Eliminating Cu–Cu Bonding Interfaces Using Electroplated Copper and (111)-Oriented Nanotwinned Copper
Abstract
1. Introduction
2. Experiment
2.1. Cu Film Electrodeposition
2.2. Sample Pretreatment
2.3. Bonding Process
2.4. Analysis Methods
3. Results and Discussion
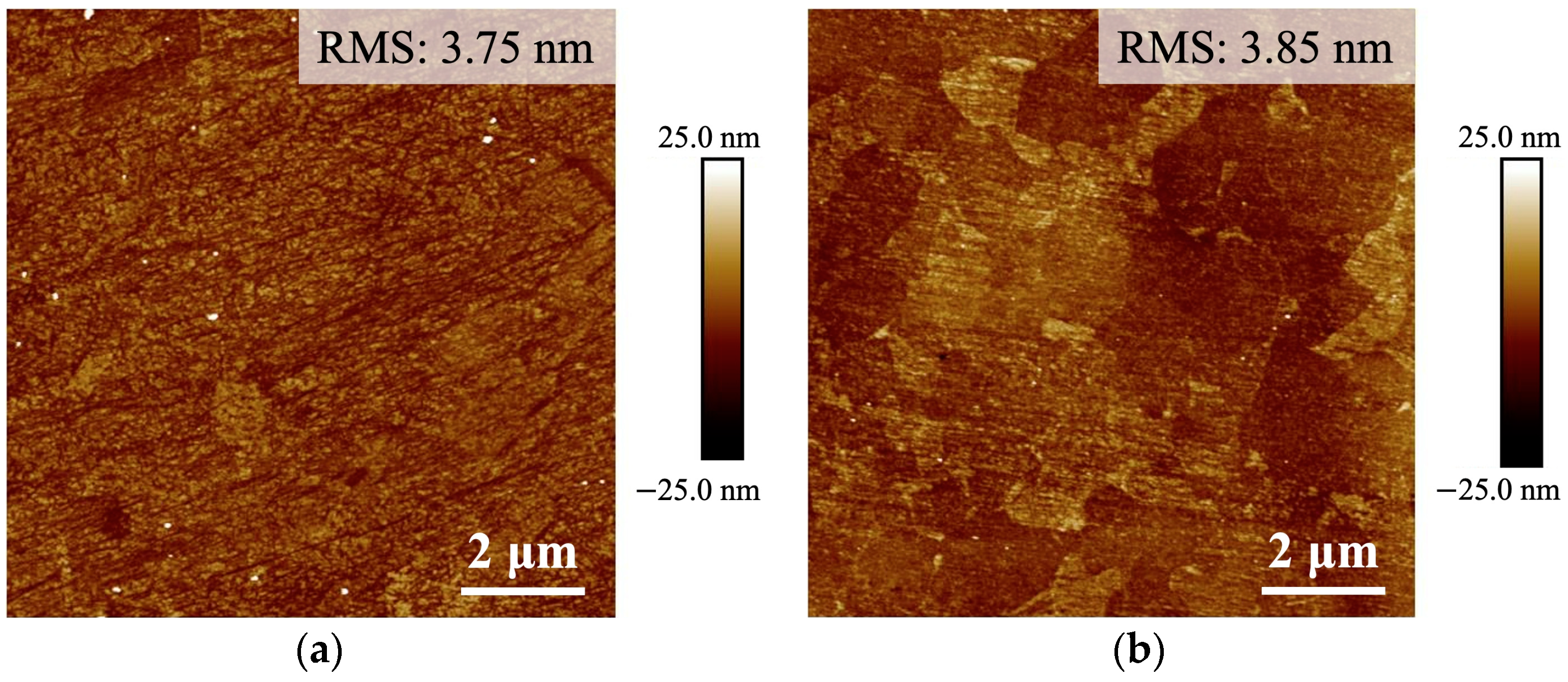
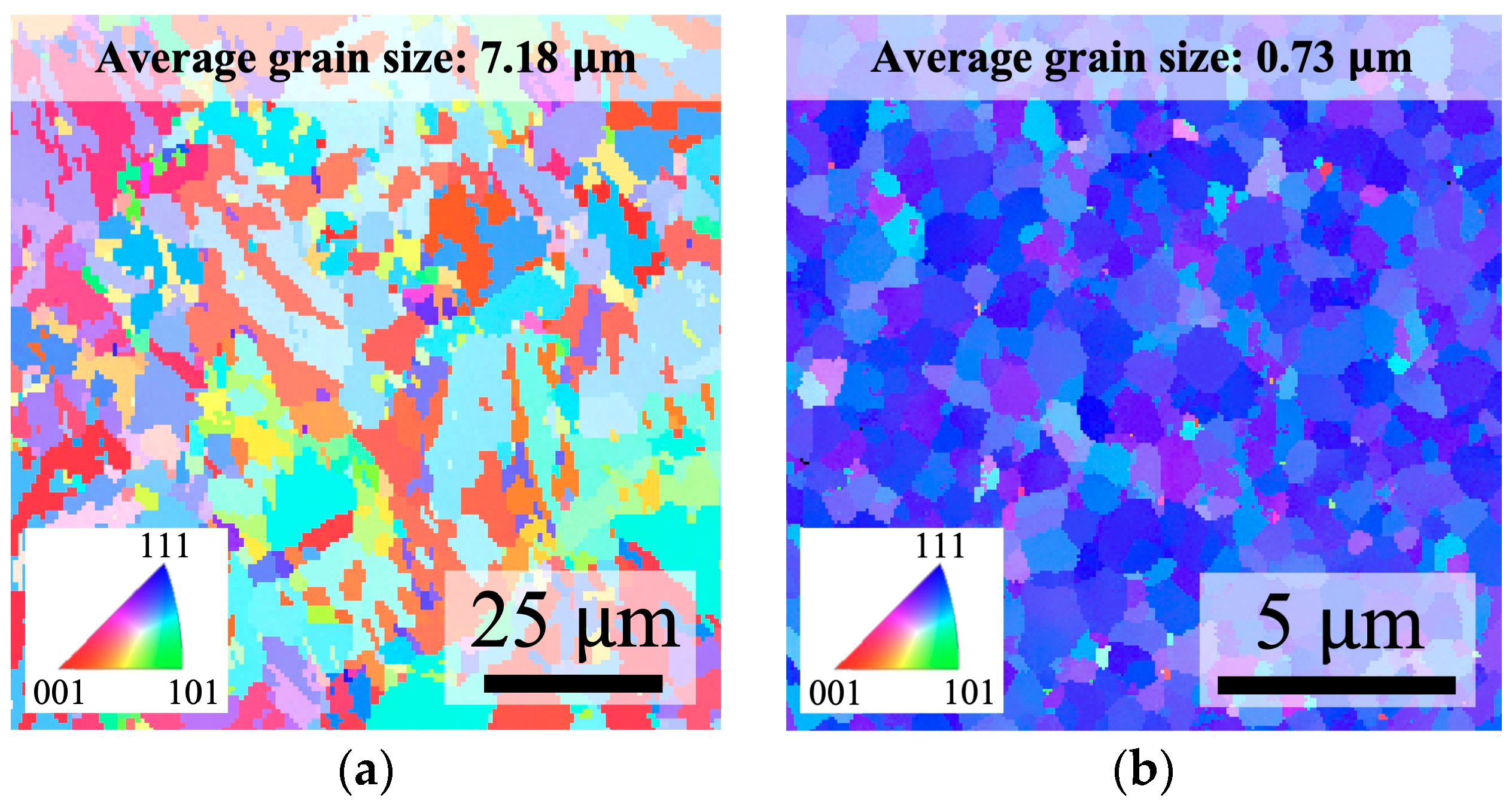
3.1. Surface Roughness and Crystallographic Information of Cu Films
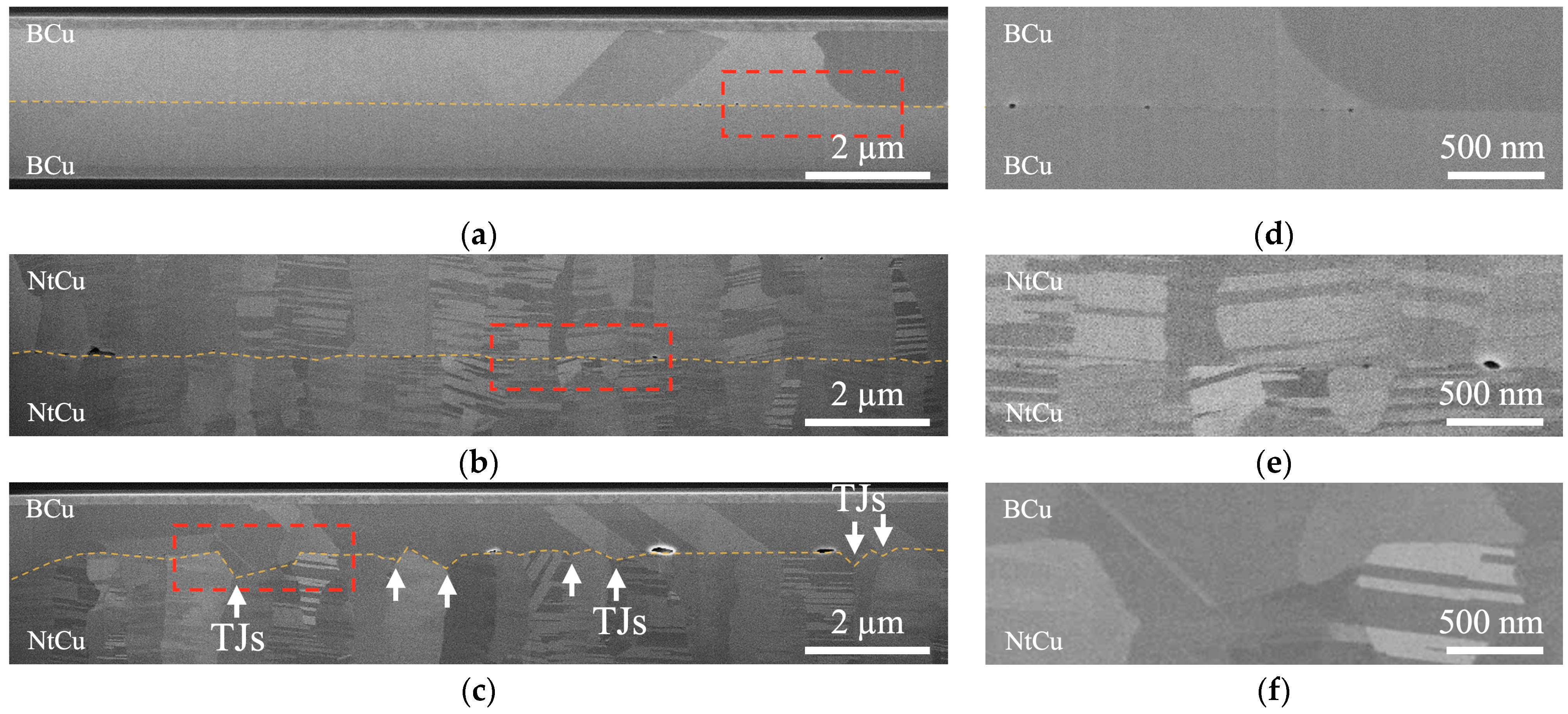
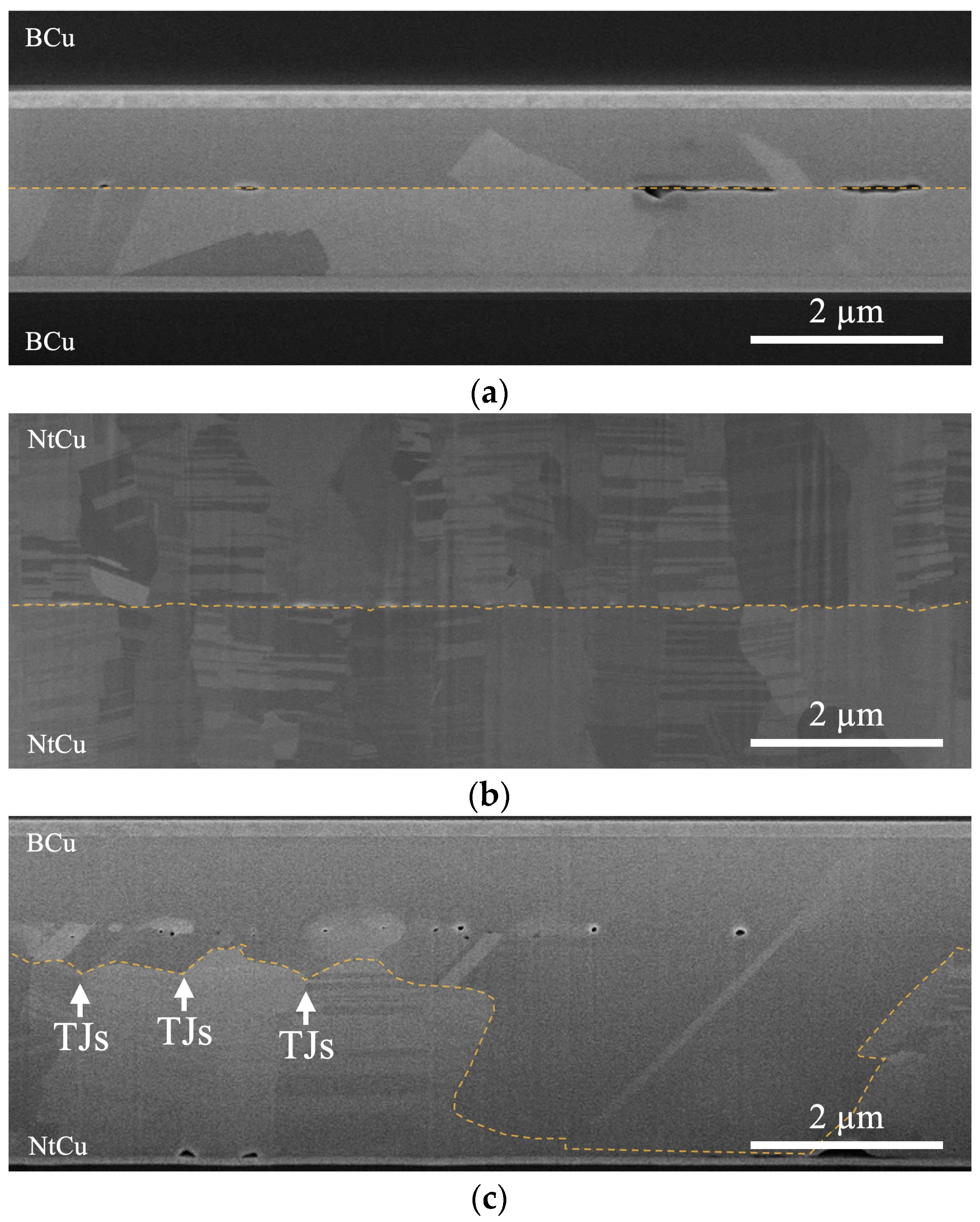
3.2. Effect of Grain Boundary Energy on Bonding Interfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, W.-W.; Chen, K.-N. Three-dimensional integrated circuit (3D IC) key technology: Through-silicon via (TSV). Nanoscale Res. Lett. 2017, 12, 56. [Google Scholar] [CrossRef]
- Huang, Z.; Hao, Y.; Li, Y.; Hu, H.; Wang, C.; Nomoto, A.; Pan, T.; Gu, Y.; Chen, Y.; Zhang, T. Three-dimensional integrated stretchable electronics. Nat. Electron. 2018, 1, 473–480. [Google Scholar] [CrossRef]
- Tu, K.-N.; Liu, Y. Recent advances on kinetic analysis of solder joint reactions in 3D IC packaging technology. Mater. Sci. Eng. R Rep. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Tseng, I.-H.; Hsu, Y.-T.; Leu, J.; Tu, K.-N.; Chen, C. Effect of thermal stress on anisotropic grain growth in nano-twinned and un-twinned copper films. Acta Mater. 2021, 206, 116637. [Google Scholar] [CrossRef]
