Investigating the Effects of the Physicochemical Properties of Cellulose-Derived Biocarbon on Direct Carbon Solid Oxide Fuel Cell Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Preparation of Biochar Samples by Thermal Processing Cellulose Raw Materials in an Inert Gas Atmosphere
2.2. Chemical and Structure Analyses of All Samples
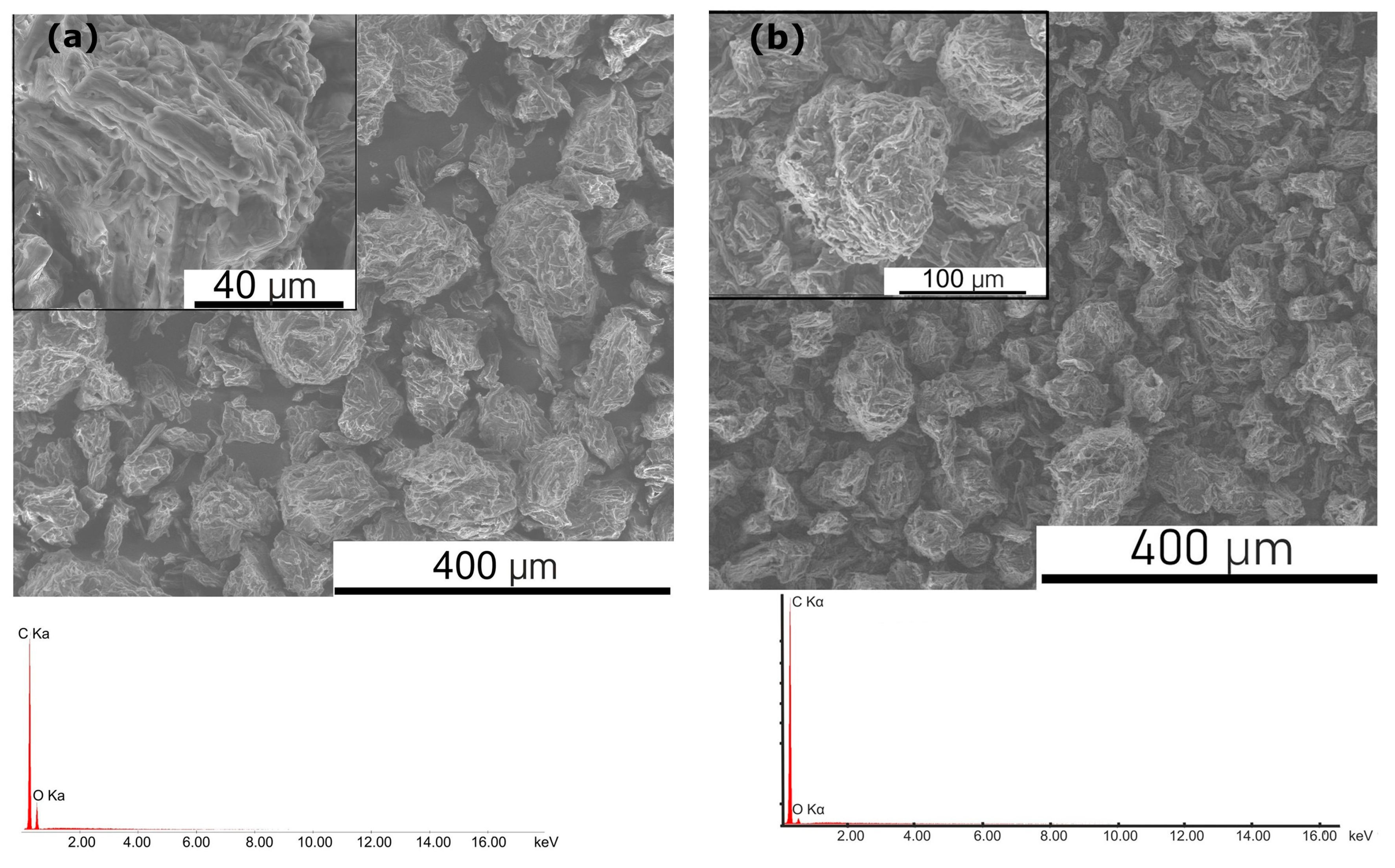
