Research on Improving Moisture Resistance of Asphalt Mixture with Compounded Recycled Metallurgical Slag Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Experimental Methods
2.3.1. Powders’ Characteristics
2.3.2. Pull-Off Test
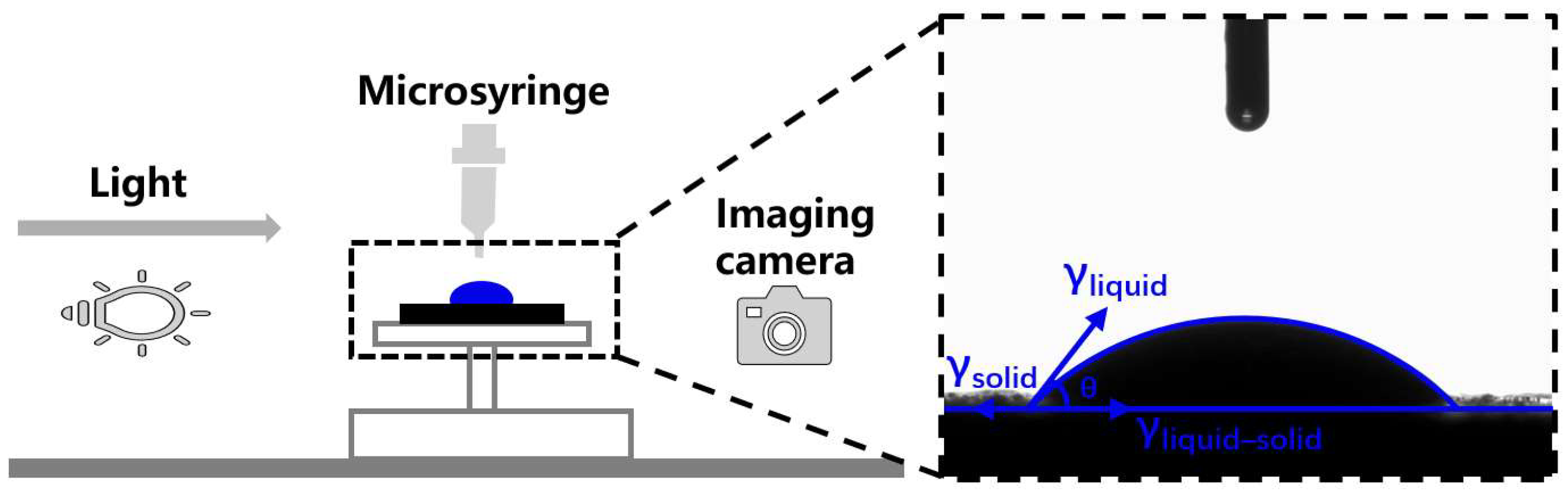
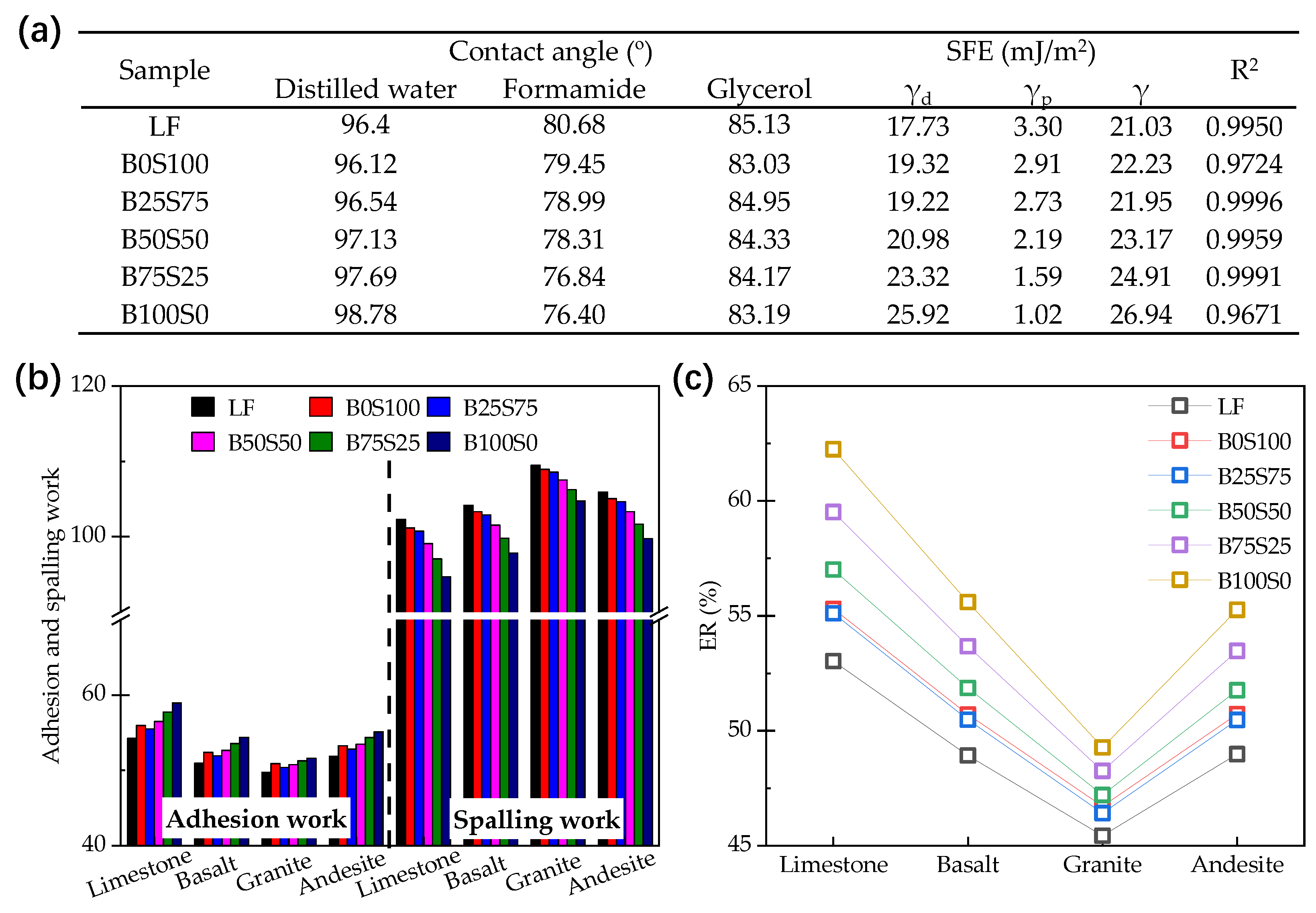
2.3.3. Contact Angle Test and Surface Free Energy (SFE) Calculation
2.3.4. Moisture Resistance of Asphalt Mixture
3. Results and Discussions
3.1. Material Characteristics
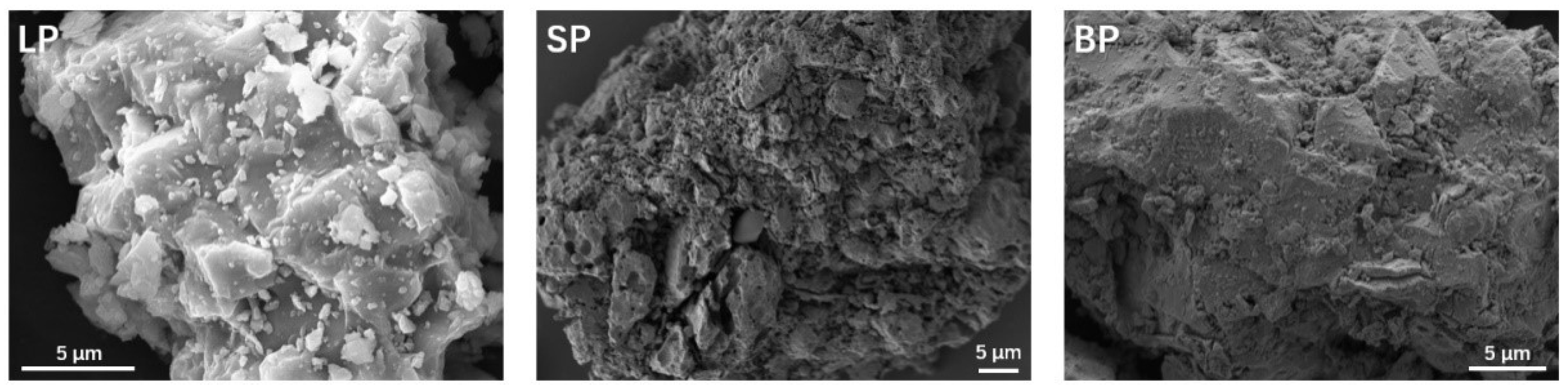
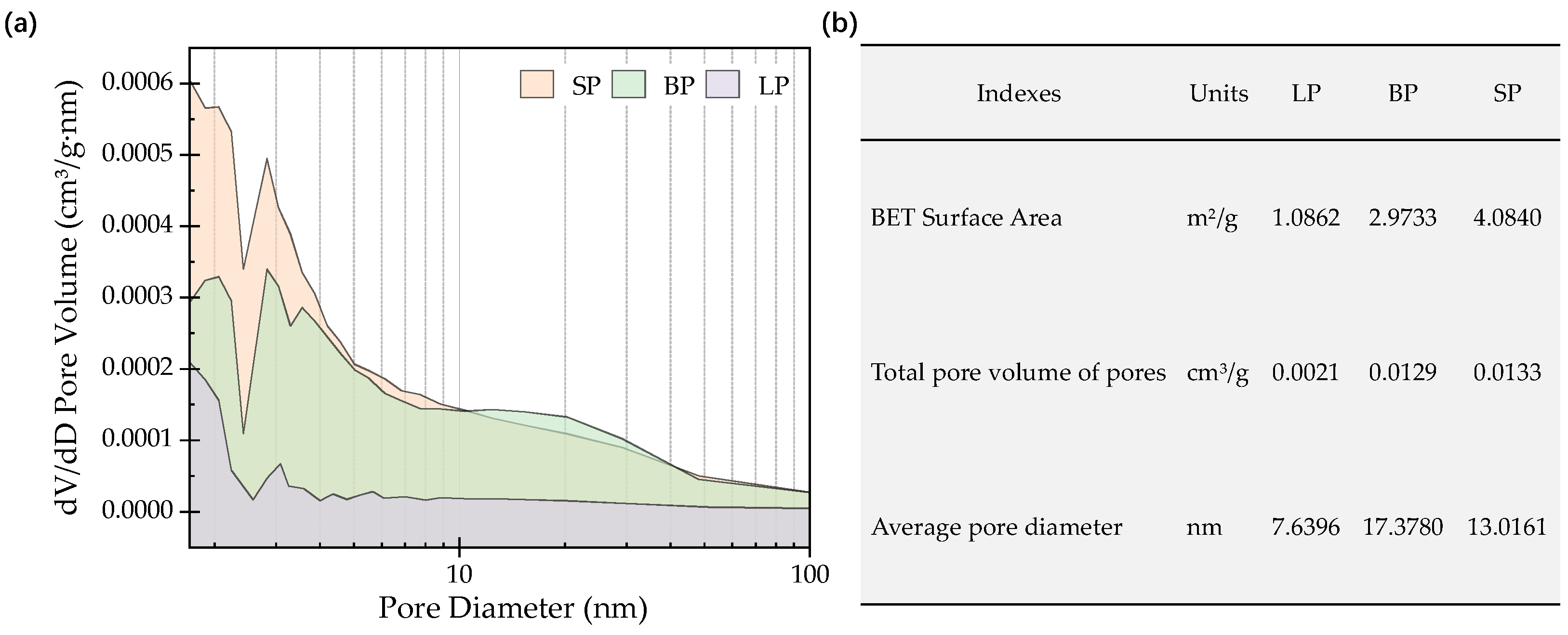
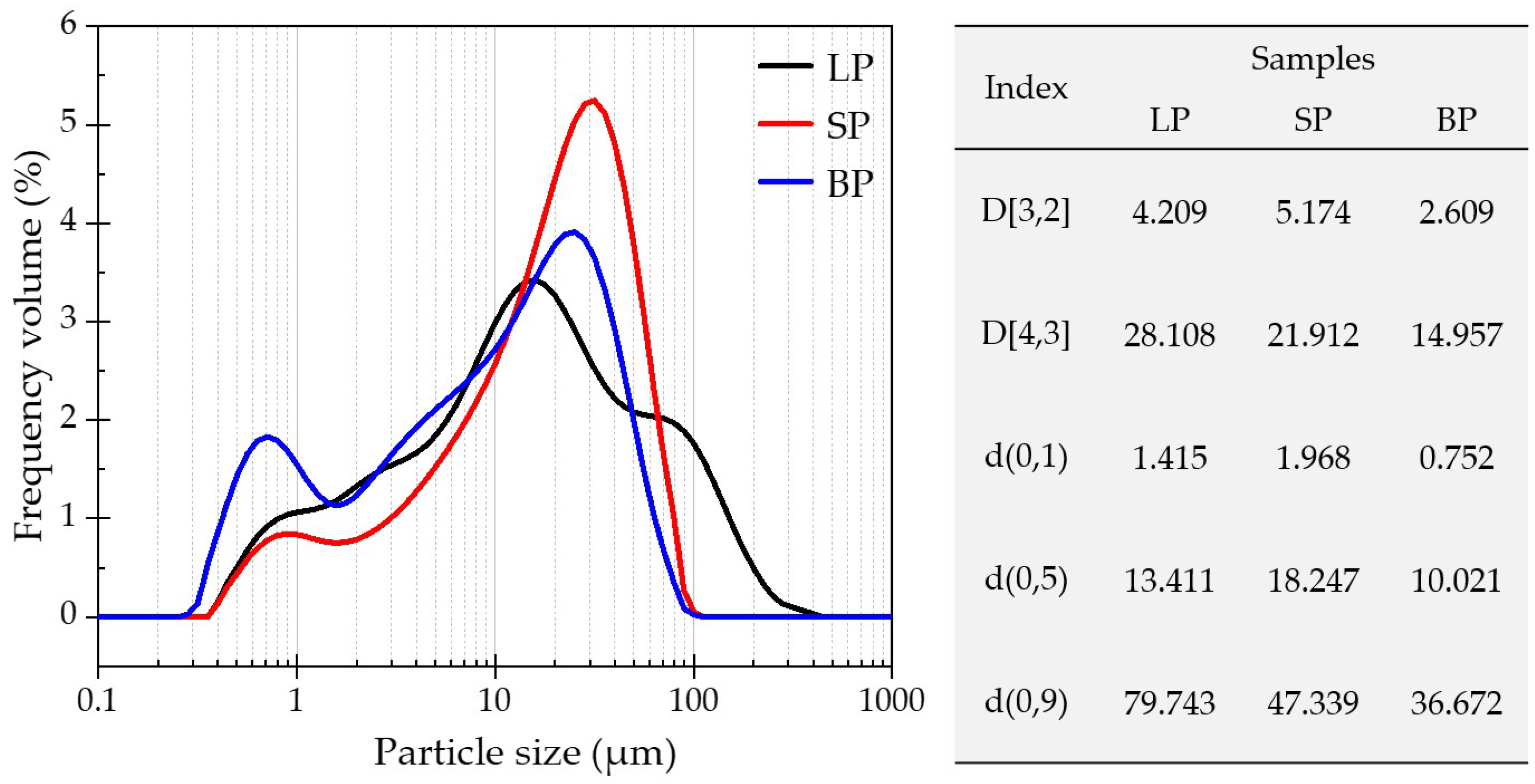
3.1.1. Appearance, Pores, and Particle Size Distribution
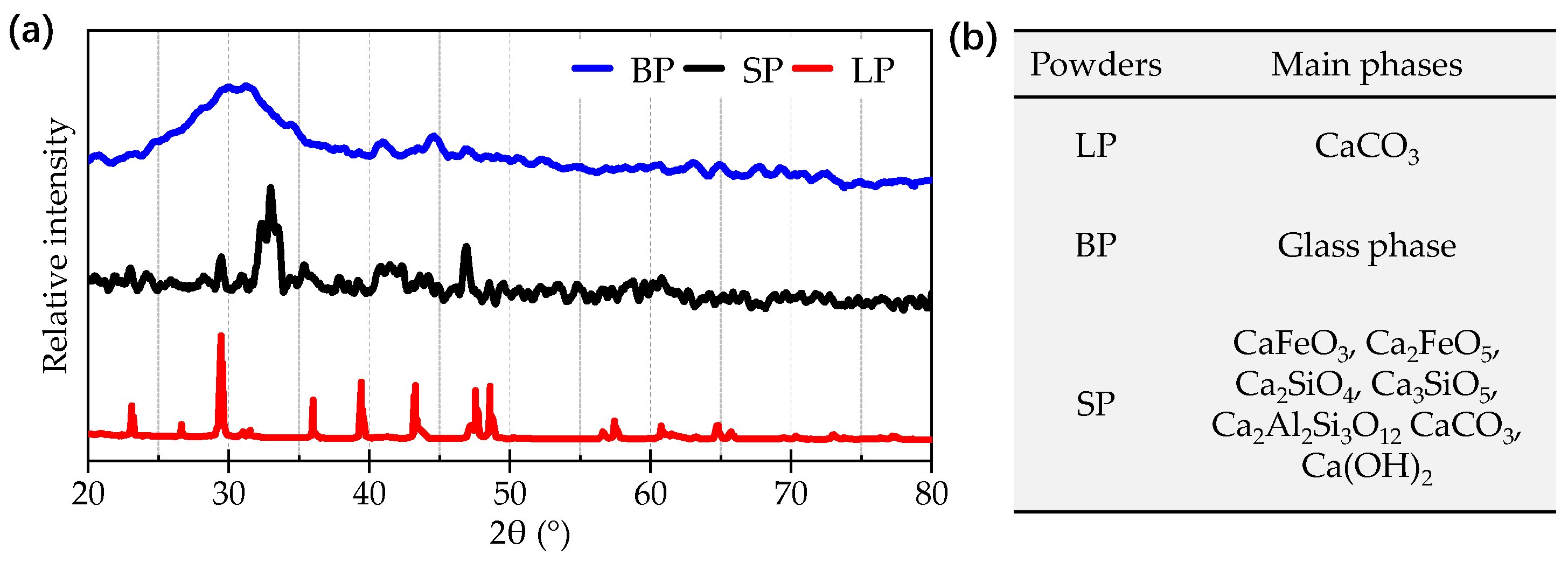
3.1.2. Chemical Compositions and Phase Distributions
3.2. Adhesion Property
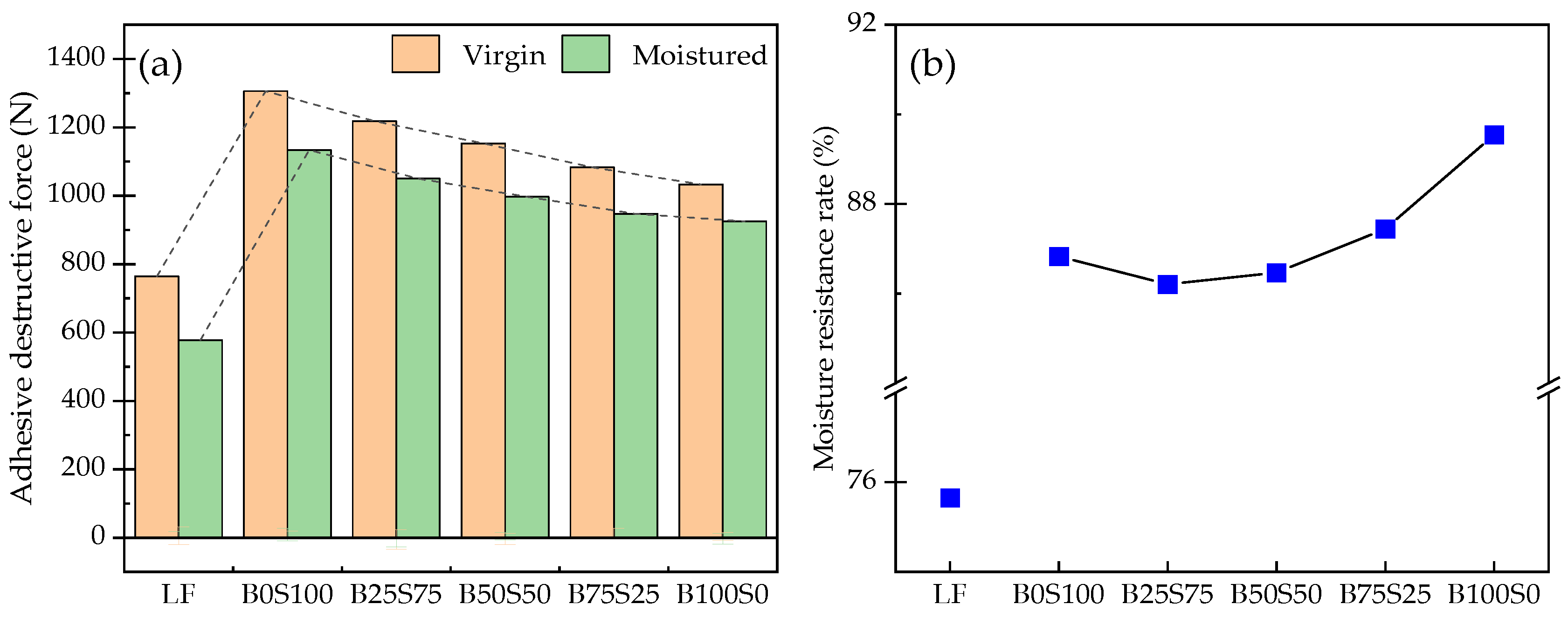
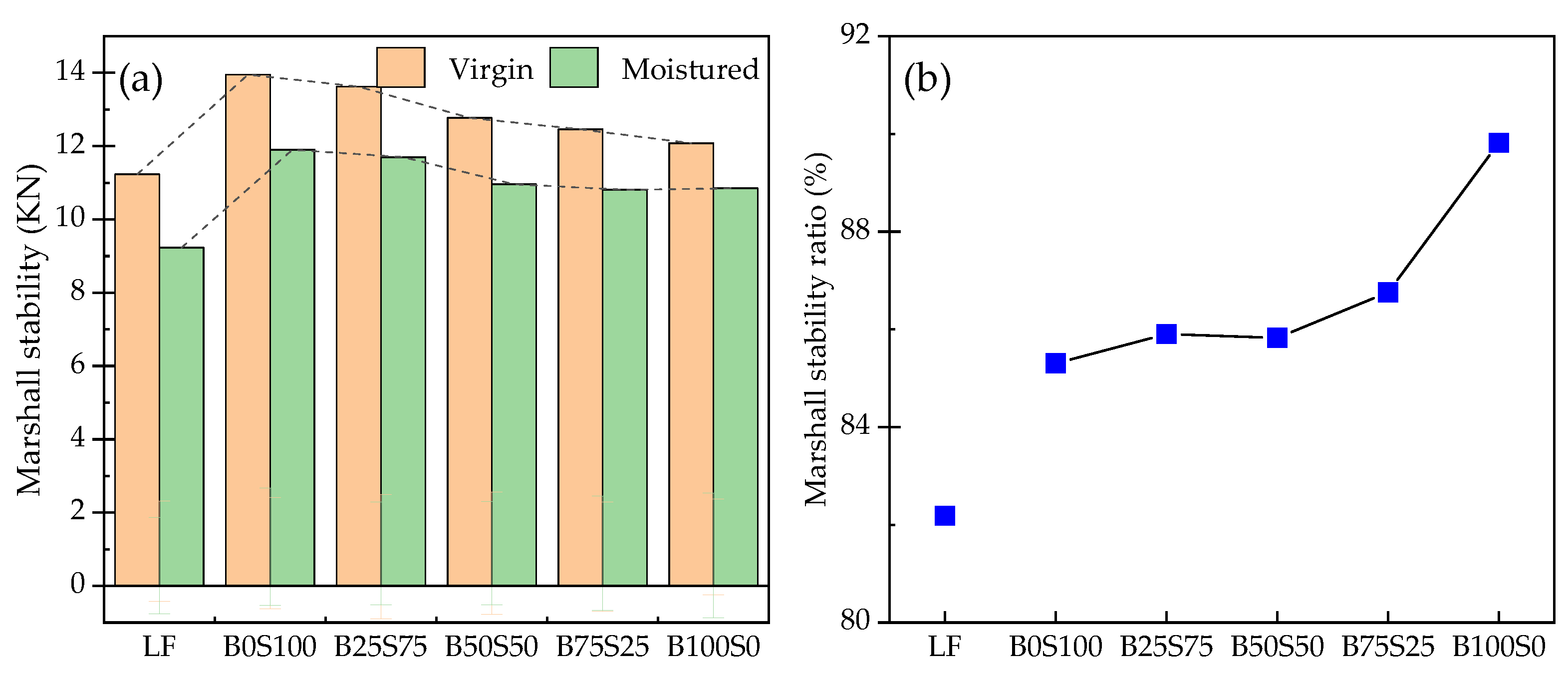
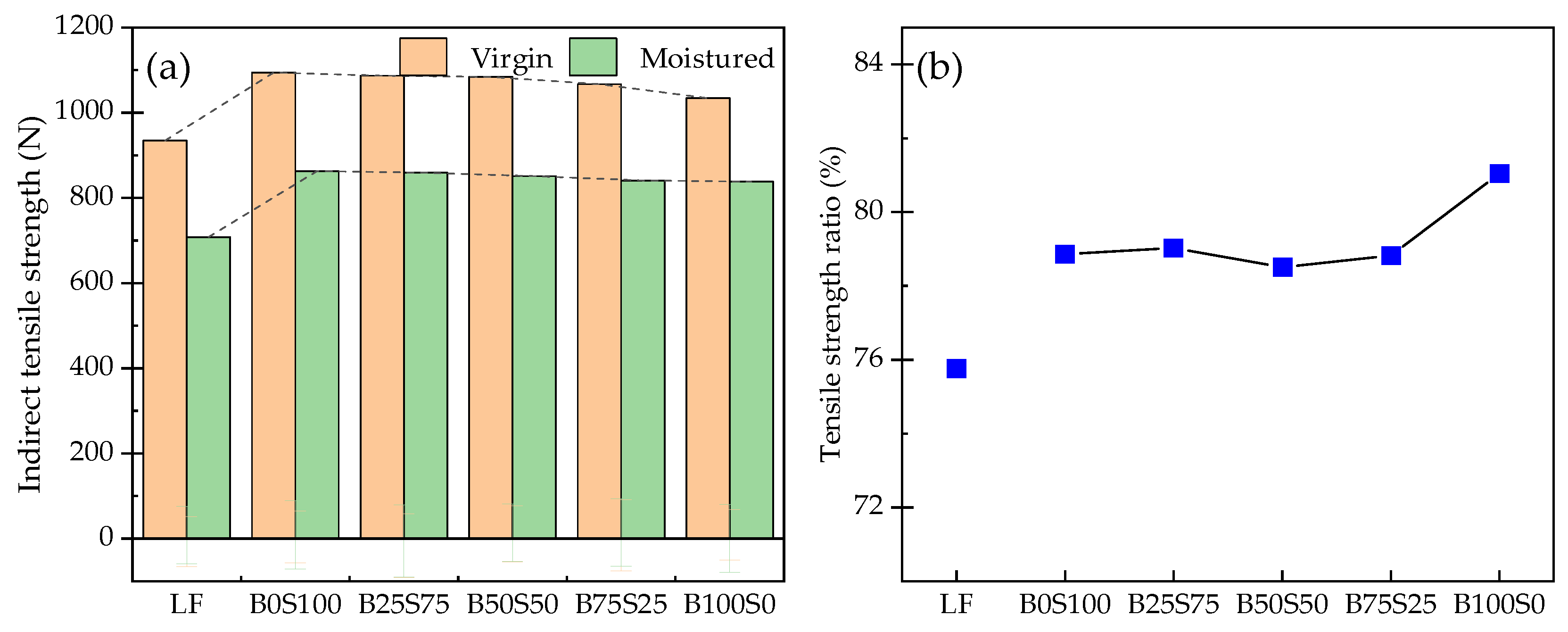
3.3. Moisture Susceptibility
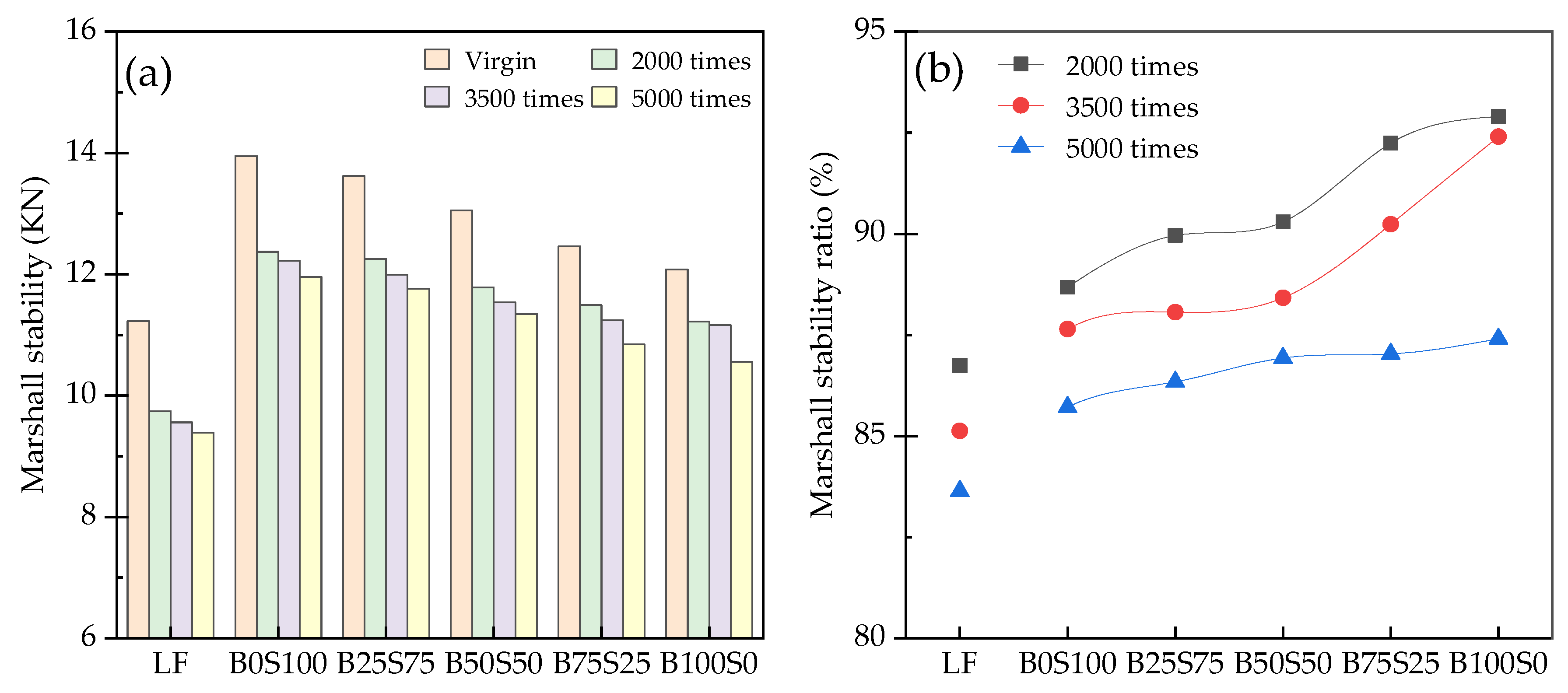
