Duplex Fluorinated and Atomic Layer Deposition-Derived ZrO2 Coatings Improve the Corrosion Resistance and Mechanical Properties of Mg-2Zn-0.46Y-0.5Nd (wt.%) Alloy Plates and Screws
Abstract
:1. Introduction
2. Materials and Methods
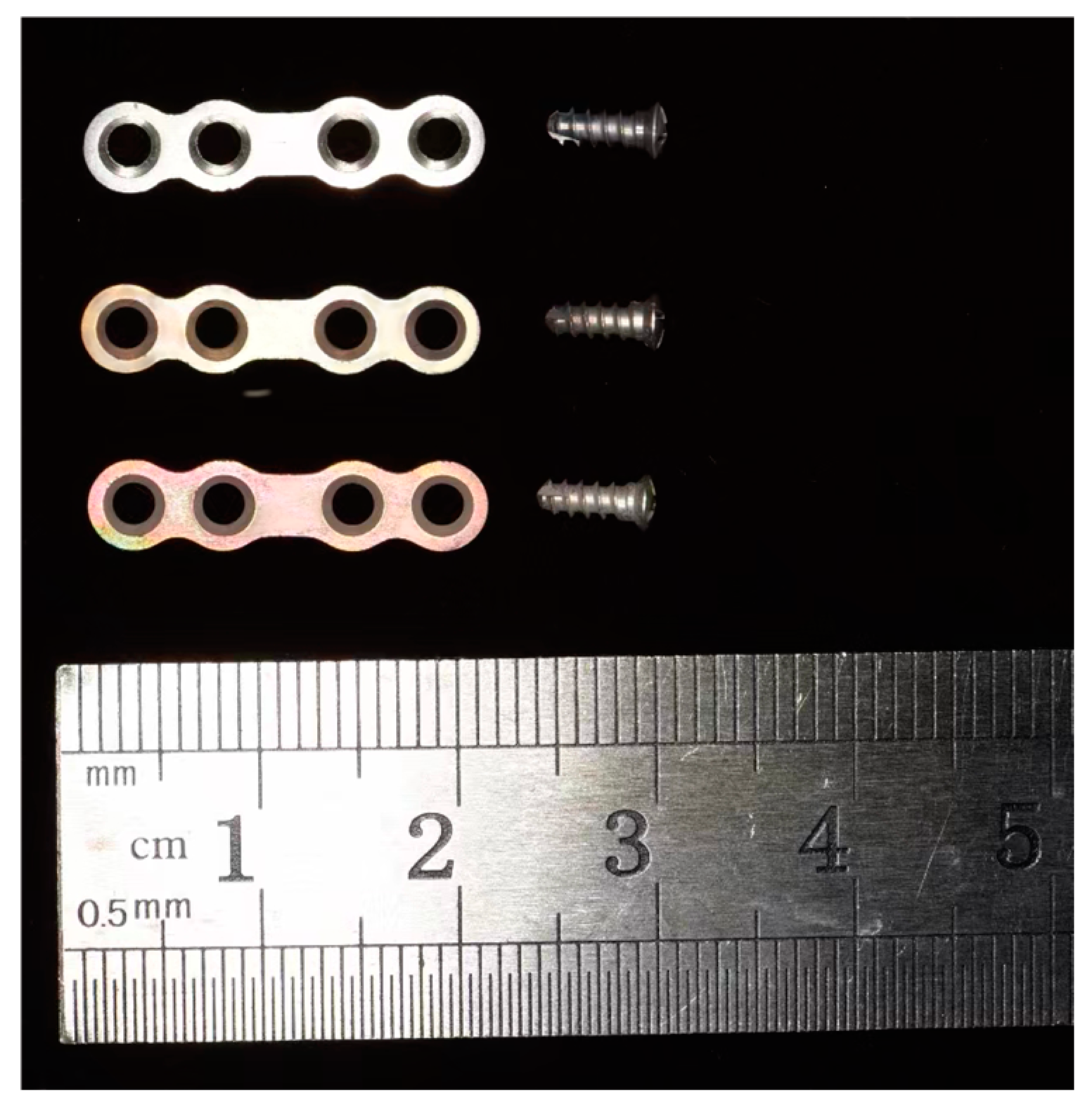
2.1. Specimen Preparation
2.2. Microstructure and Surface Characterization
2.3. In Vitro Experiment
2.4. Micro-Computed Tomography Analysis
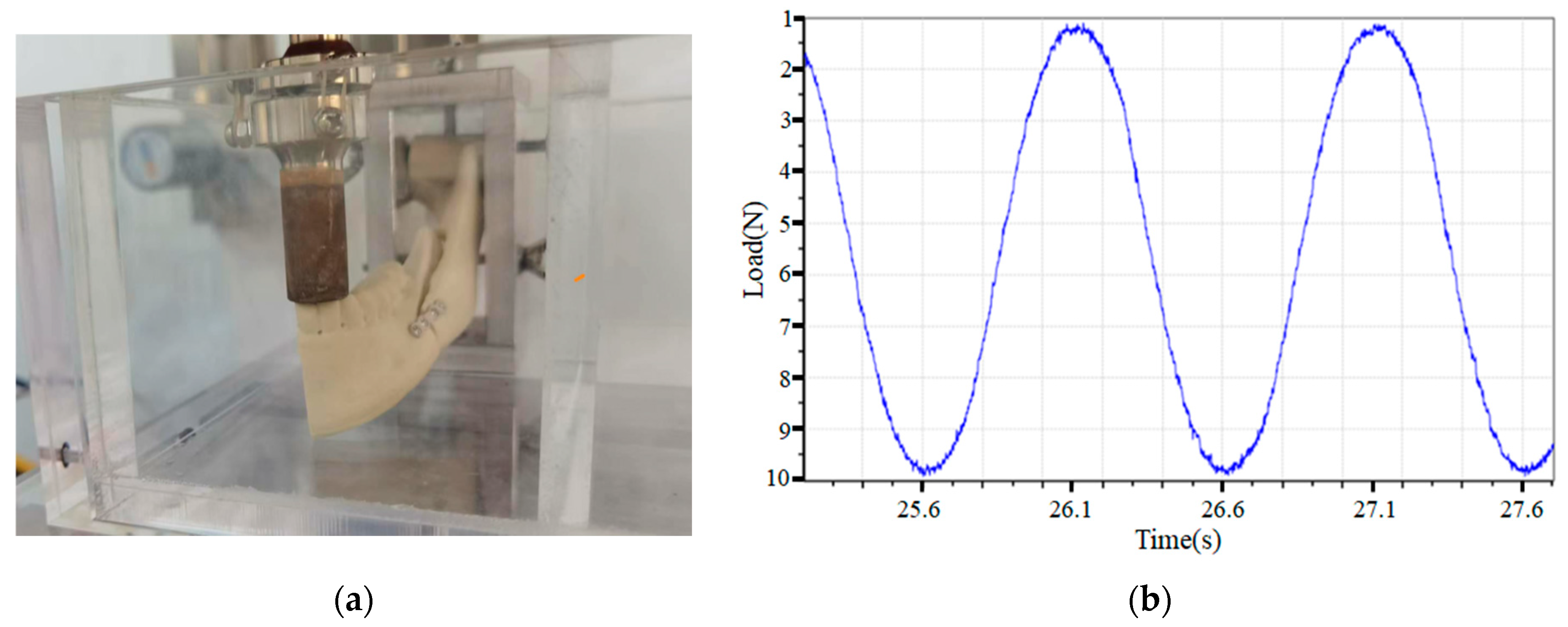
2.5. Loading Test
2.6. Statistical Analysis
3. Results
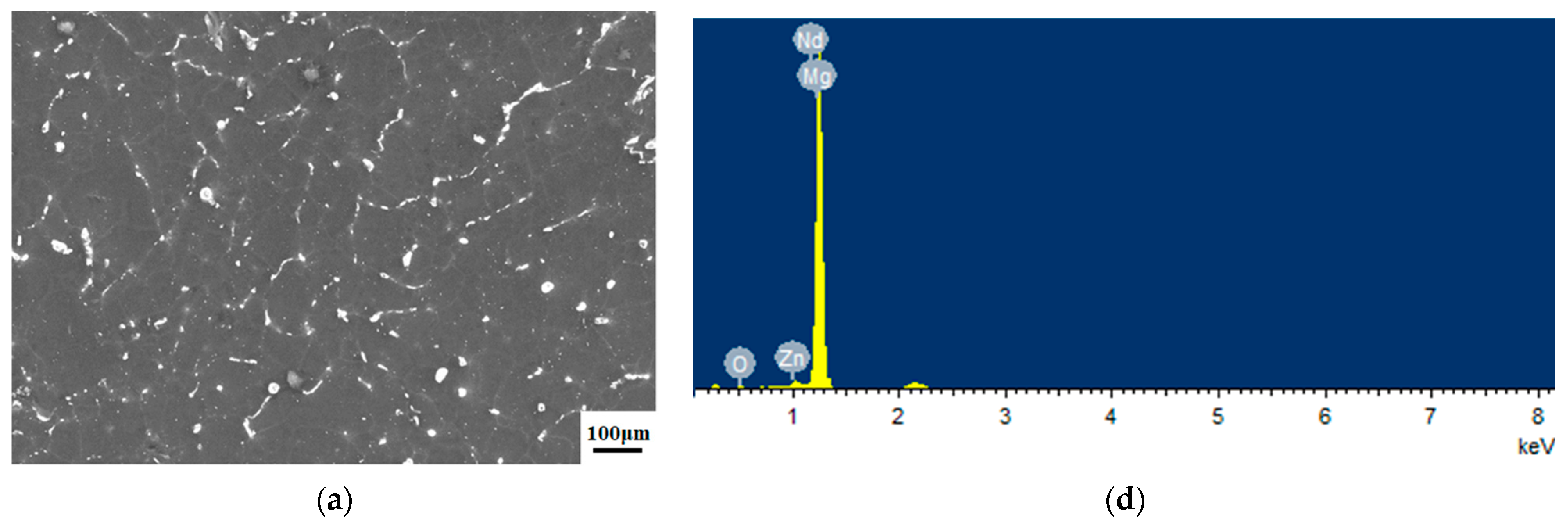
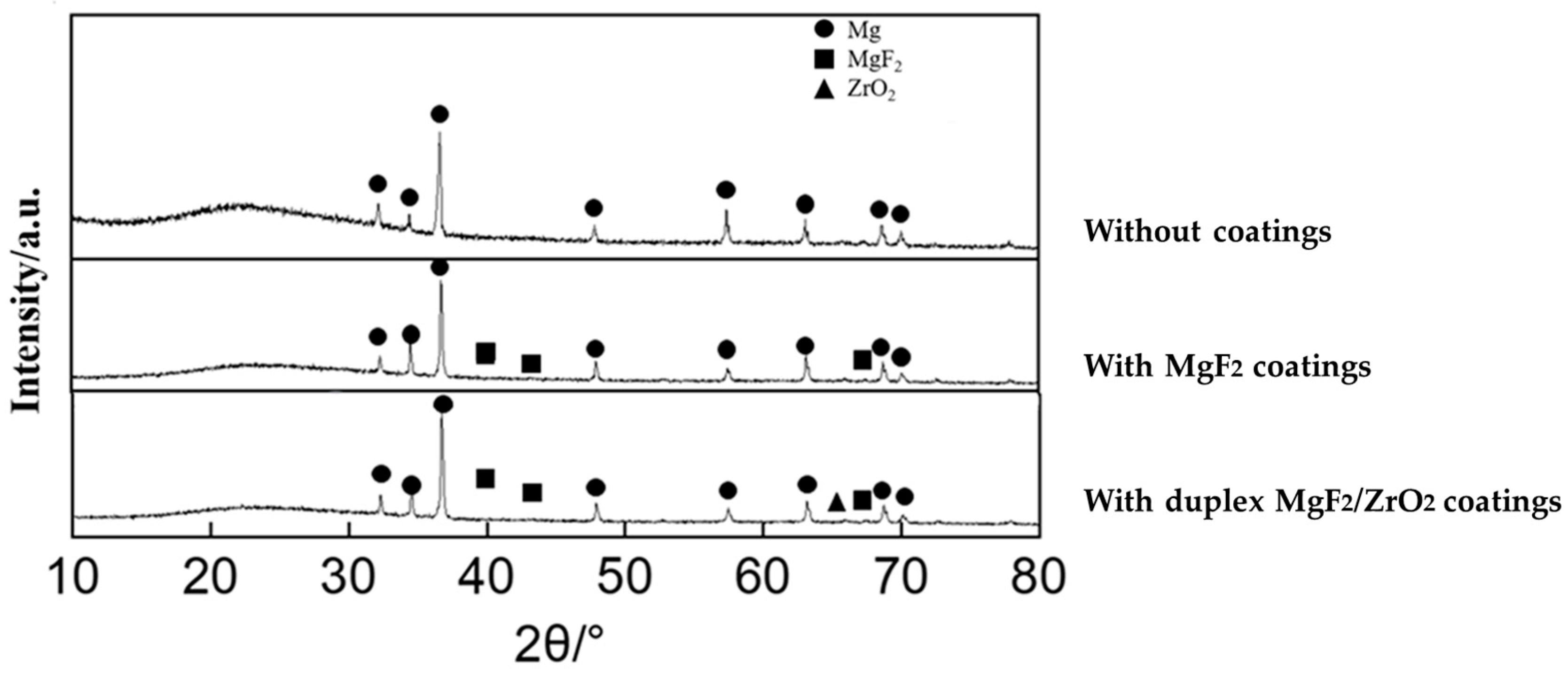
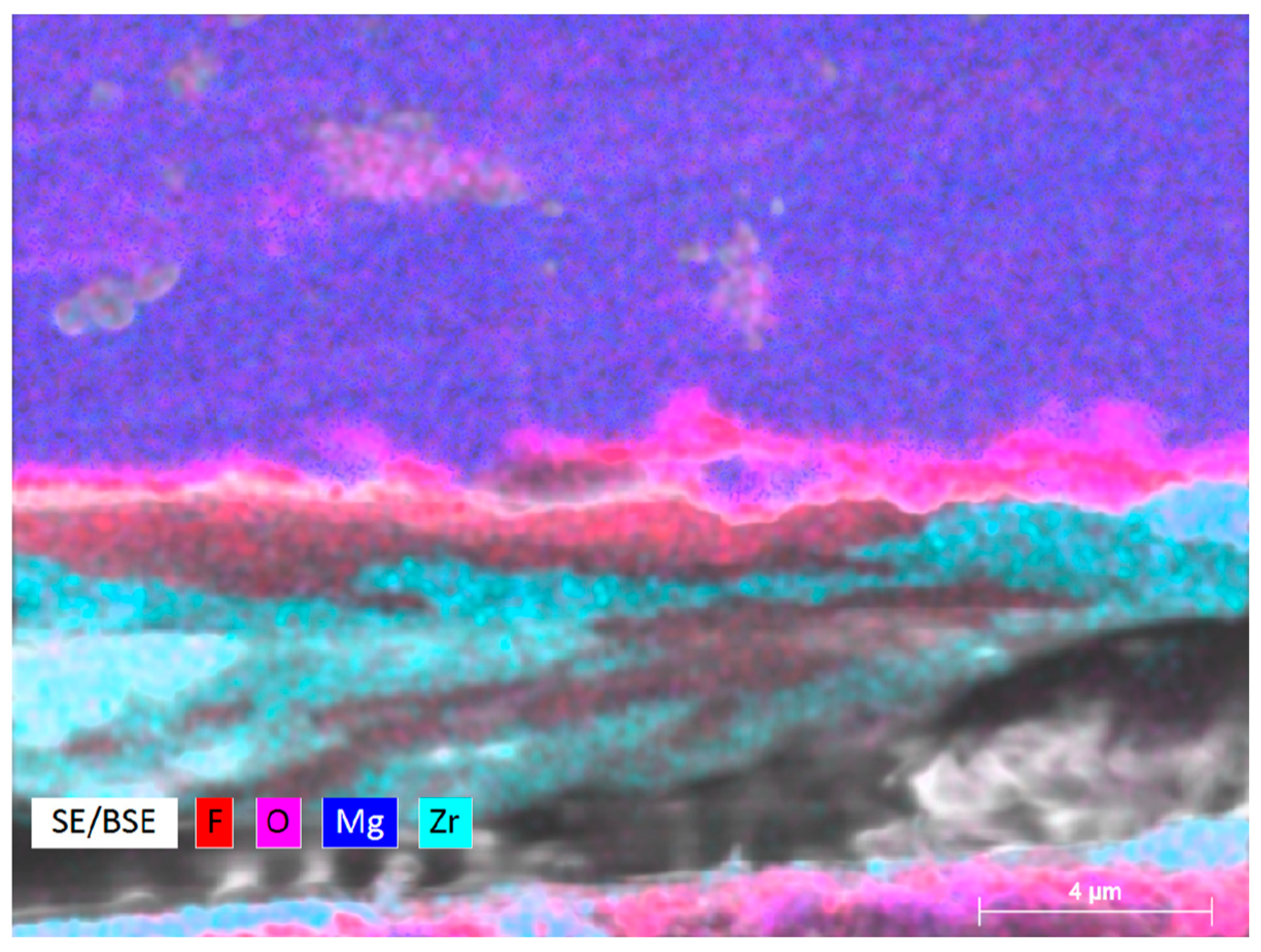
3.1. Microstructure of the Samples
3.2. Degradation of the Plate–Screw System
3.3. Loading Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, E., 3rd. Rigid skeletal fixation of fractures. J. Oral Maxillofac. Surg. 1993, 51, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, S.; Schon, R.; Otten, J.E.; Schmelzeisen, R.; Gutwald, R. The development of plate osteosynthesis for the treatment of fractures of the mandibular body—A literature review. J. Cranio-Maxillofac. Surg. 2008, 36, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Telha, W.; Wu, Y.; Abotaleb, B.; Jiang, N.; Zhu, S. Evaluation of the Properties of 3D-Printed Ti Alloy Plates: In Vivo and In Vitro Comparative Experimental Study. J. Clin. Med. 2023, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable Osteofixation Materials for Maxillofacial Bone Surgery: A Review on Polymers and Magnesium-Based Materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Kennady, M.C.; Tucker, M.R.; Lester, G.E.; Buckley, M.J. Histomorphometric evaluation of