Quantitative Analysis of the Synergy of Doping and Nanostructuring of Oxide Photocatalysts
Abstract
:1. Introduction
2. Computational Methods
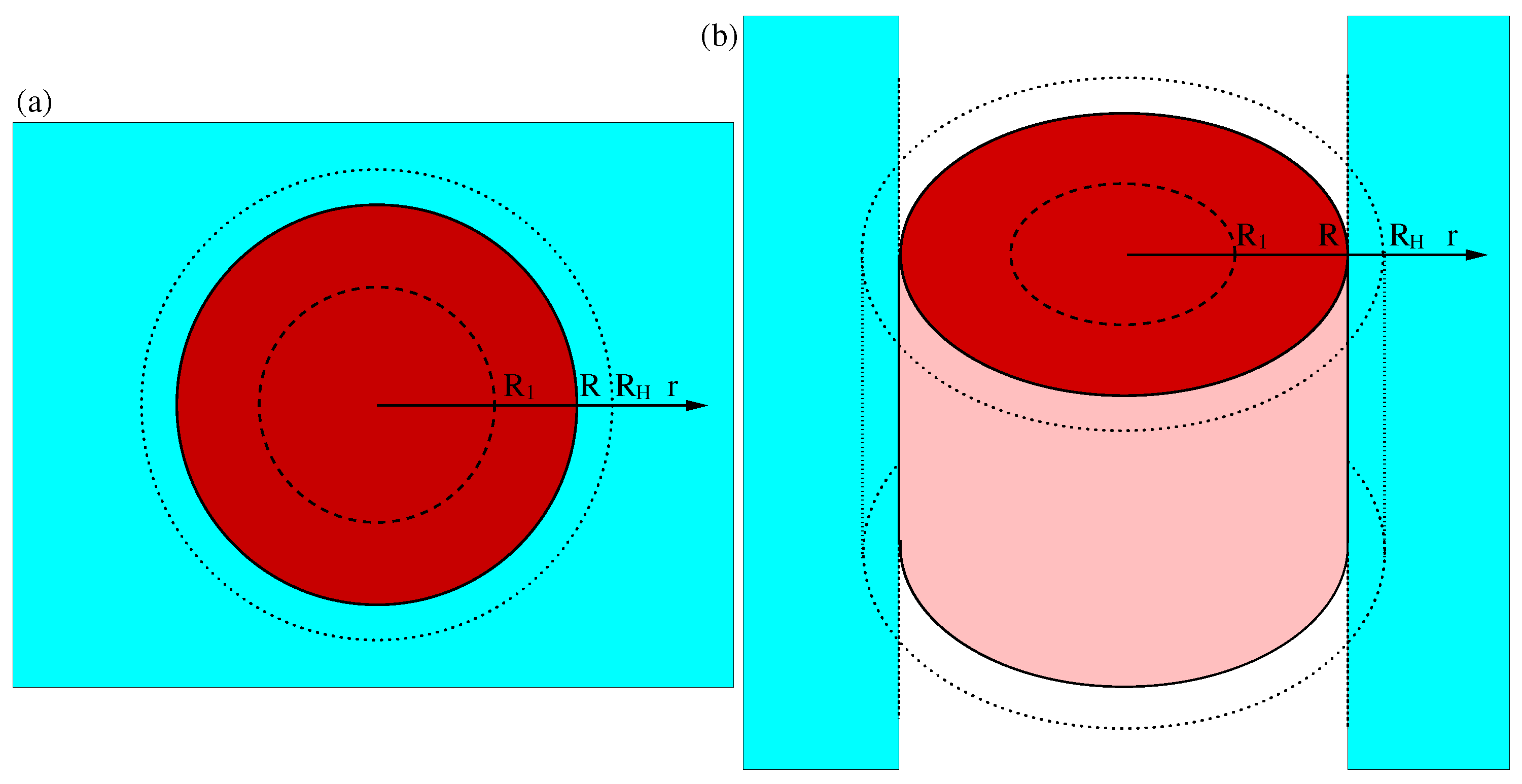
2.1. Poisson–Boltzmann Equations in the Spherical Case
2.2. Poisson–Boltzmann Equations in the Cylindrical Case
2.3. Calculation Parameters
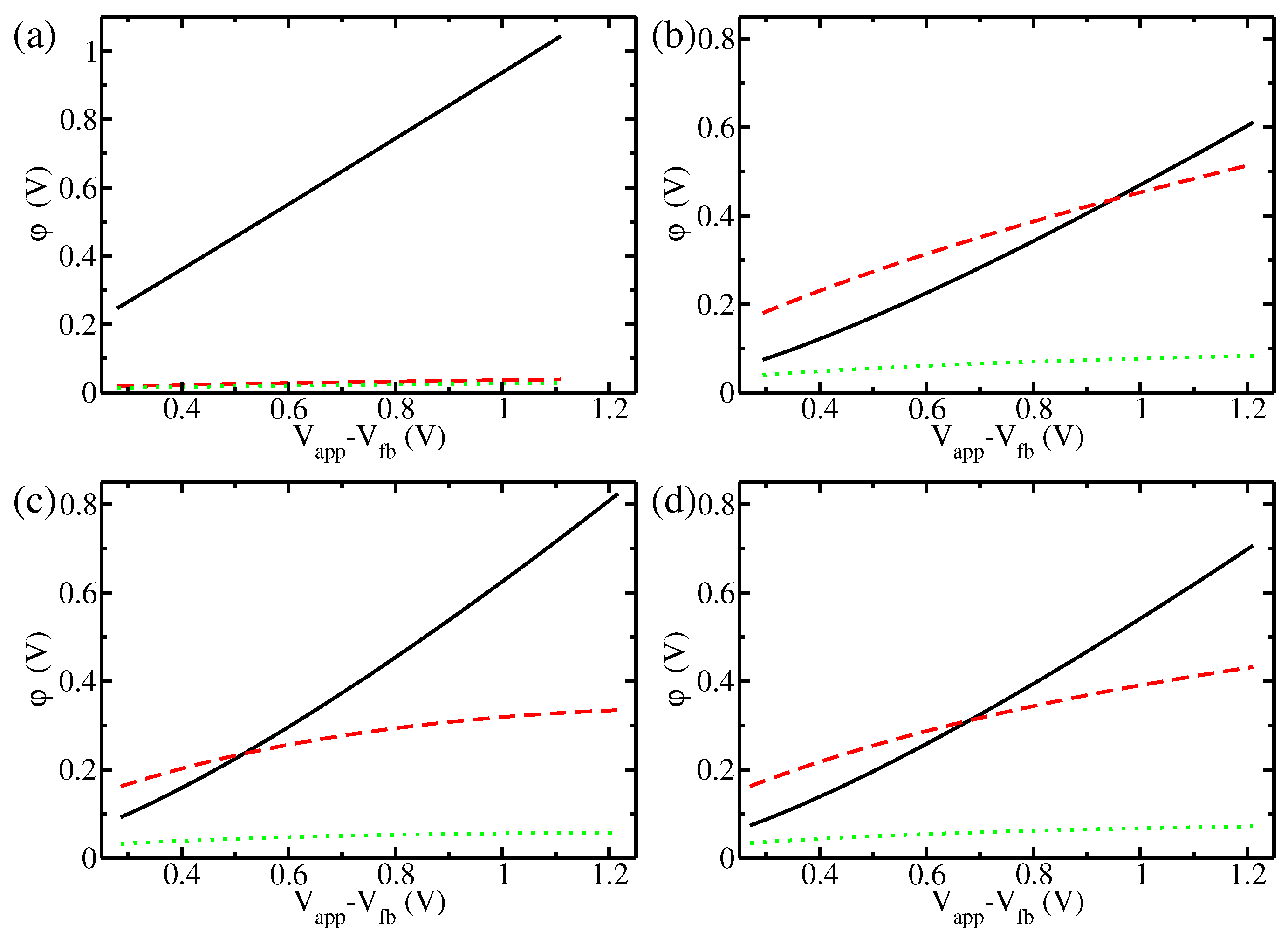
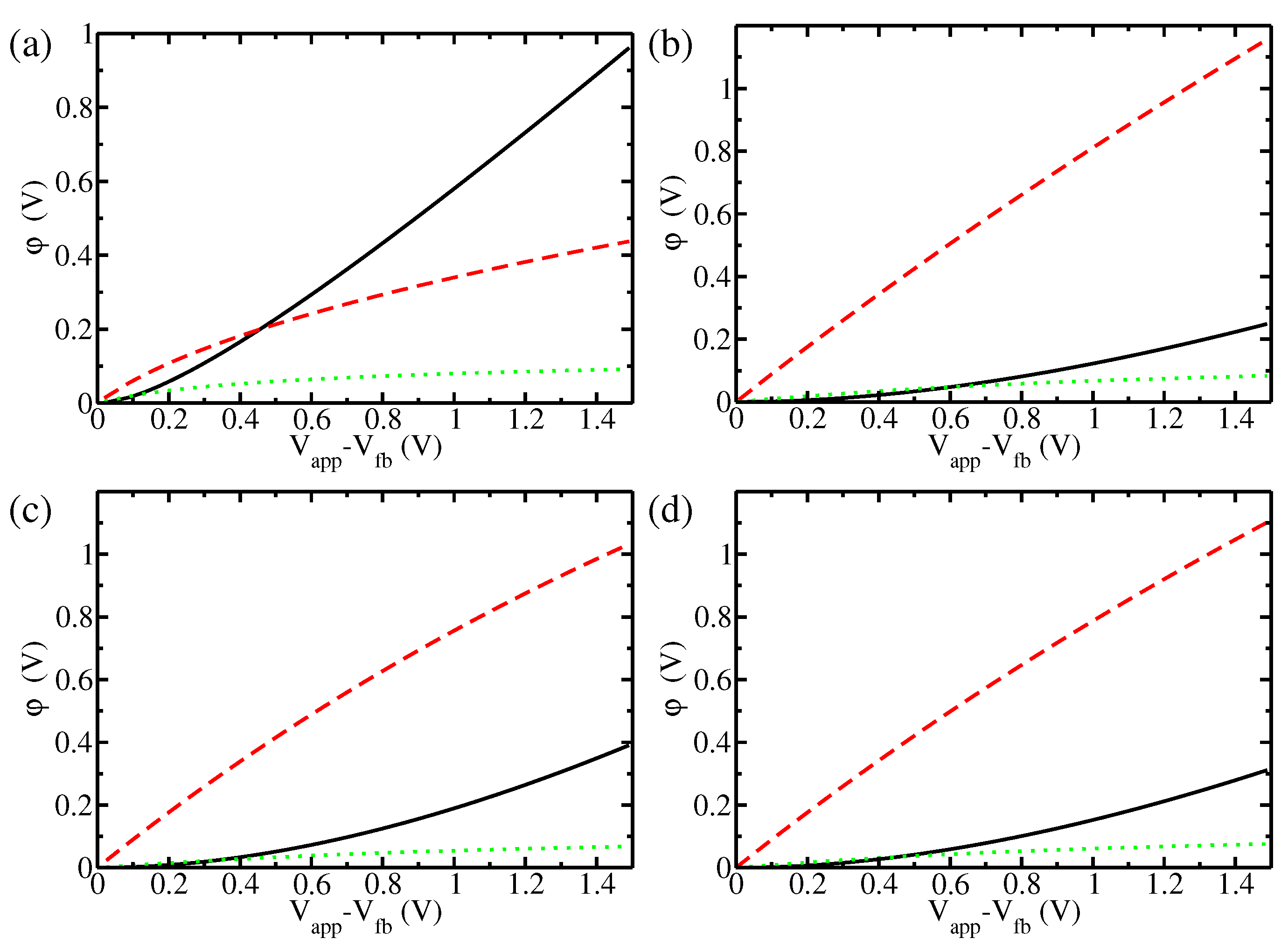
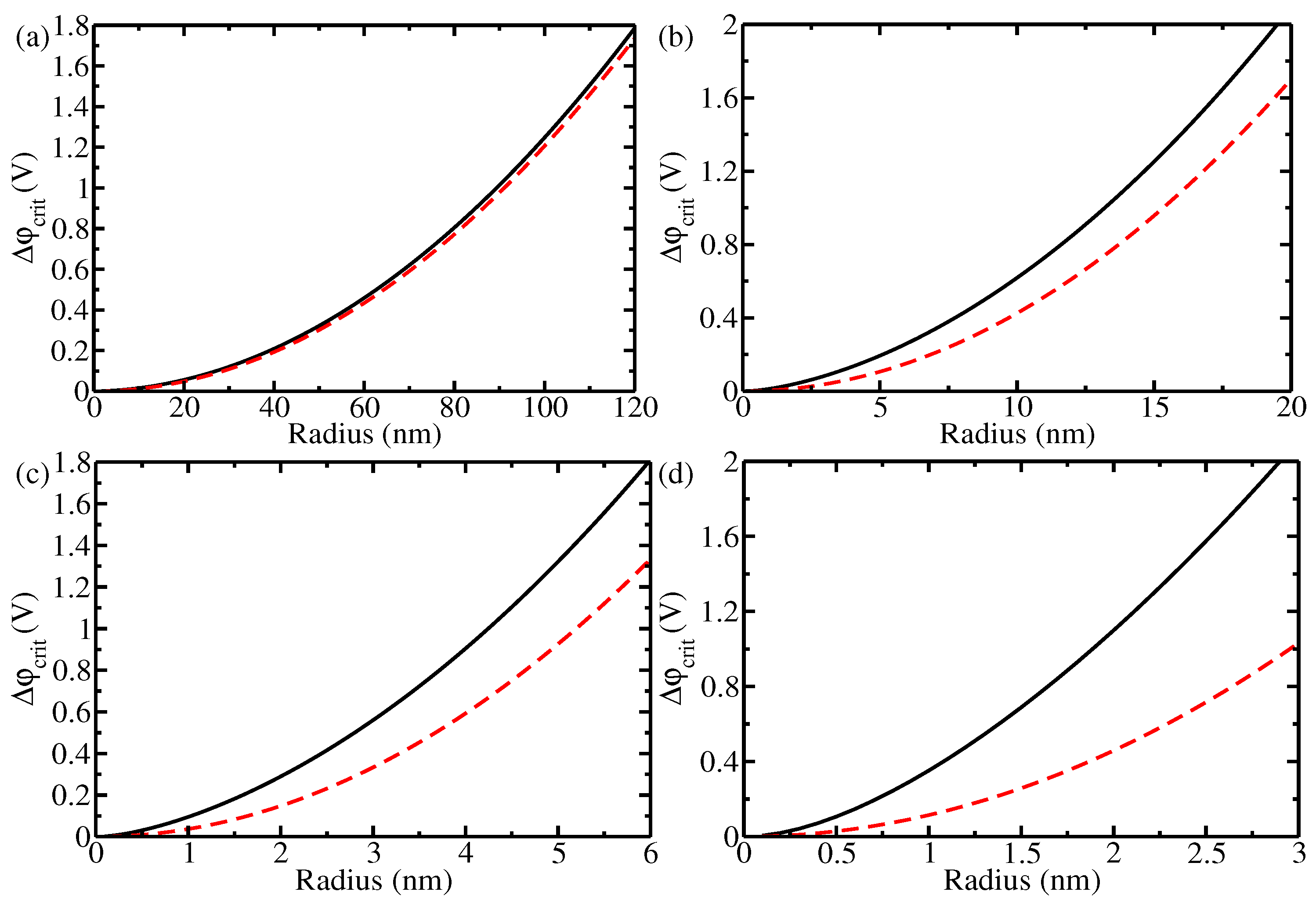
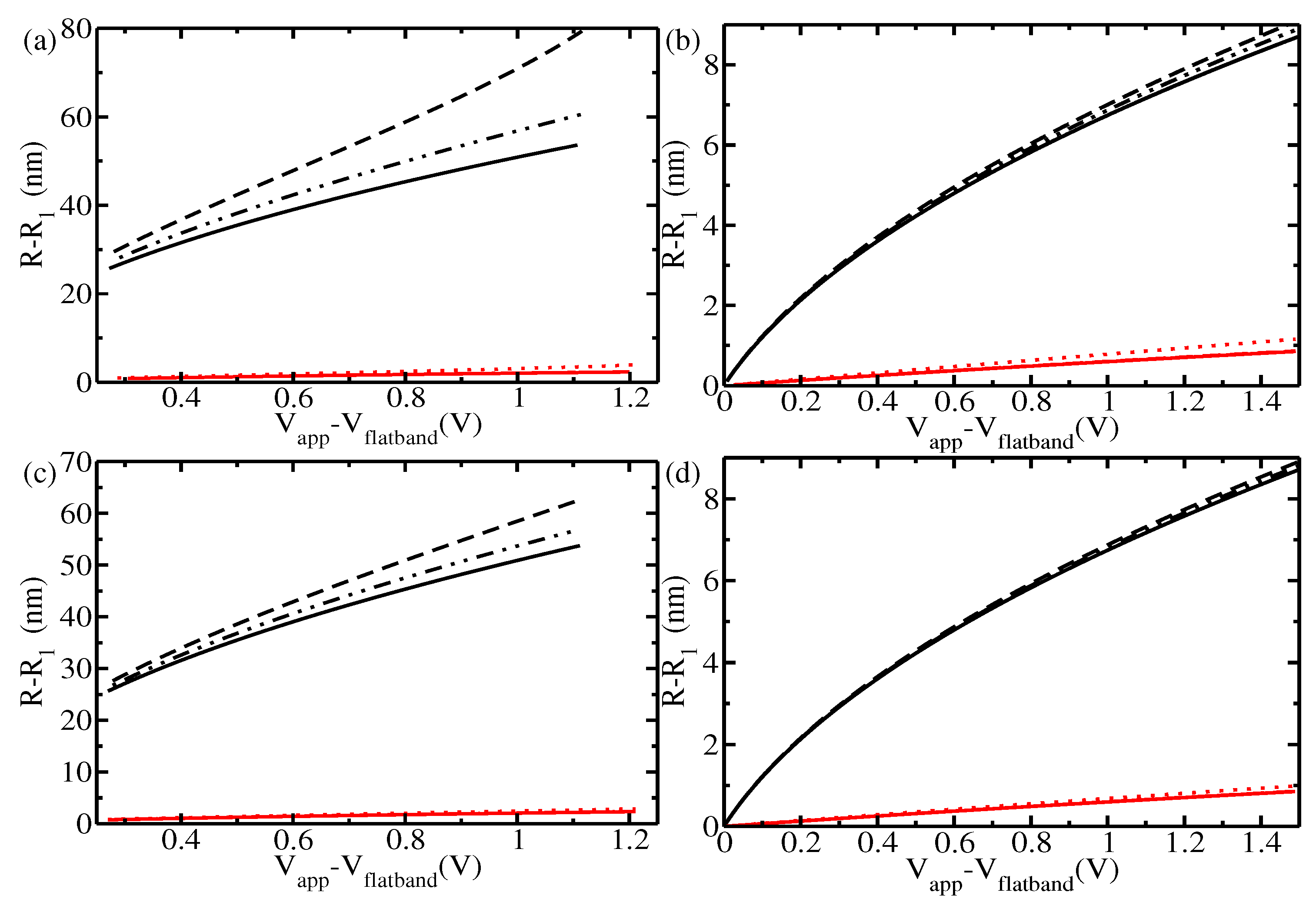
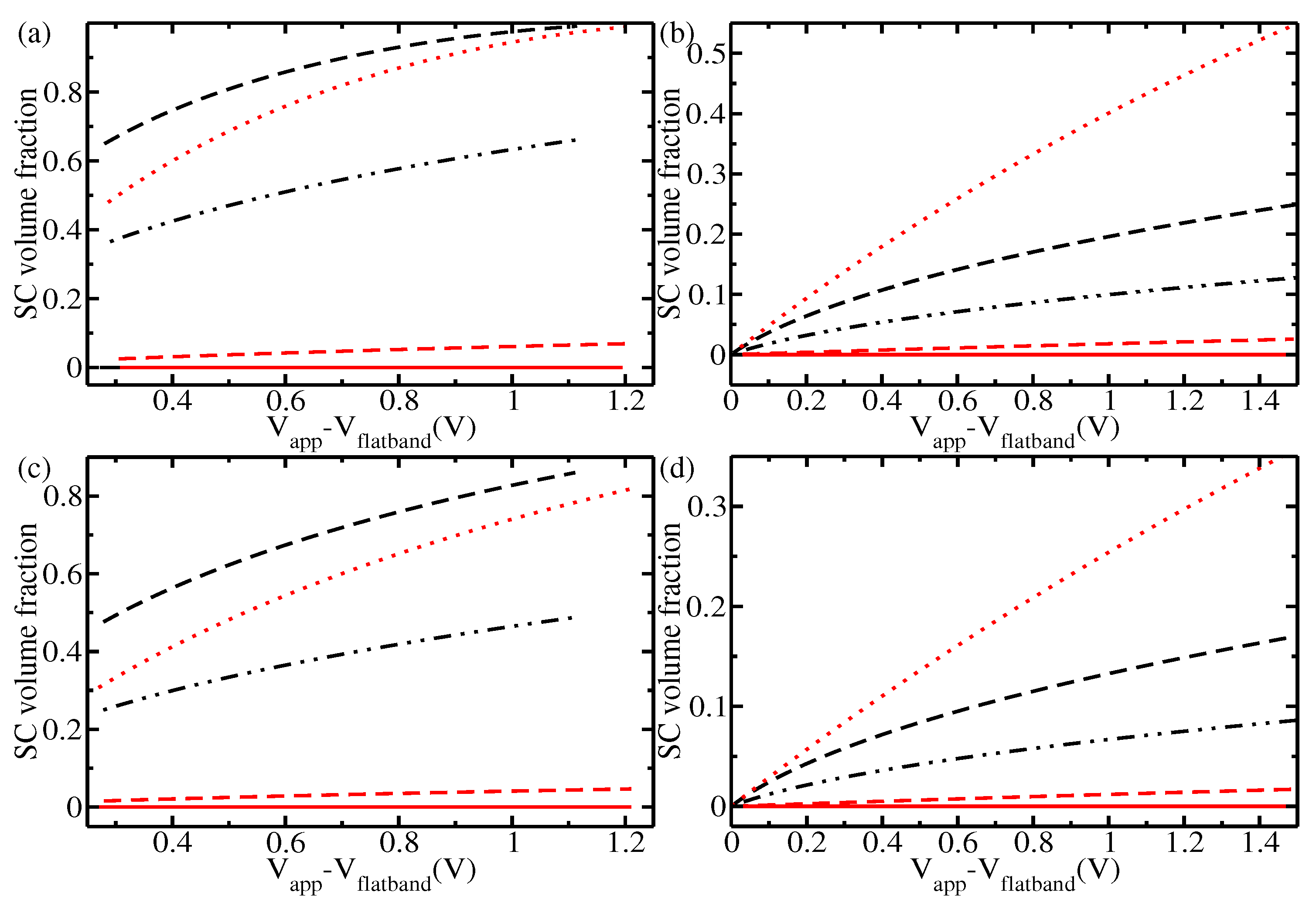
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helmholtz, H.V. Studien über elektrische Grenzschichten. Ann. Phys. 1879, 243, 337–382. [Google Scholar] [CrossRef]
- Gouy, M. Sur la constitution de la charge électrique à la surface d’un électrolyte. J. Phys. Theor. Appl. 1910, 9, 457–468. [Google Scholar] [CrossRef]
- Chapman, D.L. A contribution to the theory of electrocapillarity. Philos. Mag. J. Sci. 1913, 25, 475–481. [Google Scholar] [CrossRef]
- Stern, O. Zur Theorie der elektrolytischen Doppelschicht. Z. Elektrochem. Angew. Phys. Chem. 1924, 30, 508–516. [Google Scholar] [CrossRef]
- Sivula, K. Mott–Schottky analysis of photoelectrodes: Sanity checks are needed. ACS Energy Lett. 2021, 6, 2549–2551. [Google Scholar] [CrossRef]
- Hankin, A.; Bedoya-Lora, F.E.; Alexander, J.C.; Regoutz, A.; Kelsall, G.H. Flat band potential determination: Avoiding the pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. [Google Scholar] [CrossRef]
