Stress-Corrosion-Cracking Sensitivity of the Sub-Zones in X80 Steel Welded Joints at Different Potentials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Solution
2.2. Microhardness
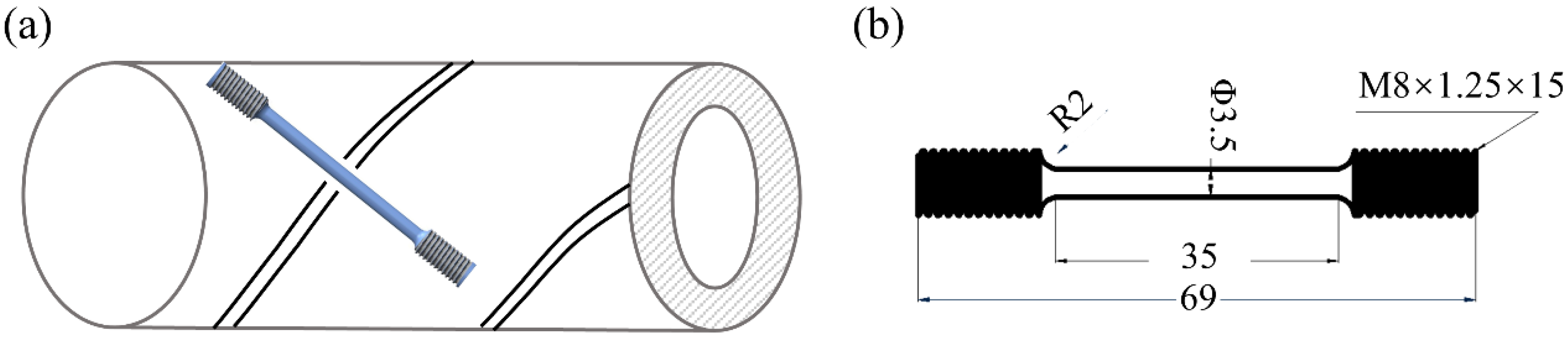
2.3. Intermittent SSRT
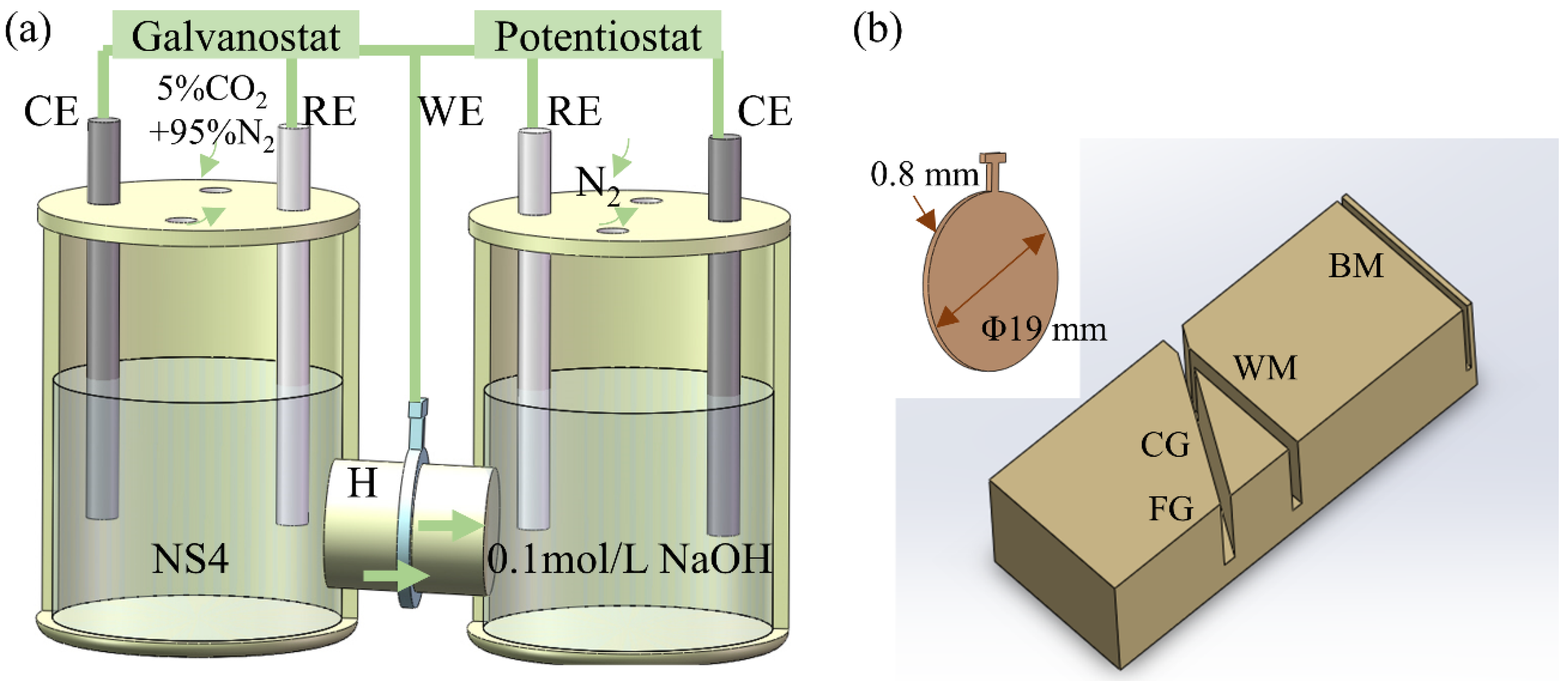
2.4. The Electrochemical Test
2.5. Hydrogen Permeation Test
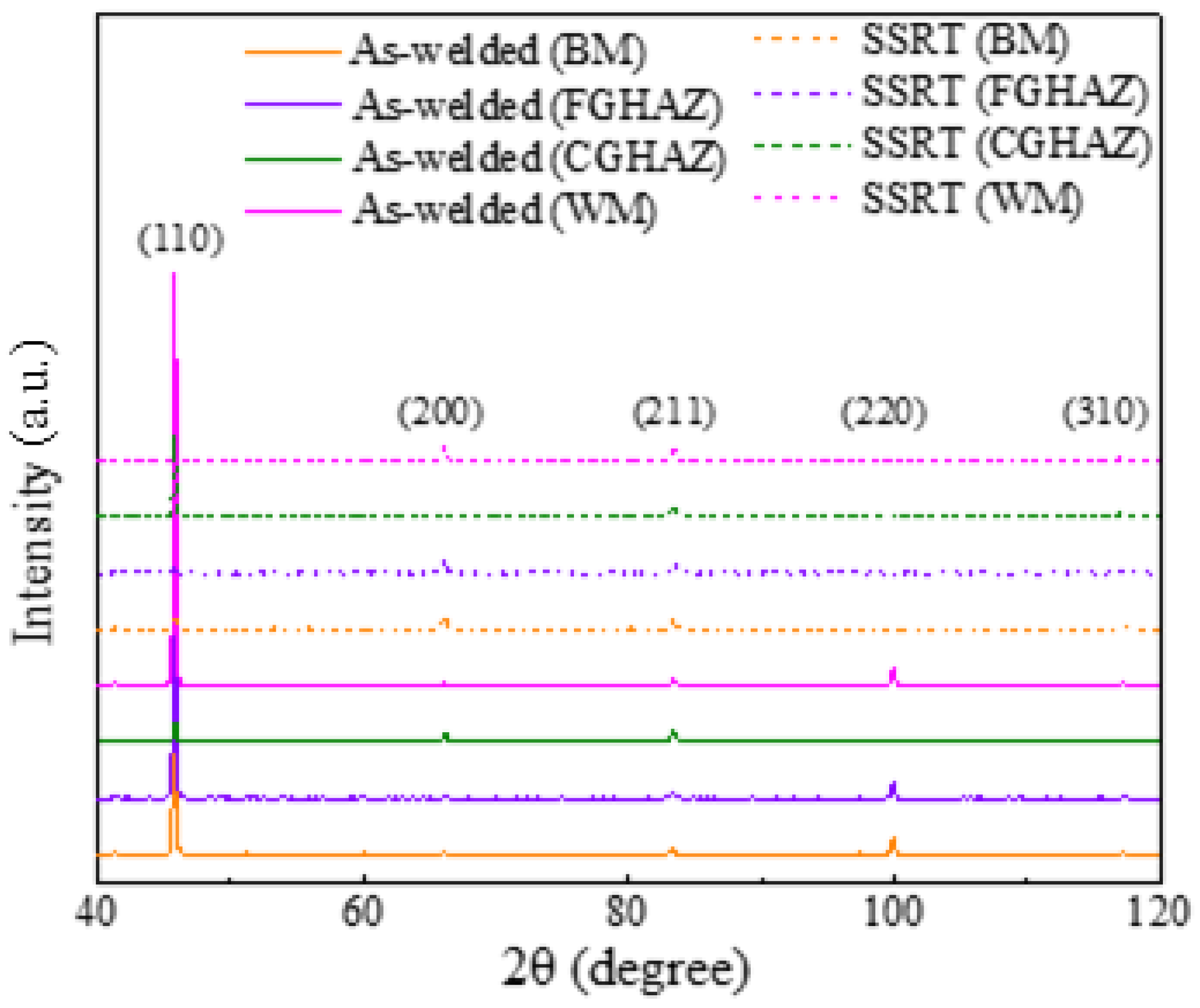
2.6. XRD Measurements
2.7. Microstructural Characterization
3. Results
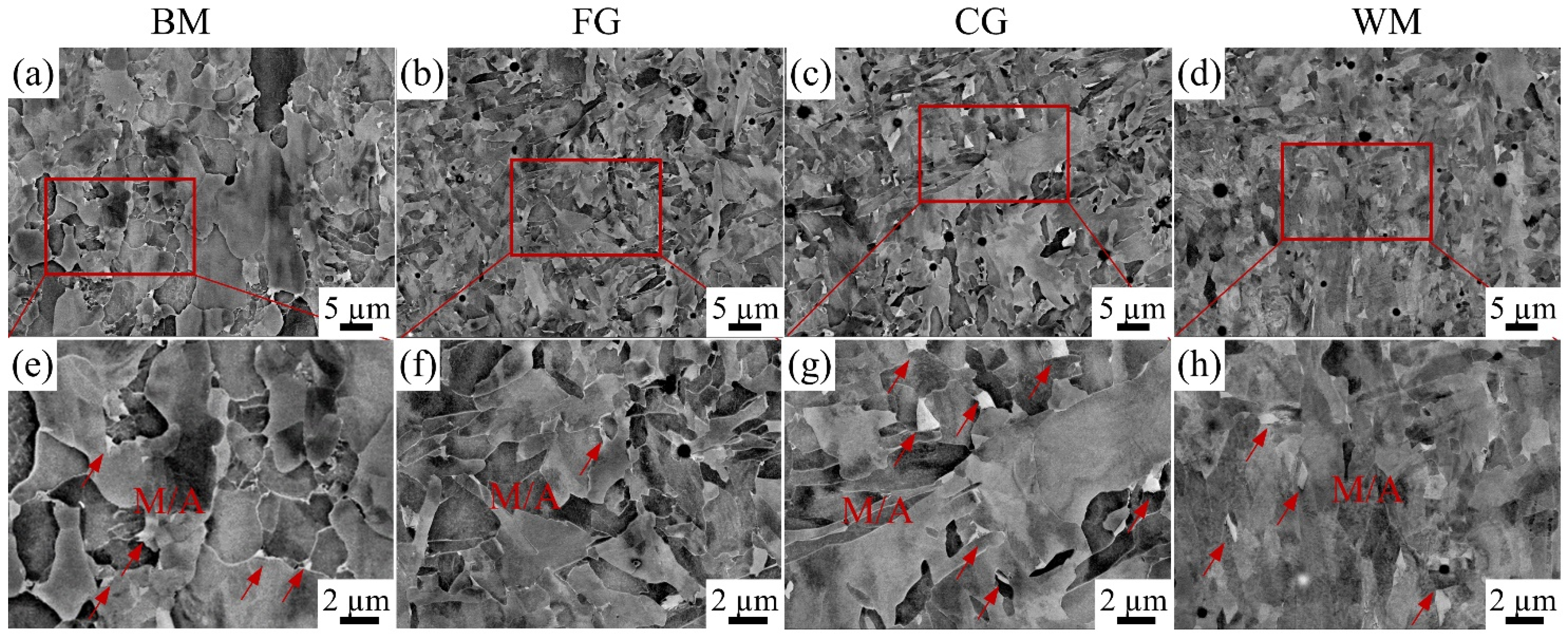
3.1. Microstructure of the Sub-Zones of X80 Steel Welded Joint
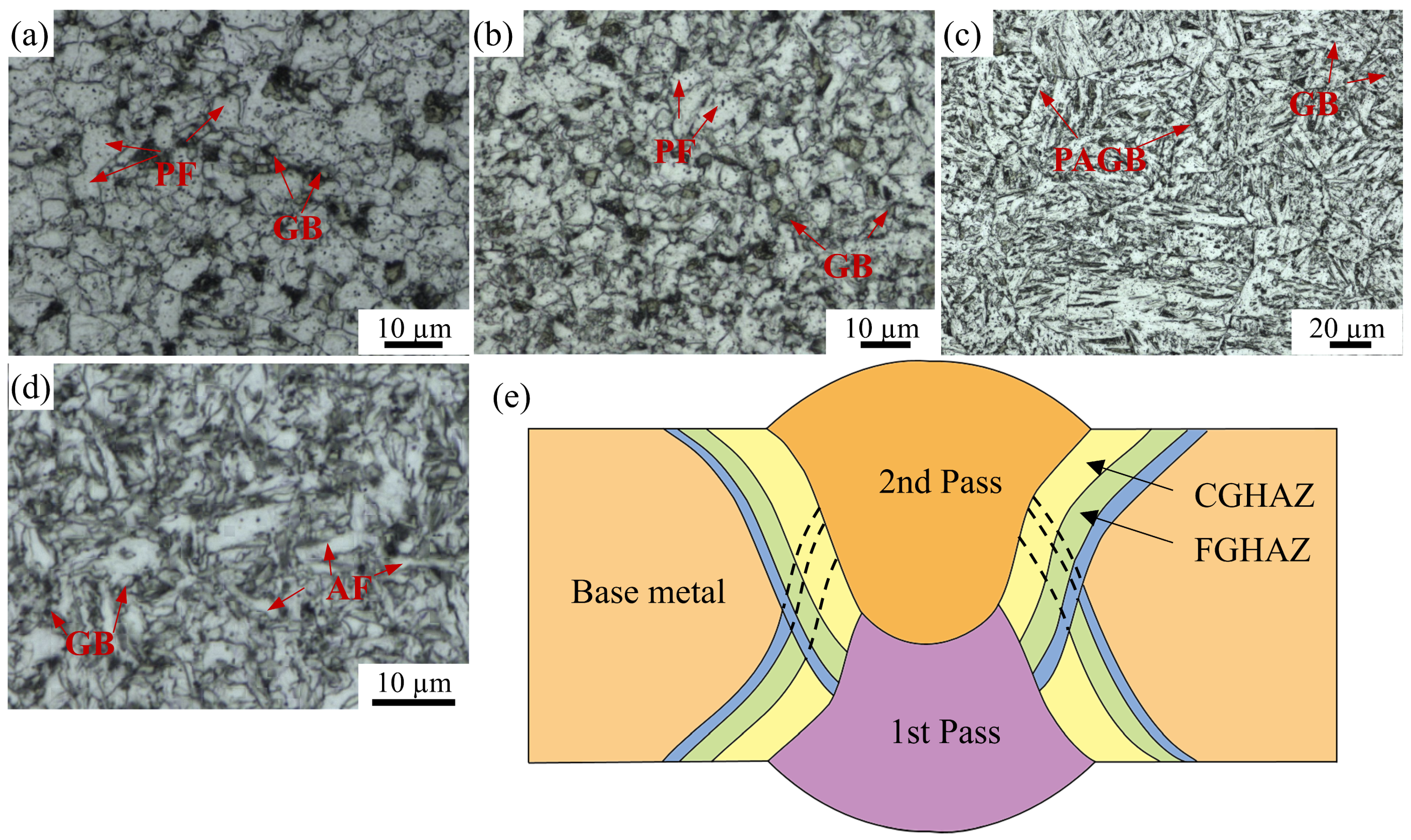
3.1.1. Macroscopic Microstructure Analysis
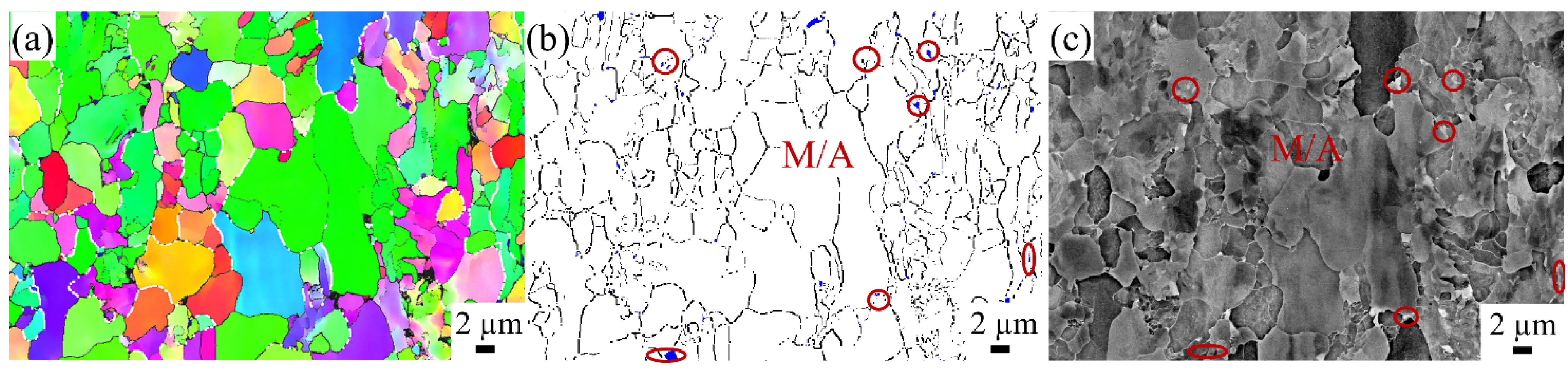
3.1.2. M/A Constituents
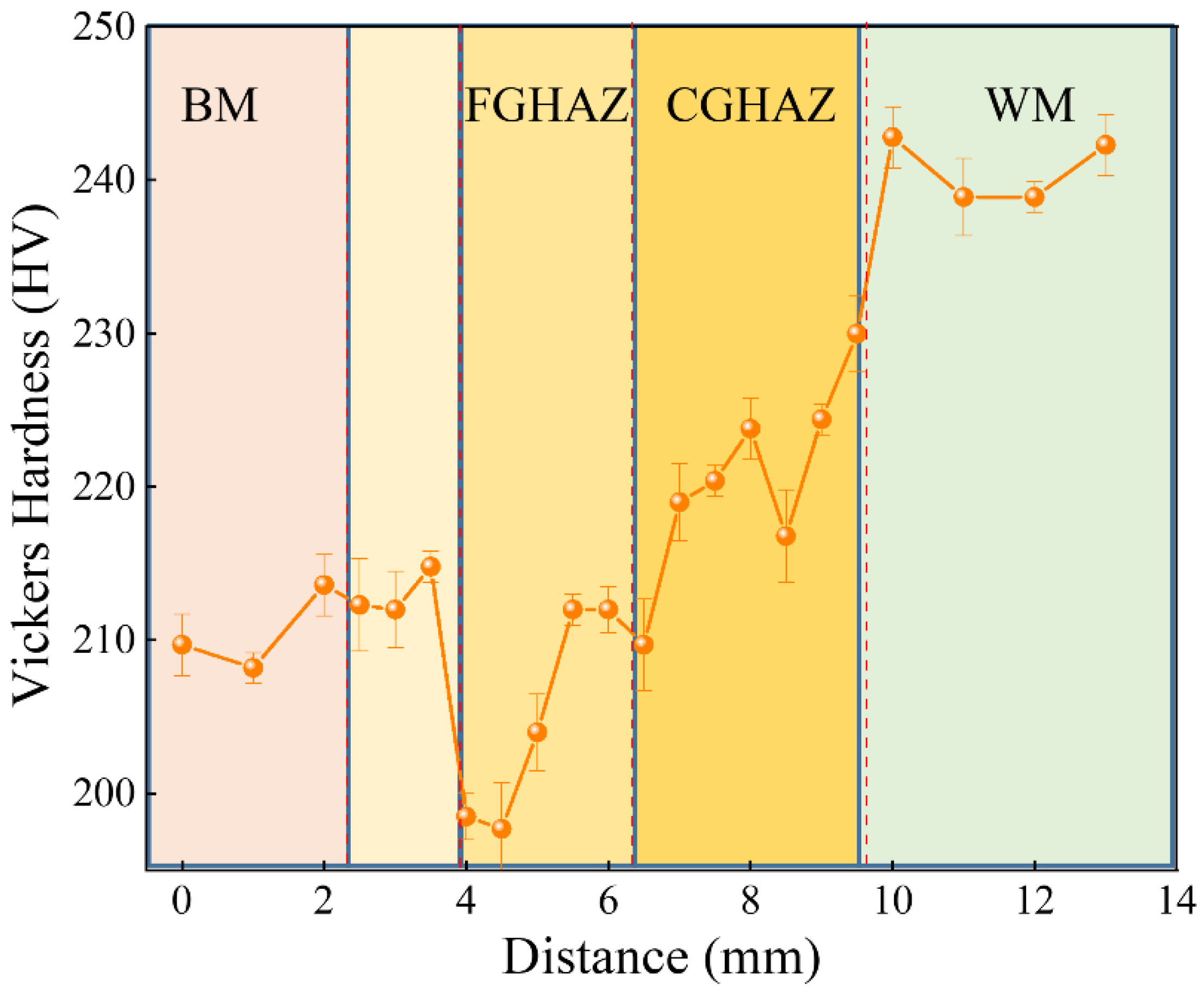
3.2. Vickers Microhardness of Sub-Zones within the X80 Steel Welded Joint
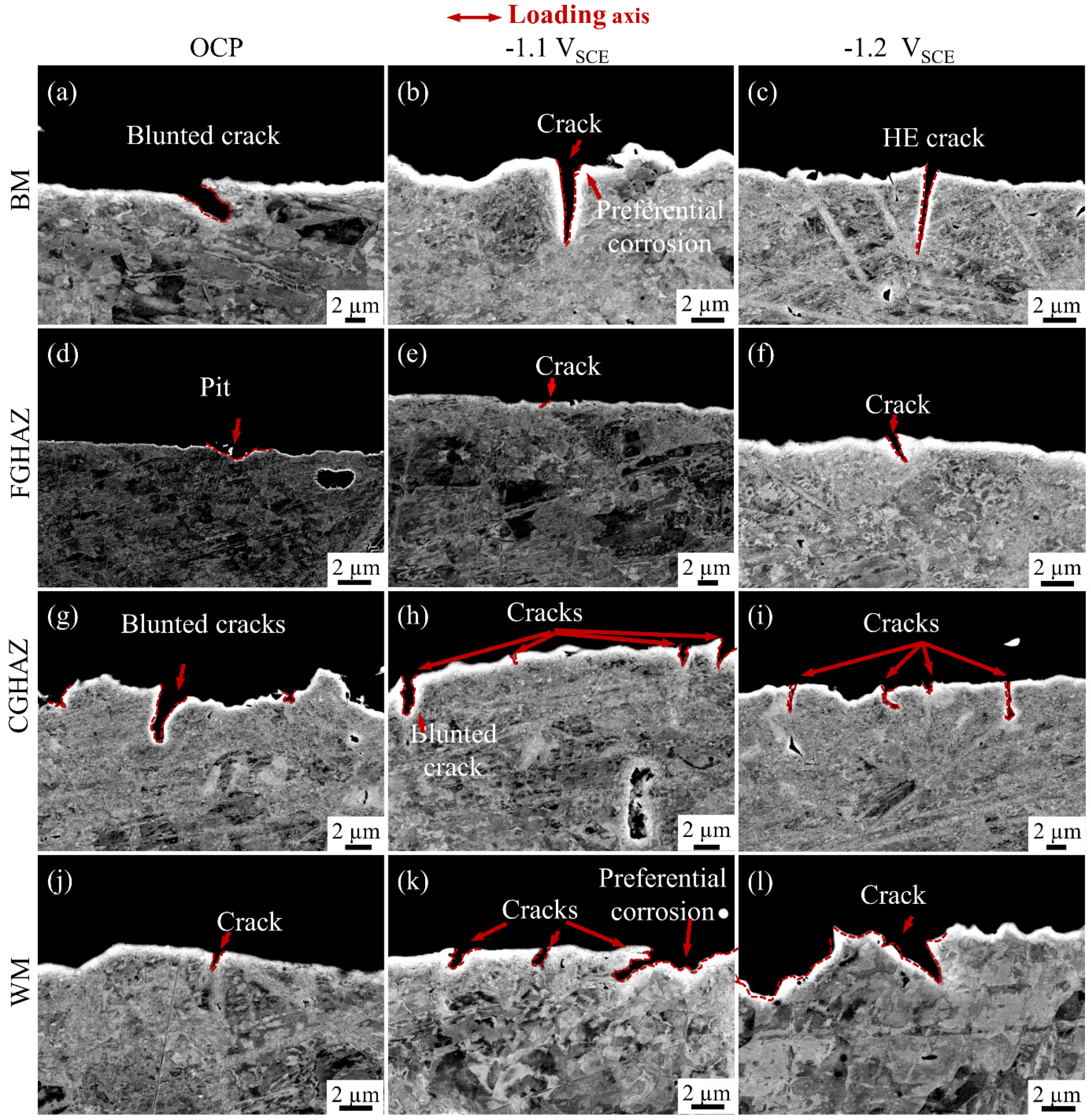
3.3. SCC Behavior
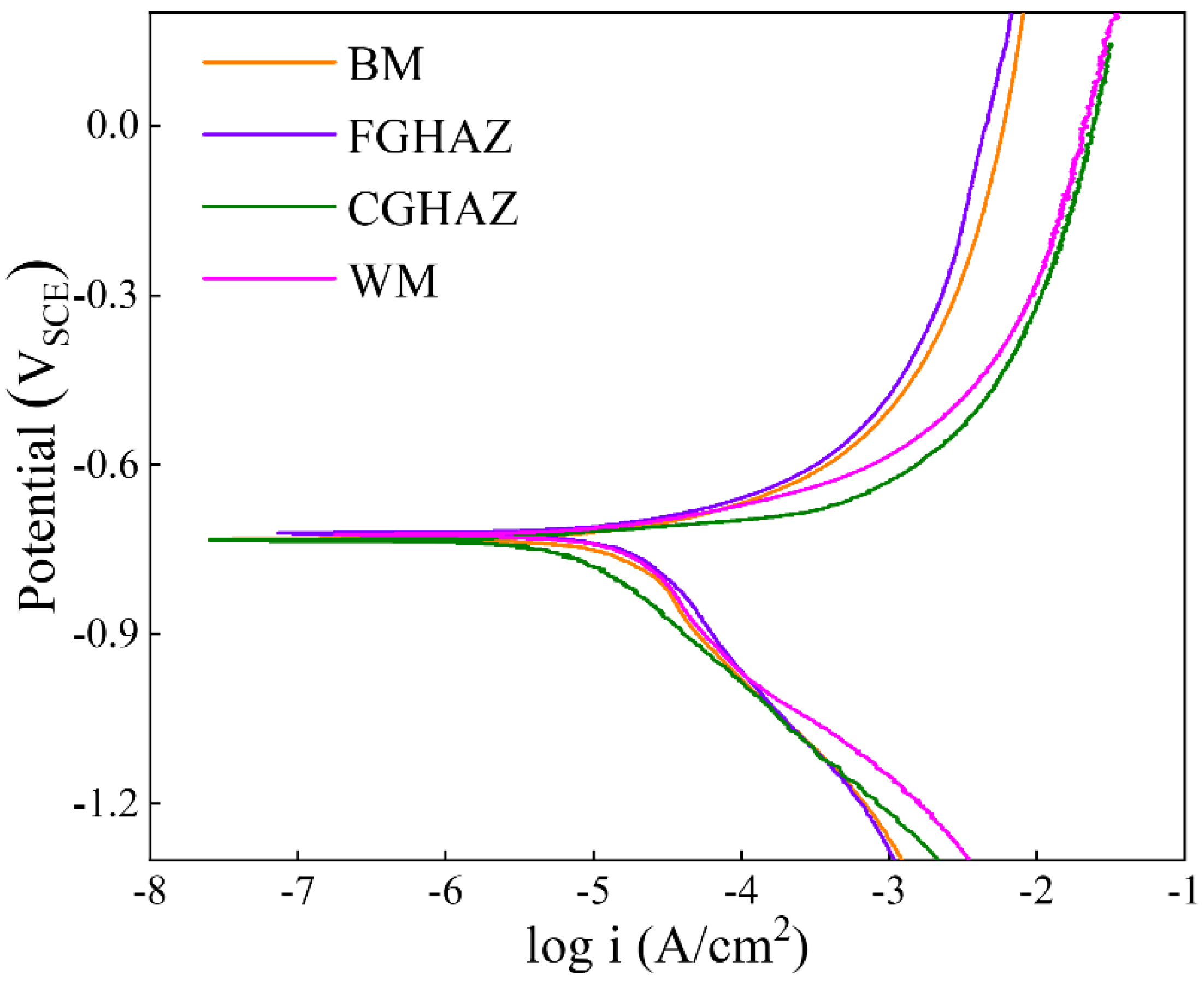
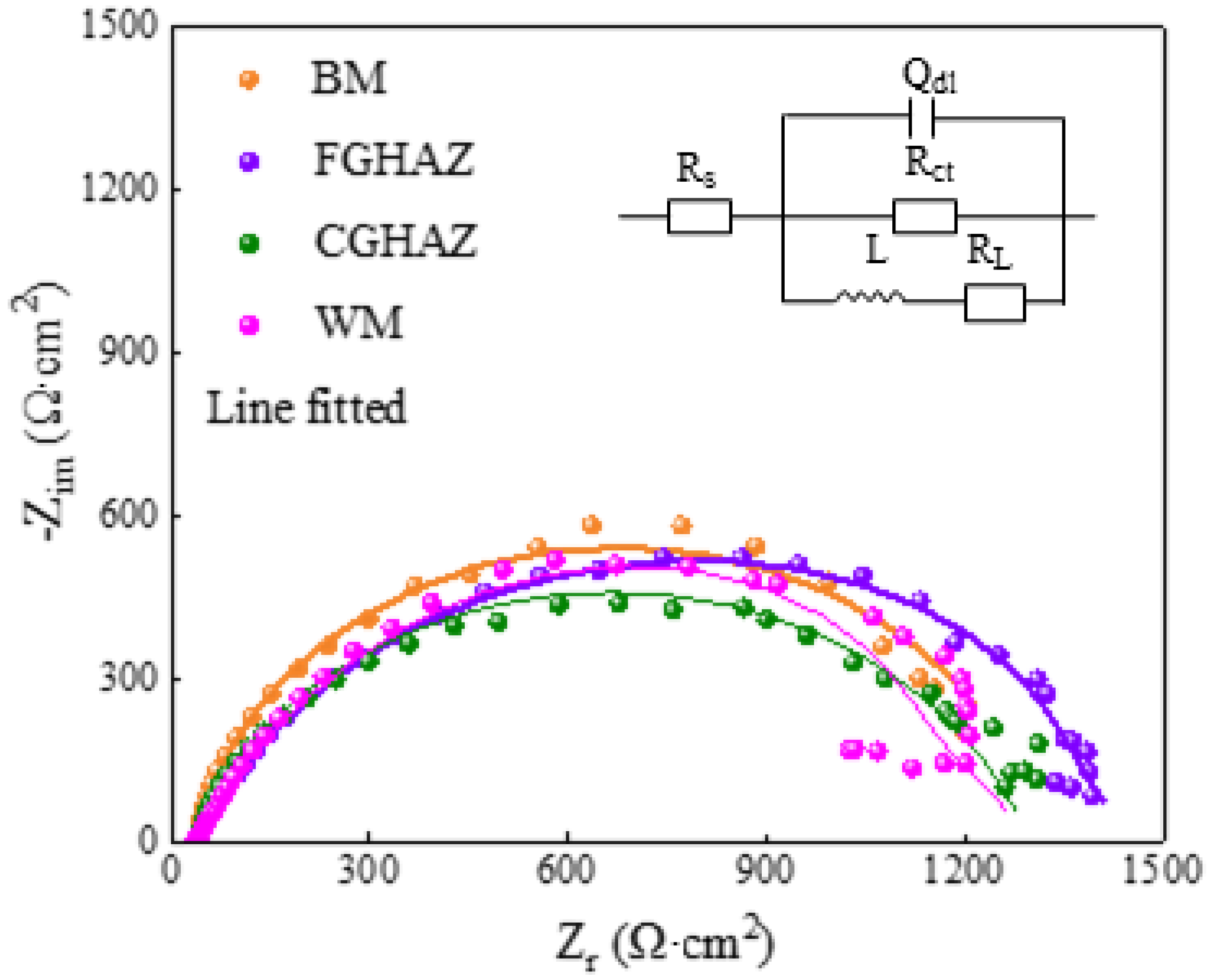
3.4. Electrochemical Behavior
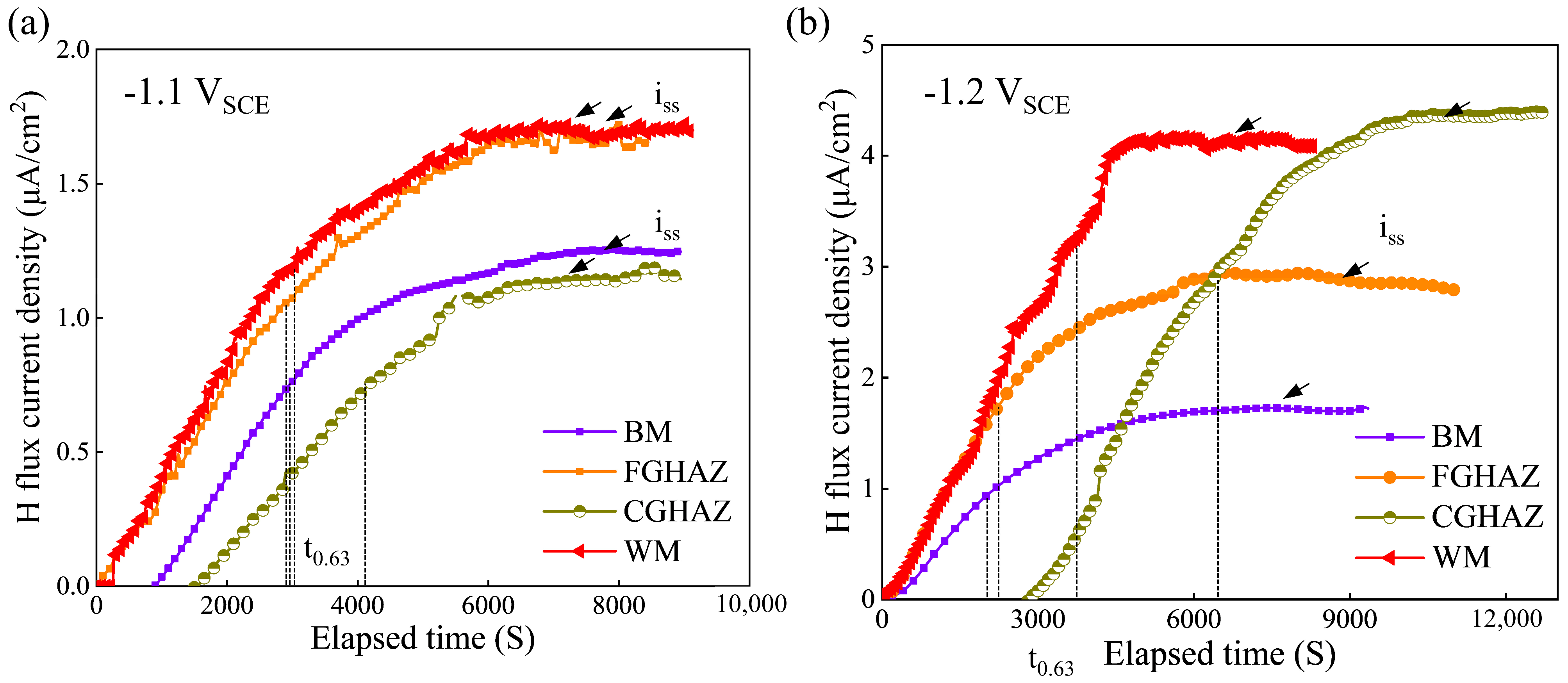
3.5. Hydrogen Permeation Behavior
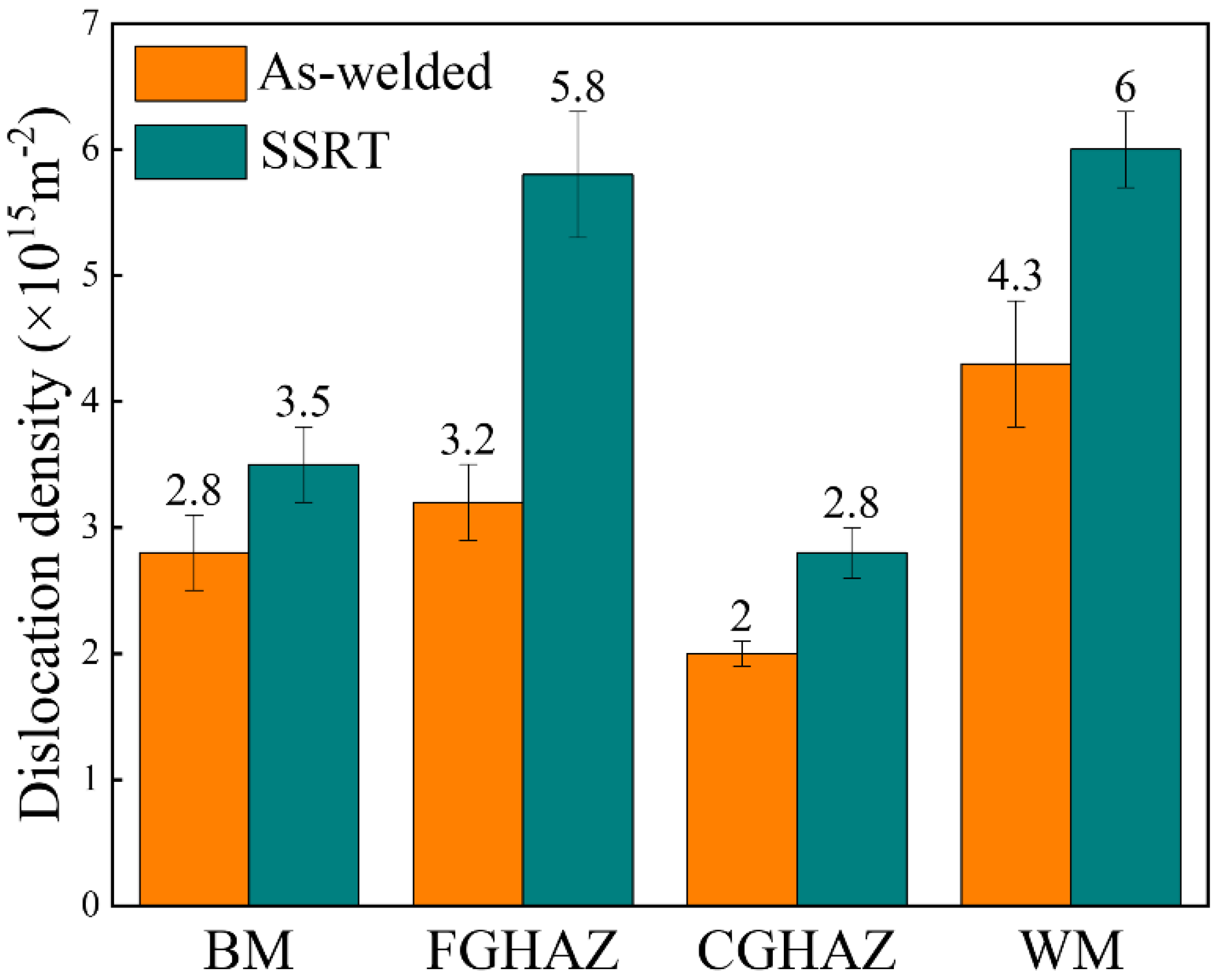
3.6. Analysis of Dislocation in Each Sub-Zone of X80 Steel Welded Joint
4. Discussion
4.1. Effect of Microstructure on Corrosion Properties of Sub-Zones within the X80 Steel Welded Joint
4.2. Effect of Microstructure on Hydrogen Permeation of Sub-Zones of the X80 Steel Welded Joint
4.3. The SCC Behavior and Mechanism of the Sub-Zones within the X80 Steel Welded Joint
