. Precipitation Hardening. Metall. Trans. A 1985, 16, 2131–2165. [Google Scholar] [CrossRef]
- Gladman, T. Precipitation Hardening in Metals. Mater. Sci. Technol. 1999, 15, 30–36. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels; Elsevier: Amsterdam, The Netherlands, 1995; ISBN 9780750680844. [Google Scholar]
- Svoboda, J.; Kocich, R.; Gamanov, Š.; Kunčická, L.; Luptáková, N.; Dymáček, P. Processing Window for Hot Consolidation by Rolling and Rotary Swaging of Fe-10Al-4Cr-4Y2O3 ODS Nanocomposite. Mater. Today Commun. 2023, 34, 105393. [Google Scholar] [CrossRef]
- Williams, C.A.; Unifantowicz, P.; Baluc, N.; Smith, G.D.W.; Marquis, E.A. The Formation and Evolution of Oxide Particles in Oxide-Dispersion-Strengthened Ferritic Steels during Processing. Acta Mater. 2013, 61, 2219–2235. [Google Scholar] [CrossRef]
- Svoboda, J.; Ecker, W.; Razumovskiy, V.I.; Zickler, G.A.; Fischer, F.D. Kinetics of Interaction of Impurity Interstitials with Dislocations Revisited. Prog. Mater. Sci. 2019, 101, 172–206. [Google Scholar] [CrossRef]
- Mayer, M.; Ressel, G.; Svoboda, J. The Effect of Cryogenic Mechanical Alloying and Milling Duration on Powder Particles’ Microstructure of an Oxide Dispersion Strengthened FeCrMnNiCo High-Entropy Alloy. Metall. Mater. Trans. A 2022, 53, 573–584. [Google Scholar] [CrossRef]
- Franke, P.; Heintze, C.; Bergner, F.; Weißgärber, T. Mechanical Properties of Spark Plasma Sintered Fe-Cr Compacts Strengthened by Nanodispersed Yttria Particles. Mater. Mater. Test. 2010, 52, 133–138. [Google Scholar] [CrossRef]
- Heintze, C.; Hernández-Mayoral, M.; Ulbricht, A.; Bergner, F.; Shariq, A.; Weissgärber, T.; Frielinghaus, H. Nanoscale Characterization of ODS Fe–9%Cr Model Alloys Compacted by Spark Plasma Sintering. J. Nucl. Mater. 2011, 428, 139–146. [Google Scholar] [CrossRef]
- Fu, J.; Brouwer, J.C.; Richardson, I.M.; Hermans, M.J.M. Effect of Mechanical Alloying and Spark Plasma Sintering on the Microstructure and Mechanical Properties of ODS Eurofer. Mater. Des. 2019, 177, 107849. [Google Scholar] [CrossRef]
- Suárez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R.; Kessel, H.U.; Hennicke, J.; Kirchner, R.; Kessel, T.; Suárez, M.; Fernández, A.; et al. Challenges and Opportunities for Spark Plasma Sintering: A Key Technology for a New Generation of Materials. Sinter. Appl. 2013, 13, 319–342. [Google Scholar] [CrossRef]
- Unifantowicz, P.; Oksiuta, Z.; Olier, P.; De Carlan, Y.; Baluc, N. Microstructure and Mechanical Properties of an ODS RAF Steel Fabricated by Hot Extrusion or Hot Isostatic Pressing. Fusion Eng. Des. 2011, 86, 2413–2416. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Boehm-Courjault, E.; Baluc, N. Relation between Microstructure and Charpy Impact Properties of an Elemental and Pre-Alloyed 14Cr ODS Ferritic Steel Powder after Hot Isostatic Pressing. J. Mater. Sci. 2010, 45, 3921–3930. [Google Scholar] [CrossRef]
- Khalaj, O.; Mašek, B.; Jirková, H.; Svoboda, J. Experimental Study on Thermomechanical Properties of New-Generation ODS Alloys. Int. J. Miner. Metall. Mater. 2017, 11, 497–500. [Google Scholar] [CrossRef]
- Grundy, E.; Patton, W.H. Properties and Applications of Hot Formed O.D.S. Alloys. In High Temperature Alloys; Springer: Dordrecht, The Netherlands, 1987; pp. 327–335. [Google Scholar] [CrossRef]
- Khalaj, O.; Jirková, H.; Burdová, K.; Stehlík, A.; Kučerová, L.; Vrtáček, J.; Svoboda, J. Hot Rolling vs. Forging: Newly Developed Fe-Al-O Based OPH Alloy. Metals 2021, 11, 228. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Liu, Y.; Huang, Y.; Guo, Q.; Li, H.; Wu, J. Evolution of Al-Containing Phases in ODS Steel by Hot Pressing and Annealing. Powder Technol. 2017, 311, 449–455. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Liu, Y.; Huang, Y.; Ma, Z.; Li, H. Effects of Aluminum and Titanium on the Microstructure of ODS Steels Fabricated by Hot Pressing. Int. J. Miner. Metall. Mater. 2018, 25, 1156. [Google Scholar] [CrossRef]
- Massey, C.P.; Dryepondt, S.N.; Edmondson, P.D.; Terrani, K.A.; Zinkle, S.J. Influence of Mechanical Alloying and Extrusion Conditions on the Microstructure and Tensile Properties of Low-Cr ODS FeCrAl Alloys. J. Nucl. Mater. 2018, 512, 227–238. [Google Scholar] [CrossRef]