- Cesar, I.; Sivula, K.; Kay, A.; Zboril, R.; Grätzel, M. Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J. Phys. Chem. C 2009, 113, 772–782. [Google Scholar] [CrossRef]
- Le Formal, F.; Tetreault, N.; Cornuz, M.; Moehl, T.; Grätzel, M.; Sivula, K. Passivating surface states on water splitting hematite photoanodes with alumina overlayers. Chem. Sci. 2011, 2, 737–743. [Google Scholar] [CrossRef]
- Gurudayal; Peter, L.M.; Wong, L.H.; Abdi, F.F. Revealing the influence of doping and surface treatment on the surface carrier dynamics in hematite nanorod photoanodes. ACS Appl. Mater. Interfaces 2017, 9, 41265–41272. [Google Scholar] [CrossRef]
- Peter, L.M.; Gurudayal; Wong, L.H.; Abdi, F.F. Understanding the role of nanostructuring in photoelectrode performance for light-driven water splitting. J. Electroanal. Chem. 2018, 819, 447–458. [Google Scholar] [CrossRef]
- Delcompare-Rodriguez, P.A.; Seriani, N. Ultrathin space charge layer in hematite photoelectrodes: A theoretical investigation. J. Chem. Phys. 2021, 155, 114701. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Gurudayal; Bassi, P.S.; Sritharan, T.; Wong, L.H. Recent progress in iron oxide based photoanodes for solar water splitting. J. Phys. D Appl. Phys. 2018, 51, 473002. [Google Scholar] [CrossRef]
- Bosman, A.J.; van Daal, H.J. Small-polaron versus band conduction in some transition-metal oxides. Adv. Phys. 1970, 19, 1–117. [Google Scholar]