3.4. Dynamic Moisture Damage Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, J.; Chen, J.; Hu, L.; Wu, S.; Wang, Z.; Li, M.; Yang, C. Preparation, Thermochromic Properties and Temperature Controlling Ability of Novel Pellets in Ultra-Thin Wearing Course. Constr. Build. Mater. 2023, 389, 131797. [Google Scholar] [CrossRef]
- European Union Road Federation Road Asset Management: An ERF Position Paper for Maintaining and Improving a Sustainable and Efficient Road Network. Available online: https://trid.trb.org/View/1357497 (accessed on 11 July 2024).
- European Commission. Directorate General for Regional and Urban Policy. In Road Infrastructure in Europe: Road Length and Its Impact on Road Performance; Publications Office: Luxembourg, 2022. [Google Scholar]
- Ministry of Transport of the People’s Republic of China. Statistical Bulletin on the Development of Transportation Industry in 2022. Available online: https://xxgk.mot.gov.cn/2020/jigou/zhghs/202306/t20230615_3847023.html (accessed on 18 June 2023).
- EAPA. Manifesto of the European Asphalt Pavement Association on the Occasion of Its 50th Anniversary 1973–2023. Available online: https://eapa.org/eapa-manifesto-2023-view/ (accessed on 11 July 2024).
- Xu, H.; Zou, Y.; Airey, G.; Wang, H.; Zhang, H.; Wu, S.; Chen, A. Wetting of Bio-Rejuvenator Nanodroplets on Bitumen: A Molecular Dynamics Investigation. J. Clean. Prod. 2024, 444, 141140. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Xie, J.; Amirkhanian, S.; Zhao, Z.; Xu, H.; Wang, F.; Zhang, L. Development of Blending Model for RAP and Virgin Asphalt in Recycled Asphalt Mixtures via a Micron-Fe3O4 Tracer. J. Clean. Prod. 2023, 383, 135407. [Google Scholar] [CrossRef]
- Sun, J.; Luo, S.; Wang, Y.; Dong, Q.; Zhang, Z. Pre-Treatment of Steel Slag and Its Applicability in Asphalt Mixtures for Sustainable Pavements. Chem. Eng. J. 2023, 476, 146802. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive Utilization of Steel Slag: A Review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Cui, P.; Amirkhanian, S.; Zhao, Z.; Wang, F.; Zhang, L.; Wei, M.; Zhou, X.; Xie, J. Performance Characterization and Enhancement Mechanism of Recycled Asphalt Mixtures Involving High RAP Content and Steel Slag. J. Clean. Prod. 2022, 336, 130484. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Liu, Q.; Wang, S.; Yu, B. Steel Slag for Roadway Construction: A Review of Material Characteristics and Application Mechanisms. J. Mater. Civ. Eng. 2022, 34, 03122001. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, D.; Miao, Y.; Sani, B.M.; Zhang, K. Development and Characterization of a Novel Steel Slag-Based Composite Phase Change Aggregate for Snow/Ice Melting of Asphalt Pavements. Constr. Build. Mater. 2022, 341, 127769. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, I.; Lastra-González, P.; Indacoechea-Vega, I.; Castro-Fresno, D. Recyclability Potential of Asphalt Mixes Containing Reclaimed Asphalt Pavement and Industrial By-Products. Constr. Build. Mater. 2019, 195, 148–155. [Google Scholar] [CrossRef]
- Skaf, M.; Manso, J.M.; Aragon, A.; Fuente-Alonso, J.A.; Ortega-Lopez, V. EAF Slag in Asphalt Mixes: A Brief Review of Its Possible Re-Use. Resour. Conserv. Recycl. 2017, 120, 176–185. [Google Scholar] [CrossRef]
- Xu, H.; Chen, A.; Wu, S.; Li, Y.; Li, J.; Zhu, Y.; Wu, J.; Zhou, Y.; Feng, J. Mechanism of Asphalt Concrete Reinforced with Industrially Recycled Steel Slag from the Perspectives of Adhesion and Skeleton. Constr. Build. Mater. 2024, 424, 135899. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, H.M.; Suh, Y.C.; Lee, J.-S. Performance Evaluation of Asphalt Mixtures with 100% Eaf and Bof Steel Slag Aggregates Using Laboratory Tests and Mechanistic Analyses. Ksce J. Civ. Eng. 2022, 26, 4542–4551. [Google Scholar] [CrossRef]
- Huang, Y.; Bird, R.N.; Heidrich, O. A Review of the Use of Recycled Solid Waste Materials in Asphalt Pavements. Resour. Conserv. Recycl. 2007, 52, 58–73. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Shen, A.; Yang, X.; Cui, T. Study of the Long-Term Water Stability of Asphalt Mixtures Containing Steel Slag Aggregate. J. Adhes. Sci. Technol. 2020, 34, 877–902. [Google Scholar] [CrossRef]
- Chen, Z.; Leng, Z.; Jiao, Y.; Xu, F.; Lin, J.; Wang, H.; Cai, J.; Zhu, L.; Zhang, Y.; Feng, N.; et al. Innovative Use of Industrially Produced Steel Slag Powders in Asphalt Mixture to Replace Mineral Fillers. J. Clean. Prod. 2022, 344, 131124. [Google Scholar] [CrossRef]
- Riz de Moura, B.L.; Sudo Lutif Teixeira, J.E.; Simao, R.A.; Khedmati, M.; Kim, Y.-R.; Moreira Pires, P.J. Adhesion between Steel Slag Aggregates and Bituminous Binder Based on Surface Characteristics and Mixture Moisture Resistance. Constr. Build. Mater. 2020, 264, 120685. [Google Scholar] [CrossRef]
- Coomarasamy, A.; Walzak, T.L. Effects of Moisture on Surface Chemistry of Steel Slags and Steel Slag-Asphalt Paving Mixes. Transp. Res. Rec. 1995, 85–95. Available online: https://trid.trb.org/view/452574 (accessed on 11 July 2024).
