The Chemistry–Process–Structure Relationships of a Functionally Graded Ti-6Al-4V/Ti-1B Alloy Processed with Laser-Engineered Net Shaping Creates Borlite
Abstract
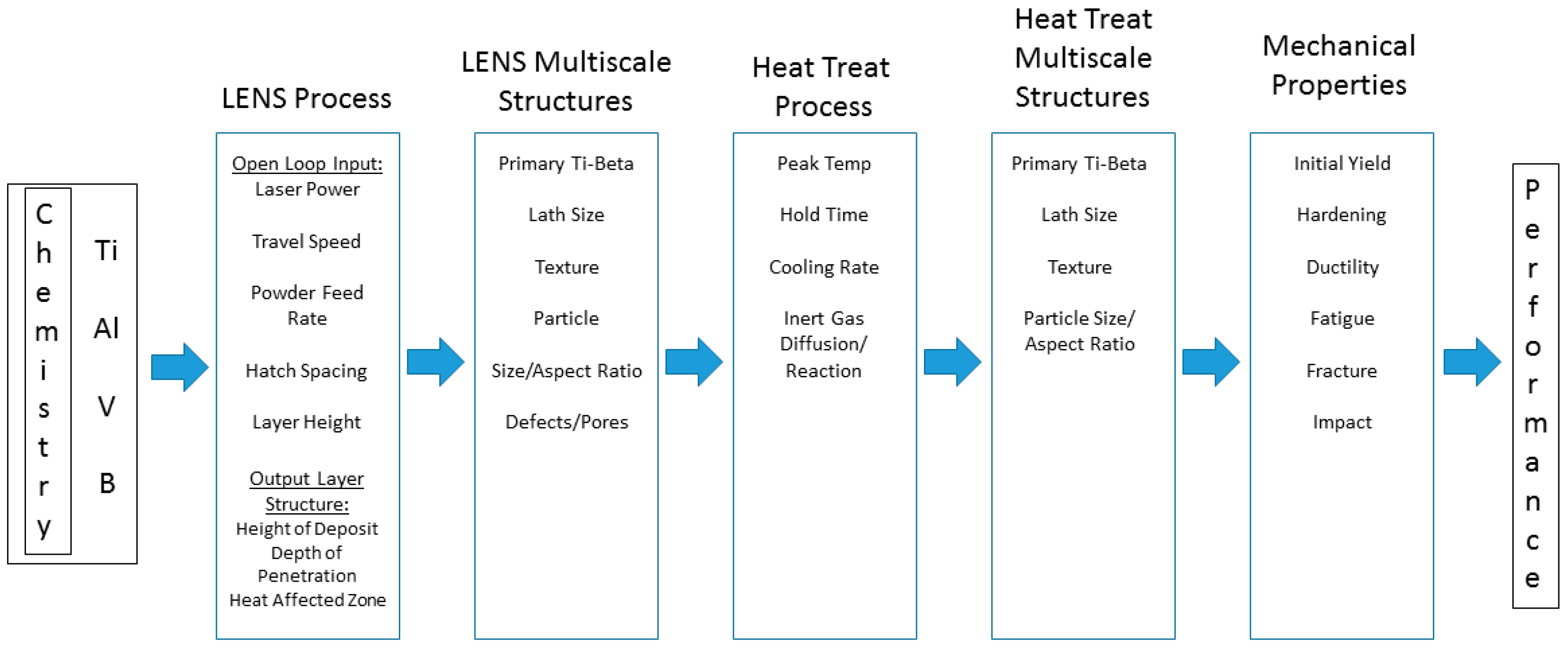
:1. Introduction
1.1. Adding Multiple Powder Chemistries Is Difficult
1.2. Laser-Engineered Net Shaping (LENS) Processing Method
1.3. Functionally Graded Material via the LENS Method
2. Processing Method of LENS for the Titanium-Aluminum-Vanadium-Boron Functionally Graded Materials
3. Results
3.1. Process–Structure Relationships Related to Porosity
3.2. Process–Structure Relationships Related to Titanium Microstructures
3.3. Chemistry–Process–Structure Relationship Related to Boron Additions
4. Discussion
4.1. Initial Titanium Beta Grain Size
4.2. Borlite Titanium/TiB Eutectic Region
4.3. Phase Transformations
4.4. The Impact of Successive LENS Thermal Cycles through α + β-Titanium Region
5. Conclusions
- A new term, “Borlite”, is proposed related to the liquid–solid transformation eutectic composition of TiB/Ti. The boron affected the solidification characteristics, which in turn determined the primary β-titanium grain size. When the boron wt.% was 0.25%, the primary β-titanium grain size saturated at a minimum level for the process variables in this study.