- Iordanova, N.; Dupuis, M.; Rosso, K.M. Charge transport in metal oxides: A theoretical study of hematite α-Fe2O3. J. Chem. Phys. 2005, 122, 144305. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Liston, D.B.; Lovejoy, J.A.; Deng, H.; Zhang, J.Z. Ultrafast studies of photoexcited electron dynamics in γ- and α-Fe2O3 semiconductor nanoparticles. J. Phys. Chem. B 1998, 102, 770–776. [Google Scholar] [CrossRef]
- Kay, A.; Cesar, I.; Grätzel, M. New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 2006, 128, 15714–15721. [Google Scholar] [CrossRef]
- Brillet, J.; Grätzel, M.; Sivula, K. Decoupling feature size and functionality in solution-processed, porous hematite electrodes for solar water splitting. Nano Lett. 2010, 10, 4155–4160. [Google Scholar] [CrossRef]
- Townsend, T.K.; Sabio, E.M.; Browning, N.D.; Osterloh, F.E. Photocatalytic water oxidation with suspended α-Fe2O3 particles-effects of nanoscaling. Energy Environ. Sci. 2011, 4, 4270–4275. [Google Scholar] [CrossRef]
- Souza, F.L.; Lopes, K.P.; Longo, E.; Leite, E.R. The influence of the film thickness of nanostructured α-Fe2O3 on water photooxidation. Phys. Chem. Chem. Phys. 2009, 11, 1215–1219. [Google Scholar] [CrossRef]
- Le Formal, F.; Grätzel, M.; Sivula, K. Controlling photoactivity in ultrathin hematite films for solar water-splitting. Adv. Funct. Mater. 2010, 20, 1099–1107. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, W.; Li, Z.; Yu, T.; Zou, Z. Improved photoelectrochemical responses of Si and Ti codoped α-Fe2O3 photoanode films. Appl. Phys. Lett. 2010, 97, 042105. [Google Scholar] [CrossRef]
- Warren, S.C.; Voitchovsky, K.; Dotan, H.; Leroy, C.M.; Cornuz, M.; Stellacci, F.; Hébert, C.; Rothschild, A.; Grätzel, M. Identifying champion nanostructures for solar water-splitting. Nat. Mater. 2013, 12, 842–849. [Google Scholar] [CrossRef]