- Ko, C.-T.; Chen, K.-N. Low temperature bonding technology for 3D integration. Microelectron. Reliab. 2012, 52, 302–311. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.E. Two-step plasma treatment on copper surface for low-temperature Cu thermo-compression bonding. IEEE Trans. Compon. Packag. Manuf. Technol. 2019, 10, 332–338. [Google Scholar] [CrossRef]
- Tu, K.-N. Recent advances on electromigration in very-large-scale-integration of interconnects. J. Appl. Phys. 2003, 94, 5451–5473. [Google Scholar] [CrossRef]
- Lu, T.-F.; Lee, P.-Y.; Wu, Y.S. Effect of Compressive Stress on Copper Bonding Quality and Bonding Mechanisms in Advanced Packaging. Materials 2024, 17, 2236. [Google Scholar] [CrossRef] [PubMed]
- Shie, K.-C.; Hsu, P.-N.; Li, Y.-J.; Tu, K.-N.; Chen, C. Reliability of Instant Bonding of Cu-Cu joints: Thermal Cycling and Electromigration Tests. In Proceedings of the 2020 15th International Microsystems, Packaging, Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 21–23 October 2020; pp. 91–94. [Google Scholar]
- Kim, T.; Howlader, M.; Itoh, T.; Suga, T. Room temperature Cu–Cu direct bonding using surface activated bonding method. J. Vac. Sci. Technol. A Vac. Surf. Film. 2003, 21, 449–453. [Google Scholar] [CrossRef]
- Rebhan, B.; Hingerl, K. Physical mechanisms of copper-copper wafer bonding. J. Appl. Phys. 2015, 118, 135301. [Google Scholar] [CrossRef]
- Jung, M.; Mitra, J.; Pan, D.Z.; Lim, S.K. TSV stress-aware full-chip mechanical reliability analysis and optimization for 3D IC. Commun. ACM 2014, 57, 107–115. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Chu, Y.-C.; Tu, K.-N.; Chen, C. Effect of anisotropic grain growth on improving the bonding strength of <111>-oriented nanotwinned copper films. Mater. Sci. Eng. A 2021, 804, 140754. [Google Scholar]
- Lu, T.-F.; Wang, P.-W.; Cheng, Y.-F.; Yen, Y.-T.; Wu, Y.S. Enhanced Nanotwinned Copper Bonding through Epoxy-Induced Copper Surface Modification. Nanomaterials 2024, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-F.; Yen, Y.-T.; Wang, P.-W.; Cheng, Y.-F.; Chen, C.-H.; Wu, Y.S. Enhanced Copper Bonding Interfaces by Quenching to Form Wrinkled Surfaces. Nanomaterials 2024, 14, 861. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-C.; Liu, C.