- Chlupová, A.; Šulák, I.; Kunčická, L.; Kocich, R.; Svoboda, J. Microstructural Aspects of New Grade ODS Alloy Consolidated by Rotary Swaging. Mater. Charact. 2021, 181, 111477. [Google Scholar] [CrossRef]
- Svoboda, J.; Kunčická, L.; Luptáková, N.; Weiser, A.; Dymáček, P. Fundamental Improvement of Creep Resistance of New-Generation Nano-Oxide Strengthened Alloys via Hot Rotary Swaging Consolidation. Materials 2020, 13, 5217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Prakash, U.; Dabhade, V.; Laha, K.; Sakthivel, T. Development of Oxide Dispersion Strengthened (ODS) Ferritic Steel Through Powder Forging. J. Mater. Eng. Perform. 2017, 26, 1817–1824. [Google Scholar] [CrossRef]
- Eiselt, C.C.; Schendzielorz, H.; Seubert, A.; Hary, B.; de Carlan, Y.; Diano, P.; Perrin, B.; Cedat, D. ODS-Materials for High Temperature Applications in Advanced Nuclear Systems. Nucl. Mater. Energy 2016, 9, 22–28. [Google Scholar] [CrossRef]
- Deng, L.; Wang, C.; Luo, J.; Tu, J.; Guo, N.; Xu, H.; He, P.; Xia, S.; Yao, Z. Preparation and Property Optimization of FeCrAl-Based ODS Alloy by Machine Learning Combined with Wedge-Shaped Hot-Rolling. Mater. Charact. 2022, 188, 111894. [Google Scholar] [CrossRef]
- Hadraba, H.; Kazimierzak, B.; Stratil, L.; Dlouhy, I. Microstructure and Impact Properties of Ferritic ODS ODM401 (14%Cr-ODS of MA957 Type). J. Nucl. Mater. 2011, 417, 241–244. [Google Scholar] [CrossRef]
- Svoboda, J.; Horník, V.; Riedel, H. Modelling of Processing Steps of New Generation ODS Alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 5296–5305. [Google Scholar] [CrossRef]
- Toualbi, L.; Cayron, C.; Olier, P.; Malaplate, J.; Praud, M.; Mathon, M.-H.; Bossu, D.; Rouesne, E.; Montani, A.; Logé, R.; et al. Assessment of a New Fabrication Route for Fe-9Cr-1W ODS Cladding Tubes. J. Nucl. Mater. 2011, 428, 47–53. [Google Scholar] [CrossRef]
- Dai, C.; Kurmanaeva, L.; Schade, C.; Lavernia, E.; Apelian, D. Microstructure and Mechanical Behavior of ODS Stainless Steel Fabricated Using Cryomilling. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2019, 50, 5767–5781. [Google Scholar] [CrossRef]
- Kimura, Y.; Takaki, S.; Suejima, S.; Uemori, R.; Tamehiro, H. Ultra Grain Refining and Decomposition of Oxide during Super-Heavy Deformation in Oxide Dispersion Ferritic Stainless Steel Powder. ISIJ Int. 1999, 39, 176–182. [Google Scholar] [CrossRef]
- Chen, C.-L.; Richter, A.; Kögler, R.; Talut, G. Dual Beam Irradiation of Nanostructured FeCrAl Oxide Dispersion Strengthened Steel. J. Nucl. Mater. 2011, 412, 350–358. [Google Scholar] [CrossRef]
- He, P.; Liu, T.; Möslang, A.; Lindau, R.; Ziegler, R.; Hoffmann, J.; Kurinskiy, P.; Commin, L.; Vladimirov, P.; Nikitenko, S.; et al. XAFS and TEM Studies of the Structural Evolution of Yttrium-Enriched Oxides in Nanostructured Ferritic Alloys Fabricated by a Powder Metallurgy Process. Mater. Chem. Phys. 2012, 136, 990–998. [Google Scholar] [CrossRef]
- Brocq, M.; Radiguet, B.; Le Breton, J.-M.; Cuvilly, F.; Pareige, P.; Legendre, F. Nanoscale Characterisation and Clustering Mechanism in an Fe-Y2O3 Model ODS Alloy Processed by Reactive Ball Milling and Annealing. Acta Mater. 2010, 58, 1806–1814. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Kasada, R.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. TEM and HRTEM Study of Oxide Particles in an Al-Alloyed High-Cr Oxide Dispersion Strengthened Steel with Zr Addition. J. Nucl. Mater. 2014, 444, 441–453. [Google Scholar] [CrossRef]
- Chauhan, A.; Bergner, F.; Etienne, A.; Aktaa, J.; de Carlan, Y.; Heintze, C.; Litvinov, D.; Hernandez-Mayoral, M.; Oñorbe, E.; Radiguet, B.; et al. Microstructure Characterization and Strengthening Mechanisms of Oxide Dispersion Strengthened (ODS) Fe–9%Cr and Fe–14%Cr Extruded Bars. J. Nucl. Mater. 2017, 495, 6–19. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.S.; Jang, C.; Kimura, A. Irradiation Hardening and Embrittlement in High-Cr Oxide Dispersion Strengthened Steels. J. Nucl. Mater. 2009, 386–388, 503–506. [Google Scholar] [CrossRef]
- Šćepanović, M.; de Castro, V.; Leguey, T.; Auger, M.A.; Lozano-Perez, S.; Pareja, R. Microstructural Stability of ODS Fe–14Cr (–2W–0.3Ti) Steels after Simultaneous Triple Irradiation. Nucl. Mater. Energy 2016, 9, 490–495. [Google Scholar] [CrossRef]