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, M., Jr. Stress Corrosion Cracking Studies; Integrity Management Program DTRS56-02-D-70036; Department of Transportation, Office and Pipeline Safety: Washington, DC, USA, 2004. [Google Scholar]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization of corrosion of welded X70 pipeline steel in near-neutral pH solution. Corros. Sci. 2009, 51, 1714–1724. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Xu, K.; Xu, P.F.; Wang, B. CO2 corrosion behavior of simulated heat-affected zones for X80 pipeline steel. Mater. Charact. 2021, 171, 110772. [Google Scholar] [CrossRef]
- McMurtrey, M.D.; Cui, B.; Robertson, I.; Farkas, D.; Was, G.S. Mechanism of dislocation channel-induced irradiation assisted stress corrosion crack initiation in austenitic stainless steel. Curr. Opin. Solid State Mater. Sci. 2015, 19, 305–314. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The detrimental effect of hydrogen at dislocations on the hydrogen embrittlement susceptibility of Fe-C-X alloys: An experimental proof of the HELP mechanism. Int. J. Hydrogen. Energ. 2018, 43, 3050–3061. [Google Scholar] [CrossRef]
- Li, X.D.; Liu, J.H.; Sun, J.B.; Lin, X.Q.; Li, C.Y.; Cao, N. Effect of microstructural aspects in the heat-affected zone of high strength pipeline steels on the stress corrosion cracking mechanism: Part I. In acidic soil environment. Corros. Sci. 2019, 160, 108167. [Google Scholar] [CrossRef]
