Abstract
GaN is more stable than most metal oxide semiconductors for the photocatalytic degradation of organic pollutants in harsh conditions, while its catalytic efficiency has been difficult to be substantially improved. In this study, the tribocatalytic degradation of organic dyes by GaN nanoparticles has been investigated. Stimulated through magnetic stirring using homemade Teflon magnetic rotary disks in glass beakers, the GaN nanoparticles were found to induce negligible degradation in rhodamine B (RhB) and methyl orange (MO) solutions. Surprisingly, the degradation was greatly enhanced in beakers with Ti and Al2O3 coatings on their bottoms: 99.2% and 99.8% of the 20 mg/L RhB solutions were degraded in 3 h for the Ti and Al2O3 coatings, respectively, and 56% and 60.2% of the 20 mg/L MO solutions were degraded in 24 h for the Ti and Al2O3 coatings, respectively. Moreover, the MO molecules were only broken into smaller organic molecules for the Ti coating, while they were completely degraded for the Al2O3 coating. These findings are important for the catalytic degradation of organic pollutants by GaN in harsh environments and for achieving a better understanding of tribocatalysis as well.
1. Introduction
The extensive use of fossil fuels by human society has not only consumed vast amounts of natural resources but has also generated huge amounts of exhaust gases, wastewater, and solid waste [1,2]. Due to the growing shortage of fossil fuels and environmental pollution, there has been an increasing focus on clean energy technologies to collect and utilize clean energies from the natural environment [3,4,5,6,7,8], such as thermal, solar, chemical, mechanical, and biological energies [9]. Photocatalytic oxidation, using semiconductors as catalysts and light as the energy source to degrade organic compounds, is an efficient, clean, and environmentally friendly technology [10]. To date, not only have various photocatalysts with novel catalytic performances emerged [11], such as Mie-resonant cuprous oxide particles with enhanced photocatalysis [12] and with tuned catalytic activity and selectivity [13], Fe-Cd co-modified ZnO with enhanced visible light photodegradation activity [14], PDMS−TiO2−Au sponge with highly enhanced photocatalytic activity [15], and nanostructured TiO2/ZnO heterojunctions with highly enhanced ultraviolet photocatalytic activity [16], but some valuable insights into the photo-redox mechanisms have also been revealed, such as the photocarrier recombination dynamics in Cu2O nanocatalyst clusters [17] and size- and shape-dependent charge-carrier dynamics in submicron cuprous oxide nanoparticles [18]. However, photocatalysis cannot operate beyond daylight hours, which significantly limits its practical environmental applications.
Apart from solar energy, mechanical energy is also a clean and sustainable energy in nature [19]. In recent years, there have been more and more papers on harvesting mechanical energy from nature for environmental remediation [2]. In 2019, Li et al. reported the tribocatalytic degradation of organic dyes using Ba0.75Sr0.25TiO3 (BST) nanoparticles under magnetic stirring [5], in which the BST nanoparticles absorbed mechanical energy through friction and converted it into chemical energy for degrading organic dyes. This was the first time that tribocatalysis appeared in environmental remediation. Previously, tribocatalysis was mainly investigated in the context of tribochemistry [20], especially for achieving ultralow friction and wear [21,22,23]. For environmental remediation, tribocatalysis does not require light irradiation within a specific intensity and wave-length range and thus has fewer environmental limitations than photocatalysis [24]. Tribocatalysis soon attracted great attention for environmental remediation, and, in the following couple of years, many materials, such as Bi2WO6 [25], BaTiO3 [26], FeS2 [27], Si [28], Ba(Zr0.05Ti0.95)O3 [29], NiCO2O4 [30], TiO2 [31], Fe2O3 [32], ZnO [33], SrTiO3 [34], CoFe2O4 [35], and CdS [36], have all been investigated for the tribocatalytic degradation of organic pollutants.
It is worth noting that some important photocatalysts, such as TiO2 [31], ZnO [33], and SrTiO3 [34], have also been employed in studies on the tribocatalytic degradation of organic pollutants. These metal oxides are well-known for their advantages of low cost, nontoxicity, and high chemical stability, which have made them important candidates in catalytic environmental remediation. With particles stimulated through magnetic stirring at relatively low rotating speeds (mostly around 400 rpm) in water, the heating effect in these studies has been mostly avoided, and a mechanism based on the excitation of electron–hole pairs in semiconductors by the mechanical energy absorbed through friction has been proposed for tribocatalysis [5], which is quite similar to that of photocatalysis. All these facts suggest that semiconducting materials are of special importance for the tribocatalytic degradation of organic pollutants. Together with numerous semiconducting metal oxides, Si as an elementary semiconductor and CdS as a typical II–VI semiconductor have also been investigated for the tribocatalytic degradation of organic pollutants and some interesting results have been obtained for them [36]. In contrast, no III–V semiconductors have been investigated for tribocatalytic applications to date.
With a band gap of 3.4 eV, GaN is an important III–V semiconductor due to its wide band gap, good chemical and thermal stability, and excellent optical and electrical properties [37,38,39]. Its high chemical stability makes it an ideal candidate, especially for the degradation of organic pollutants under extreme pH conditions, except that its photocatalytic efficiency has been difficult to be substantially improved through doping [40]. In our previous studies, we found that coating materials on the bottoms of beakers/reactors is an effective way to boost tribocatalysis [26,28,41,42,43]. Many materials have been studied as coatings for tribocatalysis, among which Ti and Al2O3 are very outstanding not only for their high chemical stability and surprising effects on tribocatalysis but also for their friendliness to the ambient environment: Ti is widely used as artificial bones to be implanted in human bodies, and Al2O3 itself is a component in soil. In this work, we have conducted an investigation on the tribocatalytic degradation of organic dyes by GaN nanoparticles. Although the GaN nanoparticles normally showed quite unsatisfactory tribocatalytic degradation efficiency for either the rhodamine B (RhB) or methyl orange (MO) solutions, the tribocatalytic degradation efficiency was found to be greatly improved through the Ti and Al2O3 coatings on the bottoms of the beakers. Moreover, two different types of MO degradation were observed between the Al2O3 and Ti coatings. These results should be relevant for GaN to be applied in the catalytic remediation of harsh environments and for better understanding tribocatalysis as well.
2. Materials and Methods
2.1. Materials and Characterization
A commercial GaN powder purchased from Shanghai Bide Pharmatech Ltd. (Shanghai, China) was investigated in this study. A commercial cubic boron nitride purchased from Shanghai Xingtian New Material Technology Co., Ltd. (Shanghai, China) (particle size 100 nm) was used for reference. Crystal structure was characterized through X-ray diffraction (XRD) using an X-ray diffractometer (BRUKER AXS D8 ADVANCE, Ettlingen, Germany) with Cu Kα radiation, and microstructure was analyzed using a field emission scanning electron microscope (SEM, Tescan CLARA, Brno, Czech Republic).
2.2. Forming Ti and Al2O3 Coatings on the Bottoms of Glass Beakers
Flat bottomed glass beakers with a dimension of φ 45 mm × 60 mm were used in this study. Ti and Al2O3 ceramic disks of φ 40 mm × 1 mm were first pasted on the bottoms of some beakers through a kind of strong glue (Deli super glue 502) separately. In this way, three kinds of beakers with glass, Ti, and Al2O3 bottoms were separately obtained.
2.3. Dye Degradation Tests
In a typical experiment, 300 mg of GaN powder were added into a glass beaker placed with a homemade Teflon magnetic rotary disk [26], followed by the addition of 30 mL of the 20 mg/L MO or 20 mg/L RhB solution. The suspension was then magnetically stirred at a speed of 400 rpm in dark, and the room temperature was kept at 25 °C. To monitor the degradation process, 3 mL of the suspension was sampled at fixed intervals, followed by centrifugation to collect the supernatant. The absorbance spectra were obtained using a UV–visible spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan).
2.4. Detection of Active Species
For the detection of superoxide (·) radicals, 10 mL of methanol (CH3OH), 0.15 g of GaN powder, and 0.05 mL of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were added into three glass beakers (φ 45 × 60 mm) with glass, Ti, and Al2O3 bottoms separately. For the detection of hydroxyl (·OH) radicals, 10 mL of deionized water, 0.15 g of GaN powder, and 0.05 mL of 5,5-dimethyl-1-pyrroline N-oxide (DMPO) were added into three glass beakers (φ 45 × 60 mm) with glass, Ti, and Al2O3 bottoms separately. Subsequently, the beakers were placed in dark at room temperature and stirred magnetically at 400 rpm using a homemade Teflon magnetic rotary disk for 15 min. The resulting suspensions were analyzed using an electron paramagnetic resonance (EPR) spectrometer (Bruker EPR A300-10/12) to identify the presence of superoxide (·) and hydroxyl (·OH) radicals.
