Influence of Low pH on the Microhardness and Roughness Surface of Dental Composite—A Preliminary Study
Abstract
1. Introduction
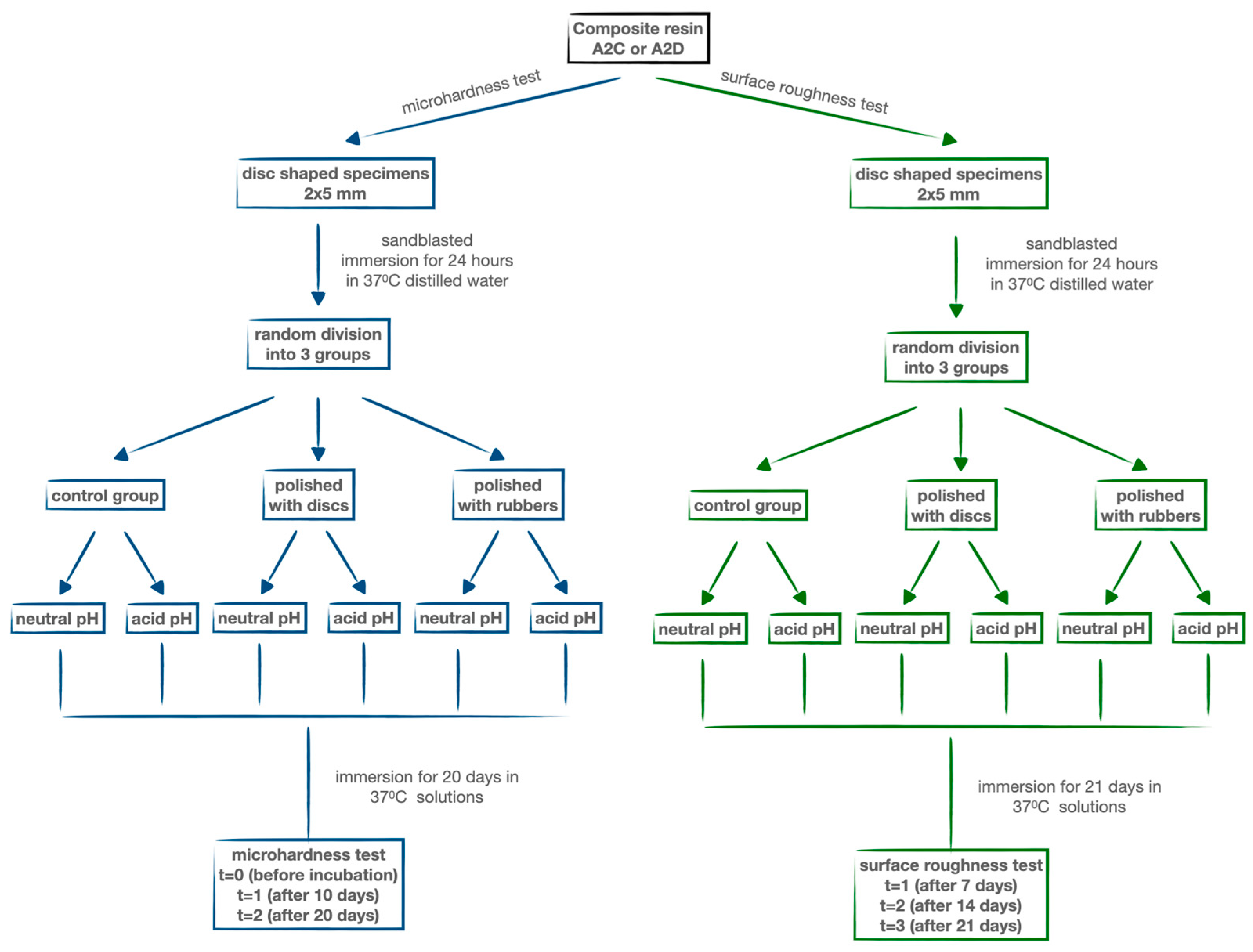
2. Materials and Methods
2.1. Specimens’ Preparation
2.2. Study Group
2.3. Vickers Microhardness Test
2.4. Roughness Test
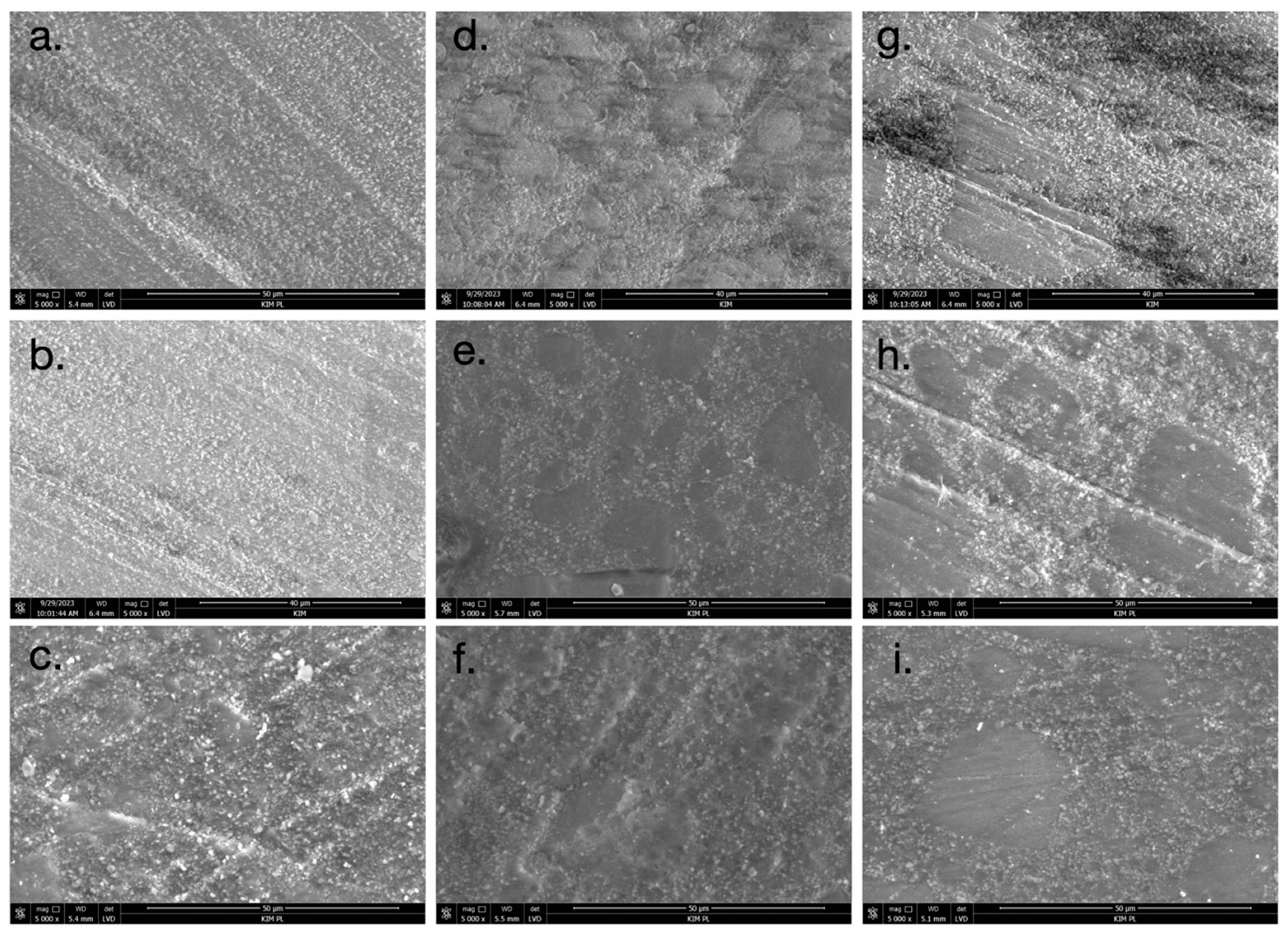
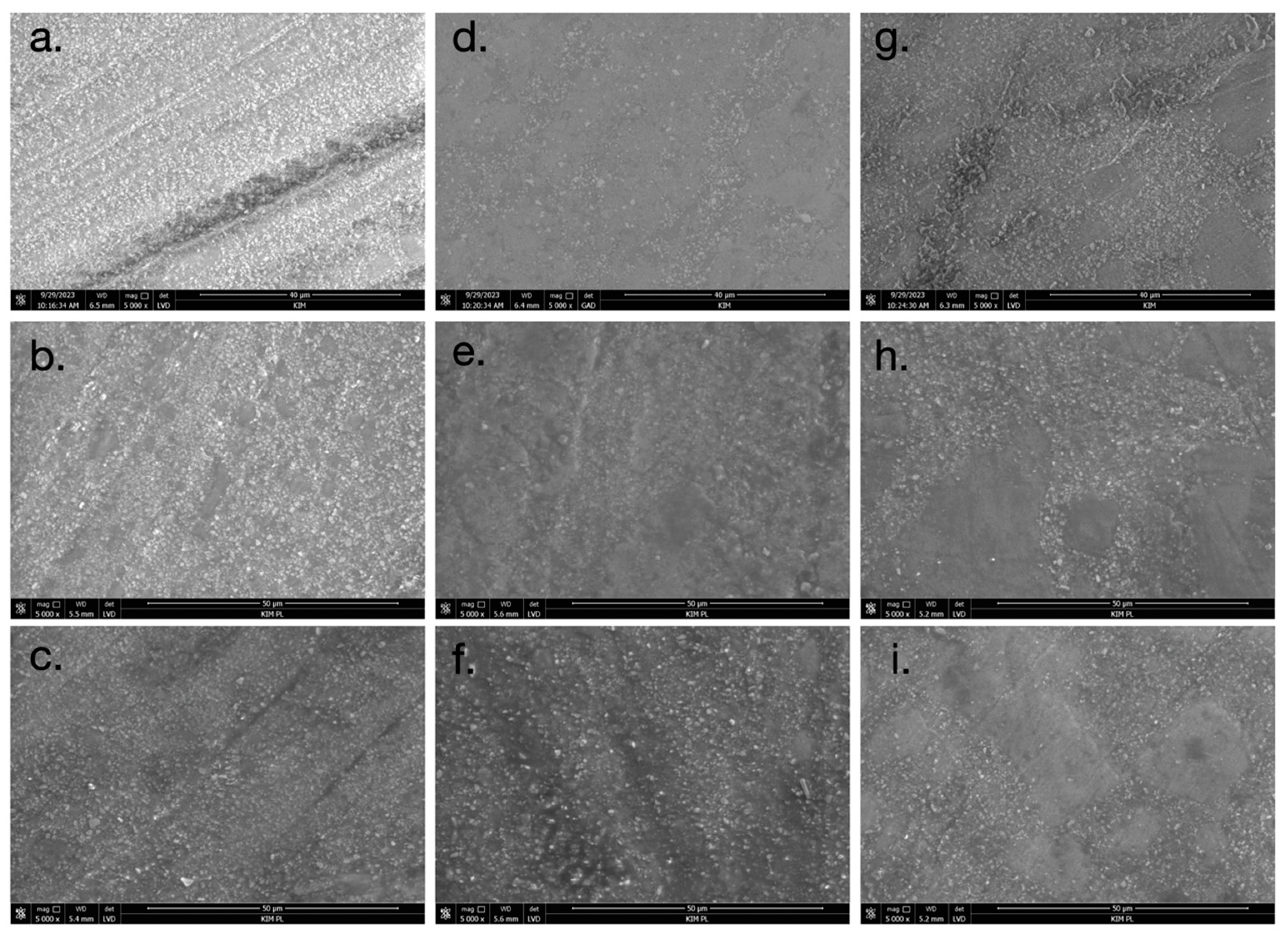
2.5. Scanning Electron Microscopy (SEM)
2.6. Statistical Methods
3. Results
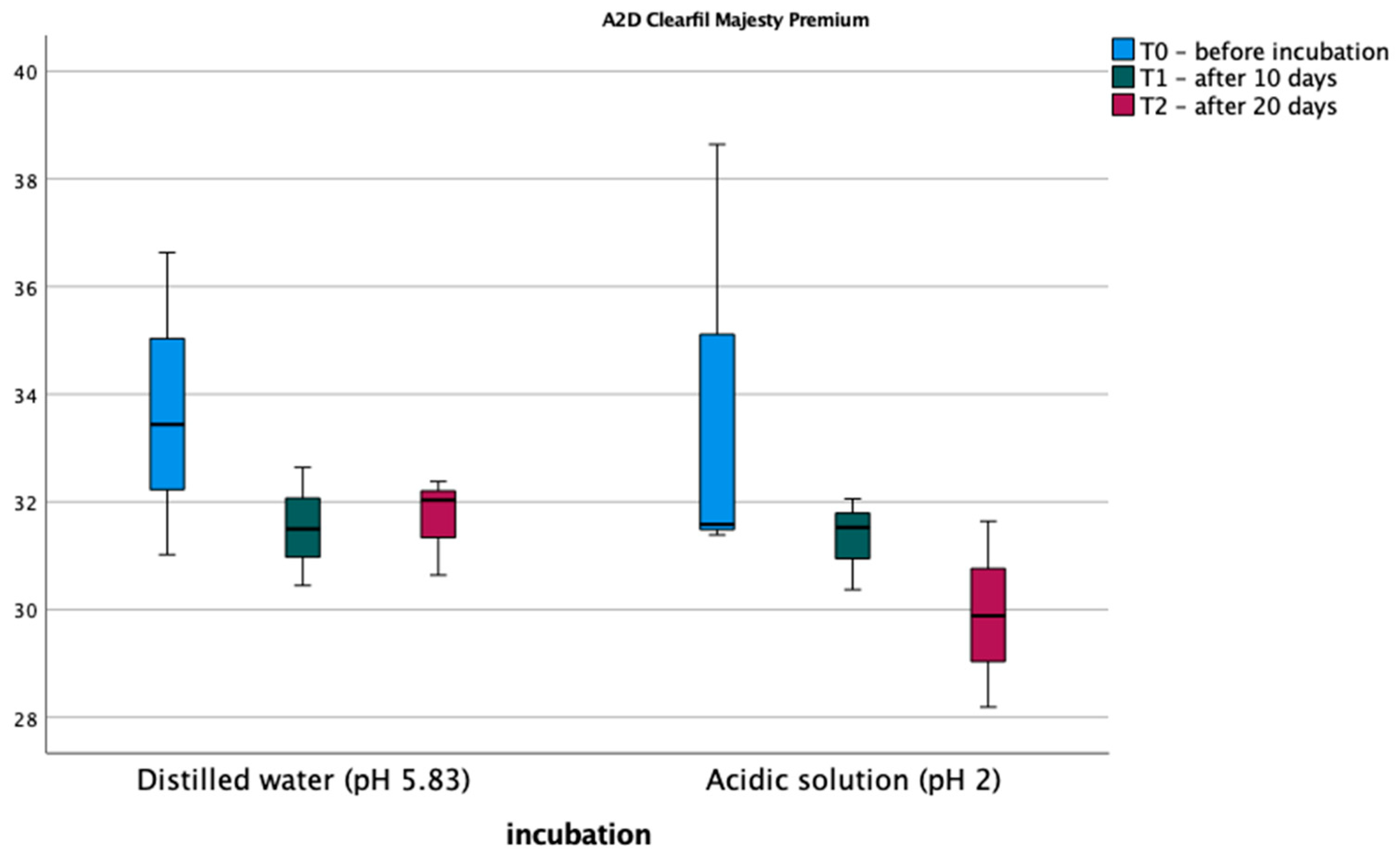
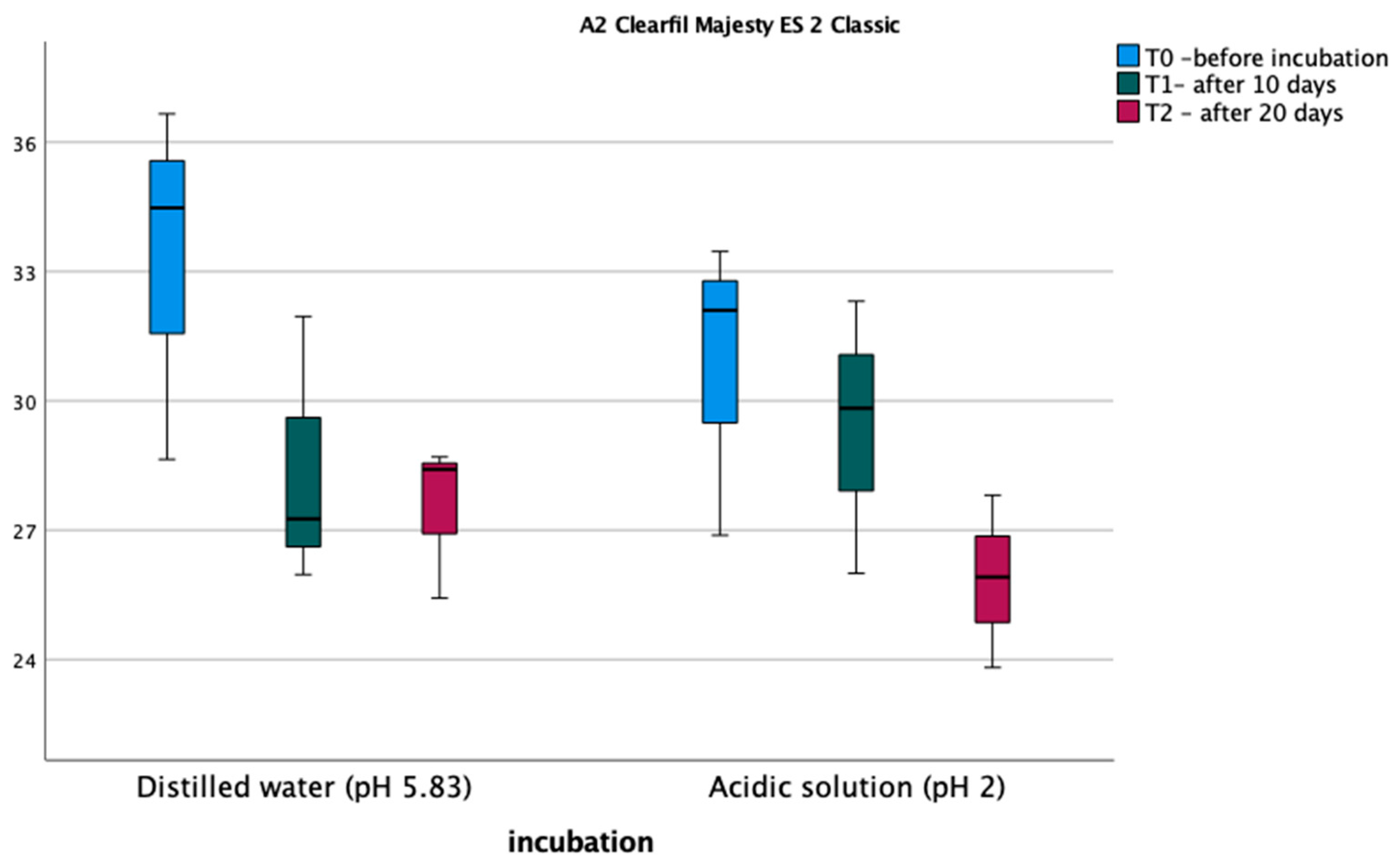
3.1. Vickers Microhardness Test
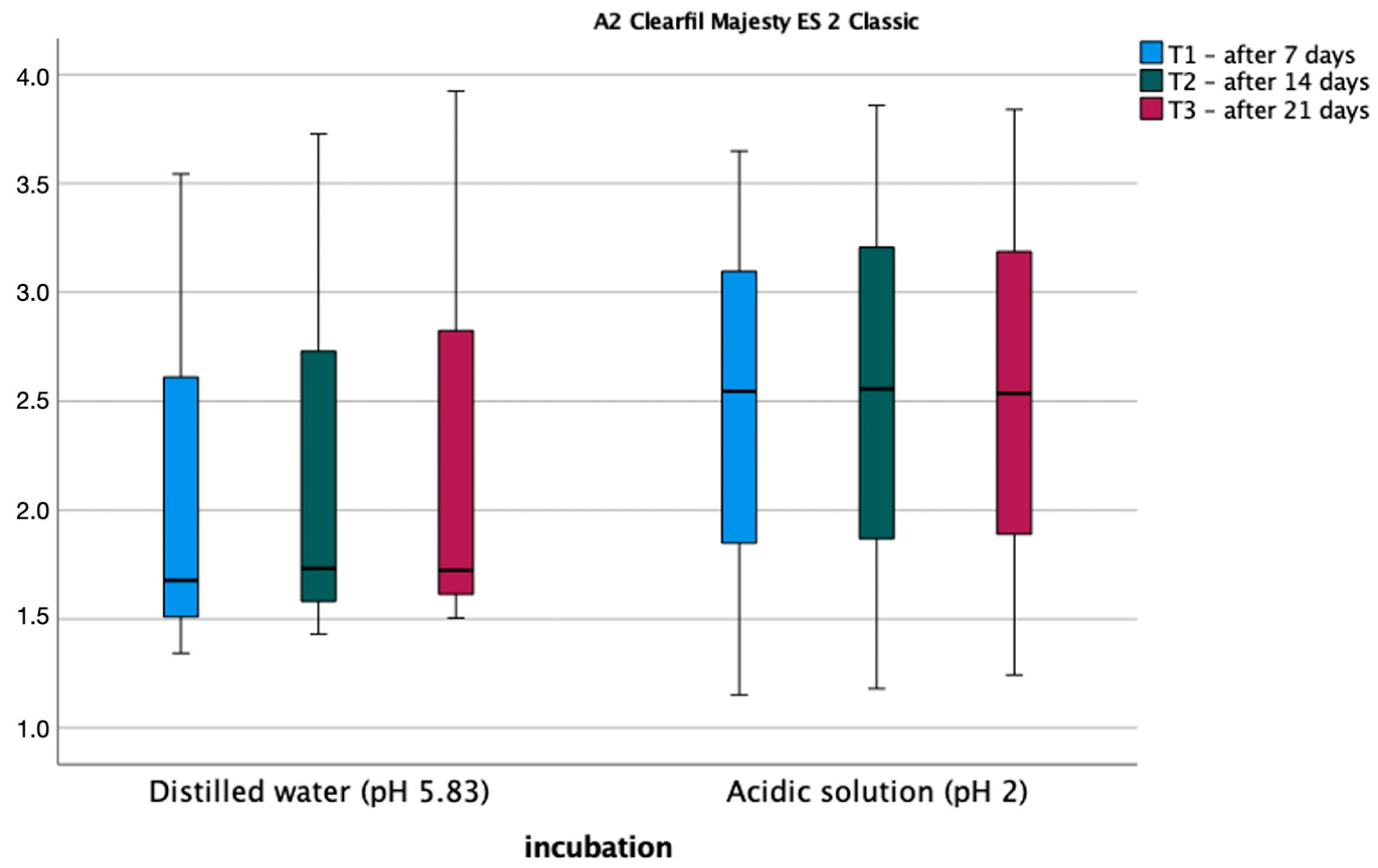
3.2. Roughness Test
3.3. Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dionysopoulos, D.; Gerasimidou, O. Wear of contemporary dental composite resin restorations: A literature review. Restor. Dent. Endod. 2021, 46, e18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Vishwanath, S.; Kadandale, S.; Kumarappan, S.K.; Ramachandran, A.; Unnikrishnan, M.; Nagesh, H.M. Finishing and Polishing of Composite Restoration: Assessment of Knowledge, Attitude and Practice Among Various Dental Professionals in India. Cureus 2022, 14, e20887. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, M.E.; Krasowski, M.; Bołtacz-Rzepkowska, E. The Effect of Various Polishing Systems on the Surface Roughness of Two Resin Composites—An In Vitro Study. Coatings 2022, 12, 916. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Sales, A.L.L.S.; Pucci, C.R.; Borges, A.B.; Torres, C.R.G. The combined effect of food-simulating solutions, brushing and staining on color stability of composite resins. Acta Biomater. Odontol. Scand. 2017, 3, 1276838. [Google Scholar] [CrossRef] [PubMed]
- Deljoo, Z.; Sadeghi, M.; Azar, M.R.; Bagheri, R. The Effect of Different Polishing Methods and Storage Media on Discoloration of Resin Composites. J. Dent. Biomater. 2016, 3, 226–232. [Google Scholar]
- Valinoti, A.C.; Neves, B.G.; da Silva, E.M.; Maia, L.C. Surface degradation of composite resins by acidic medicines and ph-cycling. J. Appl. Oral Sci. 2008, 16, 257–265. [Google Scholar] [CrossRef]
- Guo, J.; Bing, Z.; Yang, J.; Tsoi, J.K.H.; Wang, Y. Effect of roughness and acidic medium on wear behavior of dental resin composite. BMC Oral Health 2022, 22, 470. [Google Scholar] [CrossRef]