-M.; Hsiao, H.-Y.; Lu, J.-L.; Huang, Y.-S.; Chen, C. Fabrication and characterization of (111)-oriented and nanotwinned Cu by DC electrodeposition. Cryst. Growth Des. 2012, 12, 5012–5016. [Google Scholar] [CrossRef]
- Liu, C.-M.; Lin, H.-W.; Huang, Y.-S.; Chu, Y.-C.; Chen, C.; Lyu, D.-R.; Chen, K.-N.; Tu, K.-N. Low-temperature direct copper-to-copper bonding enabled by creep on (111) surfaces of nanotwinned Cu. Sci. Rep. 2015, 5, 9734. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.-J.; Tran, D.-P.; Yang, S.-C.; Shie, K.-C.; Chen, C. Shearing characteristics of Cu-Cu joints fabricated by two-step process using highly <111>-oriented nanotwinned Cu. Metals 2021, 11, 1864. [Google Scholar] [CrossRef]
- Juang, J.-Y.; Lu, C.-L.; Li, Y.-J.; Tu, K.-N.; Chen, C. Correlation between the microstructures of bonding interfaces and the shear strength of Cu-to-Cu joints using (111)-oriented and nanotwinned Cu. Materials 2018, 11, 2368. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Matsunaga, K.; Murakami, M. The effect of strain on abnormal grain growth in Cu thin films. J. Electron. Mater. 2003, 32, 261–267. [Google Scholar] [CrossRef]