3. Results and Discussion
3.1. Materials’ Information
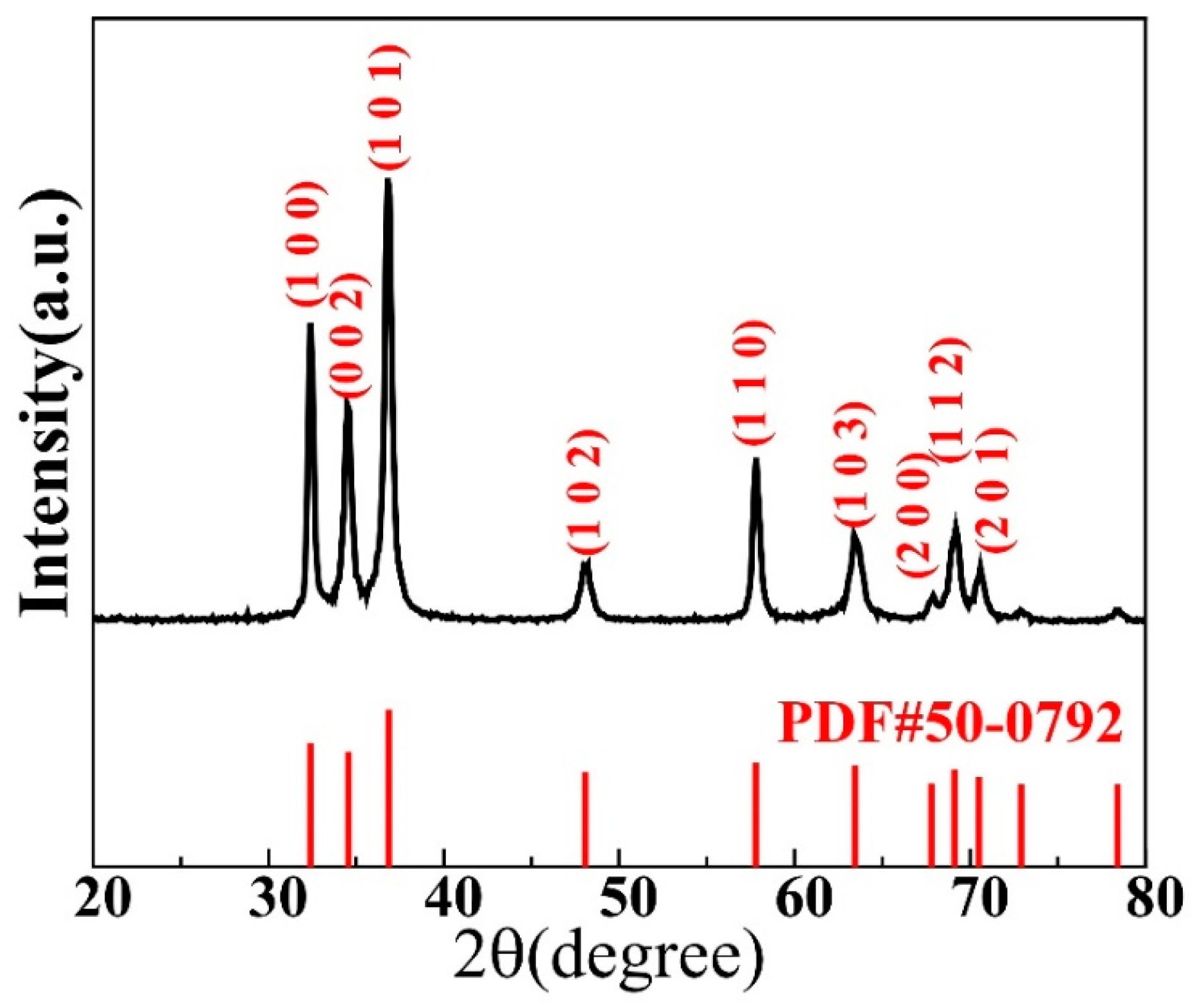
Figure 1 displays a typical XRD pattern obtained for the GaN powder used in this study. Most of the main peaks are sharp and of high intensity, indicating a high crystallinity. The peak positions in the graph match well with those of the standard PDF#50-0792 card of wurtzite GaN, as shown in the figure. No characteristic peaks of other impurities were observed, indicating the high purity of the GaN powder.
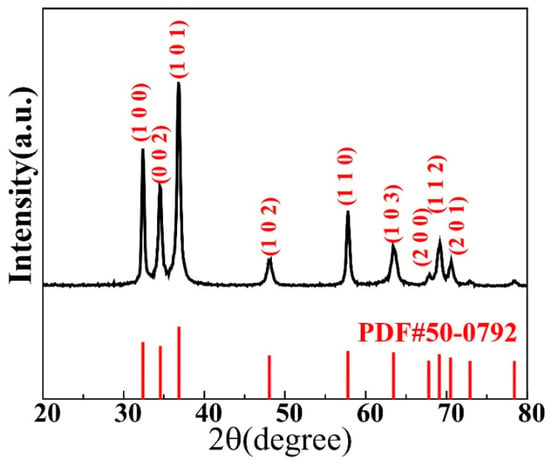
Figure 1.
X-ray diffraction pattern taken for the GaN powder investigated in this study.
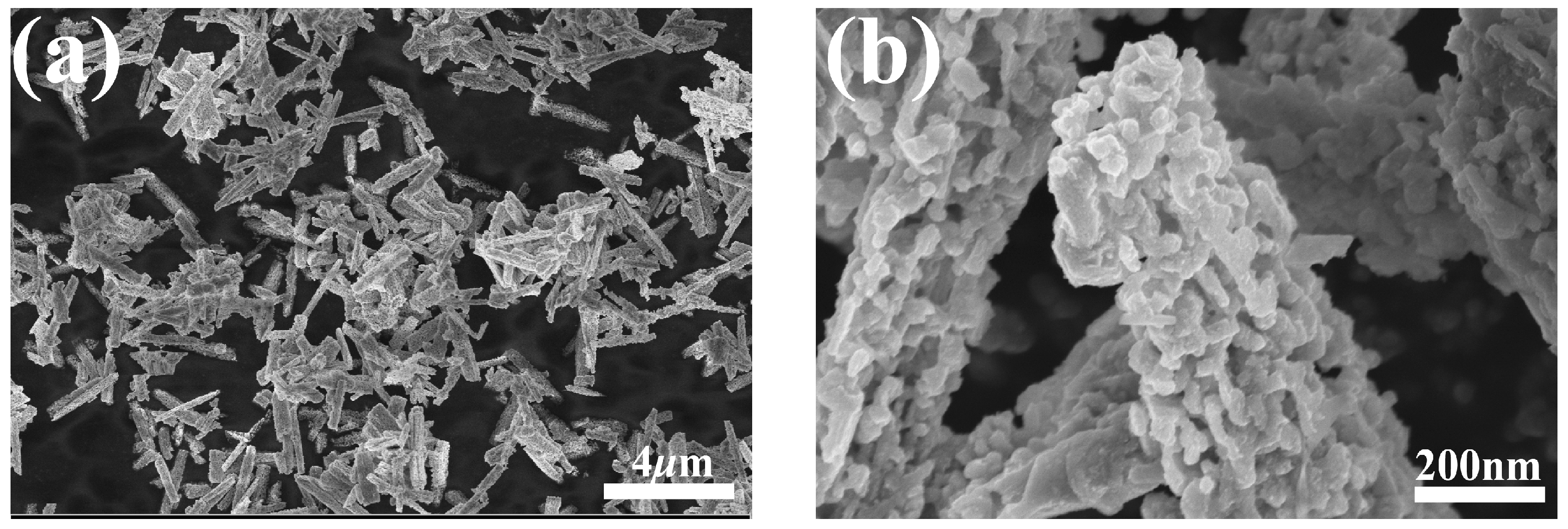
Two representative SEM images of the GaN powder are shown in Figure 2. As shown in Figure 2a, under a relatively low magnification, the GaN powder is rods a couple of micrometers long, while, under a higher magnification, it can be clearly seen that the rods are actually aggregates of numerous nanoparticles, as depicted in Figure 2b. The nanoparticles are quite irregular in shape and coalesce into micrometers-long rods with tiny voids in them.
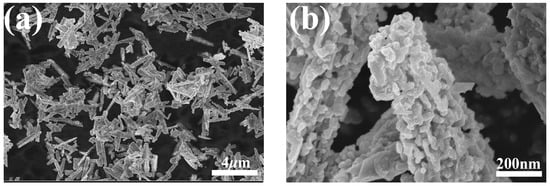
Figure 2.
SEM micrographs of the GaN powder: (a) SEM micrograph at a low magnification; (b) SEM micrograph at a higher magnification.