- Barroso, M.; Pendlebury, S.R.; Cowan, A.J.; Durrant, J.R. Charge carrier trapping, recombination and transfer in hematite (α-Fe2O3) water splitting photoanodes. Chem. Sci. 2013, 4, 2724–2734. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water oxidation at hematite photoelectrodes: The role of surface states. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef]
- Righi, G.; Plescher, J.; Schmidt, F.-P.; Campen, R.K.; Fabris, S.; Knop-Gericke, A.; Schlögl, R.; Travis, T.E.; Teschner, D.; Piccinin, S. On the origin of multihole oxygen evolution in haematite photoanodes. Nat. Catal. 2022, 5, 888–899. [Google Scholar] [CrossRef]
- Berardi, S.; Cristino, V.; Bignozzi, C.A.; Grandi, S.; Caramori, S. Hematite-based photoelectrochemical interfaces for solar fuel production. Inorg. Chim. Acta 2022, 535, 120862. [Google Scholar] [CrossRef]
- Seriani, N. Ab initio simulations of water splitting on hematite. J. Phys. Condens. Mat. 2017, 29, 463002. [Google Scholar] [CrossRef]
- Liao, P.; Toroker, M.C.; Carter, E.A. Electron transport in pure and doped hematite. Nano Lett. 2011, 11, 1775–1781. [Google Scholar] [CrossRef]
- Liao, P.; Keith, J.A.; Carter, E.A. Water oxidation on pure and doped hematite (0001) surfaces: Prediction of Co and Ni as effective dopants for electrocatalysis. J. Am. Chem. Soc. 2012, 134, 13296–13309. [Google Scholar] [CrossRef]