- Münchow, E.A.; Ferreira, A.C.A.; Machado, R.M.M.; Ramos, T.S.; Rodrigues-Junior, S.A.; Zanchi, C.H. Effect of Acidic Solutions on the Surface Degradation of a Micro-Hybrid Composite Resin. Braz. Dent. J. 2014, 25, 321–326. [Google Scholar] [CrossRef]
- Tărăboanță, I.; Buhățel, D.; Brînză Concită, C.A.; Andrian, S.; Nica, I.; Tărăboanță-Gamen, A.C.; Brânzan, R.; Stoleriu, S. Evaluation of the Surface Roughness of Bulk-Fill Composite Resins after Submission to Acidic and Abrasive Aggressions. Biomedicines 2022, 10, 1008. [Google Scholar] [CrossRef]
- Khan, A.A.; Siddiqui, A.Z.; Al-Kheraif, A.A.; Zahid, A.; Divakar, D.D. Effect of different pH solvents on micro-hardness and surface topography of dental nano-composite: An in vitro analysis. Pak. J. Med. Sci. 2015, 31, 854–859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, T.; Mahalakshmi, V.; Sahu, S.; Chatterjee, S.; Khan, A.M.; Haqh, M.F.; Singh, V. A Comparative Evaluation of the Effect of Different Beverages on Colour Stability and Surface Micromorphology of Nanocomposite Restorative Material. Cureus 2023, 15, e41905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ko, H.; Kim, D.; Park, S.