stress shielding in mandibular continuity defects treated with rigid fixation plates and bone grafts. Int. J. Oral Maxillofac. Surg. 1989, 18, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Landes, C.A.; Kriener, S. Resorbable plate osteosynthesis of sagittal split osteotomies with major bone movement. Plast. Reconstr. Surg. 2003, 111, 1828–1840. [Google Scholar] [CrossRef] [PubMed]
- Henslee, A.M.; Yoon, D.M.; Lu, B.Y.; Yu, J.; Arango, A.A.; Marruffo, L.P.; Seng, L.; Anver, T.D.; Ather, H.; Nair, M.B.; et al. Characterization of an injectable, degradable polymer for mechanical stabilization of mandibular fractures. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 529–538. [Google Scholar] [CrossRef]
- Ueki, K.; Okabe, K.; Miyazaki, M.; Mukozawa, A.; Moroi, A.; Marukawa, K.; Nakagawa, K.; Yamamoto, E. Skeletal stability after mandibular setback surgery: Comparisons among unsintered hydroxyapatite/poly-L-lactic acid plate, poly-L-lactic acid plate, and titanium plate. J. Oral Maxillofac. Surg. 2011, 69, 1464–1468. [Google Scholar] [CrossRef]
- Dolanmaz, D.; Uckan, S.; Isik, K.; Saglam, H. Comparison of stability of absorbable and titanium plate and screw fixation for sagittal split ramus osteotomy. Br. J. Oral Maxillofac. Surg. 2004, 42, 127–132. [Google Scholar] [CrossRef]
- Lopez, J.; Siegel, N.; Reategui, A.; Faateh, M.; Manson, P.N.; Redett, R.J. Absorbable Fixation Devices for Pediatric Craniomaxillofacial Trauma: A Systematic Review of the Literature. Plast. Reconstr. Surg. 2019, 144, 685–692. [Google Scholar] [CrossRef]