- Longfei, S.; Zhiyong, L.; Xiaogang, L.; Cuiwei, D. Stress Corrosion Cracking of Simulated Weld Heat-Affected Zone on X100 Pipeline Steel in Carbonate/Bicarbonate Solution. J. Mater. Eng. Perform. 2020, 29, 2574–2585. [Google Scholar] [CrossRef]
- Jiang, Y.B.; Li, C.N.; Wang, D.P.; Di, X.J. Effect of cyclic plastic deformation on hydrogen diffusion behavior and embrittlement susceptibility of reeling-pipeline steel weldments. Int. J. Hydrog. Energy 2021, 46, 30158–30172. [Google Scholar] [CrossRef]
- Lan, L.; Kong, X.; Hu, Z.; Qiu, C.; Zhao, D.; Du, L. Hydrogen permeation behavior in relation to microstructural evolution of low carbon bainitic steel weldments. Corros. Sci. 2016, 112, 180–193. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, Y.; Matsuda, K.; Zou, Z. Corrosion behavior of reheated CGHAZ of X80 pipeline steel in H2S-containing environments. Mater. Des. 2016, 99, 44–56. [Google Scholar] [CrossRef]
- Azevedo, C.R.F. Failure analysis of a crude oil pipeline. Eng. Fail. Anal. 2007, 14, 978–994. [Google Scholar] [CrossRef]
- Zhu, L.K.; Yan, Y.; Li, J.X.; Qiao, L.J.; Volinsky, A.A. Stress corrosion cracking under low stress: Continuous or discontinuous cracks? Corros. Sci. 2014, 80, 350–358. [Google Scholar] [CrossRef]
- Javidi, M.; Bahalaou Horeh, S. Investigating the mechanism of stress corrosion cracking in near-neutral and high pH environments for API 5L X52 steel. Corros. Sci. 2014, 80, 213–220. [Google Scholar] [CrossRef]
- Chu, R.; Chen, W.; Wang, S.H.; King, F.; Jack, T.R.; Fessler, R.R. Microstructure dependence of stress corrosion cracking initiation in X-65 pipeline steel exposed to a near-neutral pH soil environment. Corrosion 2004, 60, 275–283. [Google Scholar] [CrossRef]