-H. Homogeneity of dental curing unit beam profile and its effect on microhardness of dental composites with varying thicknesses. Dent. Mater. 2022, 38, e231–e243. [Google Scholar] [CrossRef] [PubMed]
- Hasanain, F.A.; Nassar, H.M.; Ajaj, R.A. Effect of Light Curing Distance on Microhardness Profiles of Bulk-Fill Resin Composites. Polymers 2022, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Marcos, A.; Salazar-Correa, G.; Castro-Ramirez, L.; Ladera-Castañeda, M.; López-Gurreonero, C.; Cachay-Criado, H.; Aliaga-Mariñas, A.; Cornejo-Pinto, A.; Cervantes-Ganoza, L.; Cayo-Rojas, C.F. The Microhardness and Surface Roughness Assessment of Bulk-Fill Resin Composites Treated with and without the Application of an Oxygen-Inhibited Layer and a Polishing System: An In Vitro Study. Polymers 2022, 14, 3053. [Google Scholar] [CrossRef] [PubMed]
- Szalewski, L.; Wójcik, D.; Bogucki, M.; Szkutnik, J.; Różyło-Kalinowska, I. The Influence of Popular Beverages on Mechanical Properties of Composite Resins. Materials 2021, 14, 3097. [Google Scholar] [CrossRef] [PubMed]