- Turvey, T.A.; Proffit, W.P.; Phillips, C. Biodegradable fixation for craniomaxillofacial surgery: A 10-year experience involving 761 operations and 745 patients. Int. J. Oral Maxillofac. Surg. 2011, 40, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, P.; Yao, J.; Li, M.; Wang, B.; Han, L.; Huang, Z.; Guo, C.; Bai, J.; Xue, F.; et al. pH/NIR-responsive and self-healing coatings with bacteria killing, osteogenesis, and angiogenesis performances on magnesium alloy. Biomaterials 2023, 301, 122237. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Jun, I.; Seok, H.K.; Lee, K.S.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.C.; Glyn-Jones, S.; Edwards, J.R. Biodegradable Magnesium Alloys Promote Angio-Osteogenesis to Enhance Bone Repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.; Qin, L. Biodegradable Magnesium-Based Implants in Orthopedics-A General Review and Perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Haslhofer, D.J.; Gotterbarm, T.; Klasan, A. High Complication Rate and High Percentage of Regressing Radiolucency in Magnesium Screw Fixation in 18 Consecutive Patients. J. Pers. Med. 2023, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef]
- Rendenbach, C.; Fischer, H.; Kopp, A.; Schmidt-Bleek, K.; Kreiker, H.; Stumpp, S.; Thiele, M.; Duda, G.; Hanken, H.; Beck-Broichsitter, B.; et al. Improved in vivo osseointegration and degradation behavior of PEO surface-modified WE43 magnesium plates and screws after 6 and 12 months. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112380. [Google Scholar] [CrossRef]
- Naujokat, H.; Ruff, C.B.; Kluter, T.; Seitz, J.M.; Acil, Y.; Wiltfang, J. Influence of surface modifications on the degradation of standard-sized magnesium plates and healing of mandibular osteotomies in miniature pigs. Int. J. Oral Maxillofac. Surg. 2020, 49, 272–283. [Google Scholar] [CrossRef]