- Juang, J.-Y.; Lu, C.-L.; Li, Y.-J.; Hsu, P.-N.; Tsou, N.-T.; Tu, K.-N.; Chen, C. A solid state process to obtain high mechanical strength in Cu-to-Cu joints by surface creep on (111)-oriented nanotwins Cu. J. Mater. Res. Technol. 2021, 14, 719–730. [Google Scholar] [CrossRef]
- Shie, K.-C.; Gusak, A.; Tu, K.-N.; Chen, C. A kinetic model of copper-to-copper direct bonding under thermal compression. J. Mater. Res. Technol. 2021, 15, 2332–2344. [Google Scholar] [CrossRef]
- Gottstein, G.; Shvindlerman, L.; Zhao, B. Thermodynamics and kinetics of grain boundary triple junctions in metals: Recent developments. Scr. Mater. 2010, 62, 914–917. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- German, R.M. Coarsening in sintering: Grain shape distribution, grain size distribution, and grain growth kinetics in solid-pore systems. Crit. Rev. Solid State Mater. Sci. 2010, 35, 263–305. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G.; Blicblau, A.; Bruggeman, K.; Cortie, M.; Long, J.; Hart, J.; Marceau, R.; Mitchell, R. Materials Science and Engineering: An Introduction; John Wiley & Sons: New York, NY, USA, 2007; Volume 7. [Google Scholar]
