Mechanism Study on the Effect of Surface Electrical Property on Microbial Membrane Formation Efficiency of TiO2-SiC Composite Filler in Recirculating Aquaculture System
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of TiO2-SiC Composite Filler
2.3. Determination of Chemical Oxygen Demand
2.4. Determination of Ammonia Nitrogen
2.5. Simulation of Aquaculture Wastewater Purification Experiment
2.6. Characterization of the Surface Potential and X-ray Diffraction
3. Results and Discussion
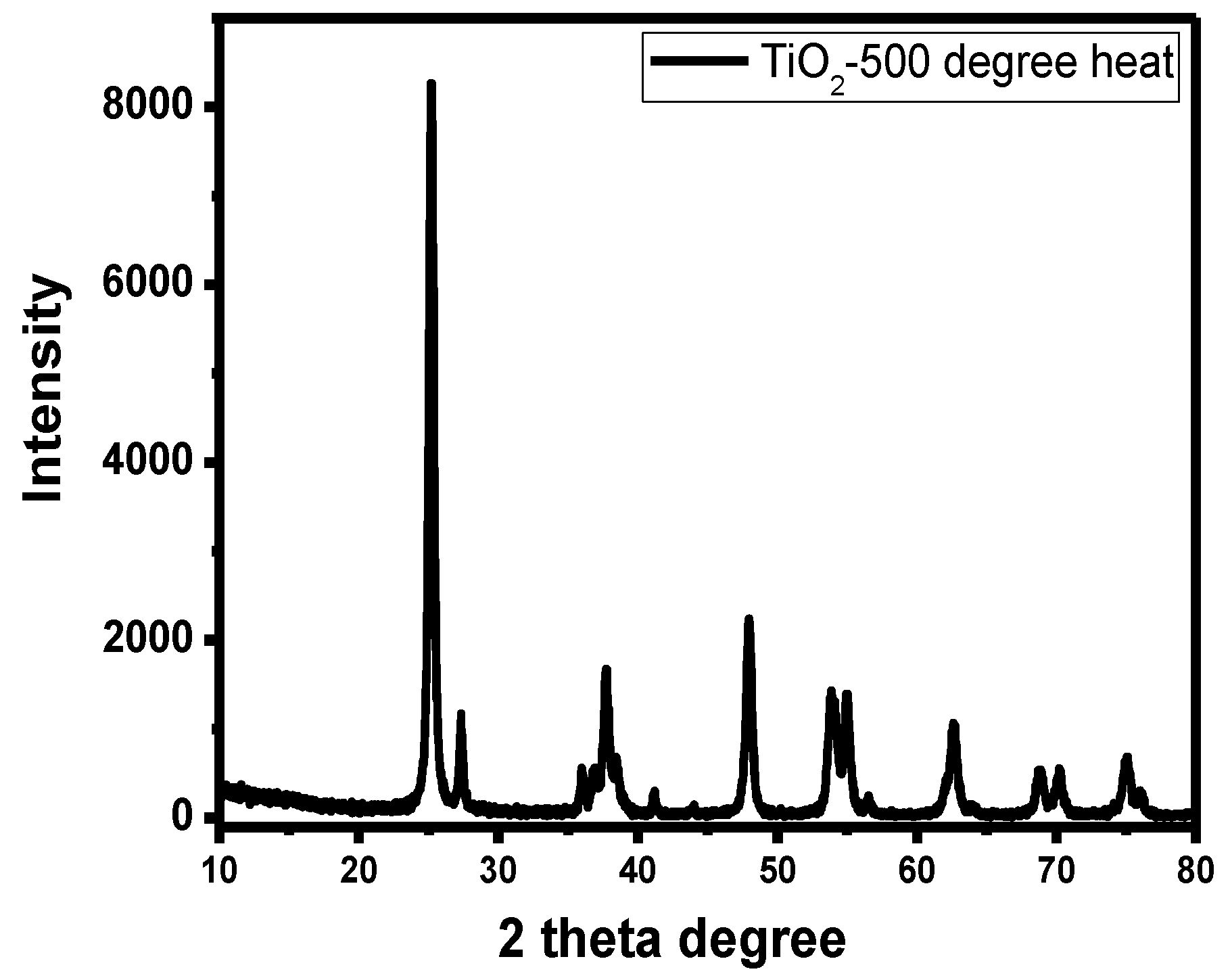
3.1. X-ray Diffraction (XRD) Characterization of Titanium Dioxide Thin Film
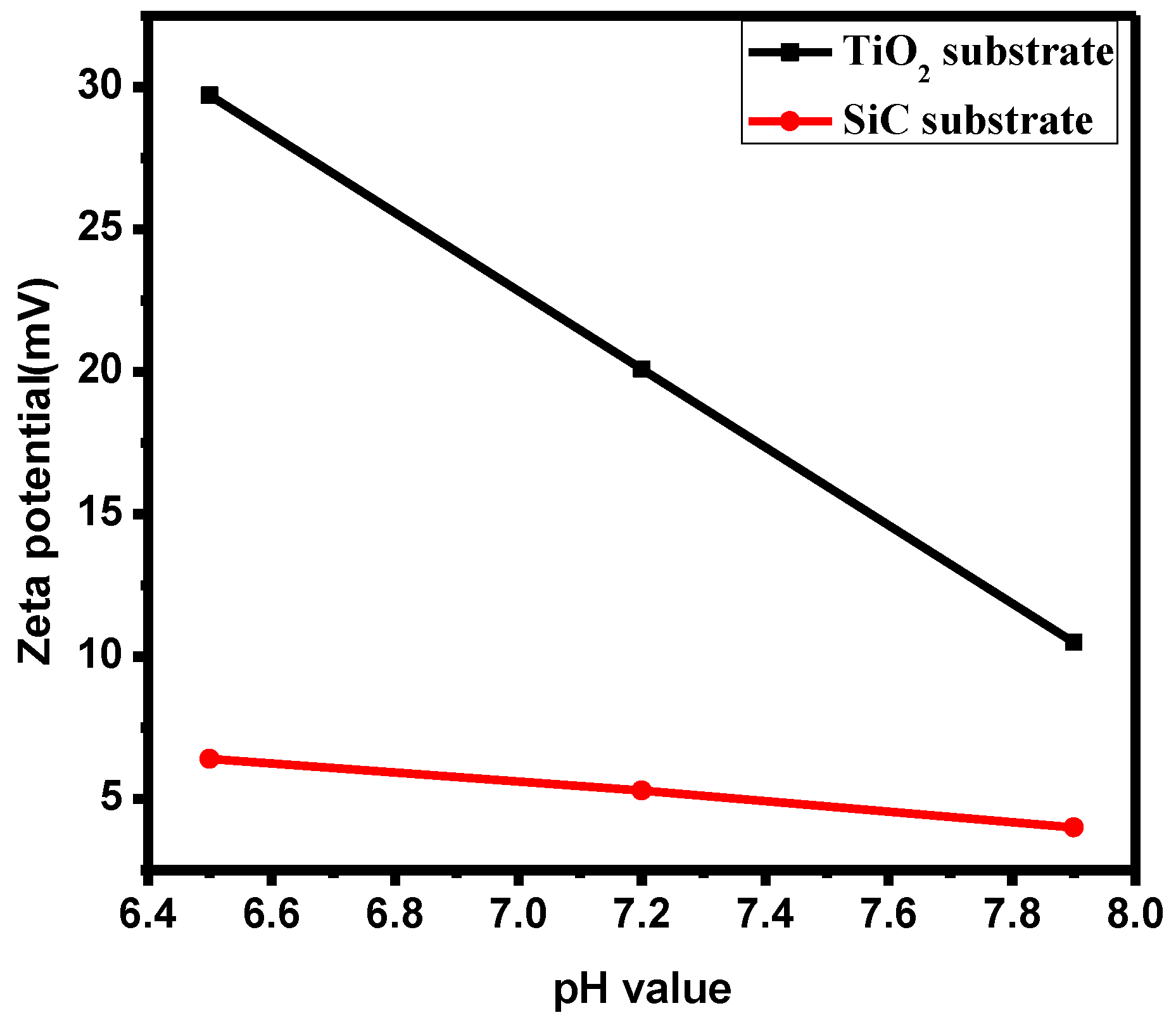
3.2. Surface Electrical Potential Test of Titanium Dioxide Film at Different pH Values
3.3. Total Bacteria Number Record by Fluorescence Microscope Direct Count Method
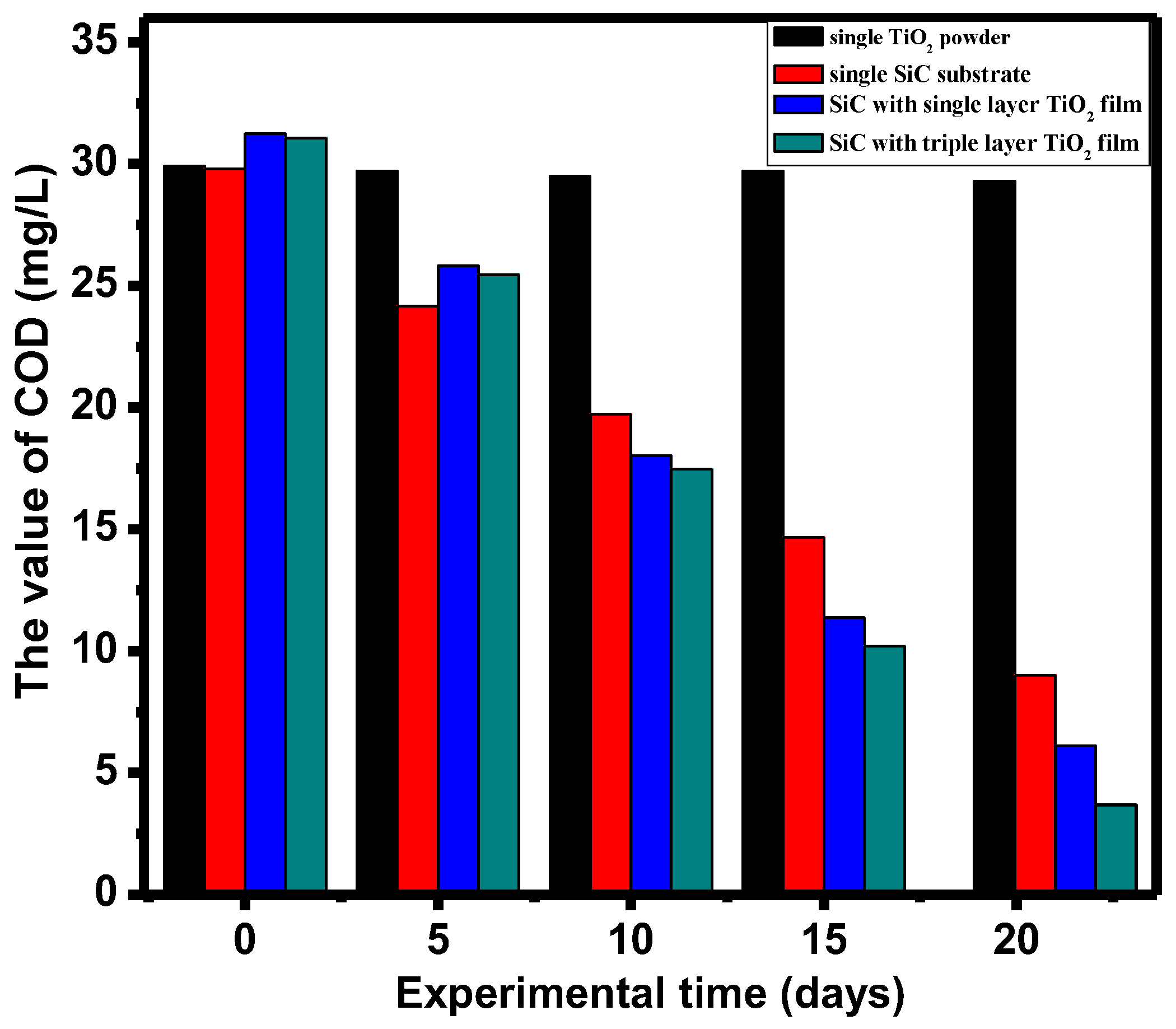
3.4. Chemical Oxygen Demand (COD) Removal Rate
3.5. Ammonia Nitrogen Removal Rate
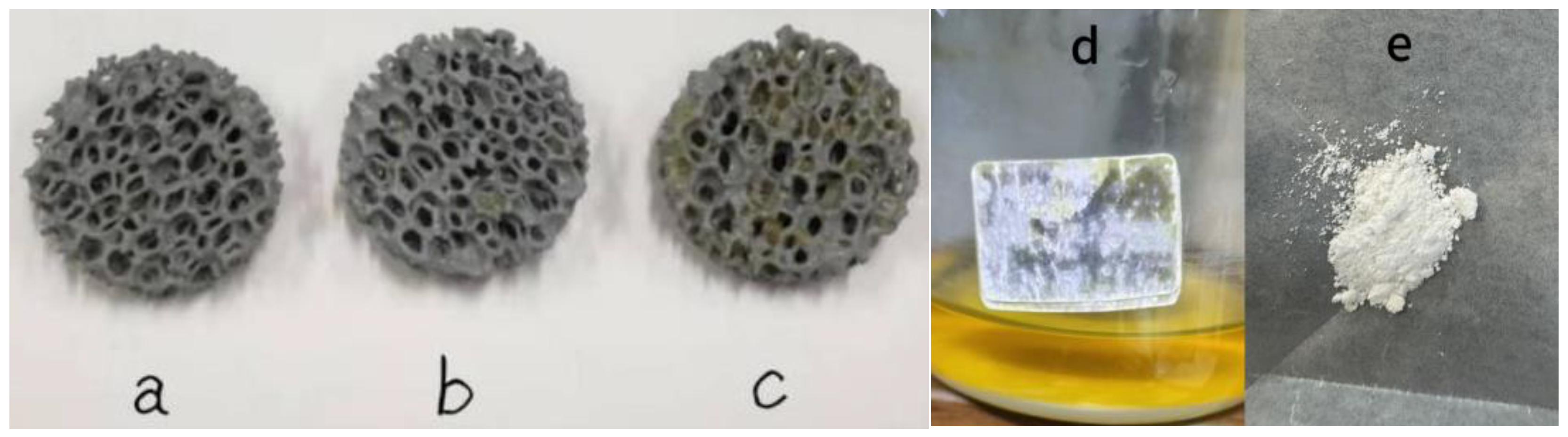
3.6. Membrane Hanging Effects of Three Kinds of TiO2-SiC Composite Fillers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, Z.W.; Wang, W.; Zhao, B.C. Advances in Biological Treatment Technology for Mariculture Wastewater. J. Qingdao Univ. Technol. 2006, 27, 13–17. (In Chinese) [Google Scholar]
- Che, Z.L.; Ren, X.Z.; Zhang, Q. Research Advances on Hydrodynamic Characteristics and Their Interaction with Fish in Recirculating Aquaculture System. J. Dalian Fish. Univ. 2021, 36, 886–898. (In Chinese) [Google Scholar]