- Hol, P.J.; Molster, A.; Gjerdet, N.R. Should the galvanic combination of titanium and stainless steel surgical implants be avoided? Injury 2008, 39, 161–169. [Google Scholar] [CrossRef]
- Byun, S.H.; Lim, H.K.; Cheon, K.H.; Lee, S.M.; Kim, H.E.; Lee, J.H. Biodegradable magnesium alloy (WE43) in bone-fixation plate and screw. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dou, J.; Yu, H.; Chen, C. Degradable magnesium-based alloys for biomedical applications: The role of critical alloying elements. J. Biomater. Appl. 2019, 33, 1348–1372. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Han, J.; Yu, X.; Yuan, S.; Nie, Z.; Qiu, T.; Yan, Z.; Tan, C.; Guo, C. Influences of Extrusion and Silver Content on the Degradation of Mg-Ag Alloys In Vitro and In Vivo. Bioinorg. Chem. Appl. 2022, 2022, 2557518. [Google Scholar] [CrossRef] [PubMed]
- Bryla, K.; Horky, J.; Krystian, M.; Litynska-Dobrzynska, L.; Mingler, B. Microstructure, mechanical properties, and degradation of Mg-Ag alloy after equal-channel angular pressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110543. [Google Scholar] [CrossRef] [PubMed]
- Weizbauer, A.; Modrejewski, C.; Behrens, S.; Klein, H.; Helmecke, P.; Seitz, J.M.; Windhagen, H.; Mohwald, K.; Reifenrath, J.; Waizy, H. Comparative in vitro study and biomechanical testing of two different magnesium alloys. J. Biomater. Appl. 2014, 28, 1264–1273. [Google Scholar] [CrossRef]