- Rahaman, M.N. Ceramic Processing and Sintering; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Juang, J.-Y.; Lu, C.-L.; Chen, K.-J.; Chen, C.-C.A.; Hsu, P.-N.; Chen, C.; Tu, K.-N. Copper-to-copper direct bonding on highly (111)-oriented nanotwinned copper in no-vacuum ambient. Sci. Rep. 2018, 8, 13910. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.-F.; Cheng, Y.-F.; Wang, P.-W.; Yen, Y.-T.; Wu, Y.S. Eliminating Cu–Cu Bonding Interfaces Using Electroplated Copper and (111)-Oriented Nanotwinned Copper. Materials 2024, 17, 3467. https://doi.org/10.3390/ma17143467
Lu T-F, Cheng Y-F, Wang P-W, Yen Y-T, Wu YS. Eliminating Cu–Cu Bonding Interfaces Using Electroplated Copper and (111)-Oriented Nanotwinned Copper. Materials. 2024; 17(14):3467. https://doi.org/10.3390/ma17143467
Chicago/Turabian StyleLu, Tsan-Feng, Yuan-Fu Cheng, Pei-Wen Wang, Yu-Ting Yen, and YewChung Sermon Wu. 2024. "Eliminating Cu–Cu Bonding Interfaces Using Electroplated Copper and (111)-Oriented Nanotwinned Copper" Materials 17, no. 14: 3467. https://doi.org/10.3390/ma17143467
APA StyleLu, T.-F., Cheng, Y.-F., Wang, P.-W., Yen, Y.-T., & Wu, Y. S. (2024). Eliminating Cu–Cu Bonding Interfaces Using Electroplated Copper and (111)-Oriented Nanotwinned Copper. Materials, 17(14), 3467. https://doi.org/10.3390/ma17143467