- Eslami, A.; Fang, B.; Kania, R.; Worthingham, B.; Been, J.; Eadie, R.; Chen, W. Stress corrosion cracking initiation under the disbonded coating of pipeline steel in near-neutral pH environment. Corros. Sci. 2010, 52, 3750–3756. [Google Scholar] [CrossRef]
- Contreras, A.; Hernández, S.L.; Orozco-Cruz, R.; Galvan-Martínez, R. Mechanical and environmental effects on stress corrosion cracking of low carbon pipeline steel in a soil solution. Mater. Des. 2012, 35, 281–289. [Google Scholar] [CrossRef]
- Tang, X.; Cheng, Y.F. Quantitative characterization by micro-electrochemical measurements of the synergism of hydrogen, stress and dissolution on near-neutral pH stress corrosion cracking of pipelines. Corros. Sci. 2011, 53, 2927–2933. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Li, T.; Zhao, Y.; Deng, Q.; Wang, Y.; Jiang, W. Comparison of hydrogen embrittlement susceptibility of three cathodic protected subsea pipeline steels from a point of view of hydrogen permeation. Corros. Sci. 2018, 131, 104–115. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; He, Y.; Wang, Y.; Zheng, W. Electrochemical potential dependence of SCC initiation in X60 pipeline steel in near-neutral pH environment. J. Mater. Res. Technol. 2023, 27, 4950–4961. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Szpunar, J.A.; Razavi-Tousi, S.S. A comparative study of hydrogen induced cracking behavior in API 5L X60 and X70 pipeline steels. Eng. Fail. Anal. 2013, 33, 163–175. [Google Scholar] [CrossRef]