- Wang, X.K.; Zhao, T.; Yue, Y. Evaluation of Heavy Metal Pollution Status and Health Risk of Recirculating Aquaculture of Red Fin Oriental Puffer. J. Dalian Fish. Univ. 2022, 37, 303–650. (In Chinese) [Google Scholar]
- Liu, Y. Research Progress of Industrialized Recirculating Aquaculture Technology in Seawater. Rev. China Agric. Sci. Technol. 2011, 13, 50–53. (In Chinese) [Google Scholar]
- Lu, S.Z.; Meng, F.; Li, W.C. Research Progress of Biological Nitrogen Removal Technology in Cultured Water Bodies. J. Fish. Sci. 2006, 4, 210–213. (In Chinese) [Google Scholar]
- Shen, J.Z. Study of Biofilm Growth Regulation and Water Circulation Optimization in Seawater Recirculating Aquaculture System. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2016. (In Chinese). [Google Scholar]
- Yautey, E.; Ten-Hage, L.; Lacoste, B. Analysis of bacterial diversity in fiver biofilms using 16SbrDNA PCR-DGGE: Methodological settings and fingerprints interpretation. Water Res. 2005, 39, 380–388. [Google Scholar]
- Bu, X.F.; Qu, K.M.; Ma, S.S. Treatment Technology of Mariculture Wastewater 1702 No. 8 Zhang Yanqing et al.: Study on Nitrogen Removal Performance and Nitrification Kinetics of Seawater Biofilter and Application Prospects. Mar. Fish. Res. 2003, 24, 85–90. (In Chinese) [Google Scholar]
- Liu, Y.H.; Luo, G.Z.; Zhu, X.B. Microbial Characterization of Biofilters for Seawater Closed-cycle System. J. Agro-Environ. Sci. 2004, 23, 540–544. (In Chinese) [Google Scholar]