- Kim, B.J.; Piao, Y.; Wufuer, M.; Son, W.C.; Choi, T.H. Biocompatibility and Efficiency of Biodegradable Magnesium-Based Plates and Screws in the Facial Fracture Model of Beagles. J. Oral Maxillofac. Surg. 2018, 76, 1055.e1–1055.e9. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Chen, J.; Zhan, J.; Kolawole, S.K.; Tan, L.; Yang, K.; Wang, J.; Su, X. Effects of Different Rare Earth Elements on the Degradation and Mechanical Properties of the ECAP Extruded Mg Alloys. Materials 2022, 15, 627. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Zhu, J.H.; Liu, G.Q.; Liu, Z.C.; Guo, C.B.; Cui, N.H.; Han, J.M. Feasibility and Efficacy of a Degradable Magnesium-Alloy GBR Membrane for Bone Augmentation in a Distal Bone-Defect Model in Beagle Dogs. Bioinorg. Chem. Appl. 2022, 2022, 4941635. [Google Scholar] [CrossRef]
- Makkar, P.; Kang, H.J.; Padalhin, A.R.; Park, I.; Moon, B.G.; Lee, B.T. Development and properties of duplex MgF2/PCL coatings on biodegradable magnesium alloy for biomedical applications. PLoS ONE 2018, 13, e0193927. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chang, R.; Webster, T.J. Atomic Layer Deposition Coating of TiO2 Nano-Thin Films on Magnesium-Zinc Alloys to Enhance Cytocompatibility for Bioresorbable Vascular Stents. Int. J. Nanomed. 2019, 14, 9955–9970. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Kim, S.G. In vitro biomechanical evaluation of fixation methods of sagittal split ramus osteotomy in mandibular setback. J. Cranio-Maxillofac. Surg. 2015, 43, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Sonego, C.L.; Scheffer, M.A.R.; Chagas Junior, O.L.; Vetromilla, B.M.; Fernandes, L.P.; Ozkomur, A.; Silva Junior, A.N.; Miguens Junior, S.A.Q.; Hernandez, P.A.G. In vitro study of a modified sagittal split osteotomy fixation technique of the