3.2. Tribocatalytic Degradation of RhB and MO
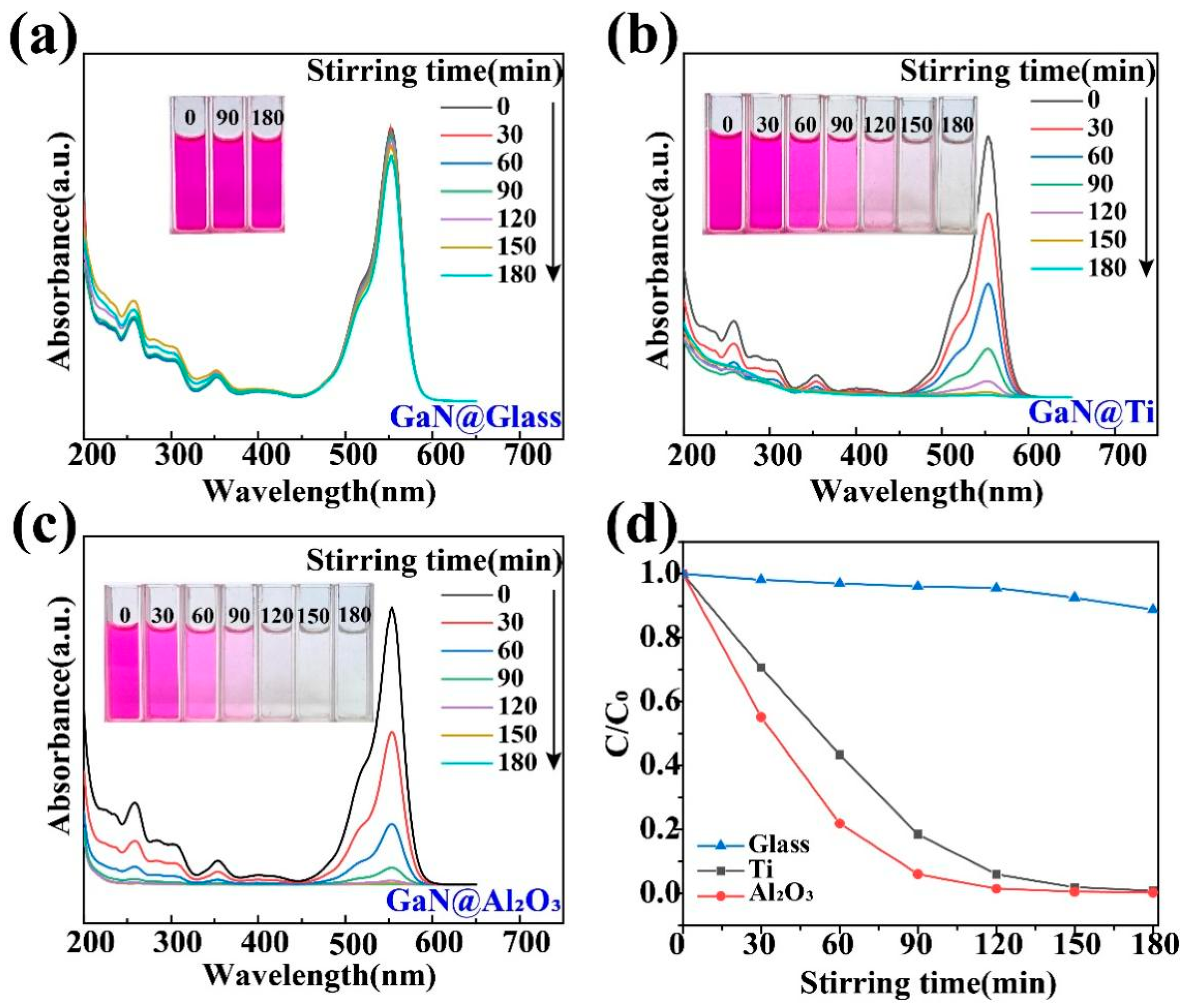
First of all, it should be pointed out that the GaN powder was found to show a very poor performance in the tribocatalytic degradation of the organic dyes in a normal way, namely in usual glass beakers. As shown in Figure 3a, after the GaN powder was stirred magnetically for 3 h in a glass beaker containing the 20 mg/L RhB solution, there was almost no change in the color of the RhB solution or in its absorbance of light, indicating a very low degradation efficiency. As a matter of fact, due to its relatively large band gap (3.4 eV), a very low photocatalytic degradation efficiency of the organic dyes was also observed for the GaN nanowires even under UV light irradiation [40]. It has been a great challenge to substantially increase the catalytic activity of GaN. Surprisingly, quite a different result was observed for the GaN powder when glass beakers with Ti and Al2O3 coatings were used, as shown in Figure 3b,c. In both cases, the RhB solution became colorless and the absorption peak at 554 nm disappeared after 3 h of magnetic stirring, indicating a degradation rate close to 100%.
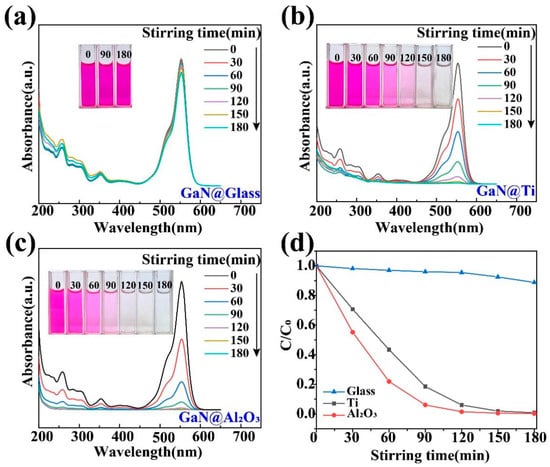
Figure 3.
Tribocatalytic degradation of RhB (20 mg/L) solution by GaN nanoparticles characterized through UV–vis absorption spectra (inset: evolution of solution color): (a) GaN @ glass; (b) GaN @ Ti; (c) GaN @ Al2O3; (d) C/C0 vs. stirring time.
To quantify the degradation efficiency of organic dyes, the formula D = 1 − C/C0 = 1 − A/A0 is used, where A and A0 represent the sustained and initial intensities of the characteristic absorption peaks of the dyes (RhB: 554 nm; MO: 464 nm), respectively. In this way, a more detailed comparison is shown in Figure 3d. After 3 h of magnetic stirring, only 3% of the 20 mg/L RhB solution was degraded in the glass beaker, while 99.2% and 99.8% of the RhB solution were degraded in the beakers with Ti and Al2O3 coatings, respectively.