- Ogalla, F.; Bourbigot, M. New developments in complete nitrogen removal with biological aerated filters. Water Sci. Technol. 1990, 22, 273–280. [Google Scholar] [CrossRef]
- Eguret, F.; Racault, Y. Hydrodynamic behavior of a full-scale submerged biofilter and its possible influence on performances. Water Sci. Technol. 1998, 38, 249–256. [Google Scholar] [CrossRef]
- Kui, C.Z.; Liu, Y.; Ren, J.F. Effect of Aeration Conditions on Denitrification Performance and Bacterial Population Changes in Iron-based Composite Filler Biofilters. J. Dalian Fish. Univ. 2021, 36, 470–477. (In Chinese) [Google Scholar]
- Liu, D.; Li, J.; Li, C. Poly(butylene succinate)/bamboo powder blends as solid-phase carbon source and biofilm carrier for denitrifying biofilters treating wastewater from recirculating aquaculture system. Sci. Rep. 2018, 8, 3289. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Liu, Z.; Gao, J. Nitrate removal efficiency and bacterial community of polycaprolactone-packed bioreactors treating water from a recirculating aquaculture system. Aquac. Int. 2018, 26, 773–784. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Shen, J.Q.; Zhang, H.G. Study on the Application of PVA-PVP Blended Filler in Recirculating Aquaculture Water Treatment. J. Guangdong Agric. Sci. 2018, 45, 115–121. (In Chinese) [Google Scholar]
- Chang, H.; Wang, J.G. Application of Nano Titanium Dioxide in the Field of Environmental Protection. Mining and metallurgy 2002, 4, 73–76. (In Chinese) [Google Scholar]