- Neufeld, O.; Yatom, N.; Toroker, M.C. A first-principles study on the role of an Al2O3 overlayer on Fe2O3 for water splitting. ACS Catal. 2015, 5, 7237–7243. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Seriani, N.; Piccinin, S.; Gebauer, R. Photo-driven oxidation of water on α-Fe2O3 surfaces: An ab initio study. J. Chem. Phys. 2014, 140, 064703. [Google Scholar] [CrossRef] [PubMed]
- Ulman, K.; Nguyen, M.T.; Seriani, N.; Gebauer, R. Passivation of surface states of α-Fe2O3 (0001) surface by deposition of Ga2O3 overlayers: A density functional theory study. J. Chem. Phys. 2016, 140, 094701. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.; Ulman, K.; Camellone, M.F.; Seriani, N.; Gebauer, R.; Piccinin, S. Hole localization in Fe2O3 from density functional theory and wave-function-based methods. Phys. Rev. Mater. 2017, 1, 035404. [Google Scholar] [CrossRef]
- Ulman, K.; Poli, E.; Seriani, N.; Piccinin, S.; Gebauer, R. Understanding the electrochemical double layer at the hematite/water interface: A first principles molecular dynamics study. J. Chem. Phys. 2019, 150, 041707. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Wang, D.; Sheng, T.; Chen, J.; Wang, H.F.; Hu, P. Identifying the key obstacle in photocatalytic oxygen evolution on rutile TiO2. Nat. Catal. 2018, 1, 291–299. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting–superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterisation of oxygen defects and nitrogen impurities in TiO2 photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 661. [Google Scholar] [CrossRef]