mandible: A mechanical test. Int. J. Oral Maxillofac. Surg. 2018, 47, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Wolters, L.; Besdo, S.; Angrisani, N.; Wriggers, P.; Hering, B.; Seitz, J.M.; Reifenrath, J. Degradation behaviour of LAE442-based plate-screw-systems in an in vitro bone model. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 305–315. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Gambaro, S.; Nascimento, M.L.; Shekargoftar, M.; Ravanbakhsh, S.; Sales, V.; Paternoster, C.; Bartosch, M.; Witte, F.; Mantovani, D. Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications. Materials 2022, 15, 8245. [Google Scholar] [CrossRef]
- Lu, X.; Cai, H.; Li, Y.R.; Zheng, X.; Yun, J.; Li, W.; Geng, X.; Kwon, J.S.; Jiang, H.B. A Systematic Review and Network Meta-Analysis of Biomedical Mg Alloy and Surface Coatings in Orthopedic Application. Bioinorg. Chem. Appl. 2022, 2022, 4529520. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Vogt, C.; Vogt, J.; Donath, T.; Beckmann, F. In vivo corrosion and corrosion protection of magnesium alloy LAE442. Acta Biomater. 2010, 6, 1792–1799. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, W.; Liu, X.; Zheng, Y.; Cui, Z.; Yang, X.; Pan, H.; Wu, S. Atomic layer deposited ZrO2 nanofilm on Mg-Sr alloy for enhanced corrosion resistance and biocompatibility. Acta Biomater. 2017, 58, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Daubert, J.S.; Hill, G.T.; Gotsch, H.N.; Gremaud, A.P.; Ovental, J.S.; Williams, P.S.; Oldham, C.J.; Parsons, G.N. Corrosion Protection of Copper Using Al2O3, TiO2, ZnO, HfO2, and ZrO2 Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 4192–4201. [Google Scholar] [CrossRef] [PubMed]