- The aluminum and vanadium affected the characteristics of the β- to α-titanium transformation. The transformed titanium α-lath size decreased from 2 μm to 0.2 μm as the aluminum and vanadium content increased from 0 to 6% and 0 to 4%, respectively.
- The chemistry composition influenced the proper heat depth of penetration to alleviate process pores during deposition. In this case, the chemistry gradients of titanium, aluminum, vanadium, and boron throughout the multilayered LENS build process induced a continuously changing depth of penetration, causing complexities in the control loop of the LENS process and, hence, uncertainties in the multiscale heterogeneous structures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffith, M.L.; Keicher, D.L.; Romero, J.A.; Atwood, C.L.; Harwell, L.D.; Greene, D.L.; Smugeresky, J.E. Laser Engineered net Shaping (LENS) for the Fabrication of Metallic Components (No. SAND–96-1293C; CONF-9605172–1); Sandia National Labs.: Albuquerque, NM, USA, 1996. [Google Scholar]
- Xue, Y.; Pascu, A.; Horstemeyer, M.F.; Wang, L.; Wang, P.T. Microporosity effects on cyclic plasticity and fatigue of LENS-processed steel. Acta Mater. 2010, 58, 4029–4038. [Google Scholar] [CrossRef]
- Grujicic, M.; Hu, Y.; Fadel, G.M.; Keicher, D.M. Optimization of the LENS Rapid Fabrication Process for In-Flight Melting of Feed Powder. J. Mater. Synth. Process. 2001, 9, 223. [Google Scholar] [CrossRef]
- Kobryn, P.A.; Semiatin, S.L. The laser additive manufacture of Ti-6Al-4V. JOM 2001, 53, 40–42. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, C.T. Design of titanium alloys by additive manufacturing: A critical review. Adv. Powder Mater. 2022, 1, 100014. [Google Scholar] [CrossRef]
- Griffith, M.L.; Schliengeral, M.E.; Harwell, D.; Olivera, M.S.; Baldwin, M.D.; Ensza, T.E.; Essiena, M.; Brooks, J.; Robinoa, V.R.; Smugeresky, J.E.; et al. Understanding Thermal Behavior in the LENS Process. Mater. Des. 1999, 20, 107–113. [Google Scholar] [CrossRef]
- Wang, L.; Felicelli, S.; Gooroochurn, Y.; Wang, P.T.; Horstemeyer, M.F. Optimization of the LENS Process for Steady Molten Pool Size. Mater. Sci. Eng. A 2008, 474, 148–156. [Google Scholar] [CrossRef]
- Bhatia, A.; Sehgal, A.K. Additive manufacturing materials, methods and applications: A review. Mater. Today Proc. 2023, 81, 1060–1067. [Google Scholar] [CrossRef]
- Gardner, L. Metal additive manufacturing in structural engineering–review, advances, opportunities and outlook. Structures 2023, 47, 2178–2193. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. J. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Atwood, C.; Griffith, M.; Harwell, L.; Schlienger, E.; Ensz, M.; Smugeresky, J.; Romero, T.; Greene, D.; Reckaway, D. Laser engineered net shaping (LENSTM): A tool for direct fabrication of metal parts. In Proceedings of the 17th International Congress on Applications of Lasers and Elector-Optics, Orlando, FL, USA, 16–19 November 1998; Available online: https://www.osti.gov/biblio/1549 (accessed on 28 May 2024).