- Stevenson, M.; Bullock, K.; Lin, W.Y. Sonolytic enhancement of the bactericidal activity of irradiated titanium dioxide suspensions in water. Res. Chem. Intermed. 1997, 23, 311–323. [Google Scholar] [CrossRef]
- Li, X.P.; Xu, B.K.; Liu, G.F. Research and Development of Nanometer Titanium Dioxide Photocatalytic Degradation of Organic Pollutants in Water. J. Funct. Mater. 1999, 3, 19–22+25. (In Chinese) [Google Scholar]
- Song, X.H.; Su, P.B.; Ju, P.F.; Wang, E.T.; Li, C.G. Research Status of Nanometer Titanium Dioxide Self-Cleaning Coating. Hot Work. Technol. 2017, 6, 20–23. (In Chinese) [Google Scholar]
- Zhang, X.; Zhao, Y.; Zhang, C.B. Preparation and Photocatalytic Performance of Titanium Dioxide Nanomaterials Under the Control of Organic Molecules. Hot Work. Technol. 2003, 4, 85–87. (In Chinese) [Google Scholar]
- She, J.H.; Jiang, D.L. Development and Application of Silicon Carbide Ceramics. Ceram. Eng. 1998, 3, 3–11+28. (In Chinese) [Google Scholar]
- Li, D.; Zhang, J.; Yuan, C.J. Preparation of Nitrogen-doped Titanium Dioxide Nanofilms by Ultrasonic Spray Pyrolysis and Its Photocatalytic Properties. Phys. Exp. 2010, 7, 17–21. (In Chinese) [Google Scholar]
- Li, D.; Zhang, J.; Shao, L.X.; Bian, H.F.; Li, D.K.; Yang, Y.Z. Preparation of Titanium Dioxide Photocatalytic Self-cleaning Ceramics by Ultrasonic Spray Pyrolysis and Its Performance. Mater. Res. Appl. 2010, 4, 613–617. (In Chinese) [Google Scholar]