- Sandim, M.J.R.; Souza Filho, I.R.; Bredda, E.H.; Kostka, A.; Raabe, D.; Sandim, H.R.Z. Short Communication on “Coarsening of Y-Rich Oxide Particles in 9%Cr-ODS Eurofer Steel Annealed at 1350 °C. ” J. Nucl. Mater. 2017, 484, 283–287. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Sandim, M.J.R.; Stamopoulos, D.; Renzetti, R.A.; Santos, A.D.; Sandim, H.R.Z. Annealing Effects on the Microstructure and Coercive Field of Two Ferritic–Martensitic Eurofer Steels: A Comparative Study. J. Nucl. Mater. 2013, 435, 189–195. [Google Scholar] [CrossRef]
- Renzetti, R.A.; Sandim, H.R.Z.; Sandim, M.J.R.; Santos, A.D.; Möslang, A.; Raabe, D. Annealing Effects on Microstructure and Coercive Field of Ferritic-Martensitic ODS Eurofer Steel. Mater. Sci. Eng. A 2011, 528, 1442–1447. [Google Scholar] [CrossRef]
- Cayron, C.; Rath, E.; Chu, I.; Launois, S. Microstructural Evolution of Y2O3 and MgAl2O4 ODS EUROFER Steels during Their Elaboration by Mechanical Milling and Hot Isostatic Pressing. J. Nucl. Mater. 2004, 335, 83–102. [Google Scholar] [CrossRef]
- García-Junceda, A.; Campos, M.; García-Rodríguez, N.; Torralba, J.M. On the Role of Alloy Composition and Sintering Parameters in the Bimodal Grain Size Distribution and Mechanical Properties of ODS Ferritic Steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 5325–5333. [Google Scholar] [CrossRef]
- Macía, E.; García-Junceda, A.; Serrano, M.; Hong, S.J.; Campos, M. Effect of Mechanical Alloying on the Microstructural Evolution of a Ferritic ODS Steel with (Y–Ti–Al–Zr) Addition Processed by Spark Plasma Sintering (SPS). Nucl. Eng. Technol. 2021, 53, 2582–2590. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Sallez, N.; Boulnat, X.; Borbély, A.; Béchade, J.L.; Fabrègue, D.; Perez, M.; de Carlan, Y.; Hennet, L.; Mocuta, C.; Thiaudière, D.; et al. In Situ Characterization of Microstructural Instabilities: Recovery, Recrystallization and Abnormal Growth in Nanoreinforced Steel Powder. Acta Mater. 2015, 87, 377–389. [Google Scholar] [CrossRef]
- Klimenkov, M.; Jäntsch, U.; Rieth, M.; Dürrschnabel, M.; Möslang, A.; Schneider, H.C. Post-Irradiation Microstructural Examination of EUROFER-ODS Steel Irradiated at 300 °C and 400 °C. J. Nucl. Mater. 2021, 557, 153259. [Google Scholar] [CrossRef]
- Materna-Morris, E.; Lindau, R.; Schneider, H.C.; Möslang, A. Tensile Behavior of EUROFER ODS Steel after Neutron Irradiation up to 16.3 Dpa between 250 and 450 °C. Fusion Eng. Des. 2015, 98–99, 2038–2041. [Google Scholar] [CrossRef]
- Kolluri, M.; Edmondson, P.D.; Luzginova, N.V.; Berg, F.A.V.D. A Structure-Property Correlation Study of Neutron Irradiation Induced Damage in EU Batch of ODS Eurofer97 Steel. Mater. Sci. Eng. A 2013, 597, 111–116. [Google Scholar] [CrossRef]
- Kolluri, M.; Edmondson, P.D.; Luzginova, N.V.; Berg, F.A.V.D. Influence of Irradiation Temperature on Microstructure of EU Batch of ODS Eurofer97 Steel Irradiated with Neutrons. Mater. Sci. Technol. 2014, 9, 1697–1702. [Google Scholar] [CrossRef]
- Klimenkov, M. Effect of Irradiation Temperature on Microstructure of Ferritic-Martensitic ODS Steel. J. Nucl. Mater. 2017, 493, 426–435. [Google Scholar] [CrossRef]
- Capdevila, C.; Bhadeshia, H.K.D.H. Manufacturing and Microstructural Evolution of Mechanuically Alloyed Oxide Dispersion Strengthened Superalloys. Adv. Eng. Mater. 2001, 3, 647. [Google Scholar] [CrossRef]
- Hosemann, P.; Thau, H.T.; Johnson, A.L.; Maloy, S.A.; Li, N. Corrosion of ODS Steels in Lead-Bismuth Eutectic. J. Nucl. Mater. 2008, 373, 246–253. [Google Scholar] [CrossRef]
- Takaya, S.; Furukawa, T.; Aoto, K.; Müller, G.; Weisenburger, A.; Heinzel, A.; Inoue, M.; Okuda, T.; Abe, F.; Ohnuki, S.; et al. Corrosion Behavior of Al-Alloying High Cr-ODS Steels in Lead-Bismuth Eutectic. J. Nucl. Mater. 2009, 386–388, 507–510. [Google Scholar] [CrossRef]
- Jung, H.J.; Edwards, D.J.; Kurtz, R.J.; Yamamoto, T.; Wu, Y.; Odette, G.R. Structural and Chemical Evolution in Neutron Irradiated and Helium-Injected Ferritic ODS PM2000 Alloy. J. Nucl. Mater. 2017, 484, 68–80. [Google Scholar] [CrossRef]