- Seriani, N.; Pinilla, C.; Crespo, Y. Presence of gap states at Cu/TiO2 anatase surfaces: Consequences for the photocatalytic activity. J. Phys. Chem. C. 2015, 119, 6696–6702. [Google Scholar] [CrossRef]
- Choi, E.M.; Yoon, Y.H.; Lee, S.; Kang, H. Freezing transition of interfacial water at room temperature under electric fields. Phys. Rev. Lett. 2005, 95, 085701. [Google Scholar] [CrossRef]
- Pleskov, J.V.; Gurevich, Y.Y.; Bartlett, P.N. Semiconductor Photoelectrochemistry; Consultants Bureau: New York, NY, USA, 1986. [Google Scholar]
- Bisquert, J. Nanostructured Energy Devices: Equilibrium Concepts and Kinetics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Yao, Z.; Bowick, M.J.; Ma, X. The electric double layer structure around charged spherical interfaces. J. Chem. Phys. 2012, 136, 044106. [Google Scholar] [CrossRef] [PubMed]
- Glasscock, J.A.; Barnes, P.R.F.; Plumb, I.C.; Bendavid, A.; Martin, P.J. Structural, optical and electrical properties of undoped polycrystalline hematite thin films produced using filtered arc deposition. Thin Solid Films. 2008, 516, 1716–1724. [Google Scholar] [CrossRef]
- Buchner, R.; Hefter, G.; May, P.M.; Sipos, P. Dielectric relaxation of dilute aqueous NaOH, NaAl(OH)4, and NaB(OH)4. J. Phys. Chem. B. 1999, 103, 11186–11190. [Google Scholar] [CrossRef]
- Iandolo, B.; Zhang, H.; Wickman, B.; Zorić, I.; Conibeer, G.; Hellman, A. Correlating flat band and onset potentials for solar water splitting on model hematite photoanodes. RSC Adv. 2015, 5, 61021–61030. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Cardenas-Morcoso, D.; García-Tecedor, M.; Fabregat-Santiago, F.; Bisquert, J.; Al-Mayouf, A.M.; Gimenez, S. TiO2 nanotubes for solar water splitting: Vacuum annealing and Zr doping enhance water oxidation kinetics. ACS Omega 2019, 4, 16095–16102. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Zhang, Y.; Wang, J.; Zhao, Y.; Chen, Y.T. Correlation of the depletion layer with the Helmholtz layer in the anatase TiO2–H2O interface via molecular dynamics simulations. Phys. Chem. Chem. Phys. 2016, 18, 15427–15435. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.; Hidalgo, D.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Cauda, V.; Tresso, E.; Saracco, G. Comparison of photocatalytic and transport properties of TiO2 and ZnO nanostructures for solar-driven water splitting. Phys. Chem. Chem. Phys. 2015, 17, 7775–7786. [Google Scholar] [CrossRef]
- Zhang, Z.; Nagashima, H.; Tachikawa, T. Ultra-narrow depletion layers in a hematite mesocrystal-based photoanode for boosting multihole water oxidation. Angew. Chem. Int. Ed. 2020, 59, 9047–9054. [Google Scholar] [CrossRef]
- Cristino, V.; Marinello, S.; Molinari, A.; Caramori, S.; Carli, S.; Boaretto, R.; Argazzi, R.; Meda, L.; Bignozzi, C.A. Some aspects of the charge transfer dynamics in nanostructured WO3 films. J. Mater. Chem. A. 2016, 4, 2995–3006. [Google Scholar] [CrossRef]
- Shaddad, M.N.; Arunachalam, P.; Hezam, M.; AL-Saeedan, N.M.; Gimenez, S.; Bisquert, J.; Al-Mayouf, A.M. Unprecedented solar water splitting of dendritic nanostructured Bi2O3 films by combined oxygen vacancy formation and Na2MoO4 doping. Int. J. Hydrogen Energy 2021, 46, 23702–23714. [Google Scholar] [CrossRef]