- Raji, S.A.; Popoola, A.P.I. Microstructural, microhardness and electrochemical properties of heat-treated LENS in-situ synthesized Ti–Al-x (Mo, Si) alloys. Heliyon 2024, 10, e25519. [Google Scholar] [CrossRef]
- Antolak-Dudka, A.; Czujko, T.; Durejko, T.; Stępniowski, W.J.; Ziętala, M.; Łukasiewicz, J. Comparison of the Microstructural, Mechanical and Corrosion Resistance Properties of Ti6Al4V Samples Manufactured by LENS and Subjected to Various Heat Treatments. Materials 2024, 17, 1166. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Foucks, N.; Geva, D.; Oren, S.; Shriki, N.; Vaknin, D.; Fishman, D.; Levi, O. Comparative quality control of titanium alloy Ti–6Al–4V, 17–4 pH stainless steel, and aluminum alloy 4047 either manufactured or repaired by Laser Engineered Net Shaping (LENS). Materials 2020, 13, 4171. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Farzaneh, A.; Mohammed, M.; Gibson, I.; Rolfe, B. A review of laser engineered net shaping (LENS) build and process parameters of metallic parts. Rapid Prototyp. J. 2020, 26, 1059–1078. [Google Scholar] [CrossRef]
- Kumar, S.A.; Prasad, R.V.S. Basic principles of additive manufacturing: Different additive manufacturing technologies. In Additive Manufacturing: A Tool for Industrial Revolution 4.0; Woodhead Publishing: Duxford, UK; Cambridge, MA, USA; Kidlington, UK, 2021; pp. 17–35. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, F.; Huang, Z.; Jia, M.; Chen, G.; Ye, Y.; Lin, Y.; Liu, W.; Chen, B.; Shen, Q.; et al. Additive manufacturing of functionally graded materials: A review. Mater. Sci. Eng. A 2019, 764, 138209. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Lu, J.; Xu, L.; Lin, Y.; Chen, P.; Sheng, Q.; Chen, F. Additive manufacturing and mechanical properties of martensite/austenite functionally graded materials by laser engineered net shaping. J. Mater. Res. Technol. 2022, 17, 1570–1581. [Google Scholar] [CrossRef]
- Noda, N. Thermal stresses in functionally graded materials. J. Therm. Stress. 1999, 22, 477–512. [Google Scholar] [CrossRef]
- Mantri, S.A.; Torgerson, T.; Ivanov, E.; Scharf, T.W.; Banerjee, R. Effect of Boron Addition on the Mechanical Wear Resistance of Additively Manufactured Biomedical Titanium Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2018, 49, 806–810. [Google Scholar] [CrossRef]
- Cui, Y.; Aoyagi, K.; Zhao, Y.; Yamanaka, K.; Hayasaka, Y.; Koizumi, Y.; Fujieda, T.; Chiba, A. Manufacturing of a nanosized TiB strengthened Ti-based alloy via electron beam powder bed fusion. Addit. Manuf. 2020, 36, 101472. [Google Scholar] [CrossRef]
- Tshephe, T.S.; Akinwamide, S.O.; Olevsky, E.; Olubambi, P.A. Additive manufacturing of titanium-based alloys- A review of methods, properties, challenges, and prospects. Heliyon 2022, 8, e09041. [Google Scholar] [CrossRef]
- Lepakova, O.K.; Raskolenko, L.G.; Maksimov, Y.M. Titanium borides prepared by self-propagating high-temperature synthesis. Inorg. Mater. 2000, 36, 568–575. [Google Scholar] [CrossRef]
- Wang, L.; Thompson, L.T. Self-Propagating High-Temperature Synthesis and Dynamic Compaction of Titanium Boride and Titanium Carbide; Army Research Laboratory: Adelphi, MD, USA, 1999; p. 51. [Google Scholar]
- Feng, H.; Meng, Q.; Zhou, Y.; Jia, D. Spark plasma sintering of functionally graded material in the Ti-TiB2-B system. Mater. Sci. Eng. A 2005, 397, 92–97. [Google Scholar] [CrossRef]
- Gupta, N.; Basu, B.; Bhanu Prasad, V.V.; Vemuri, M. Ballistic Studies on TiB2-Ti Functionally Graded Armor Ceramics. Def. Sci. J. 2012, 62, 382–389. [Google Scholar] [CrossRef]
- Galvan, D.; Ocelík, V.; Pei, Y.; Kooi, B.J.; De Hosson, J.T.M.; Ramous, E. Microstructure and properties of TiB/Ti-6Al-4V coatings produced with laser treatments. J. Mater. Eng. Perform. 2004, 13, 406–412. [Google Scholar] [CrossRef]
- Genç, A.; Banerjee, R.; Hill, D.; Fraser, H.L. Structure of TiB precipitates in laser deposited in situ, Ti-6Al-4V–TiB composites. Mater. Lett. 2006, 60, 859–863. [Google Scholar] [CrossRef]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in situ titanium–titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater. 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Bermingham, M.J.; Kent, D.; Zhan, H.; Stjohn, D.H.; Dargusch, M.S. Controlling the microstructure and properties of wire arc additive manufactured Ti–6Al–4V with trace boron additions. Acta Mater. 2015, 91, 289–303. [Google Scholar] [CrossRef]