- Chen, C.-L.; Richter, A.; Kögler, R. The Effect of Dual Fe+/He+ Ion Beam Irradiation on Microstructural Changes in FeCrAl ODS Alloys. J. Alloys Compd. 2014, 586, S173–S179. [Google Scholar] [CrossRef]
- Hsiung, L.L.; Fluss, M.J.; Tumey, S.J.; Choi, B.W.; Serruys, Y.; Willaime, F.; Kimura, A. Formation Mechanism and the Role of Nanoparticles in Fe-Cr ODS Steels Developed for Radiation Tolerance. Phys. Rev. B 2010, 82, 184103. [Google Scholar] [CrossRef]
- de Castro, V.; Briceno, M.; Jenkins, M.L.; Kirk, M.; Lozano-Perez, S.; Roberts, S.G. In-Situ Fe+ Ion Irradiation of an Oxide Dispersion Strengthened Steel. J. Phys. Conf. Ser. 2014, 522, 012032. [Google Scholar] [CrossRef]
- Chen, J.; Jung, P.; Hoffelner, W.; Ullmaier, H. Dislocation Loops and Bubbles in Oxide Dispersion Strengthened Ferritic Steel after Helium Implantation under Stress. Acta Mater. 2008, 56, 250–258. [Google Scholar] [CrossRef]
- Miller, M.K.; Hoelzer, D.T.; Kenik, E.A.; Russell, K.F. Stability of Ferritic MA/ODS Alloys at High Temperatures. Intermetallics 2005, 13, 387–392. [Google Scholar] [CrossRef]
- Fischer, J.J. Dispersion Strengthened Ferritic Alloy for Use in Liquid-Metal Fast Breeder Reactors (LMFBRS). U.S. Patent 4,075,010, 21 February 1978. [Google Scholar]
- Bailey, G.; Price, R.; Voelkl, E.; Musselman, I.; Kenik, E.; Hoelzer, D.; Maziasz, P.; Miller, M. Characterization of Nanoscale Clusters in Ods Iron-Based Alloys. Microsc. Microanal. 2001, 7, 550–551. [Google Scholar] [CrossRef]
- Miller, M.K.; Kenik, E.A.; Russell, K.F.; Heatherly, L.; Hoelzer, D.T.; Maziasz, P.J. Atom Probe Tomography of Nanoscale Particles in ODS Ferritic Alloys. Mater. Sci. Eng. A 2003, 353, 140–145. [Google Scholar] [CrossRef]
- Miller, M.K.; Russell, K.F.; Hoelzer, D.T. Characterization of Precipitates in MA/ODS Ferritic Alloys. J. Nucl. Mater. 2006, 351, 261–268. [Google Scholar] [CrossRef]
- Sakasegawa, H.; Chaffron, L.; Legendre, F.; Boulanger, L.; Cozzika, T.; Brocq, M.; De Carlan, Y. Correlation between Chemical Composition and Size of Very Small Oxide Particles in the MA957 ODS Ferritic Alloy. J. Nucl. Mater. 2009, 384, 115–118. [Google Scholar] [CrossRef]
- Miller, M.K.; Parish, C.M.; Li, Q. Materials Science and Technology Advanced Oxide Dispersion Strengthened and Nanostructured Ferritic Alloys Advanced Oxide Dispersion Strengthened and Nanostructured Ferritic Alloys. Mater. Sci. Technol. 2013, 29, 1174–1178. [Google Scholar] [CrossRef]
- Wu, Y.; Ciston, J.; Kräemer, S.; Bailey, N.; Odette, G.R.; Hosemann, P. The Crystal Structure, Orientation Relationships and Interfaces of the Nanoscale Oxides in Nanostructured Ferritic Alloys. Acta Mater. 2016, 111, 108–115. [Google Scholar] [CrossRef]
- London, A.J.; Lozano-Perez, S.; Santra, S.; Amirthapandian, S.; Panigrahi, B.K.; Sundar, C.S.; Grovenor, C.R.M. Comparison of Atom Probe Tomography and Transmission Electron Microscopy Analysis of Oxide Dispersion Strengthened Steels. J. Phys. Conf. Ser. 2014, 522, 012028. [Google Scholar] [CrossRef]
- Spartacus, G.; Malaplate, J.; De Geuser, F.; Mouton, I.; Sornin, D.; Perez, M.; Guillou, R.; Arnal, B.; Rouesne, E.; Deschamps, A. Chemical and Structural Evolution of Nano-Oxides from Mechanical Alloying to Consolidated Ferritic Oxide Dispersion Strengthened Steel. Acta Mater. 2022, 233, 117992. [Google Scholar] [CrossRef]
- Edmondson, P.D.; Parish, C.M.; Zhang, Y.; Hallén, A.; Miller, M.K. Helium Bubble Distributions in a Nanostructured Ferritic Alloy. J. Nucl. Mater. 2013, 434, 210–216. [Google Scholar] [CrossRef]
- Auger, M.A.; Hoelzer, D.T.; Field, K.G.; Moody, M.P. Nanoscale Analysis of Ion Irradiated ODS 14YWT Ferritic Alloy. J. Nucl. Mater. 2020, 528, 151852. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Z.; Mo, K.; Miao, Y.; Xu, S.; Jia, H.; Liu, X.; Stubbins, J.F. The Effect of Thermal-Aging on the Microstructure and Mechanical Properties of 9Cr Ferritic/Martensitic ODS Alloy. J. Nucl. Mater. 2019, 522, 212–219. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, S.; Zou, L.; Schneider, Y.; Schmauder, S.; Wang, M. Enhanced Strength and High Temperature Resistance of 25Cr20Ni ODS Austenitic Alloy through Thermo-Mechanical Treatment and Addition of Mo. Fusion Eng. Des. 2019, 138, 175–182. [Google Scholar] [CrossRef]