- Fan, C.Z.; Xiao, J.P.; Ding, Y.W. Progress in the Preparation and Photocatalytic Reaction of Nanometer Titanium Dioxide. Sci. Bull. 2001, 4, 265–273. (In Chinese) [Google Scholar]
- Tao, N.; Kang, B.; Wang, L.W. Nanometer Titanium Dioxide Preparation and Application Technology Development. Sichuan Metall. 2002, 2, 52–56. (In Chinese) [Google Scholar]
- Ding, H.C.; Chai, Y.H.; Zhang, Z.H. Determination of Chemical Oxygen Demand in Surface Water by Photocatalytic Oxidation. Chinese. J. Chem. 2005, 2, 56–60+3. (In Chinese) [Google Scholar]
- Zhao, H.P.; Li, Q.X.; Tao, J.H. Fluorescent Microscopic Counting of Marine Bacteria and Its Application. J. Hebei Univ. Eng. (Nat. Sci. Ed.) 2007, 1, 59–62. (In Chinese) [Google Scholar]
- Song, B.B. Comparative Study on the Efficiency of Biofilter with Four Fillers for Simulated Mariculture Wastewater Treatment. Master’s Thesis, Graduate School of Oceanology, Chinese Academy of Sciences, Beijing, China, 2009. (In Chinese). [Google Scholar]
- GB17378.7-2007; Ecological Survey for Offshore Pullution and Biological Monitoring. National Standards of People’s Republic of China: Beijing, China, 2007; part 7.
- Yang, X.F.; Dan, D.Z. Current Status and Recent Progress of Chemical Oxygen Demand (COD) Determination Method. J. Chongqing Environ. Sci. 1997, 4, 57–61. (In Chinese) [Google Scholar]
- Xu, B.; Xu, H.S. Application and Development of Fluorescent Microscopic Counting Method for Aquatic Bacteria. Microbiology 1993, 6, 36–41. (In Chinese) [Google Scholar]