- Gawriołek, M.; Sikorska, E.; Ferreira, L.F.V.; Costa, A.I.; Khmelinskii, I.; Krawczyk, A.; Sikorski, M.; Koczorowski, R. Color and Luminescence Stability of Selected Dental Materials In Vitro: Color and Luminescence Stability. J. Prosthodont. 2012, 21, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Kumari, C.; Bhat, K.; Bansal, R.; Singh, N.; Anupama, A.; Lavanya, T. Evaluation of surface roughness and hardness of newer nanoposterior composite resins after immersion in food-simulating liquids. Contemp. Clin. Dent. 2019, 10, 289–293. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Abdelnabi, A.; Othman, M.S.; Bayoumi, R.E.; Abdelraouf, R.M. Effect of Different Mouthwashes on the Surface Microhardness and Color Stability of Dental Nanohybrid Resin Composite. Polymers 2023, 15, 815. [Google Scholar] [CrossRef]
- Barve, D.; Dave, P.N.; Gulve, M.N.; Sahib, M.A.M.; Naz, F.; Shahabe, S.A. Effect of Commonly Consumed Beverages on Microhardness of Two Types of Composites. Int. J. Clin. Pediatr. Dent. 2020, 13, 663–667. [Google Scholar] [CrossRef]
- Ünal, M.; Candan, M.; İpek, I.; Küçükoflaz, M.; Özer, A. Evaluation of the microhardness of different resin-based dental restorative materials treated with gastric acid: Scanning electron microscopy–energy dispersive X-ray spectroscopy analysis. Microsc. Res. Tech. 2021, 84, 2140–2148. [Google Scholar] [CrossRef]
- Vecek, N.N.; Par, M.; Sever, E.K.; Miletic, I.; Krmek, S.J. The Effect of a Green Smoothie on Microhardness, Profile Roughness andng Color Change of Dental Restorative Materials. Polymers 2022, 14, 2067. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Strazzi-Sahyon, H.B.; Suzuki, T.Y.U.; Briso, A.L.F.; dos Santos, P.H. Effect of dental bleaching on the microhardness and surface roughness of sealed composite resins. Restor. Dent. Endod. 2020, 45, e12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Camilotti, V.; Detogni, A.C.; Ambrosano, G.M.B.; Mendonça, M.J.; Ueda, J.K.; De Goes, M.F. Effect of acidic solutions present in the diet on the surface roughness of microhybrid composite resins. Res. Soc. Dev. 2022, 11, e46111426588. [Google Scholar] [CrossRef]