- Pal, S.; Alam, M.E.; Maloy, S.A.; Hoelzer, D.T.; Odette, G.R. Texture Evolution and Microcracking Mechanisms in As-Extruded and Cross-Rolled Conditions of a 14YWT Nanostructured Ferritic Alloy. Acta Mater. 2018, 152, 338–357. [Google Scholar] [CrossRef]
- Moghadasi, M.A.; Nili-Ahmadabadi, M.; Forghani, F.; Kim, H.S. Development of an Oxide-Dispersion-Strengthened Steel by Introducing Oxygen Carrier Compound into the Melt Aided by a General Thermodynamic Model. Sci. Rep. 2016, 6, 38621. [Google Scholar] [CrossRef] [PubMed]
- Gradl, P.; Mireles, O.R.; Katsarelis, C.; Smith, T.M.; Sowards, J.; Park, A.; Chen, P.; Tinker, D.C.; Protz, C.; Teasley, T.; et al. Advancement of Extreme Environment Additively Manufactured Alloys for next Generation Space Propulsion Applications. Acta Astronaut. 2023, 211, 483–497. [Google Scholar] [CrossRef]
- Svoboda, J.; Horník, V.; Stratil, L.; Hadraba, H.; Mašek, B.; Khalaj, O.; Jirková, H. Microstructure Evolution in ODS Alloys with a High-Volume Fraction of Nano Oxides. Metals 2018, 8, 1079. [Google Scholar] [CrossRef]
- Benjamin, J.S. Dispersion Strengthened Superalloys by Mechanical Alloying. Metall. Trans. 1970, 1, 2943–2951. [Google Scholar] [CrossRef]
- Dymácek, P.; Kocich, R.; Kuncická, L.; Jarý, M.; Luptáková, N.; Holzer, J.; Mašek, B.; Svoboda, J. Processing of Top Creep and Oxidation Resistant Fe-Al Based ODS Alloys. Procedia Struct. Integr. 2022, 42, 1576–1583. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Sun, H.; Hu, H.; Li, S. Effects of Plastic Deformations on Microstructure and Mechanical Properties of ODS-310 Austenitic Steel. J. Nucl. Mater. 2012, 430, 259–263. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, S.; Chen, W.; Liao, L.; Xu, Y. Processing and Characterization of a Hipped Oxide Dispersion Strengthened Austenitic Steel. J. Nucl. Mater. 2012, 428, 31–34. [Google Scholar] [CrossRef]
- Svoboda, J.; Bořil, P.; Holzer, J.; Luptáková, N.; Jarý, M.; Mašek, B.; Dymáček, P. Substantial Improvement of High Temperature Strength of New-Generation Nano-Oxide-Strengthened Alloys by Addition of Metallic Yttrium. Materials 2022, 15, 504. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.C. The Superalloys; Cambridge University Press: Cambridge, UK, 2006; ISBN 9780521859042. [Google Scholar]
- Malaplate, J.; Mompiou, F.; Béchade, J.L.; Van Den Berghe, T.; Ratti, M. Creep Behavior of ODS Materials: A Study of Dislocations/Precipitates Interactions. J. Nucl. Mater. 2011, 417, 205–208. [Google Scholar] [CrossRef]
- Rösler, J.; Arzt, E. A New Model-Based Creep Equation for Dispersion Strengthened Materials. Acta Metall. Mater. 1990, 38, 671–683. [Google Scholar] [CrossRef]
- Gamanov, Š.; Holzer, J.; Roupcová, P.; Svoboda, J. High Temperature Oxidation Kinetics of Fe-10Al-4Cr-4Y2O3 ODS Alloy at 1200–1400 °C. Corros. Sci. 2022, 206, 110498. [Google Scholar] [CrossRef]
- Gamanov, Š.; Luptáková, N.; Bořil, P.; Jarý, M.; Mašek, B.; Dymáček, P.; Svoboda, J. Mechanisms of Plastic Deformation and Fracture in Coarse Grained Fe–10Al–4Cr–4Y2O3 ODS Nanocomposite at 20–1300 °C. J. Mater. Res. Technol. 2023, 24, 4863–4874. [Google Scholar] [CrossRef]
- Oka, K.; Ohnuki, S.; Yamashita, S.; Akasaka, N.; Ohtsuka, S.; Tanigawa, H. Structure of Nano-Size Oxides in ODS Steels and Its Stability under Electron Irradiation. Mater. Trans. 2007, 48, 2563–2566. [Google Scholar] [CrossRef]
- Bowen, A.W.; Leak, G.M. Solute Diffusion in Alpha- and Gamma-Iron. Metall. Trans. 1970, 1, 1695–1700. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, L.; Liu, Y.; Ma, Z.; Li, H.; Liu, C.; Wu, J. Microstructures and Tensile Properties of an Austenitic ODS Heat Resistance Steel. Mater. Sci. Eng. A 2019, 767, 138419. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Sun, H.; Hu, H.; Li, S. Microstructural Observation and Tensile Properties of ODS-304 Austenitic Steel. Mater. Sci. Eng. A 2013, 559, 287–292. [Google Scholar] [CrossRef]
- Miao, Y.; Mo, K.; Zhou, Z.; Liu, X.; Lan, K.C.; Zhang, G.; Miller, M.K.; Powers, K.A.; Almer, J.; Stubbins, J.F. In Situ Synchrotron Tensile Investigations on the Phase Responses within an Oxide Dispersion-Strengthened (ODS) 304 Steel. Mater. Sci. Eng. A 2015, 625, 146–152. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Z.; Li, M.; He, P. Fabrication and Characterization of ODS Austenitic Steels. J. Nucl. Mater. 2011, 417, 283–285. [Google Scholar] [CrossRef]