- Ramunni, V.P.; Coelho, T.D.P.; Miranda, P.E.V.D. Interaction of hydrogen with the microstructure of low-carbon steel. Mater. Sci. Eng. A 2006, 435, 504–514. [Google Scholar] [CrossRef]
- Chakraborty, G.; Rejeesh, R.; Albert, S.K. Study on hydrogen assisted cracking susceptibility of HSLA steel by implant test. Def. Technol. 2016, 12, 490–495. [Google Scholar] [CrossRef]
- Park, G.T.; Koh, S.U.; Jung, H.G.; Kim, K.Y. Effect of microstructure on the hydrogen trapping efficiency and hydrogen induced cracking of linepipe steel. Corros. Sci. 2008, 50, 1865–1871. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, G.; Zhao, W.L.; Zhang, B.; Rao, W.F. Microstructure and Corrosion Resistance to H2S in the Welded Joints of X80 Pipeline Steel. Metals 2019, 9, 1325. [Google Scholar] [CrossRef]
- Cao, X.; Wu, T.; Liu, M.; Wang, B.; Zhang, M.; Li, C.; Yin, F. Corrosion behavior of the weld joint of X80 pipeline steel in an acidic red soil. Mater. Res. Express 2020, 7, 036527. [Google Scholar] [CrossRef]
- Xu, L.Y.; Cheng, Y.F. Corrosion of X100 pipeline steel under plastic strain in a neutral pH bicarbonate solution. Corros. Sci. 2012, 64, 145–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, F.; Lu, S.; Huang, C.; Liu, J. Effect of Micro-phase Electrochemical Activity on the Initial Corrosion Dynamics of Weathering Steel. Mater. Chem. Phys. 2019, 241, 122045. [Google Scholar] [CrossRef]