- Tavangar, M.; Bagheri, R.; Kwon, T.; Mese, A.; Manton, D.J. Influence of beverages and surface roughness on the color change of resin composites. J. Investig. Clin. Dent. 2018, 9, e12333. [Google Scholar] [CrossRef] [PubMed]
- Patnana, A.K.; Vaidya, N.; Kumar, P.; Pathak, K.; Punia, S.K.; Choudhary, A. Comparative evaluation of the influence of different sports/energy drinks and alcoholic beverages on the surface roughness of three different flowable esthetic restorative materials: An in vitro Analysis. J. Int. Soc. Prev. Community Dent. 2020, 10, 585–590. [Google Scholar] [CrossRef]
- Lepri, C.P.; Palma-Dibb, R.G. Surface roughness and color change of a composite: Influence of beverages and brushing. Dent. Mater. J. 2012, 31, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dent. Mater. 2011, 27, 598–607. [Google Scholar] [CrossRef]
- Szalewski, L.; Szalewska, M.; Jarosz, P.; Woś, M.; Szymańska, J. Temperature Changes in Composite Materials during Photopolymerization. Appl. Sci. 2021, 11, 474. [Google Scholar] [CrossRef]
- Szalewski, L.; Wójcik, D.; Sofińska-Chmiel, W.; Kuśmierz, M.; Różyło-Kalinowska, I. How the Duration and Mode of Photopolymerization Affect the Mechanical Properties of a Dental Composite Resin. Materials 2023, 16, 113. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szalewski, L.; Wójcik, D.; Sowa, M.; Vivcharenko, V.; Pałka, K. Influence of Low pH on the Microhardness and Roughness Surface of Dental Composite—A Preliminary Study. Materials 2024, 17, 3443. https://doi.org/10.3390/ma17143443
Szalewski L, Wójcik D, Sowa M, Vivcharenko V, Pałka K. Influence of Low pH on the Microhardness and Roughness Surface of Dental Composite—A Preliminary Study. Materials. 2024; 17(14):3443. https://doi.org/10.3390/ma17143443
Chicago/Turabian StyleSzalewski, Leszek, Dorota Wójcik, Monika Sowa, Vladyslav Vivcharenko, and Krzysztof Pałka. 2024. "Influence of Low pH on the Microhardness and Roughness Surface of Dental Composite—A Preliminary Study" Materials 17, no. 14: 3443. https://doi.org/10.3390/ma17143443
APA StyleSzalewski, L., Wójcik, D., Sowa, M., Vivcharenko, V., & Pałka, K. (2024). Influence of Low pH on the Microhardness and Roughness Surface of Dental Composite—A Preliminary Study. Materials, 17(14), 3443. https://doi.org/10.3390/ma17143443