- Miao, Y.; Mo, K.; Zhou, Z.; Liu, X.; Lan, K.C.; Zhang, G.; Park, J.S.; Almer, J.; Stubbins, J.F. Load-Partitioning in an Oxide Dispersion-Strengthened 310 Steel at Elevated Temperatures. Mater. Des. 2016, 111, 622–630. [Google Scholar] [CrossRef]
- Leo, J.R.O.; Pirfo Barroso, S.; Fitzpatrick, M.E.; Wang, M.; Zhou, Z. Microstructure, Tensile and Creep Properties of an Austenitic ODS 316L Steel. Mater. Sci. Eng. A 2019, 749, 158–165. [Google Scholar] [CrossRef]
- Gräning, T.; Klimenkov, M.; Rieth, M.; Heintze, C.; Möslang, A. Long-Term Stability of the Microstructure of Austenitic ODS Steel Rods Produced with a Carbon-Containing Process Control Agent. J. Nucl. Mater. 2019, 523, 111–120. [Google Scholar] [CrossRef]
- Zhang, H.K.; Yao, Z.; Zhou, Z.; Wang, M.; Kaitasov, O.; Daymond, M.R. Radiation Induced Microstructures in ODS 316 Austenitic Steel under Dual-Beam Ions. J. Nucl. Mater. 2014, 455, 242–247. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, K.; Guo, X.; Zhang, L. A Study on the Corrosion and Stress Corrosion Cracking Susceptibility of 310-ODS Steel in Supercritical Water. J. Nucl. Mater. 2019, 514, 56–65. [Google Scholar] [CrossRef]
- Baker, I.; Iliescu, B.; Li, J.; Frost, H.J. Experiments and Simulations of Directionally Annealed ODS MA 754. Mater. Sci. Eng. A 2008, 492, 353–363. [Google Scholar] [CrossRef]
- Cong, S.; Liu, Z.; Dang, Y.; Zhang, L.; Guo, X. Effects of Cold Work on the Corrosion Behavior of Alloy 800H Exposed to Aerated Supercritical Water. J. Nucl. Mater. 2022, 559, 153408. [Google Scholar] [CrossRef]
- Kim, T.-K.; Bae, C.-S.; Kim, D.-H.; Jang, J.-S.; Kim, S.-H.; Lee, C.-B.; Hahn, D.-H. Microstructural Observation and Tensile Isotropy of an Austenitic ODS Steel. Nucl. Eng. Technol. 2008, 40, 305–310. [Google Scholar] [CrossRef]
- Sandim, H.R.Z.; Renzetti, R.A.; De Oliveira Zimmermann, A.J.; Padilha, A.F.; Möslang, A. Annealing Behavior of Ferritic-Martensitic ODS-Eurofer Steel: Effect of Y2O3 Particles on Recrystallization. Mater. Sci. Forum 2012, 715–716, 629–634. [Google Scholar] [CrossRef]
- Rollett, A.; Humphreys, F.; Rohrer, G.S.; Hatherly, M. Recrystallization and Related Annealing Phenomena, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–628. [Google Scholar] [CrossRef]
- Auger, M.A.; Leguey, T.; Muñoz, A.; Monge, M.A.; De Castro, V.; Fernández, P.; Garcés, G.; Pareja, R. Microstructure and Mechanical Properties of Ultrafine-Grained Fe–14Cr and ODS Fe–14Cr Model Alloys. J. Nucl. Mater. 2011, 417, 213–216. [Google Scholar] [CrossRef]
- Gräning, T.; Rieth, M.; Hoffmann, J.; Seils, S.; Edmondson, P.D.; Möslang, A. Microstructural Investigation of an Extruded Austenitic Oxide Dispersion Strengthened Steel Containing a Carbon-Containing Process Control Agent. J. Nucl. Mater. 2019, 516, 335–346. [Google Scholar] [CrossRef]
- Balázsi, C.; Gillemot, F.; Horváth, M.; Wéber, F.; Balázsi, K.; Sahin, F.C.; Onüralp, Y.; Horváth, Á. Preparation and Structural Investigation of Nanostructured Oxide Dispersed Strengthened Steels. J. Mater. Sci. 2011, 46, 4598–4605. [Google Scholar] [CrossRef]
- Gräning, T.; Rieth, M.; Hoffmann, J.; Möslang, A. Production, Microstructure and Mechanical Properties of Two Different Austenitic ODS Steels. J. Nucl. Mater. 2017, 487, 348–361. [Google Scholar] [CrossRef]
- Ressel, G.; Parz, P.; Primig, S.; Leitner, H.; Clemens, H.; Puff, W. New Findings on the Atomistic Mechanisms Active during Mechanical Milling of a Fe-Y2O3 Model Alloy. J. Appl. Phys. 2014, 115, 124313. [Google Scholar] [CrossRef]
- Alinger, M.J.; Glade, S.C.; Wirth, B.D.; Odette, G.R.; Toyama, T.; Nagai, Y.; Hasegawa, M. Positron Annihilation Characterization of Nanostructured Ferritic Alloys. Mater. Sci. Eng. A 2009, 518, 150–157. [Google Scholar] [CrossRef]
- Suresh, K.; Nagini, M.; Vijay, R.; Ramakrishna, M.; Gundakaram, R.C.; Reddy, A.V.; Sundararajan, G. Microstructural Studies of Oxide Dispersion Strengthened Austenitic Steels. Mater. Des. 2016, 110, 519–525. [Google Scholar] [CrossRef]
- Suryanarayana, C. Synthesis of Nanocomposites by Mechanical Alloying. J. Alloys Compd. 2011, 509, S229–S234. [Google Scholar] [CrossRef]