- Hesami, S.; Ameri, M.; Goli, H.; Akbari, A. Laboratory Investigation of Moisture Susceptibility of Warm-Mix Asphalt Mixtures Containing Steel Slag Aggregates. Int. J. Pavement Eng. 2015, 16, 745–759. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Y.; Rahman, A.; Ding, H. Application of Steel Slag Powder to Enhance the Low-Temperature Fracture Properties of Asphalt Mastic and Its Corresponding Mechanism. J. Clean. Prod. 2018, 184, 21–31. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, M.; Wu, S.; Xie, J.; Kong, D.; Qiao, Z.; Niu, C. Moisture Susceptibility Evaluation of Asphalt Mixtures Containing Steel Slag Powder as Filler. Materials 2019, 12, 3211. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, S.; Chen, A.; Liu, Q.; Amirkhanian, S.; Xu, S.; Yang, C.; Wan, P.; Xu, H.; Lu, Z. Comprehensive Properties Assessment of Asphalt Binder under Aqueous Solutions with Different pH Values and Its Gradient Damage Behaviors. Constr. Build. Mater. 2024, 414, 134938. [Google Scholar] [CrossRef]
- Zhang, G.; Qiu, J.; Zhao, J.; Wei, D.; Wang, H. Development of Interfacial Adhesive Property by Novel Anti-Stripping Composite between Acidic Aggregate and Asphalt. Polymers 2020, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.A.; Haghshenas, H.F.; Underwood, B.S.; Harvey, J.; Blankenship, P. Performance of Warm Asphalt Mixtures Containing Reclaimed Asphalt Pavement, an Anti-Stripping Agent, and Recycling Agents: A Study Using a Balanced Mix Design Approach. Constr. Build. Mater. 2023, 363, 129633. [Google Scholar] [CrossRef]
- Li, J.; Xiao, F.; Amirkhanian, S.N.; Xu, O. Dynamic and Rutting Characteristics of Recycled Asphalt Mixtures Containing Natural Sand and Anti-Stripping Agents. J. Clean. Prod. 2021, 280, 124365. [Google Scholar] [CrossRef]
- Mahto, S.K.; Sinha, S. Application of Marble Dust and Ground Granulated Blast-Furnace Slag in Emulsified Asphalt Warm Mixtures. J. Clean. Prod. 2022, 370, 133532. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, W.; Yuan, Q.; Chen, W.; Wan, J.; Guo, J.; Cai, J. Effect of Carbon Dioxide Mineralization Curing on Mechanical Properties and Microstructure of Portland Cement–Steel Slag–Granulated Blast Furnace Slag Ternary Paste. Constr. Build. Mater. 2024, 431, 136553. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, G.; Wang, C.; Zhang, Y.; Wang, P.; Yan, N. Activation Effects and Micro Quantitative Characterization of High-Volume Ground Granulated Blast Furnace Slag in Cement-Based Composites. Cem. Concr. Comp. 2020, 109, 103556. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Qiu, J.; Xing, J. Review: Alkali-Activated Blast Furnace Slag for Eco-Friendly Binders. J. Mater. Sci. 2022, 57, 1599–1622. [Google Scholar] [CrossRef]
- Alexander Rondon-Quintana, H.; Carlos Ruge-Cardenas, J.; de Farias, M.M. Behavior of Hot-Mix Asphalt Containing Blast Furnace Slag as Aggregate: Evaluation by Mass and Volume Substitution. J. Mater. Civ. Eng. 2019, 31, 04018364. [Google Scholar] [CrossRef]
- Gong, K.; White, C.E. Impact of Chemical Variability of Ground Granulated Blast-Furnace Slag on the Phase Formation in Alkali-Activated Slag Pastes. Cem. Concr. Res 2016, 89, 310–319. [Google Scholar] [CrossRef]
- Du, S. Mechanical Properties and Reaction Characteristics of Asphalt Emulsion Mixture with Activated Ground Granulated Blast-Furnace Slag. Constr. Build. Mater. 2018, 187, 439–447. [Google Scholar] [CrossRef]
- Nassar, A.I.; Mohammed, M.K.; Thom, N.; Parry, T. Mechanical, Durability and Microstructure Properties of Cold Asphalt Emulsion Mixtures with Different Types of Filler. Constr. Build. Mater. 2016, 114, 352–363. [Google Scholar] [CrossRef]
- Dulaimi, A.; Shanbara, H.K.; Al-Rifaie, A. The Mechanical Evaluation of Cold Asphalt Emulsion Mixtures Using a New Cementitious Material Comprising Ground-Granulated Blast-Furnace Slag and a Calcium Carbide Residue. Constr. Build. Mater. 2020, 250, 118808. [Google Scholar] [CrossRef]
- Lu, D.; Wang, Y.; Leng, Z.; Zhong, J. Influence of Ternary Blended Cementitious Fillers in a Cold Mix Asphalt Mixture. J. Clean. Prod. 2021, 318, 128421. [Google Scholar] [CrossRef]
- Shaygan, S.; Izadi, A.; Zalnezhad, M. Performance and Environmental Assessment of Microsurfacing Mixture Using the Granulated Blast-Furnace Slag Powder (GBSP) as Potential Recycled Filler. Constr. Build. Mater. 2022, 359, 129502. [Google Scholar] [CrossRef]
- Huang, J.; Xing, X.; Cai, J.; Pei, J.; Li, R.; Wen, Y. Utilization of Water-Quenching Blast Furnace Slag as Alternative Filler in Asphalt Mastic. Can. J. Civ. Eng. 2020, 47, 1075–1083. [Google Scholar] [CrossRef]
- Ou, L.; Zhu, H.; Chen, R.; Su, C.; Yang, X. Effect of Industrial Solid Waste as Fillers on the Rheology and Surface Free Energy of Asphalt Mastic. Materials 2024, 17, 1125. [Google Scholar] [CrossRef] [PubMed]
- Chegenizadeh, A.; Hanson, S.W.; Nikraz, H.; Scott Kress, C. Effects of Ground-Granulated Blast-Furnace Slag Used as Filler in Dense Graded Asphalt. Appl. Sci. 2022, 12, 2769. [Google Scholar] [CrossRef]
- Ministry of Transport of the People’s Republic of China. Technical Specification for Construction of Highway Asphalt Pavements; Ministry of Transport of the People’s Republic of China: Beijing, China, 2004.