| Sample Number | Sample Amount/mL | Count Record | Mean | Bacteria Number/ (Bacteria/0.0001 mm2) | ||||
|---|---|---|---|---|---|---|---|---|
| a | 0.2 | 6 | 7 | 6 | 11 | 11 | 7 | 3452 |
| 4 | 4 | 5 | 8 | 7 | ||||
| 3 | 6 | 3 | 16 | 6 | ||||
| 12 | 7 | 3 | 7 | 2 | ||||
| b | 0.2 | 12 | 20 | 23 | 25 | 15 | 15 | 7397 |
| 16 | 15 | 17 | 11 | 16 | ||||
| 10 | 9 | 11 | 6 | 20 | ||||
| 15 | 14 | 19 | 16 | 5 | ||||
| c | 0.2 | 36 | 20 | 16 | 12 | 17 | 21 | 10,355 |
| 14 | 8 | 18 | 15 | 20 | ||||
| 39 | 6 | 13 | 5 | 11 | ||||
| 15 | 21 | 17 | 14 | 106 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hong, Z.; Ouyang, J.; Zheng, H.; Liu, Y. Mechanism Study on the Effect of Surface Electrical Property on Microbial Membrane Formation Efficiency of TiO2-SiC Composite Filler in Recirculating Aquaculture System. Materials 2024, 17, 3501. https://doi.org/10.3390/ma17143501
Li J, Hong Z, Ouyang J, Zheng H, Liu Y. Mechanism Study on the Effect of Surface Electrical Property on Microbial Membrane Formation Efficiency of TiO2-SiC Composite Filler in Recirculating Aquaculture System. Materials. 2024; 17(14):3501. https://doi.org/10.3390/ma17143501
Chicago/Turabian StyleLi, Jiaxin, Ze Hong, Jingying Ouyang, Han Zheng, and Ying Liu. 2024. "Mechanism Study on the Effect of Surface Electrical Property on Microbial Membrane Formation Efficiency of TiO2-SiC Composite Filler in Recirculating Aquaculture System" Materials 17, no. 14: 3501. https://doi.org/10.3390/ma17143501