- Oka, H.; Watanabe, M.; Hashimoto, N.; Ohnuki, S.; Yamashita, S.; Ohtsuka, S. Morphology of Oxide Particles in ODS Austenitic Stainless Steel. J. Nucl. Mater. 2013, 442, S164–S168. [Google Scholar] [CrossRef]
- Oka, H.; Watanabe, M.; Ohnuki, S.; Hashimoto, N.; Yamashita, S.; Ohtsuka, S. Effects of Milling Process and Alloying Additions on Oxide Particle Dispersion in Austenitic Stainless Steel. J. Nucl. Mater. 2014, 447, 248–253. [Google Scholar] [CrossRef]
- Maziasz, P.J. Developing an Austenitic Stainless Steel for Improved Performance in Advanced Fossil Power Facilities. JOM 1989, 41, 14–20. [Google Scholar] [CrossRef]
- Baloch, M.M.; Bhadeshia, H.K.D.H. Directional Recrystallisation in Inconel MA 6000 Nickel Base Oxide Dispersion Strengthened Superalloy. Mater. Sci. Technol. 1990, 6, 1236–1246. [Google Scholar] [CrossRef]
- Somani, M.C.; Muraleedharan, K.; Birla, N.C.; Singh, V.; Prasad, Y.V.R.K. Hot Deformation Characteristics of INCONEL Alloy MA 754 and Development of a Processing Map. Metall. Mater. Trans. A 1994, 25, 1693–1702. [Google Scholar] [CrossRef]
- Ukai, S.; Oono-Hori, N.; Ohtsuka, S. Oxide Dispersion Strengthened Steels. In Comprehensive Nuclear Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 255–292. [Google Scholar] [CrossRef]
- Kalinin, G.; Barabash, V.; Cardella, A.; Dietz, J.; Ioki, K.; Matera, R.; Santoro, R.; Tivey, R.; Garching JWS, I. Assessment and Selection of Materials for ITER In-Vessel Components. J. Nucl. Mater. 2000, 283-287, 10–19. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Maziasz, P.J.; Stoller, R.E. Dose Dependence of the Microstructural Evolution in Neutron-Irradiated Austenitic Stainless Steel. J. Nucl. Mater. 1993, 206, 266–286. [Google Scholar] [CrossRef]
- Harries, D.R. Review Neutron Irradiation-Induced Embrittlement in Type 316 and Other Austenitic Steels and Alloys. J. Nucl. Mater. 1979, 82, 2–21. [Google Scholar] [CrossRef]
- Liu, C.; Yu, C.; Hashimoto, N.; Ohnuki, S.; Ando, M.; Shiba, K.; Jitsukawa, S. Micro-Structure and Micro-Hardness of ODS Steels after Ion Irradiation. J. Nucl. Mater. 2011, 417, 270–273. [Google Scholar] [CrossRef]
- Kritzer, P. Corrosion in High-Temperature and Supercritical Water and Aqueous Solutions: A Review. J. Supercrit. Fluids 2004, 29, 1–29. [Google Scholar] [CrossRef]
- Was, G.S.; Teysseyre, S.; McKinley, J.; Jiao, Z. Corrosion of Austenitic Alloys in Supercritical Water. Corrosion 2006, 62, 989–1005. [Google Scholar] [CrossRef]
- Behnamian, Y.; Mostafaei, A.; Kohandehghan, A.; Amirkhiz, B.S.; Zahiri, R.; Zheng, W.; Guzonas, D.; Chmielus, M.; Chen, W.; Luo, J.L. A Comparative Study on the Oxidation of Austenitic Alloys 304 and 304-Oxide Dispersion Strengthened Steel in Supercritical Water at 650 °C. J. Supercrit. Fluids 2017, 119, 245–260. [Google Scholar] [CrossRef]
- Yetisir, M.; Xu, R.; Gaudet, M.; Movassat, M.; Hamilton, H.; Nimrouzi, M.; Goldak, J.A. Various Design Aspects of the Canadian Supercritical Water-Cooled Reactor Core. J. Nucl. Eng. Radiat. Sci. 2016, 2, 011007. [Google Scholar] [CrossRef]
- Was, G.S. Fundamentals of Radiation Materials Science: Metals and Alloys, 2nd ed.; Springer: Berlin, Germany, 2016; pp. 1–1002. [Google Scholar] [CrossRef]
- Dutt, B.S.; Shanthi, G.; Sasikala, G.; Babu, M.N.; Venugopal, S.; Albert, S.K.; Bhaduri, A.K.; Jayakumar, T. ScienceDirect Effect of Nitrogen Addition and Test Temperatures on Elastic-Plastic Fracture Toughness of SS 316 LN. Procedia Eng. 2014, 86, 302–307. [Google Scholar] [CrossRef]
- Hu, H.; Zhou, Z.; Li, M.; Zhang, L.; Wang, M.; Li, S.; Ge, C. Study of the Corrosion Behavior of a 18Cr-Oxide Dispersion Strengthened Steel in Supercritical Water. Corros. Sci. 2012, 65, 209–213. [Google Scholar] [CrossRef]
- Hu, H.L.; Zhou, Z.J.; Liao, L.; Zhang, L.F.; Wang, M.; Li, S.F.; Ge, C.C. Corrosion Behavior of a 14Cr-ODS Steel in Supercritical Water. J. Nucl. Mater. 2013, 437, 196–200. [Google Scholar] [CrossRef]