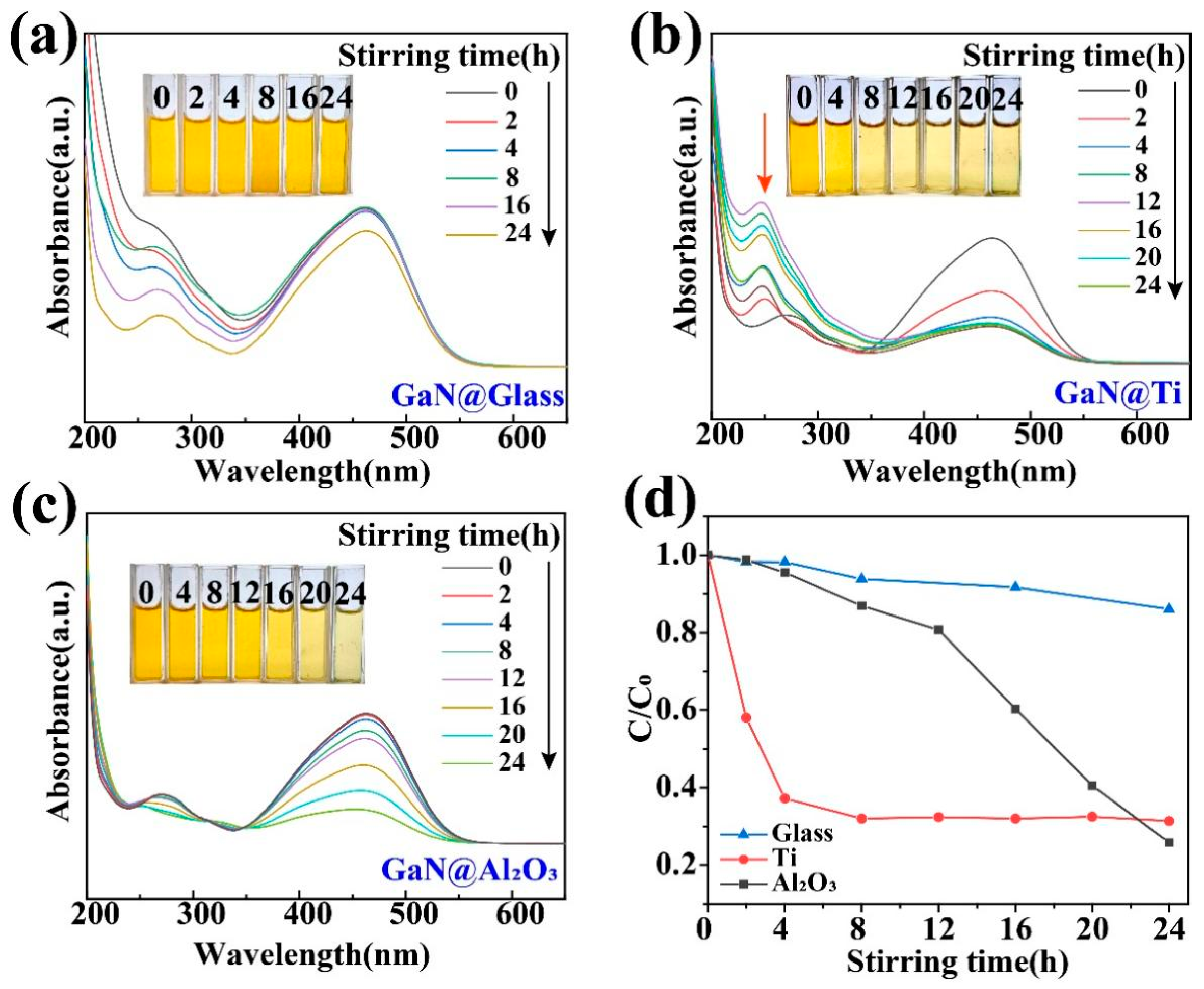
The presence of high-energy bonds in dye molecules significantly affects the degradation process [29,30,35], which greatly increases the difficulty to degrade those dyes with high-energy bonds in them, such as MO. As shown in Figure 4a, in a usual glass beaker, the absorption peak at 464 nm in the UV–vis absorption spectra almost remained unchanged even after 16 h of magnetic stirring. In contrast, when glass beakers with Ti and Al2O3 coatings were used, the absorption peak decreased significantly after 24 h of magnetic stirring, as shown in Figure 4b,c. For a more detailed comparison, as shown in Figure 4d, when glass beakers with Ti and Al2O3 coatings were used, the degradation efficiency after 24 h of magnetic stirring is increased to 5 times and 5.3 times the ordinary glass beaker, respectively. More importantly, it is worth noting that the degradation of the MO associated with the Ti coating was quite different from that with the Al2O3 coating. For the former, a peak around 250 nm was formed in the course of the degradation process, while, for the latter, such a peak was not observed. As a matter of fact, these two types of MO degradations had been studied in detail in some previous investigations. The appearance of the absorption peak around 250 nm actually indicates that the MO molecules were only broken into some smaller molecules like benzoic acid, succinic acid, and p-phenol and the degradation was only a partial degradation [28,44,45]. Obviously, these by-products should still be regarded as organic pollutants in the ambient environments. Fortunately, such a peak did not appear when MO molecules were degraded mostly into H2O and CO2 for the Al2O3 coating. Such a contrast between the Ti and Al2O3 coatings not only highlights the advantage of the Al2O3 coating for GaN nanoparticles but also demonstrates the importance of the coating choice in tribocatalysis. It is worth noting that, for dynamic friction between two solid materials, their surfaces are put into intimate contact by a compressive force and strong interactions can occur between them through sliding. Accordingly, the tribocatalytic behavior of the GaN must have been affected by the coating materials in a very complicated way.
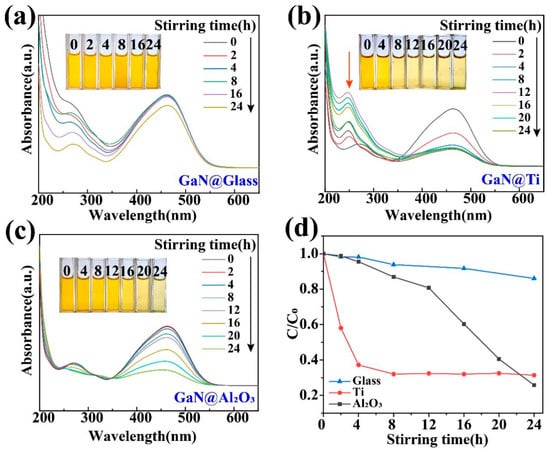
Figure 4.
Tribocatalytic degradation of MO (20 mg/L) solution by GaN nanoparticles characterized through UV–vis absorption spectra (inset: evolution of solution color): (a) GaN @ glass; (b) GaN @ Ti; (c) GaN @ Al2O3; (d) C/C0 vs. stirring time.