- Atsu, S.S.; Aksan, M.E.; Bulut, A.C.; Tamimi, F. The effect of nanocoatings of SiO2, TiO2, and ZrO2 on titanium-porcelain bonding. J. Prosthet. Dent. 2021, 126, 222.e1–222.e8. [Google Scholar] [CrossRef] [PubMed]
- Peron, M.; Bertolini, R.; Cogo, S. On the corrosion, stress corrosion and cytocompatibility performances of ALD TiO2 and ZrO2 coated magnesium alloys. J. Mech. Behav. Biomed. Mater. 2022, 125, 104945. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, C.; Lou, T.; Chen, B.; Yi, R.; Wang, W.; Zhang, R.; Zuo, M.; Xu, H.; Han, P.; et al. Crevice corrosion—A newly observed mechanism of degradation in biomedical magnesium. Acta Biomater. 2019, 98, 152–159. [Google Scholar] [CrossRef]




| Group A | Group B | Group C | p | |
|---|---|---|---|---|
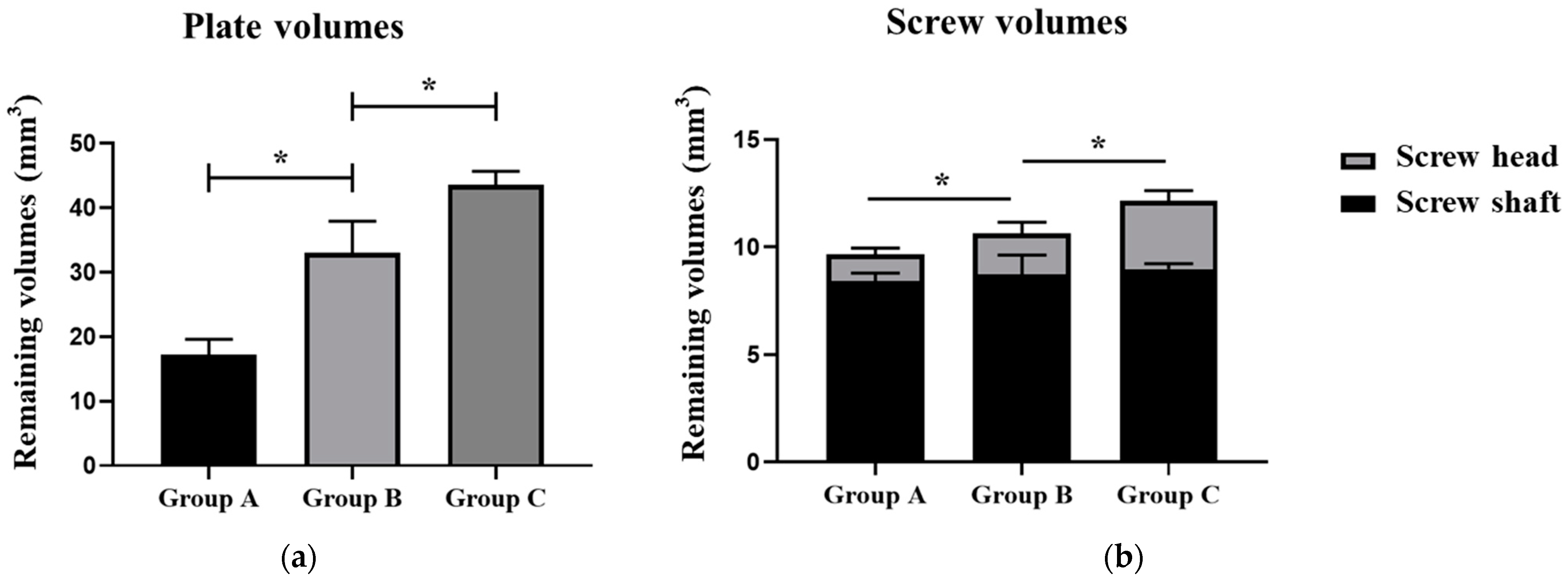
| Plate residual (mm3) | 17.18 ± 2.41 | 33.01 ± 4.94 | 43.55 ± 2.12 | <0.001 * |
| Plate residual (%) | 30.02 | 57.68 | 76.09 | |
| Screw residual (mm3) | 9.66 ± 0.41 | 10.65 ± 0.86 | 12.15 ± 0.55 | <0.001 * |
| Screw residual (%) | 73.19 | 80.69 | 92.06 | |
| Screw head residual (mm3) | 1.23 ± 0.30 | 1.91 ± 0.52 | 3.17 ± 0.48 | <0.001 * |
| Screw head residual (%) | 31.49 | 48.89 | 81.14 | |
| Screw shaft residual (mm3) | 8.43 ± 0.36 | 8.74 ± 0.89 | 8.98 ± 0.25 | 0.077 |
| Screw shaft residual (%) | 91.10 | 94.45 | 97.04 |
| Group Description | Time Point | 1 mm Displacement (N) | 3 mm Displacement (N) | 5 mm Displacement (N) | 10 mm Displacement (N) |
|---|---|---|---|---|---|
| No surface coating | T1 | 0.351 ± 0.079 | 0.898 ± 0.064 | 1.632 ± 0.141 | 3.790 ± 0.397 |
| T2 | 0.255 ± 0.051 | 0.717 ± 0.072 | 1.428 ± 0.101 | 2.837 ± 0.267 | |