- Isselin, J.; Kasada, R.; Kimura, A. Corrosion Behaviour of 16%Cr–4%Al and 16%Cr ODS Ferritic Steels under Different Metallurgical Conditions in a Supercritical Water Environment. Corros. Sci. 2010, 52, 3266–3270. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Y.; Tang, R. Selection and Corrosion Evaluation Tests of Candidate SCWR Fuel Cladding Materials. Nucl. Eng. Des. 2012, 249, 180–187. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, L.; Tang, R.; Zhang, Q. The Effect of Temperature on the SSRT Behavior of Austenitic Stainless Steels in SCW. J. Nucl. Mater. 2014, 454, 274–282. [Google Scholar] [CrossRef]
- Monnet, I.; Dubuisson, P.; Serruys, Y.; Ruault, M.O.; Kaıtasovkaıtasov, O.; Jouffrey, B. Microstructural Investigation of the Stability under Irradiation of Oxide Dispersion Strengthened Ferritic Steels. J. Nucl. Mater. 2004, 335, 311–321. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Liu, C.T.; Takeyama, M.; Maziasz, P.J.; Pint, B.A. Alumina-Forming Austenitic Stainless Steels Strengthened by Laves Phase and MC Carbide Precipitates. Metall. Mater. Trans. A 2007, 38, 2737–2746. [Google Scholar] [CrossRef]
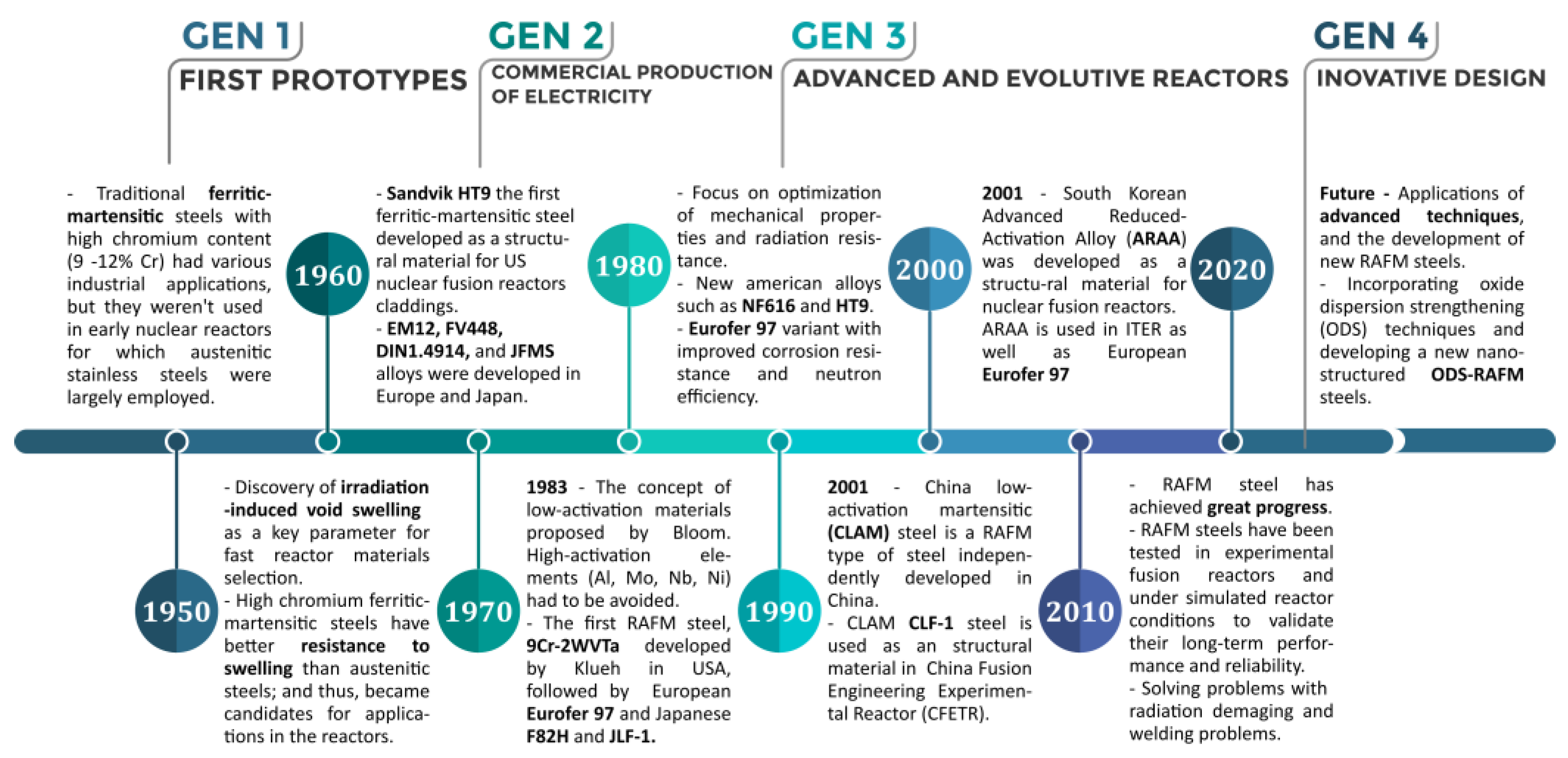
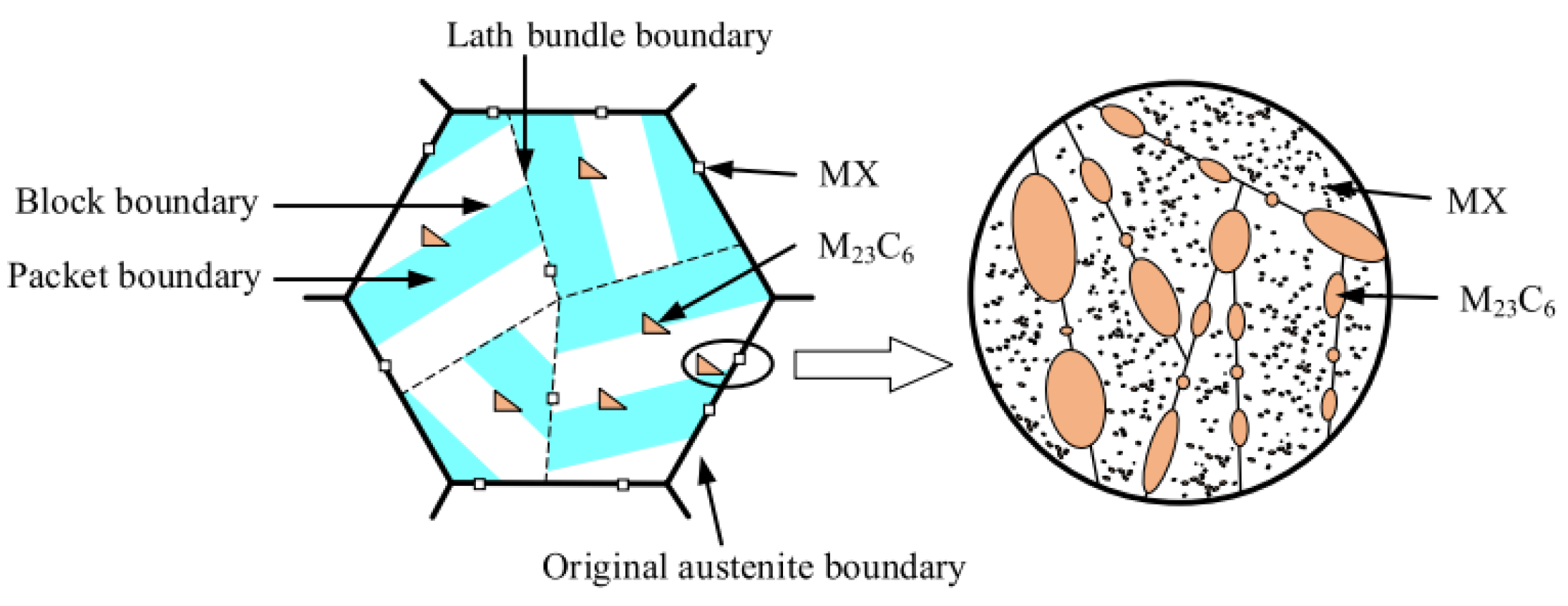
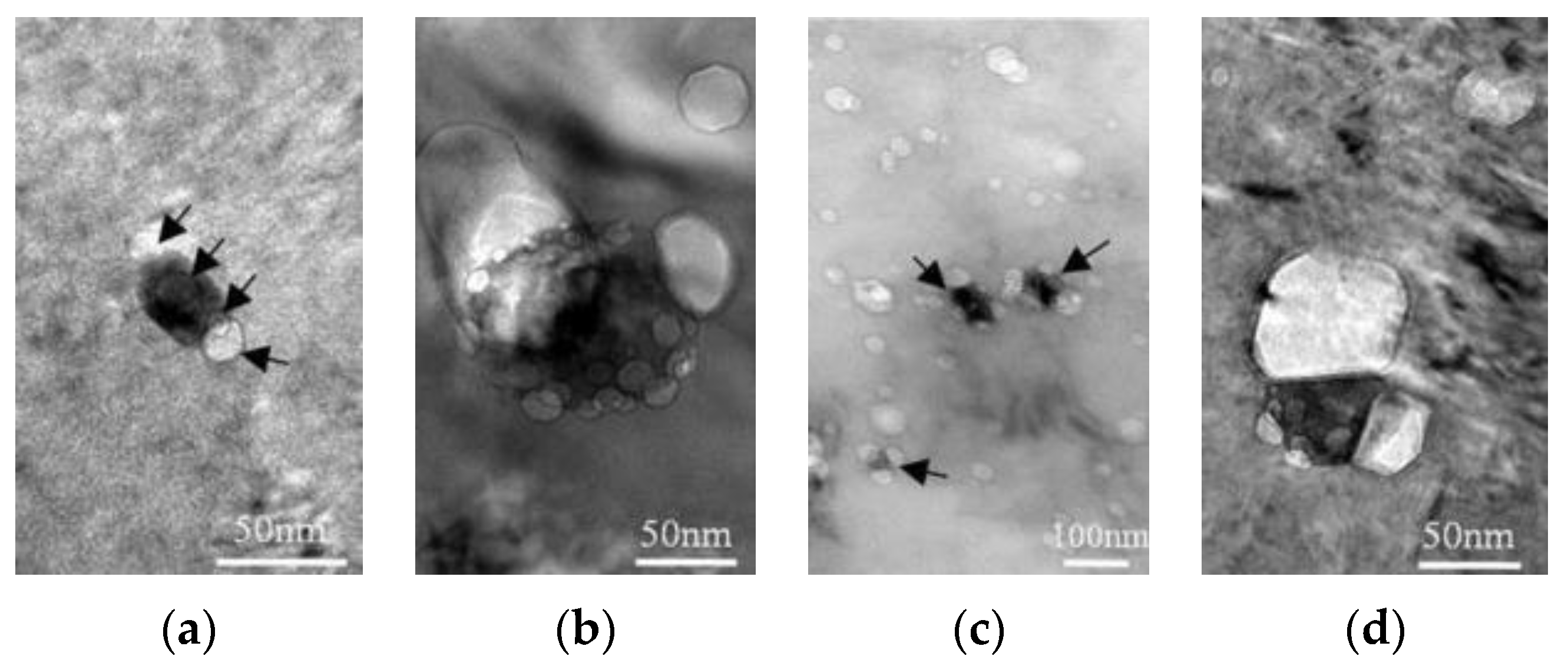
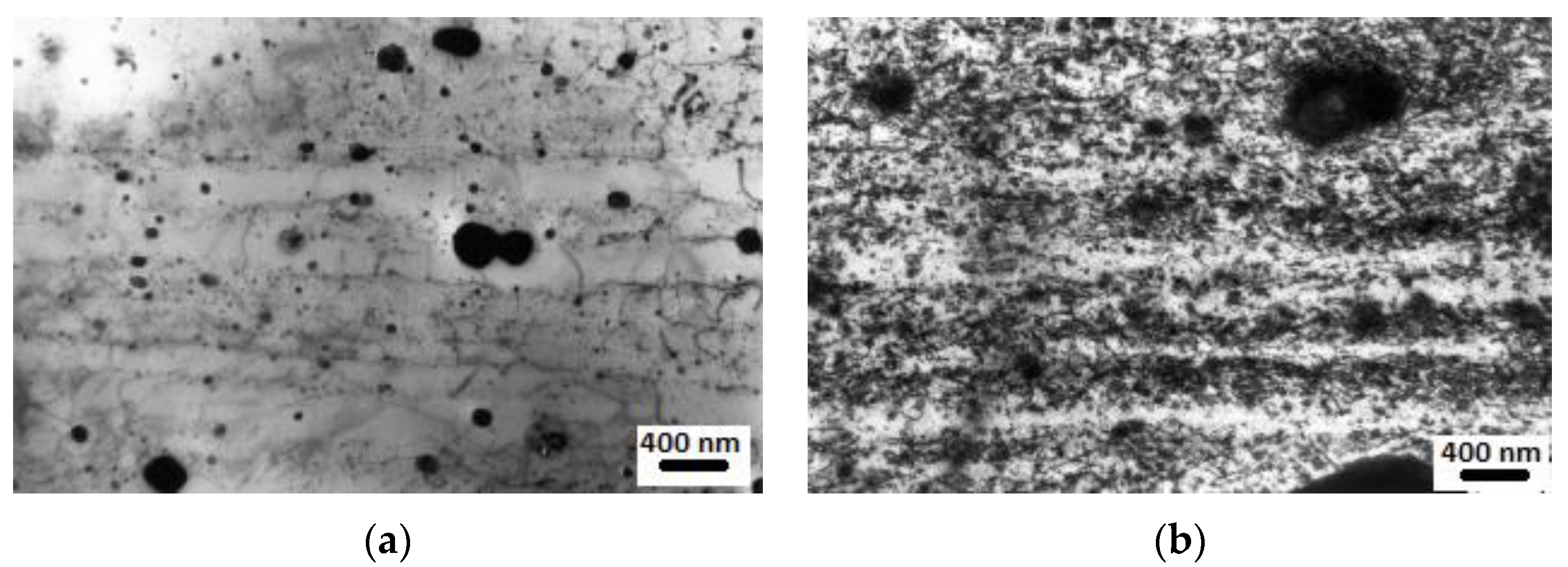


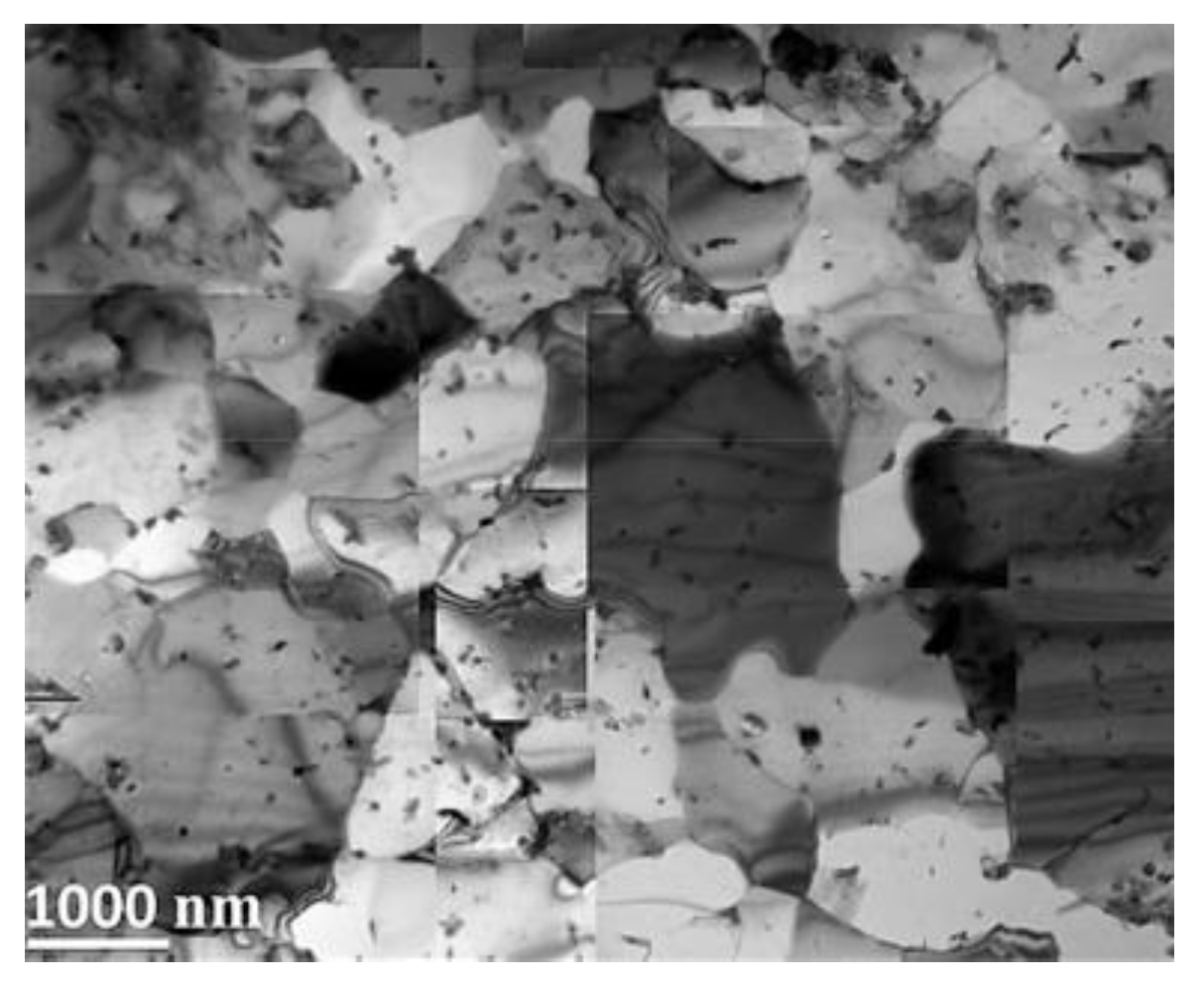
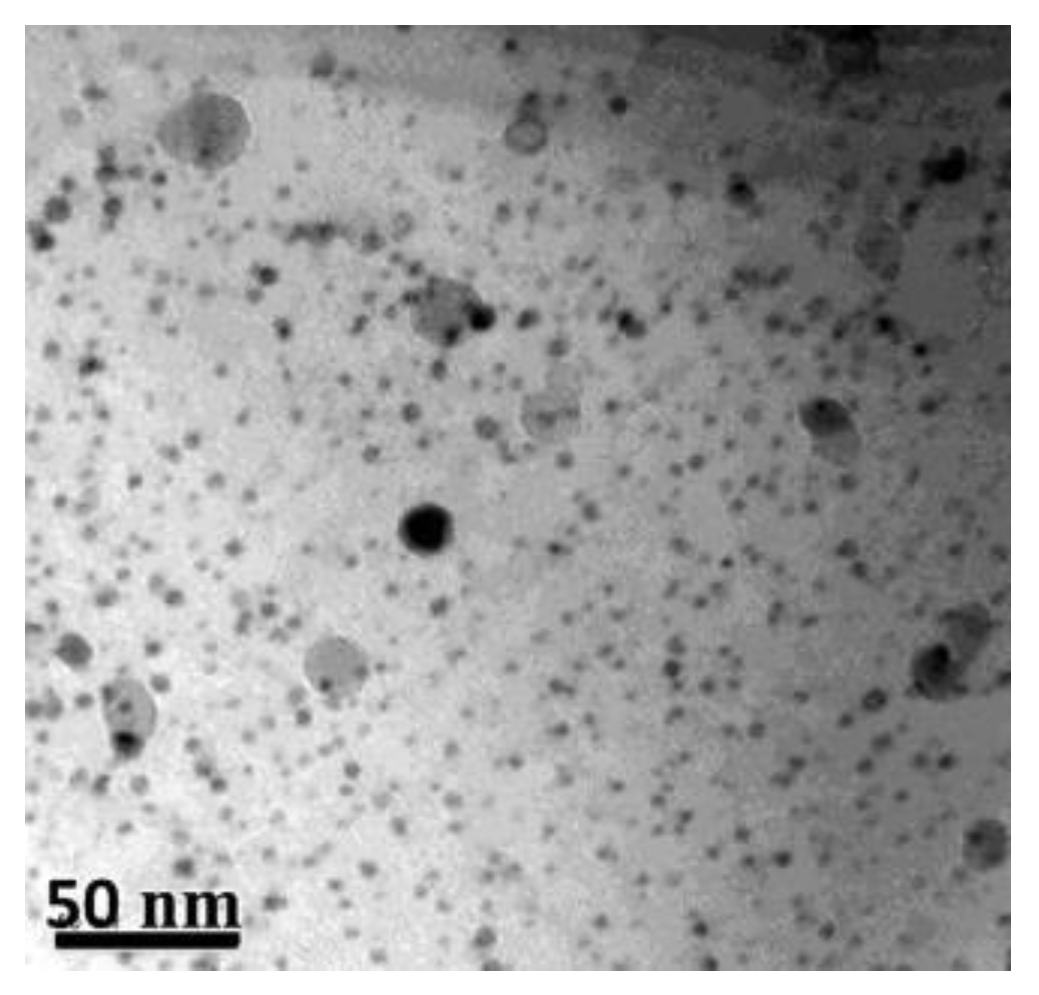
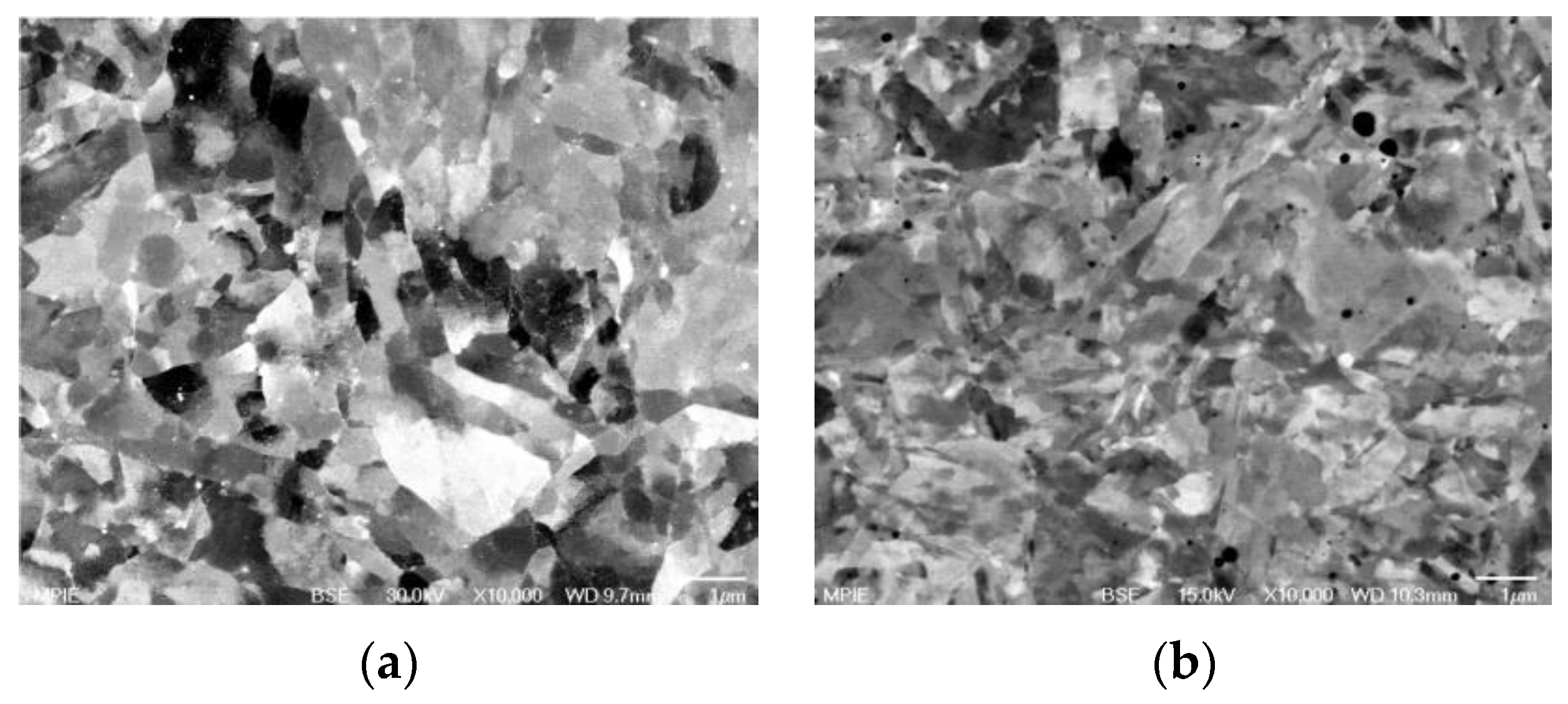
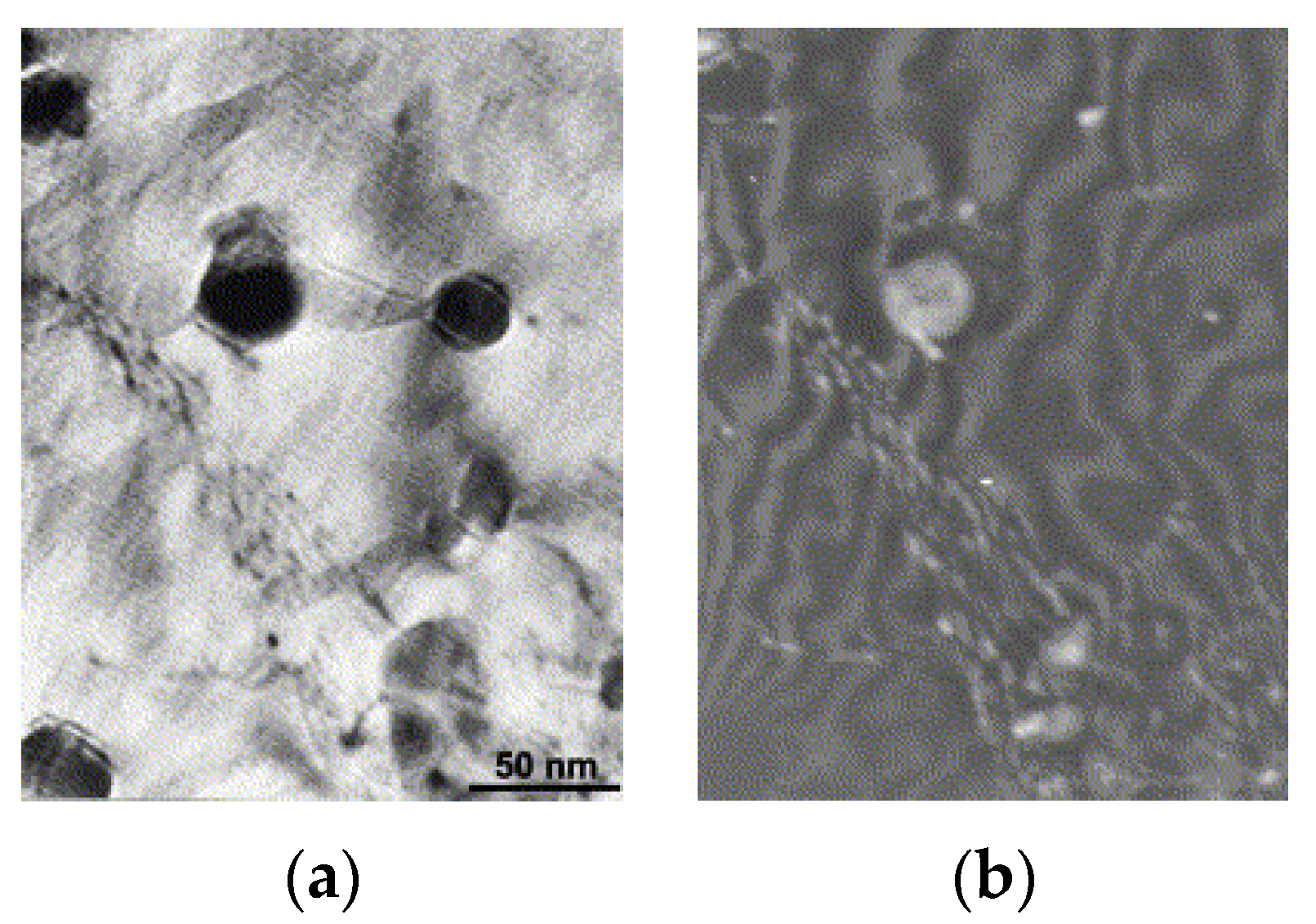
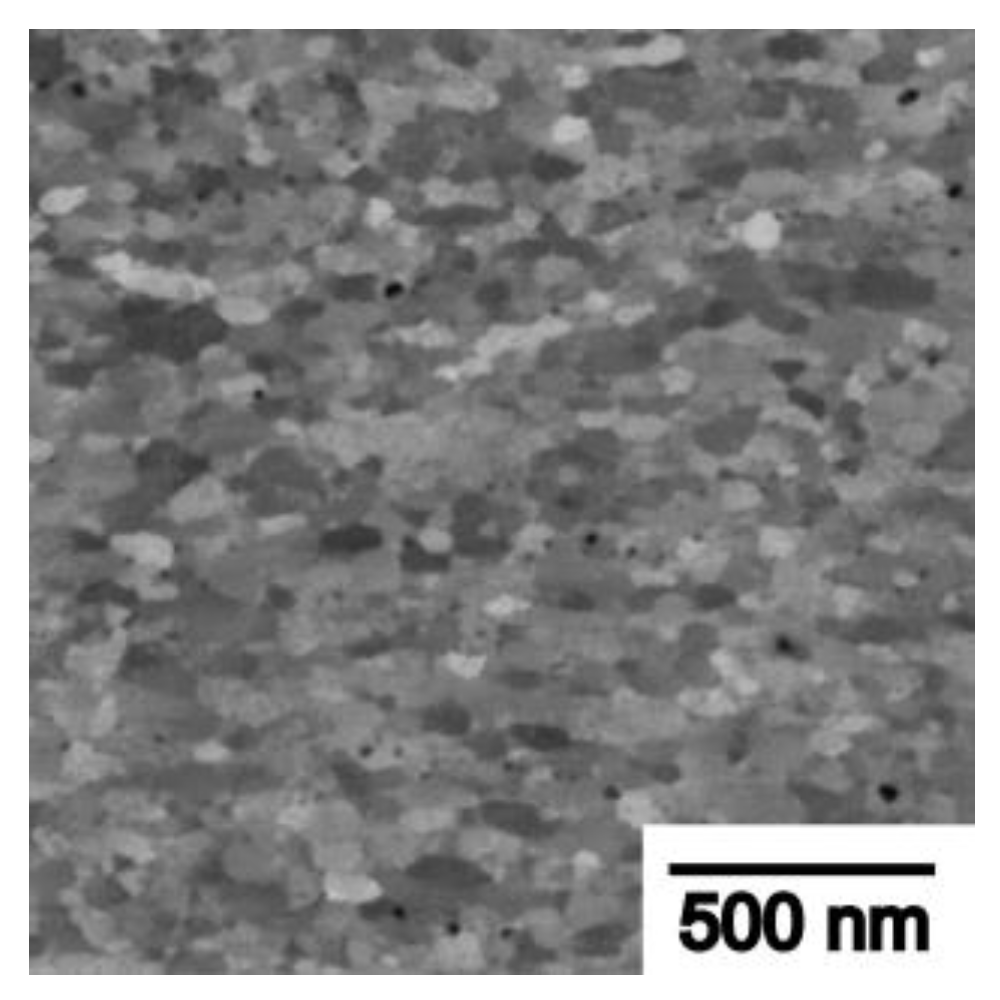


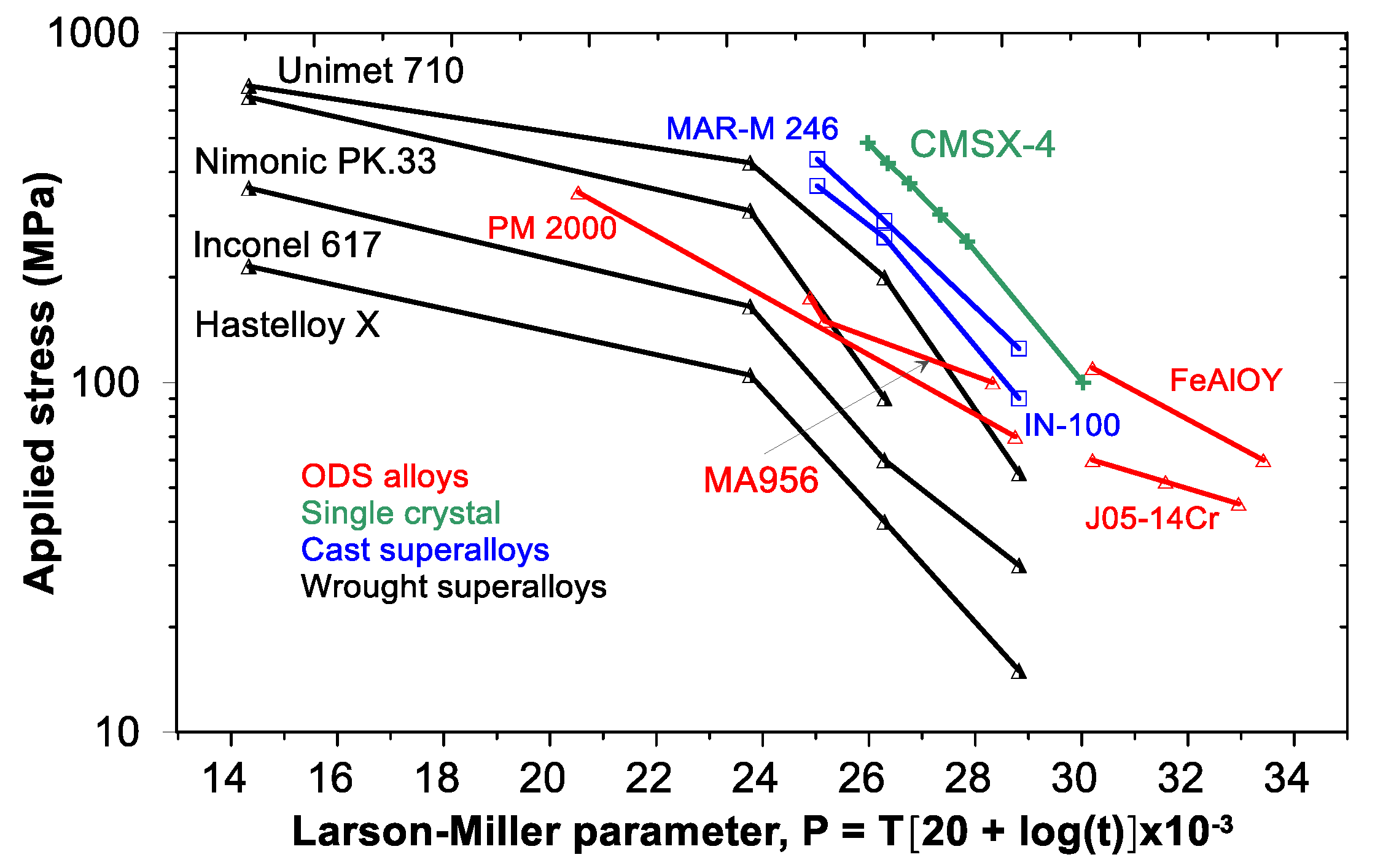

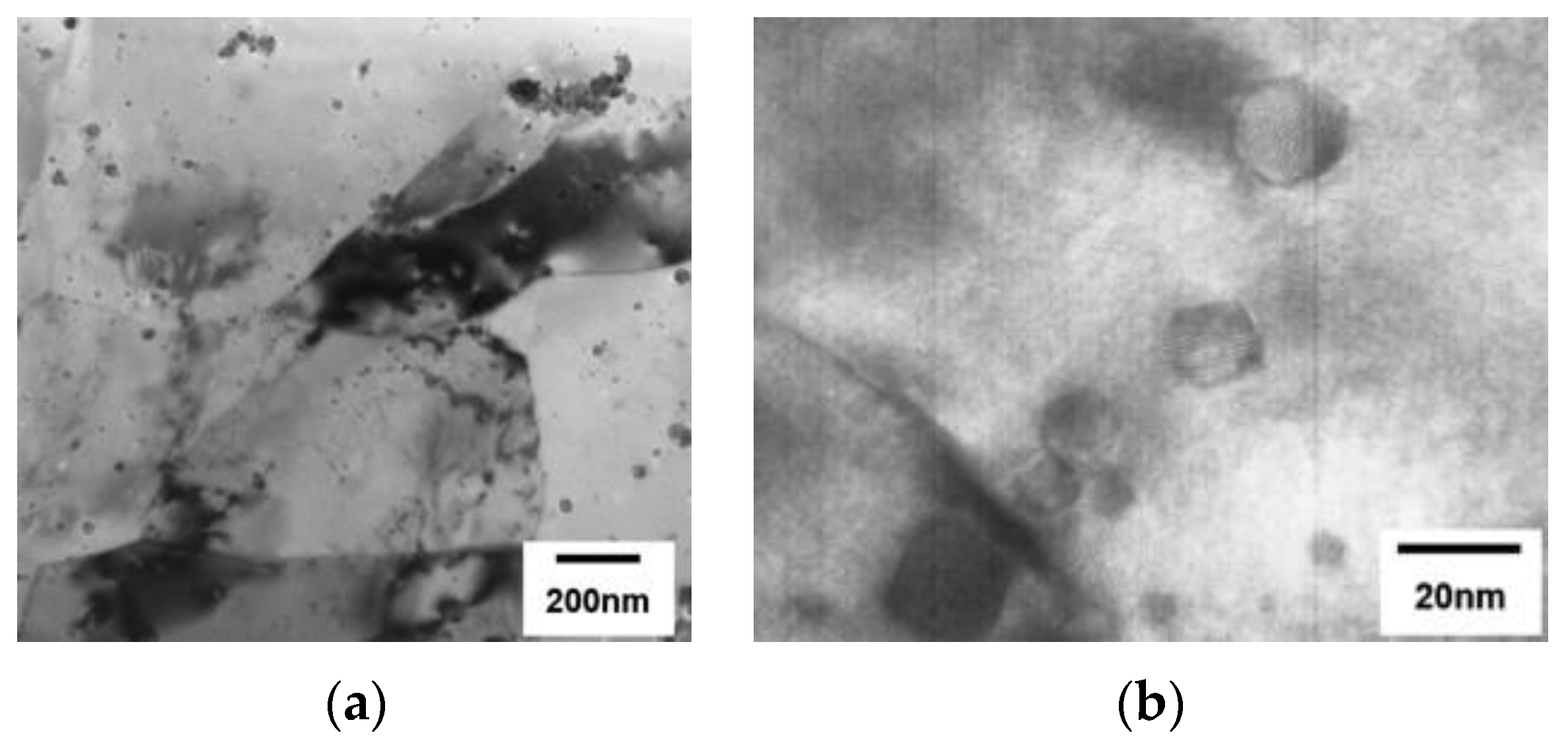
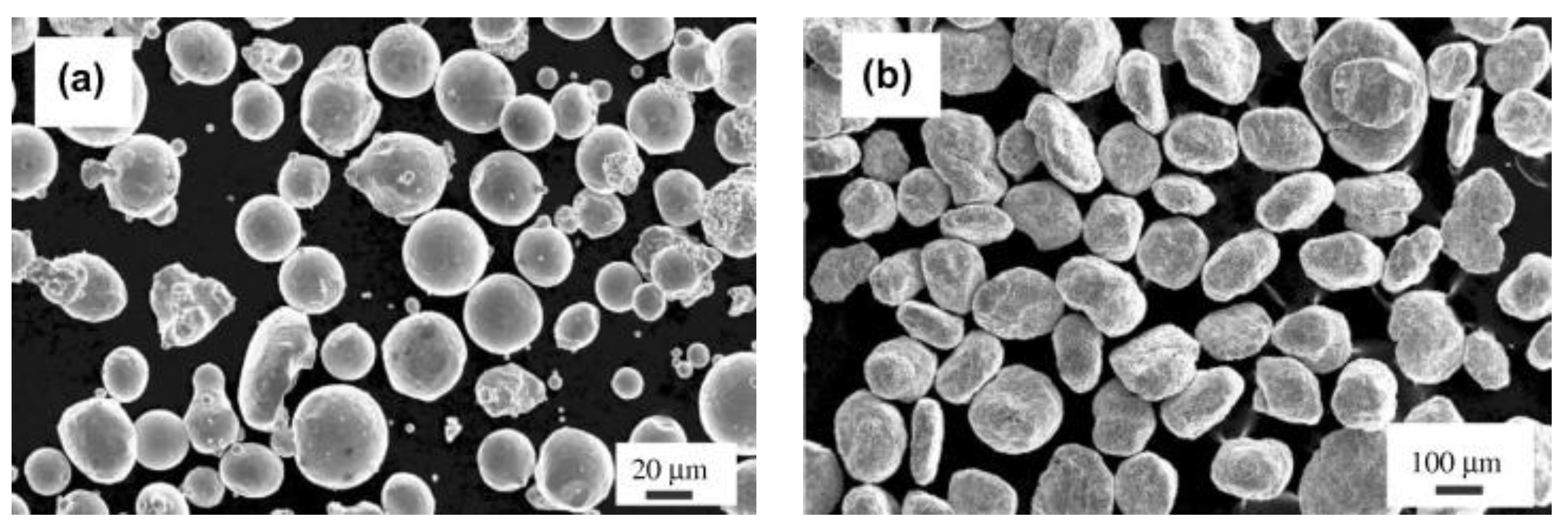
| RAFM Steel | Chemical Composition | Matrix Structure |
|---|---|---|
| Eurofer97 | (8.5–9.5)Cr-1W-0.2V-0.1Ta-0.4Mn | Complete martensite |
| 9Cr-2WVTa | 9Cr-2W-0.18V-0.08Ta | Martensite |
| F82H | 8Cr-2W-0.2V-0.004Ta-0.1C | Martensite |
| JLF-1 | 9Cr-2W-0.18V-0.08Ta | Ferrite |
| CLAM | 9Cr-1.5W-0.5Mn-0.2V-0.15Ta-0.11C | Complete martensite |
| CLF-1 | 8.5Cr-1.5W-0.5Mn-0.3V-0.1Ta-0.1C | Complete martensite |
| BATMAN II | 9Cr-1.5W-0.2Ti-0.25V-3.5Mn | Complete martensite |
| ARRA | 9Cr-0.9W-0.18V-0.06Ta-0.09Si | Martensite |
| Elements | Activation | Decay Time |
|---|---|---|
| Li, Be, B, C, O, Si, S, P | Very low | 14 days |
| Ti, V, Cr, Zr, W, Pb, Y | Low | 30 days–5 years |
| Mn, Fe. Zn, Hf | High | 10–30 years |
| Al, Ni, Cu, Nb. Mo, Sn | Very high | >100 years |
| ODS Steel | Fe | Cr | W | Ti | Mo | Mn | Al | C | Y2O3 | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| ODS Eurofer [35] | Bal. | 9 | 1.1 | - | - | 0.4 | - | 0.11 | 0.3 | 0.2 V; 0.12 Ta |
| 12Y1 [107] | Bal. | 12.85 | <0.01 | 0.03 | 0.03 | 0.04 | 0.007 | 0.045 | 0.25 | 0.03 Si; 0.24 Ni |
| 12YW [107] | Bal. | 12 | 3 | - | - | - | - | - | 0.3 | - |
| 12YWT [107] | Bal. | 12 | 3 | 0.4 | - | - | - | - | 0.25 | - |
| 14YWT [77] | Bal. | 14 | 3 | 0.4 | - | - | - | - | 0.3 | - |
| Fe-18Cr1W [108] | Bal. | 18 | 0.95 | 0.25 | - | 0.3 | - | 0.03 | 0.5 | 0.3 Si; 0.2 Ni |
| MA956 [109] | Bal. | 20 | - | 04 | - | - | 4.5 | - | 0.5 | |
| MA957 [110] | Bal. | 14 | - | 0.9 | 0.3 | - | 0.03 | 0.01 | 0.25 | |
| PM2000 [110] | Bal. | 20 | - | 0.5 | - | - | 5.5 | - | 0.5 | |
| C36M [111] | Bal. | 12 | - | - | 2 | - | 6 | - | 0.03 | 0.2 Si |
| FeCrAl-ODS-1 [112] | Bal. | 12 | - | 0.5 | - | - | 6 | - | 0.5 | 0.4 Zr |
| FeCrAl-ODS-2 [113] | Bal. | 13 | - | 0.5 | 2 | - | 5 | - | 0.35 | 0.6 Zr |
| 9CrODS [114] | Bal. | 9 | 1.5 | 0.5 | - | - | - | 0.1 | 0.35 | 0.2 V |
| 12CrODS [115] | Bal. | 12 | 1.2 | 0.5 | - | 0.5 | - | 0.1 | 0.35 | |
| 14CrODS [116] | Bal. | 14 | 2 | 0.5 | - | - | - | - | 0.35 | |
| 18CrODS [117] | Bal. | 18 | 2. | 0.5 | 4 | - | 4 | - | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luptáková, N.; Svoboda, J.; Bártková, D.; Weiser, A.; Dlouhý, A. The Irradiation Effects in Ferritic, Ferritic–Martensitic and Austenitic Oxide Dispersion Strengthened Alloys: A Review. Materials 2024, 17, 3409. https://doi.org/10.3390/ma17143409
Luptáková N, Svoboda J, Bártková D, Weiser A, Dlouhý A. The Irradiation Effects in Ferritic, Ferritic–Martensitic and Austenitic Oxide Dispersion Strengthened Alloys: A Review. Materials. 2024; 17(14):3409. https://doi.org/10.3390/ma17143409
Chicago/Turabian StyleLuptáková, Natália, Jiří Svoboda, Denisa Bártková, Adam Weiser, and Antonín Dlouhý. 2024. "The Irradiation Effects in Ferritic, Ferritic–Martensitic and Austenitic Oxide Dispersion Strengthened Alloys: A Review" Materials 17, no. 14: 3409. https://doi.org/10.3390/ma17143409