It has been a great challenge to improve the photocatalytic performance of GaN nanomaterials. While doping to GaN is rather difficult, forming composites/heterostructures with other materials, such as ZnO [46,47,48,49,50], has been widely adopted for GaN. It has to be pointed out that, however, these materials including ZnO are usually less stable in harsh environments than GaN, and their composites/heterostructures are not suitable for applications in harsh environments. On the other hand, more and more research has shown that, for tribocatalysis, coating materials on the bottoms of beakers is an effective and convenient method to achieve a better catalytic performance [26,28,41]. As for the Ti and Al2O3 coatings in this study, not only are these two kinds of materials chemically stable in harsh environments but also both disks for these two kinds of coatings are uniform in composition from the surface to interior, indicating that they can be adopted for GaN to achieve an improved degradation efficiency of organic pollutants in harsh environments and when some surface layer of the coatings is worn off after some prolonged service. As a matter of fact, both kinds of coatings had repeatedly been used in our laboratory and there were no detectable changes in their performance. It has to be pointed out that the tribocatalytic performance of GaN nanoparticles with Ti and Al2O3 coatings is still lower than that of many metal oxides [27,43]. The effects of more kinds of coatings on tribocatalysis should be further extensively explored for GaN nanoparticles.
3.3. Mechanism Studies
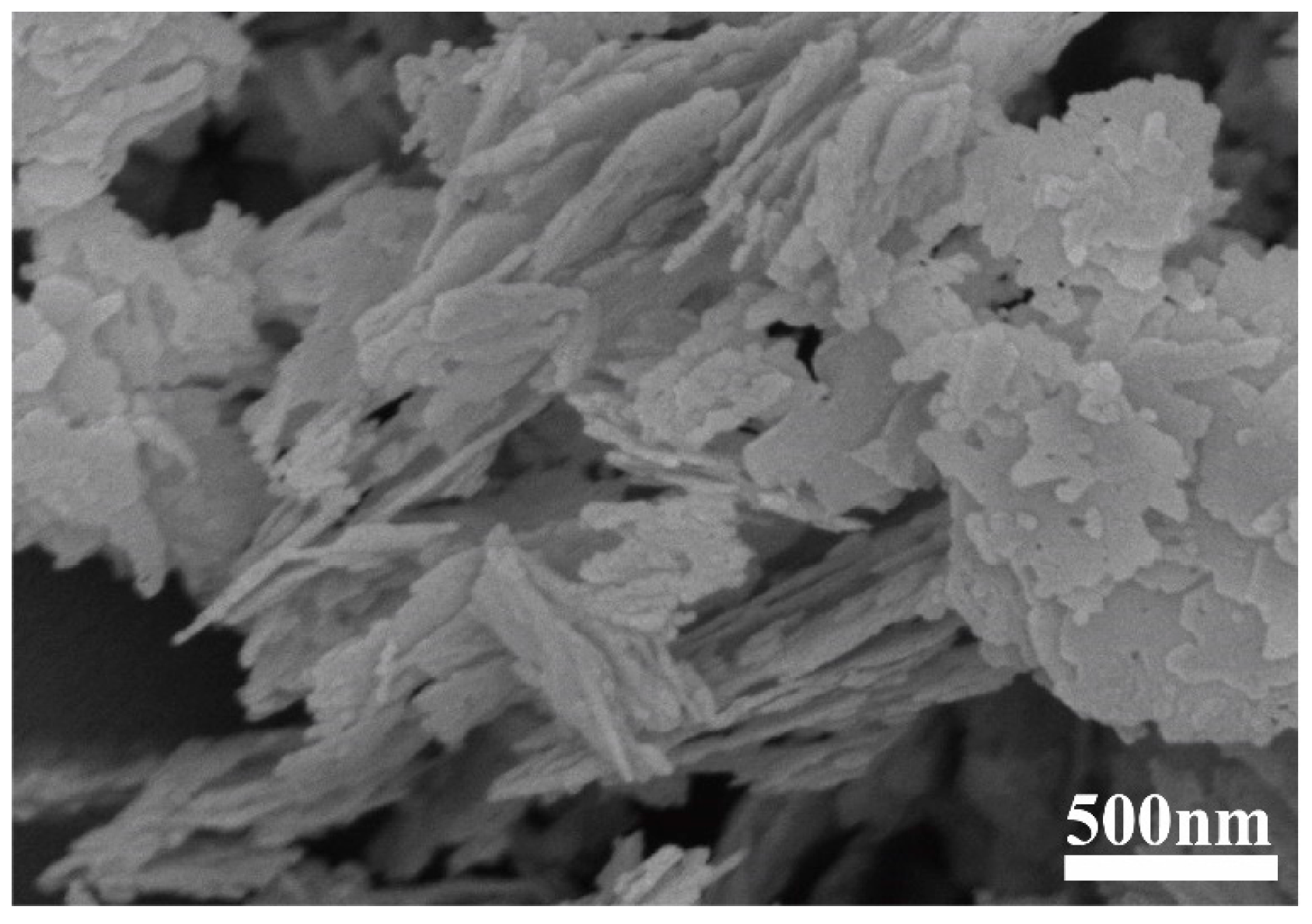
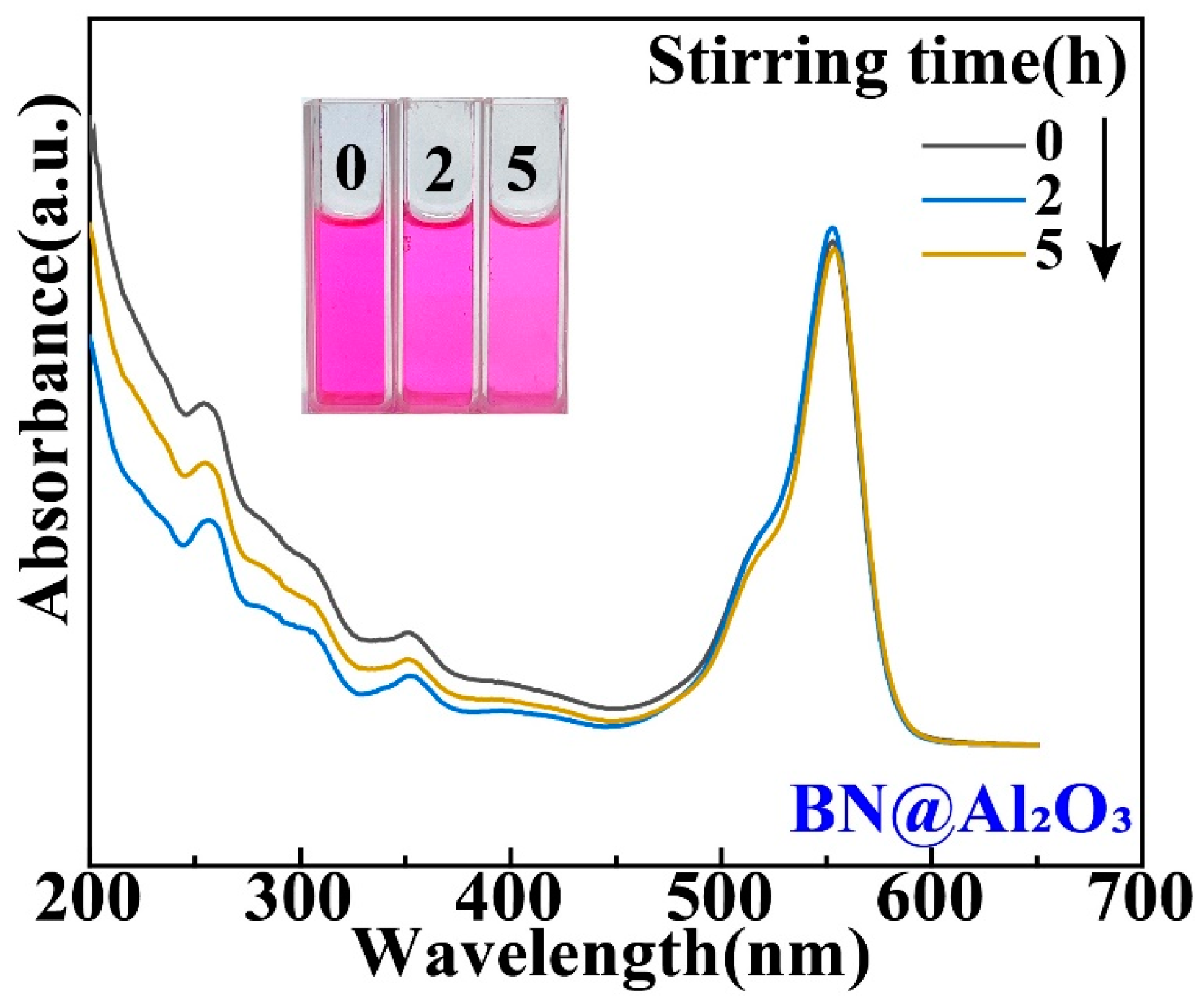
While the Ti and Al2O3 coatings were found to have surprising effects on the tribocatalytic degradation of organic dyes by GaN nanoparticles, it is necessary to examine whether the effects have mainly arisen from the coatings themselves [22]. For this purpose, we utilized a commercial cubic BN powder to replace the GaN powder as the catalyst. Figure 5 shows an SEM image of the cubic BN powder, which appears flake-shaped with a thickness around 30 nm. As shown in Figure 6, in a beaker with an Al2O3 coating, the RhB solution almost showed no degradation after the BN nanoparticles were magnetically stirred for 5 h, forming a stark contrast to the results obtained with the GaN nanoparticles. This phenomenon suggests that Al2O3 itself is chemically inert and the enhanced tribocatalytic degradation of the organic dyes by the GaN nanoparticles associated with the Al2O3 coating has resulted from the interaction, namely the friction, between the GaN nanoparticles and the Al2O3 coating.
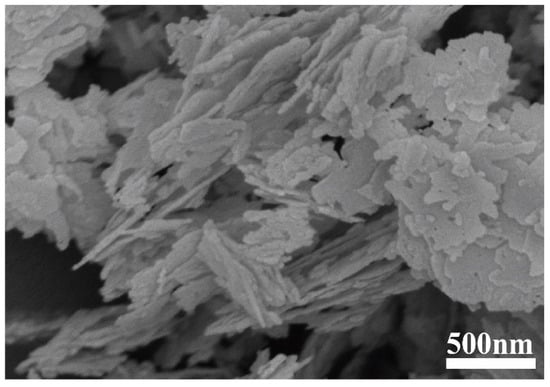
Figure 5.
SEM micrograph of as-received BN powder.
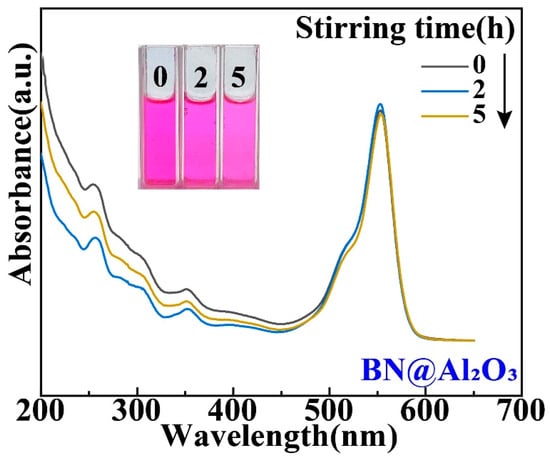
Figure 6.
Tribocatalytic degradation of RhB (20 mg/L) solution by BN nanoparticles in a beaker with Al2O3 coating characterized through UV–vis absorption spectra (inset: evolution of solution color).