- Xue, H.B.; Cheng, Y.F. Hydrogen Permeation and Electrochemical Corrosion Behavior of the X80 Pipeline Steel Weld. J. Mater. Eng. Perform. 2012, 22, 170–175. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, T.; Zhao, Y.; Sun, J.; Wang, Y. Hydrogen permeation and embrittlement susceptibility of X80 welded joint under high-pressure coal gas environment. Corros. Sci. 2016, 111, 84–97. [Google Scholar] [CrossRef]
- Han, Y.D.; Jing, H.; Xu, L.Y. Welding heat input effect on the hydrogen permeation in the X80 steel welded joints. Mater. Chem. Phys. 2012, 132, 216–222. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Zhao, H.M.; Wang, L.; Jin, X. Hydrogen trapping sites and hydrogen-induced cracking in high strength quenching & partitioning (Q&P) treated steel. Int. J. Hydrogen Energy 2014, 39, 13031–13040. [Google Scholar]
- Park, C.; Kang, N.; Liu, S.; Lee, J.S.; Chun, E.J.; Yoo, S.J. Effect of Prestrain on Hydrogen Embrittlement Susceptibility of EH 36 Steels Using In Situ Slow-Strain-Rate Testing. Met. Mater. Int. 2018, 25, 584–593. [Google Scholar] [CrossRef]
- Wang, S.H.; Luu, W.C.; Ho, K.F.; Wu, J.K. Hydrogen permeation in a submerged arc weldment of TMCP steel. Mater. Chem. Phys. 2003, 77, 447–454. [Google Scholar] [CrossRef]
- Song, L.F.; Liu, Z.Y.; Li, X.G.; Guo, X.P.; Zhang, Y.X.; Wu, W. Influence of Microstructure on Stress Corrosion Cracking of X100 Pipeline Steel in Carbonate/Bicarbonate Solution. J. Mater. Res. Technol. 2022, 17, 150–165. [Google Scholar]