- Bermingham, M.J.; McDonald, S.D.; Dargusch, M.S. Effect of trace lanthanum hexaboride and boron additions on microstructure, tensile properties and anisotropy of Ti-6Al-4V produced by additive manufacturing. Mater. Sci. Eng. A 2018, 719, 1–11. [Google Scholar] [CrossRef]
- Yang, Z.W.; Fu, L.Q.; Wang, S.L.; Zhang, M.; Wang, Y.; Ma, Z.Q.; Wang, D.P. Balance of strength and plasticity of additive manufactured Ti-6Al-4V alloy by forming TiB whiskers with cyclic gradient distribution. Addit. Manuf. 2021, 39, 101883. [Google Scholar] [CrossRef]
- Fujieda, T.; Cui, Y.; Aoyagi, K.; Koizumi, Y.; Chiba, A. Electron beam melting of boron-modified Ti–6Al–2Sn–4Zr–2Mo–0.1Si alloy with superior tensile strength and oxidation resistance at elevated temperatures. Materialia 2018, 4, 367–372. [Google Scholar] [CrossRef]
- Verma, P.K.; Warghane, S.; Nichul, U.; Kumar, P.; Dhole, A.; Hiwarkar, V. Effect of boron addition on microstructure, hardness and wear performance of Ti-6Al-4 V alloy manufactured by laser powder bed fusion additive manufacturing. Mater. Charact. 2021, 172, 110848. [Google Scholar] [CrossRef]
- Fereiduni, E.; Ghasemi, A.; Elbestawi, M. Microstructural characterization and mechanical properties of nano-scale/sub-micron TiB-reinforced titanium matrix composites fabricated by laser powder bed fusion. J. Alloys Compd. 2022, 896, 163054. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Ebel, T.; Yan, M. Cost-affordable, high-performance Ti–TiB composite for selective laser melting additive manufacturing. J. Mater. Res. 2020, 35, 1922–1935. [Google Scholar] [CrossRef]
- Liu, H.; Fang, M.; Han, Y.; Huang, G.; Sun, Z.; Zhang, L.; Lu, W. Achieving strength-ductility combination and anisotropy elimination in additively manufactured TiB/Ti6Al4V by in-situ synthesized network architecture with fine grains. Compos. Part B Eng. 2023, 262, 110822. [Google Scholar] [CrossRef]
- Zhang, L.C.; Liu, Y.; Li, S.; Hao, Y. Additive manufacturing of titanium alloys by electron beam melting: A review. Adv. Eng. Mater. 2018, 20, 1700842. [Google Scholar] [CrossRef]
- El Kadiri, H.; Wang, L.; Horstemeyer, M.F.; Yassar, R.; Shahbazian, Y.; Felicelli, S.; Wang, P.T. Phase Transformations in Low Alloy Steel Laser Deposits. Mater. Sci. Eng. A 2008, 494, 10–20. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H.J. Phase transformations during cooling in α+β titanium alloys. Mater. Sci. Eng. A 1998, 243, 206–211. [Google Scholar] [CrossRef]
- Sen, I.; Tamirisakandala, S.; Miracle, D.B.; Ramamurty, U. Microstructural effects on the mechanical behavior of B-modified Ti–6Al–4V alloys. Acta Mater. 2007, 55, 4983–4993. [Google Scholar] [CrossRef]
- Sato, T. The Effect of Solidification Conditions upon the Primary Dendrite Arm Spacing. J. Jpn. Inst. Met. Mater. 1982, 46, 232–236. [Google Scholar] [CrossRef]
- Banerjee, R.; Collins, P.C.; Bhattacharyya, D.; Banerjee, S.; Fraser, H.L. Microstructural evolution in laser deposited compositionally graded [alpha]/[beta] titanium-vanadium alloys. Acta Mater. 2003, 51, 3277–3292. [Google Scholar] [CrossRef]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seely, D.; Bagheri, M.A.; Dickel, D.; Cho, H.E.; Rhee, H.; Horstemeyer, M.F. The Chemistry–Process–Structure Relationships of a Functionally Graded Ti-6Al-4V/Ti-1B Alloy Processed with Laser-Engineered Net Shaping Creates Borlite. Materials 2024, 17, 3491. https://doi.org/10.3390/ma17143491
Seely D, Bagheri MA, Dickel D, Cho HE, Rhee H, Horstemeyer MF. The Chemistry–Process–Structure Relationships of a Functionally Graded Ti-6Al-4V/Ti-1B Alloy Processed with Laser-Engineered Net Shaping Creates Borlite. Materials. 2024; 17(14):3491. https://doi.org/10.3390/ma17143491
Chicago/Turabian StyleSeely, D., M. A. Bagheri, D. Dickel, H. E. Cho, H. Rhee, and M. F. Horstemeyer. 2024. "The Chemistry–Process–Structure Relationships of a Functionally Graded Ti-6Al-4V/Ti-1B Alloy Processed with Laser-Engineered Net Shaping Creates Borlite" Materials 17, no. 14: 3491. https://doi.org/10.3390/ma17143491