- Futera, Z.; English, N.J. Water breakup at Fe2O3-hematite/water interfaces: Influence of external electric fields from nonequilibrium ab initio molecular dynamics. J. Phys. Chem. Lett. 2021, 12, 6818–6826. [Google Scholar] [CrossRef] [PubMed]
| Reference | Dopant Type | () | (M) | |||
|---|---|---|---|---|---|---|
| Hematite | ||||||
| Iandolo et al. [48] | Oxygen vacancies | 0.73 | 1.65 | 1.28 | 0.1056 | |
| Le Formal et al. [8] | Si | 0.53 | 1.58 | 1.0 | 1.0330 | |
| Titania | ||||||
| Hernandez et al. [51] | Oxygen vacancies | 0.2 | 1.75 | 0.17 | 0.1 | |
| Shaddad et al. [49] | Oxygen vacancies | 0.11 | - | 0.13 | 1.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seriani, N.; Delcompare-Rodriguez, P.; Pandey, D.; Adak, A.K.; Mahamiya, V.; Pinilla, C.; El-Khozondar, H.J. Quantitative Analysis of the Synergy of Doping and Nanostructuring of Oxide Photocatalysts. Materials 2024, 17, 3460. https://doi.org/10.3390/ma17143460
Seriani N, Delcompare-Rodriguez P, Pandey D, Adak AK, Mahamiya V, Pinilla C, El-Khozondar HJ. Quantitative Analysis of the Synergy of Doping and Nanostructuring of Oxide Photocatalysts. Materials. 2024; 17(14):3460. https://doi.org/10.3390/ma17143460
Chicago/Turabian StyleSeriani, Nicola, Paola Delcompare-Rodriguez, Dhanshree Pandey, Abhishek Kumar Adak, Vikram Mahamiya, Carlos Pinilla, and Hala J. El-Khozondar. 2024. "Quantitative Analysis of the Synergy of Doping and Nanostructuring of Oxide Photocatalysts" Materials 17, no. 14: 3460. https://doi.org/10.3390/ma17143460