- Lu, B.T.; Luo, J.L.; Norton, P.R. Environmentally assisted cracking mechanism of pipeline steel in near-neutral pH groundwater. Corros. Sci. 2010, 52, 1787–1795. [Google Scholar] [CrossRef]
- Li, X.; Shang, C.; Ma, X.; Gault, B.; Subramanian, S.V.; Sun, J.; Misra, R.D.K. Elemental distribution in the martensite–austenite constituent in intercritically reheated coarse-grained heat-affected zone of a high-strength pipeline steel. Scr. Mater. 2017, 139, 67–70. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Guo, H.; Shang, C.; Misra, R. Isolating contribution of individual phases during deformation of high strength–high toughness multi-phase pipeline steel. Mater. Sci. Eng. A 2015, 639, 131–135. [Google Scholar] [CrossRef]
- Davis, C.L.; King, J.E. Cleavage initiation in the intercritically reheated coarse-grained heat-affected zone: Part I. Fractographic evidence. Metall. Mater. Trans. A 1994, 25, 563–573. [Google Scholar] [CrossRef]

| Material | Chemical Composition (wt. %) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | P | S | Mo | Ni | Cr | Cu | Nb | Al | Fe | |
| X80 | 0.078 | 1.60 | 0.30 | 0.009 | 0.007 | 0.11 | 0.084 | 0.18 | 0.15 | 0.025 | 0.024 | bal. |
| Zone | Ecorr (mVSCE) | Icorr (uA/cm2) | Beta A (mVSCE/dec) | Beta C (mVSCE/dec) |
|---|---|---|---|---|
| BM | −739.25 | 11.87 | 74.28 | 175.05 |
| FGHAZ | −733.80 | 7.73 | 60.16 | 177.424 |
| CGHAZ | −746.12 | 15.37 | 80.03 | 155.44 |
| WM | −745.15 | 13.55 | 86.40 | 175.53 |
| Zone | Rs (Ω·cm2) | CPEdl (μF·cm2·Sn−1) | n | Rct (Ω·cm2) | RL (Ω·cm2) | L (H·cm2) |
|---|---|---|---|---|---|---|