- Tao, G.; Xiao, Y.; Yang, L.; Cui, P.; Kong, D.; Xue, Y. Characteristics of Steel Slag Filler and Its Influence on Rheological Properties of Asphalt Mortar. Constr. Build. Mater. 2019, 201, 439–446. [Google Scholar] [CrossRef]
- ASTM D6926-20; Standard Practice for Preparation of Asphalt Mixture Specimens Using Marshall Apparatus. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM D4867/D4867M-22; Standard Test Method for Effect of Moisture on Asphalt Mixtures. ASTM: West Conshohocken, PA, USA, 2022. [CrossRef]
- ASTM D1075-07; Standard Test Method for Effect of Moisture on Asphalt Mixtures. ASTM: West Conshohocken, PA, USA, 2011. [CrossRef]


| Materials | Properties | Values | Requirements |
|---|---|---|---|
| AH-70 asphalt | Penetration (25 °C, 0.1 mm) | 65.9 | 60~80 |
| Ductility (15 °C, cm) | >100 | ≥40 | |
| Softening point (°C) | 46.1 | 43 | |
| Density (g/cm3) | 1.032 | - | |
| Basalt | Coarse aggregates density (g/cm3) | 2.89 | ≥2.5 |
| Fine aggregates density (g/cm3) | 2.87 | ≥2.6 | |
| Los Angeles abrasion | 16.5 | ≤28 | |
| Crush values | 14.9 | ≤26 |
| Properties | Powders | Requirements | ||
|---|---|---|---|---|
| LP | BP | SP | ||
| Density (g/cm3) | 2.691 | 2.912 | 3.787 | ≥2.5 |
| Hydrophilic coefficient | 0.716 | 0.742 | 0.696 | <1.0 |
| Water content (%) | 0.423 | 0.554 | 0.783 | ≤1.0 |
| Formula | Abbreviation | |||||
|---|---|---|---|---|---|---|
| LF | B0S100 | B25S75 | B50S50 | B75S25 | B100S0 | |
| Fillers–asphalt ratio | 30% | |||||
| LP | 100% | / | / | / | / | / |
| BP | / | / | 25% | 50% | 75% | 100% |
| SP | / | 100% | 75% | 50% | 25% | 0% |
| Distilled Water | Formamide | Glycerol | Glycol | |
|---|---|---|---|---|
| 1 (mJ/m2) | 21.8 | 39.0 | 34.0 | 29.3 |
| 2 (mJ/m2) | 51.0 | 19.0 | 30.0 | 19.0 |
| 3 (mJ/m2) | 72.8 | 58.0 | 64.0 | 48.3 |
| Sample | Contact Angle (°) | SFE (mJ/m2) | R2 | ||||
|---|---|---|---|---|---|---|---|
| Distilled Water | Formamide | Glycol | 1 | 2 | 3 | ||
| Limestone | 75.2 | 58.1 | 39.9 | 26.93 | 9.16 | 36.09 | 0.9947 |
| Basalt | 76.3 | 62.2 | 46.8 | 22.07 | 10.55 | 32.62 | 0.9987 |
| Granite | 72.8 | 60.7 | 48.1 | 18.65 | 14.33 | 32.98 | 0.9962 |
| Andesite | 74.1 | 59.5 | 44.6 | 22.45 | 11.61 | 34.06 | 0.9997 |
| Compound | CaO | Fe2O3 | SiO2 | MgO | Al2O3 | Others | |
|---|---|---|---|---|---|---|---|
| Proportion (wt%) | LP | 55.54 | 1.77 | 1.57 | 0.53 | 0.17 | 40.42 |
| BP | 40.42 | 0.48 | 32.22 | 7.39 | 14.99 | 4.50 | |
| SP | 33.27 | 16.73 | 13.95 | 13.55 | 12.29 | 10.21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, B.; Xu, H.; Wu, S.; Wang, H.; Yang, X.; Chen, P. Research on Improving Moisture Resistance of Asphalt Mixture with Compounded Recycled Metallurgical Slag Powders. Materials 2024, 17, 3499. https://doi.org/10.3390/ma17143499
Gao B, Xu H, Wu S, Wang H, Yang X, Chen P. Research on Improving Moisture Resistance of Asphalt Mixture with Compounded Recycled Metallurgical Slag Powders. Materials. 2024; 17(14):3499. https://doi.org/10.3390/ma17143499
Chicago/Turabian StyleGao, Bo, Haiqin Xu, Shaopeng Wu, Huan Wang, Xinkui Yang, and Pengrui Chen. 2024. "Research on Improving Moisture Resistance of Asphalt Mixture with Compounded Recycled Metallurgical Slag Powders" Materials 17, no. 14: 3499. https://doi.org/10.3390/ma17143499
APA StyleGao, B., Xu, H., Wu, S., Wang, H., Yang, X., & Chen, P. (2024). Research on Improving Moisture Resistance of Asphalt Mixture with Compounded Recycled Metallurgical Slag Powders. Materials, 17(14), 3499. https://doi.org/10.3390/ma17143499