A mechanism based on the excitation of the electron–hole pairs in semiconductors has been proposed for tribocatalysis [5]. Accordingly, for the GaN nanoparticles in this study, the tribocatalytic degradation of organic dyes can be expressed as follows:
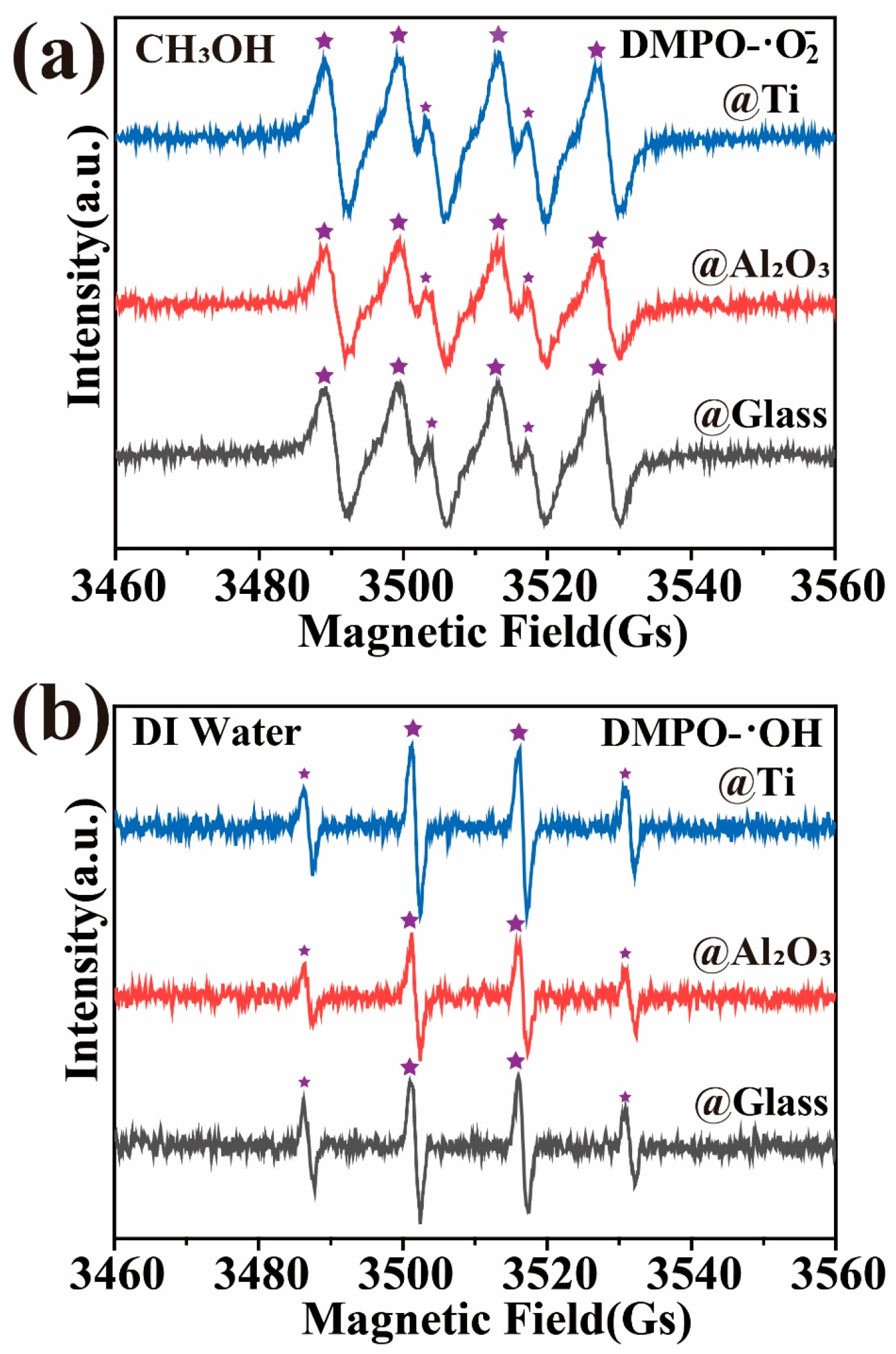
The results of the EPR experiments are shown in Figure 7, where the signals corresponding to DMPO-· (Figure 7a) and DMPO-·OH (1:2:2:1, Figure 7b) can be observed during the magnetically stirred induction process of GaN nanoparticles in beakers with glass, Ti, and Al2O3 bottoms separately. The same results have also been reported in previous studies [5,30,51], which provide strong evidence for the excitation of electron–hole pairs by the mechanical energy absorbed through friction in semiconducting materials, including GaN. However, it has to be pointed out that, as DMPO-· and DMPO-·OH radicals were found to be similarly generated for the three kinds of bottoms, the EPR results provided no evidence for understanding the surprising effects of the Ti and Al2O3 coatings on the tribocatalytic degradations of the organic dyes by the GaN nanoparticles. More analyses are highly desirable in future investigations.
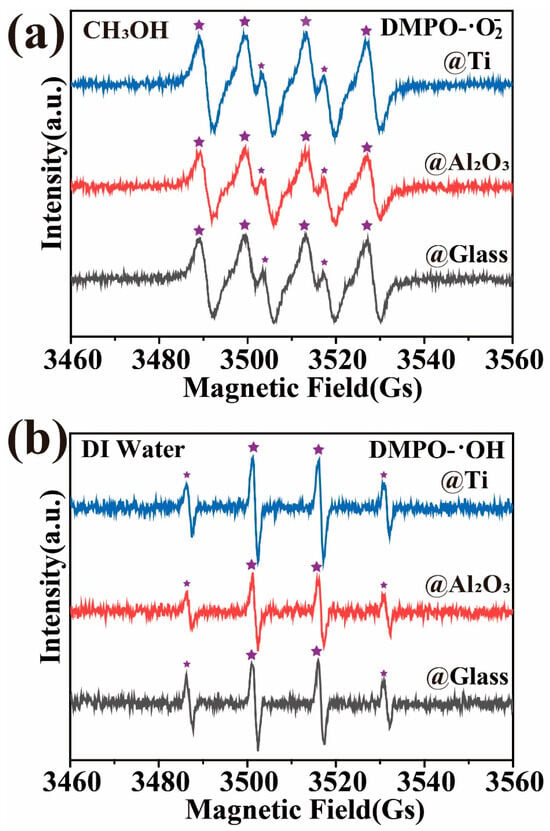
Figure 7.
EPR spectra for three different coatings: GaN nanoparticles were magnetically stirred in deionized water and DMSO separately using a PTFE magnetic rotating disk.
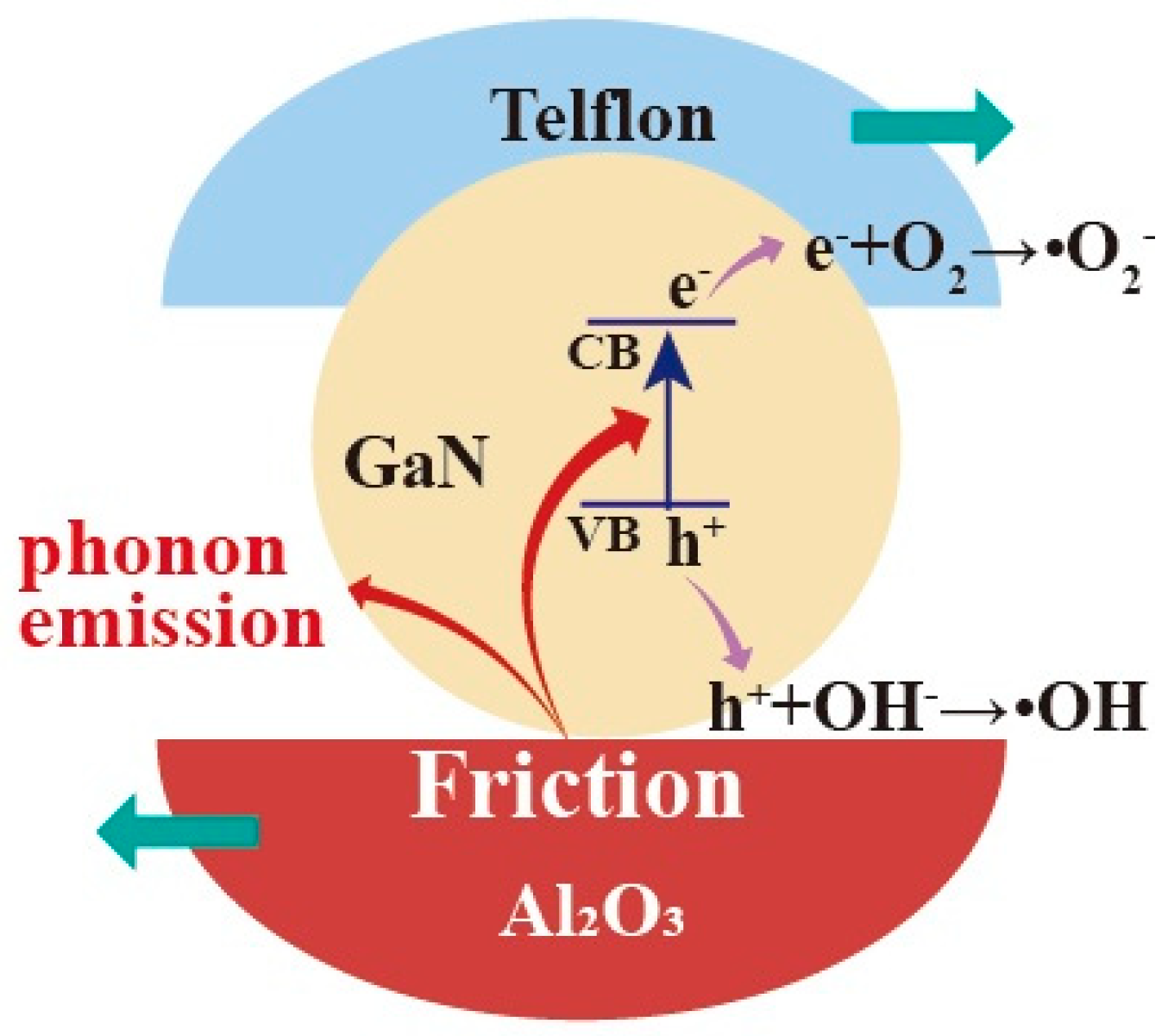
For GaN nanoparticles under magnetic stirring in beakers, the friction between the GaN nanoparticles and beaker bottoms should be mostly responsible for the excitation of the electron–hole pairs in the GaN. In this way, the dependence of the tribocatalytic behavior of the GaN nanoparticles on the coatings observed in this study can be preliminarily explained. Among the dynamic frictions between the three friction pairs (GaN and glass, GaN and Ti, and GaN and Al2O3), the one between GaN and Al2O3, as shown in Figure 8, is most outstanding in that not only is the fastest degradation of RhB observed for the Al2O3 coating in Figure 3 but also a complete degradation of the MO can only be observed for the Al2O3 coating in Figure 4. Nevertheless, it is still a great challenge to fully understand the coating dependence of the tribocatalytic behavior of GaN nanoparticles.