| BM | 37.18 | 0.00037 | 0.750 | 1576 | 2741 | 1.374 × 104 |
| FGHAZ | 36.09 | 0.00034 | 0.739 | 1865 | 2035 | 389.9 |
| CGHAZ | 38.26 | 0.00030 | 0.827 | 1237 | 6258 | 0.01074 |
| WM | 37.67 | 0.00043 | 0.897 | 1275 | 1242 | 0.01177 |
| Potential (VSCE) | Zone | iss (μA/cm2) | tlag (s) | Deff (×10−6 cm2/s) | C0 (ppm) |
|---|---|---|---|---|---|
| −1.1 | BM | 1.24 | 3062 | 3.38 | 0.14 |
| FGHAZ | 1.66 | 3020 | 3.36 | 0.52 | |
| CGHAZ | 1.13 | 4089 | 9.10 | 0.145 | |
| WM | 2.65 | 3110 | 5.4 | 0.36 | |
| −1.2 | BM | 1.86 | 2130 | 3.38 | 0.63 |
| FGHAZ | 2.85 | 2310 | 2.50 | 1.13 | |
| CGHAZ | 4.33 | 6451 | 6.48 | 0.58 | |
| WM | 3.11 | 4020 | 4.40 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; He, Y.; Zheng, W. Stress-Corrosion-Cracking Sensitivity of the Sub-Zones in X80 Steel Welded Joints at Different Potentials. Materials 2024, 17, 3481. https://doi.org/10.3390/ma17143481
Zhang C, He Y, Zheng W. Stress-Corrosion-Cracking Sensitivity of the Sub-Zones in X80 Steel Welded Joints at Different Potentials. Materials. 2024; 17(14):3481. https://doi.org/10.3390/ma17143481
Chicago/Turabian StyleZhang, Ci, Yinsheng He, and Wenyue Zheng. 2024. "Stress-Corrosion-Cracking Sensitivity of the Sub-Zones in X80 Steel Welded Joints at Different Potentials" Materials 17, no. 14: 3481. https://doi.org/10.3390/ma17143481
APA StyleZhang, C., He, Y., & Zheng, W. (2024). Stress-Corrosion-Cracking Sensitivity of the Sub-Zones in X80 Steel Welded Joints at Different Potentials. Materials, 17(14), 3481. https://doi.org/10.3390/ma17143481