| T3 | 0.134 ± 0.061 | 0.462 ± 0.030 | 0.931 ± 0.126 | 1.419 ± 0.161 | |
| Fluorinated coating | T1 | 0.555 ± 0.073 | 1.593 ± 0.262 | 2.554 ± 0.247 | 4.457 ± 0.567 |
| T2 | 0.356 ± 0.038 | 1.059 ± 0.047 | 1.977 ± 0.252 | 3.453 ± 0.573 | |
| T3 | 0.298 ± 0.045 | 0.687 ± 0.137 | 1.274 ± 0.116 | 2.085 ± 0.363 | |
| Duplex fluorinated and ALD ZrO2 coating | T1 | 0.556 ± 0.032 | 1.749 ± 0.265 | 2.764 ± 0.385 | 4.687 ± 0.617 |
| T2 | 0.514 ± 0.042 | 1.637 ± 0.221 | 2.606 ± 0.339 | 4.223 ± 0.484 | |
| T3 | 0.489 ± 0.068 | 1.351 ± 0.283 | 2.252 ± 0.389 | 3.474 ± 0.493 | |
| T4 | 0.276 ± 0.074 | 0.604 ± 0.133 | 1.377 ± 0.430 | 1.983 ± 0.372 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Yang, R.; Chen, L.; Liu, G.; Han, J.; Guo, C. Duplex Fluorinated and Atomic Layer Deposition-Derived ZrO2 Coatings Improve the Corrosion Resistance and Mechanical Properties of Mg-2Zn-0.46Y-0.5Nd (wt.%) Alloy Plates and Screws. Materials 2024, 17, 3485. https://doi.org/10.3390/ma17143485
Qiu T, Yang R, Chen L, Liu G, Han J, Guo C. Duplex Fluorinated and Atomic Layer Deposition-Derived ZrO2 Coatings Improve the Corrosion Resistance and Mechanical Properties of Mg-2Zn-0.46Y-0.5Nd (wt.%) Alloy Plates and Screws. Materials. 2024; 17(14):3485. https://doi.org/10.3390/ma17143485
Chicago/Turabian StyleQiu, Tiancheng, Rong Yang, Liangwei Chen, Guanqi Liu, Jianmin Han, and Chuanbin Guo. 2024. "Duplex Fluorinated and Atomic Layer Deposition-Derived ZrO2 Coatings Improve the Corrosion Resistance and Mechanical Properties of Mg-2Zn-0.46Y-0.5Nd (wt.%) Alloy Plates and Screws" Materials 17, no. 14: 3485. https://doi.org/10.3390/ma17143485