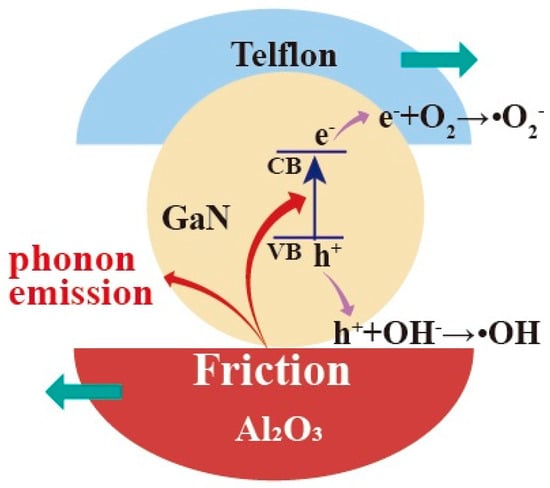
Figure 8.
Absorption of mechanical energy by GaN nanoparticles through friction under magnetic stirring in a glass beaker with Al2O3 bottom and the excitation of electron–hole pairs in GaN.
4. Conclusions
Neither the RhB nor the methyl orange MO solutions were evidently degraded by the GaN nanoparticles under magnetic stirring using homemade magnetic rotary disks in glass beakers. Surprisingly, greatly enhanced degradation was obtained after the Ti and Al2O3 disks were coated on the bottoms of the beakers separately. For the Ti coating, 99.2% of the 20 mg/L RhB was degraded in 3 h and 56% of the 20 mg/L MO solution was degraded in 24 h. For the Al2O3 coating, 99.8% of the 20 mg/L RhB solution was degraded in 3 h and 60.2% of the 20 mg/L MO solution was degraded in 24 h. Moreover, a striking contrast was observed in the MO degradation between the two coatings: the MO molecules were only broken into smaller organic molecules for the Ti coating, while they were degraded into H2O and CO2 for the Al2O3 coating. These findings provide a practicable method to improve the catalytic efficiency of GaN for the degradation of organic pollutants in harsh environments and will enrich our understanding of tribocatalysis as a whole.
Author Contributions
Conceptualization, X.X. and W.C.; methodology, X.X. and C.M.; validation, W.C., C.P. and Y.H.; formal analysis, W.C., X.X., C.M., J.S. and S.K.; investigation, X.X., C.M., J.S. and C.P.; data curation, X.X. and C.M.; writing—original draft preparation, X.X., W.C. and C.M.; writing—review and editing, X.X., W.C. and C.M.; visualization, X.X.; supervision, W.C.; project administration, W.C.; funding acquisition, Y.H. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the National Natural Science Foundation of China under Grant No. U21A20500, and the National Key R&D Program of China under Grant No. 2020YFB2008800.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lin, E.; Kang, Z.; Wu, J.; Huang, R.; Qin, N.; Bao, D. BaTiO3 nanocubes/cuboids with selectively deposited Ag nanoparticles: Efficient piezocatalytic degradation and mechanism. Appl. Catal. B Environ. 2021, 285, 119823. [Google Scholar] [CrossRef]
- Wang, Z.; Berbille, A.; Feng, Y.; Li, S.; Zhu, L.; Tang, W.; Wang, Z.L. Contact-electro-catalysis for the degradation of organic pollutants using pristine dielectric powders. Nat. Commun. 2022, 13, 130. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Yang, Y.; Wang, L.; Bian, J.; Liu, Q.; Lou, X. Insights into the tribo-/pyro-catalysis using Sr-doped BaTiO3 ferroelectric nanocrystals for efficient water remediation. Chem. Eng. J. 2021, 416, 128986. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, S.; Zhao, Z.; Li, S.; Wang, F. Piezocatalytic Effect Induced Hydrogen Production from Water over Non-noble Metal Ni Deposited Ultralong GaN Nanowires. ACS Appl. Mater. Interfaces 2021, 13, 10916–10924. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Park, J.Y.; Salmeron, M. Fundamental Aspects of Energy Dissipation in Friction. Chem. Rev. 2014, 114, 677–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tang, Q.S.; Zhang, L.L.; Xu, M.H.; Sun, L.H.; Sun, S.H.; Zhang, J.X.; Wang, S.M.; Liang, X.L. Ultrasmall Barium Titanate Nanoparticles for Highly Efficient Hypoxic Tumor Therapy via Ultrasound Triggered Piezocatalysis and Water Splitting. Acs Nano 2021, 15, 11326–11340. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiao, L.; Wu, Z.; Jia, Y.; Ye, X.; Wang, F.; Yuan, B.; Yu, Y.; Huang, H.; Zou, G. Harvesting vibration energy to piezo-catalytically generate hydrogen through Bi2WO6 layered-perovskite. Nano Energy 2020, 78, 105351. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Dong, S.; Wang, P.; Chen, W.; Lu, Z.; Ye, D.; Pan, B.; Wu, D.; Vecitis, C.D.; et al. Ultrasonic activation of inert poly(tetrafluoroethylene) enables piezocatalytic generation of reactive oxygen species. Nat. Commun. 2021, 12, 3508. [Google Scholar] [CrossRef]
- Zhao, Z. Research progress of semiconductor photocatalysis applied to environmental governance. IOP Conf. Ser. Earth Environ. Sci. 2021, 631, 012022. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Tirumala, R.T.A.; Gyawali, S.; Wheeler, A.; Ramakrishnan, S.B.; Sooriyagoda, R.; Mohammadparast, F.; Khatri, N.; Tan, S.; Kalkan, A.K.; Bristow, A.D.; et al. Structure–Property–Performance Relationships of Cuprous Oxide Nanostructures for Dielectric Mie Resonance-Enhanced Photocatalysis. ACS Catal. 2022, 12, 7975–7985. [Google Scholar] [CrossRef]
- Addanki Tirumala, R.T.; Khatri, N.; Ramakrishnan, S.B.; Mohammadparast, F.; Khan, M.T.; Tan, S.; Wagle, P.; Puri, S.; McIlroy, D.N.; Kalkan, A.K.; et al. Tuning Catalytic Activity and Selectivity in Photocatalysis on Mie-Resonant Cuprous Oxide Particles: Distinguishing Electromagnetic Field Enhancement Effect from the Heating Effect. ACS Sustain. Chem. Eng. 2023, 11, 15931–15940. [Google Scholar] [CrossRef]
- Kondamareddy, K.K.; Bin, H.; Lu, D.; Kumar, P.; Dwivedi, R.K.; Pelenovich, V.O.; Zhao, X.-Z.; Gao, W.; Fu, D. Enhanced visible light photodegradation activity of RhB/MB from aqueous solution using nanosized novel Fe-Cd co-modified ZnO. Sci. Rep. 2018, 8, 10691. [Google Scholar]
- Shang, J.; Zhao, F.; Zhu, T.; Li, J. Photocatalytic degradation of rhodamine B by dye-sensitized TiO2 under visible-light irradiation. Sci. China Chem. 2011, 54, 167–172. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Gyawali, S.; Tirumala, R.T.A.; Loh, H.; Andiappan, M.; Bristow, A.D. Photocarrier Recombination Dynamics in Highly Scattering Cu(2)O Nanocatalyst Clusters. J. Phys. Chemistry. C Nanomater. Interfaces 2024, 128, 2003–2011. [Google Scholar] [CrossRef]
- Gyawali, S.; Tirumala, R.T.A.; Andiappan, M.; Bristow, A.D. Size- and Shape-Dependent Charge-Carrier Dynamics in Sub-Micron Cuprous Oxide Nanoparticles; Frontiers in Optics + Laser Science; Optica Publishing Group: Washington, DC, USA, 2022; p. JTu4A.86. [Google Scholar]
- Zhou, Q.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Efficient removal of Bisphenol A in water via piezocatalytic degradation by equivalent-vanadium-doped SrTiO3 nanofibers. Chem. Eng. Sci. 2022, 247, 116707. [Google Scholar] [CrossRef]
- Petruschke, M. Tribochemistry. von G. HEINICKE; Akademie-Verlag: Berlin, Germany, 1984. [Google Scholar]
- Berman, D.; Erdemir, A. Achieving Ultralow Friction and Wear by Tribocatalysis: Enabled by In-Operando Formation of Nanocarbon Films. ACS Nano 2021, 15, 18865–18879. [Google Scholar] [CrossRef]
- Junbin, Y.; Junxiu, D. Tribocatalysis reaction during antiwear synergism between borates and Sn(IV) compounds in boundary lubrication. Tribol. Int. 1996, 29, 429–432. [Google Scholar] [CrossRef]
- Onodera, T.; Kawasaki, K.; Nakakawaji, T.; Higuchi, Y.; Ozawa, N.; Kurihara, K.; Kubo, M. Tribocatalytic Reaction of Polytetrafluoroethylene Sliding on an Aluminum Surface. J. Phys. Chem. C 2015, 119, 15954–15962. [Google Scholar] [CrossRef]
- Peng, K.Q.; Xu, Y.; Wu, Y.; Yan, Y.J.; Lee, S.T.; Zhu, J. Aligned single-crystalline Si nanowire arrays for photovoltaic applications. Small 2005, 1, 1062–1067. [Google Scholar] [CrossRef]
- Fan, F.-R.; Xie, S.; Wang, G.-W.; Tian, Z.-Q. Tribocatalysis: Challenges and perspectives. Sci. China Chem. 2021, 64, 1609–1613. [Google Scholar] [CrossRef]
- Mao, C.; Zhang, Y.-C.; Lei, H.; Jia, X.; Chen, F.; Yao, W.; Liu, P.; Chen, W. Boosting tribo-catalytic degradation of organic pollutants by BaTiO3 nanoparticles through metallic coatings. Appl. Surf. Sci. 2024, 663, 160172. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Song, W.; Shi, J.; Zhang, Y. Performance of tribocatalysis and tribo-photocatalysis of pyrite under agitation. J. Clean. Prod. 2023, 414, 137566. [Google Scholar] [CrossRef]
- Cui, X.; Guo, Z.; Lei, H.; Jia, X.; Mao, C.; Ruan, L.; Zhou, X.; Wang, Z.; Chen, F.; Chen, W. Tribo-Catalytic Degradation of Methyl Orange Solutions Enhanced by Silicon Single Crystals. Coatings 2023, 13, 1804. [Google Scholar] [CrossRef]
- Tang, Q.; Zhu, M.; Zhang, H.; Gao, J.; Kwok, K.W.; Kong, L.-B.; Jia, Y.; Liu, L.; Peng, B. Enhanced tribocatalytic degradation of dye pollutants through governing the charge accumulations on the surface of ferroelectric barium zirconium titanate particles. Nano Energy 2022, 100, 107519. [Google Scholar] [CrossRef]
- Ruan, L.; Jia, Y.; Guan, J.; Xue, B.; Huang, S.; Wang, Z.; Fu, Y.; Wu, Z. Tribo-electro-catalytic dye degradation driven by mechanical friction using MOF-derived NiCO2O4 double-shelled nanocages. J. Clean. Prod. 2022, 345, 131060. [Google Scholar] [CrossRef]
- Mao, C.; Lei, H.; Guo, Z.; Jia, X.; Cui, X.; Huang, J.; Fei, L.; Jia, Y.; Chen, W. Exceptional tribo-catalytic degradation of concentrated methyl orange and methylene blue solutions by DXN-RT30 TiO2 nanoparticles. Ceram. Int. 2024, 50, 4737–4745. [Google Scholar] [CrossRef]
- Yu, H.; Fu, J.; Zhu, X.; Zhao, Z.; Sui, X.; Sun, S.; He, X.; Zhang, Y.; Ye, W. Tribocatalytic Degradation of Organic Pollutants Using Fe2O3 Nanoparticles. ACS Appl. Nano Mater. 2023, 6, 14364–14373. [Google Scholar] [CrossRef]
- Lei, H.; Cui, X.; Jia, X.; Qi, J.; Wang, Z.; Chen, W. Enhanced Tribocatalytic Degradation of Organic Pollutants by ZnO Nanoparticles of High Crystallinity. Nanomaterials 2022, 13, 46. [Google Scholar] [CrossRef]
- Cao, J.; Jia, Y.; Wan, X.; Li, B.; Zhang, Y.; Huang, S.; Yang, H.; Yuan, G.; Li, G.; Cui, X.; et al. Strong tribocatalysis of strontium titanate nanofibers through harvesting friction energy for dye decomposition. Ceram. Int. 2022, 48, 9651–9657. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Wang, X.; Zhang, L.; Yuan, G.; Wu, Z. Efficient tribocatalysis of magnetically recyclable cobalt ferrite nanoparticles through harvesting friction energy. Sep. Purif. Technol. 2023, 307, 122846. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Guo, X.; Wang, L.; Xu, T.; Bian, J.; Yang, Y.; Liu, Q.; Du, Y.; Lou, X. Enhanced tribocatalytic degradation using piezoelectric CdS nanowires for efficient water remediation. J. Mater. Chem. C 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Zhang, X.H.; Han, X.Y.; Su, J.; Zhang, Q.; Gao, Y.H. Well vertically aligned ZnO nanowire arrays with an ultra-fast recovery time for UV photodetector. Appl. Phys. A 2012, 107, 255–260. [Google Scholar] [CrossRef]
- Jung, B.O.; Bae, S.Y.; Kato, Y.; Imura, M.; Lee, D.S.; Honda, Y.; Amano, H. Morphology development of GaN nanowires using a pulsed-mode MOCVD growth technique. CrystEngComm 2014, 16, 2273–2282. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary, P.H.I.V.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Jung, H.S.; Hong, Y.J.; Li, Y.; Cho, J.; Kim, Y.J.; Yi, G.C. Photocatalysis using GaN nanowires. ACS Nano 2008, 2, 637–642. [Google Scholar] [CrossRef]
- Jia, X.; Wang, H.; Lei, H.; Mao, C.; Cui, X.; Liu, Y.; Jia, Y.; Yao, W.; Chen, W. Boosting tribo-catalytic conversion of H2O and CO2 by CO3O4 nanoparticles through metallic coatings in reactors. J. Adv. Ceram. 2023, 12, 1833–1843. [Google Scholar] [CrossRef]
- Cui, X.; Wang, H.; Lei, H.; Jia, X.; Jiang, Y.; Fei, L.; Jia, Y.; Chen, W. Surprising Tribo-catalytic Conversion of H2O and CO2 into Flammable Gases utilizing Frictions of Copper in Water. ChemistrySelect 2023, 8, e202204146. [Google Scholar] [CrossRef]
- Lei, H.; Wu, Z.; Wang, H.; Mao, C.; Guo, Z.; Fei, L.; Chen, W. Converting H2O and CO2 into chemical fuels by nickel via friction. Surf. Interfaces 2024, 46, 104203. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Xu, L.; Wang, L.; Ju, Z.; Li, G.; Qian, Y. High yield synthesis of novel boron nitride submicro-boxes and their photocatalytic application under visible light irradiation. Catal. Sci. Technol. 2011, 1, 1159–1165. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Li, H. Comparative study on the mechanism in photocatalytic degradation of different-type organic dyes on SnS2 and CdS. Appl. Catal. B Environ. 2012, 123–124, 174–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.-F.; Gao, P.-F.; Fang, D.-Q.; Zhang, S.-L. Enhanced visible light absorption in ZnO/GaN heterostructured nanofilms. J. Alloys Compd. 2017, 704, 478–483. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Wu, A.; Zhang, X.; Xu, T.; Liu, B. Band-Gap Tunable 2D Hexagonal (GaN)(1-x)(ZnO)(x) Solid-Solution Nanosheets for Photocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, B.; Zhang, J.; Lin, W.; Wang, J.; Xu, Y.; Xiang, Y.; Hisatomi, T.; Domen, K.; Ma, G. Synthesis of Narrow-Band-Gap GaN:ZnO Solid Solution for Photocatalytic Overall Water Splitting. ACS Catal. 2022, 12, 14637–14646. [Google Scholar] [CrossRef]
- Patil, S.S.; Johar, M.A.; Hassan, M.A.; Patil, D.R.; Ryu, S.-W. Anchoring MWCNTs to 3D honeycomb ZnO/GaN heterostructures to enhancing photoelectrochemical water oxidation. Appl. Catal. B Environ. 2018, 237, 791–801. [Google Scholar] [CrossRef]
- Wang, D.-T.; Zhang, M.; Zhuang, H.; Chen, X.H.; Wang, X.; Zheng, X.; Yang, J.J.A.S.S. The photocatalytic properties of hollow (GaN)1-x(ZnO)x composite nanofibers synthesized by electrospinning. Appl. Surf. Sci. 2017, 396, 888–896. [Google Scholar] [CrossRef]
- Cui, X.; Li, P.; Lei, H.; Tu, C.; Wang, D.; Wang, Z.; Chen, W. Greatly enhanced tribocatalytic degradation of organic pollutants by TiO2 nanoparticles through efficiently harvesting mechanical energy. Sep. Purif. Technol. 2022, 289, 120814. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).