2.3. Scanning Electron Microscopy Investigations of the Obtained Samples
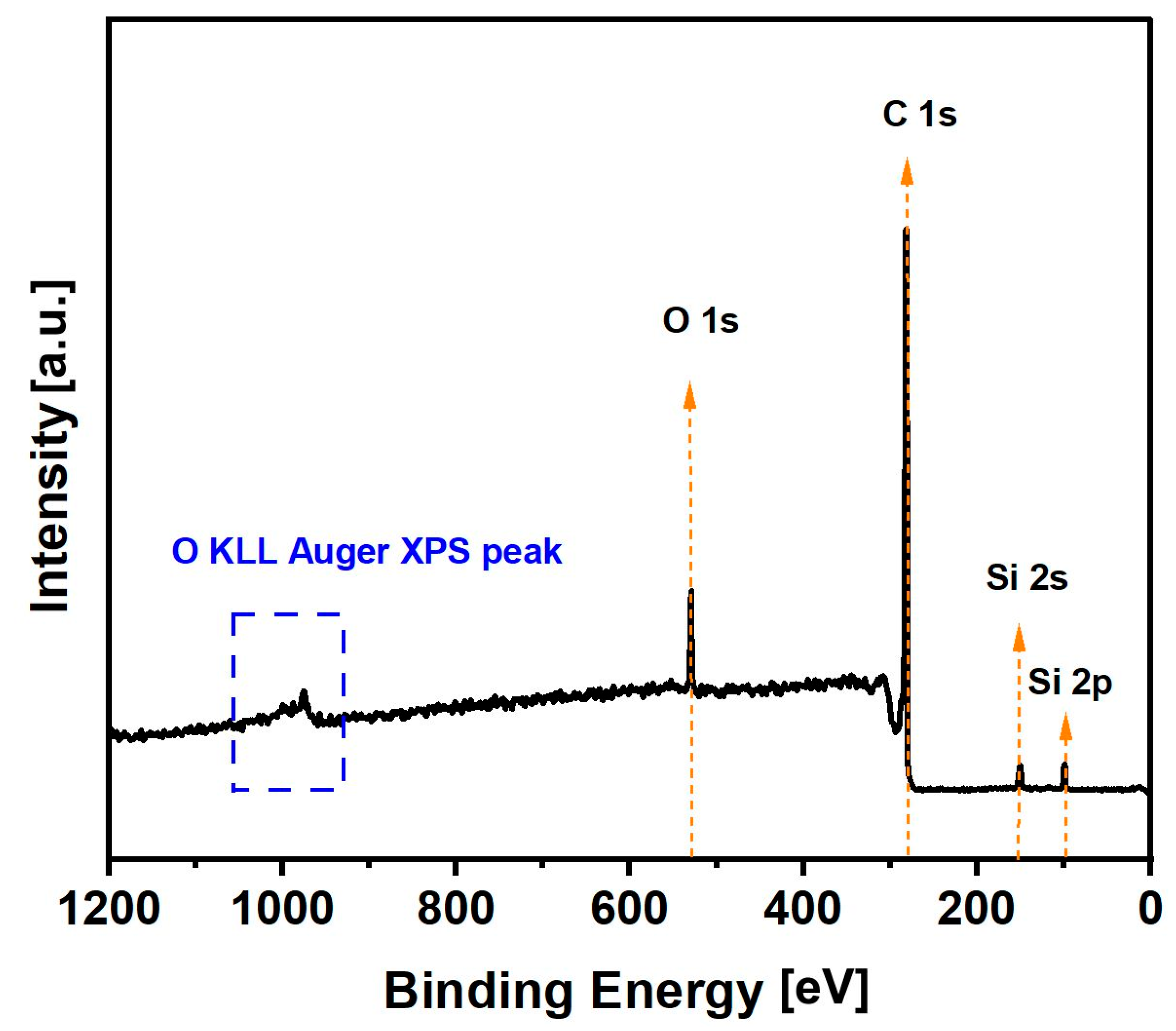
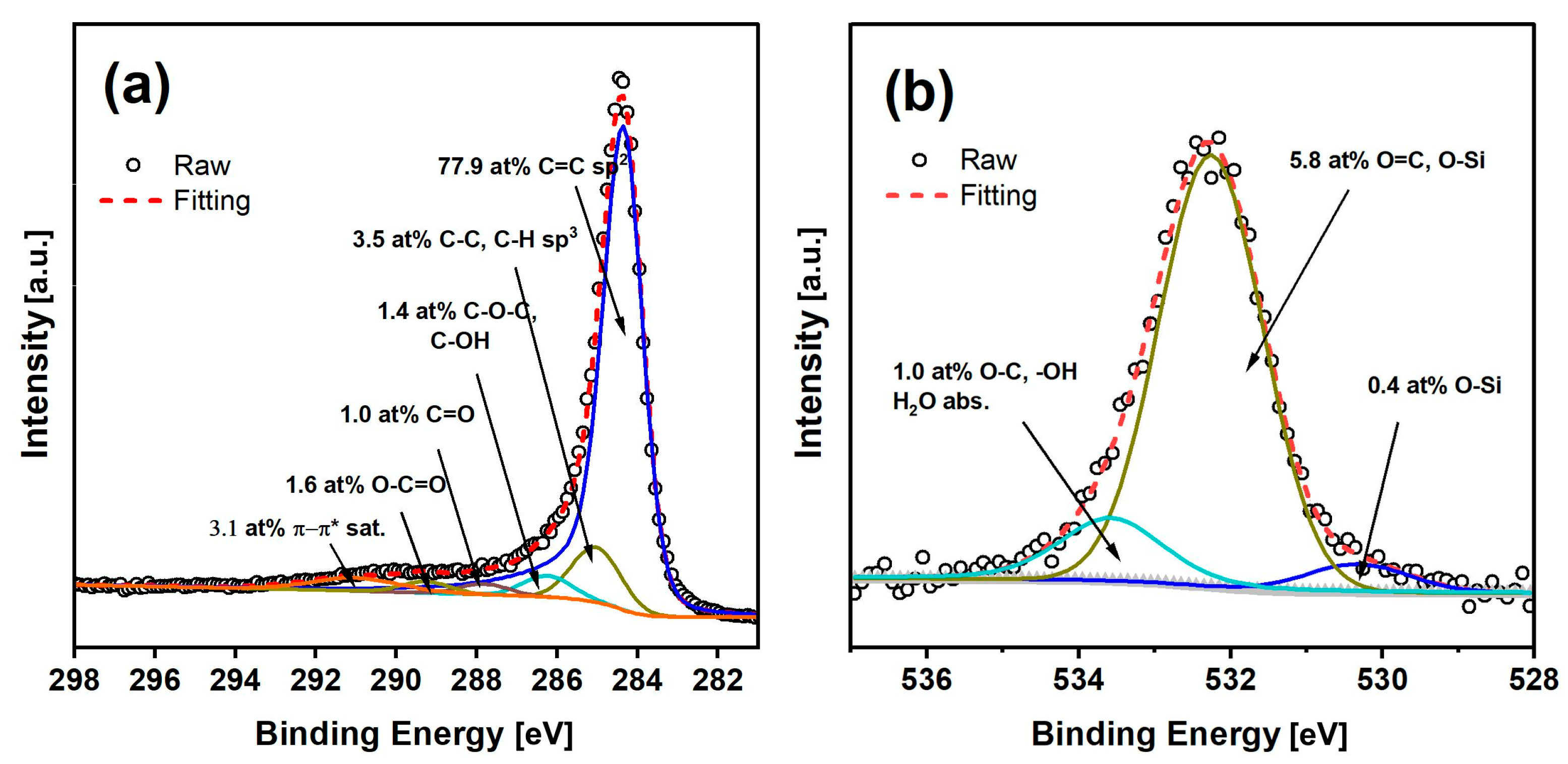
2.4. X-ray Photoelectron Spectroscopy Analyses of Biochar Derived from Cellulose
2.5. Investigations Using Thermal Analysis Methods Performed on the Biochar Samples
2.6. Analysis of Carbon Oxide Production during the Thermal Processes Occurring in the Carbon Bed Formed in a Direct Carbon Solid Oxide Fuel Cell Anode Chamber
2.7. Analysis of the Electrochemical Performance of Direct Carbon Solid Oxide Fuel Cells Supplied by Solid Fuels Derived from Cellulose
- (A)
- C|8YSZ|LSM-GDC|LSM|O2
- (B)
- C|Ni-YSZ|YSZ|LSM-GDC|LSM|O2
3. Results and Discussion
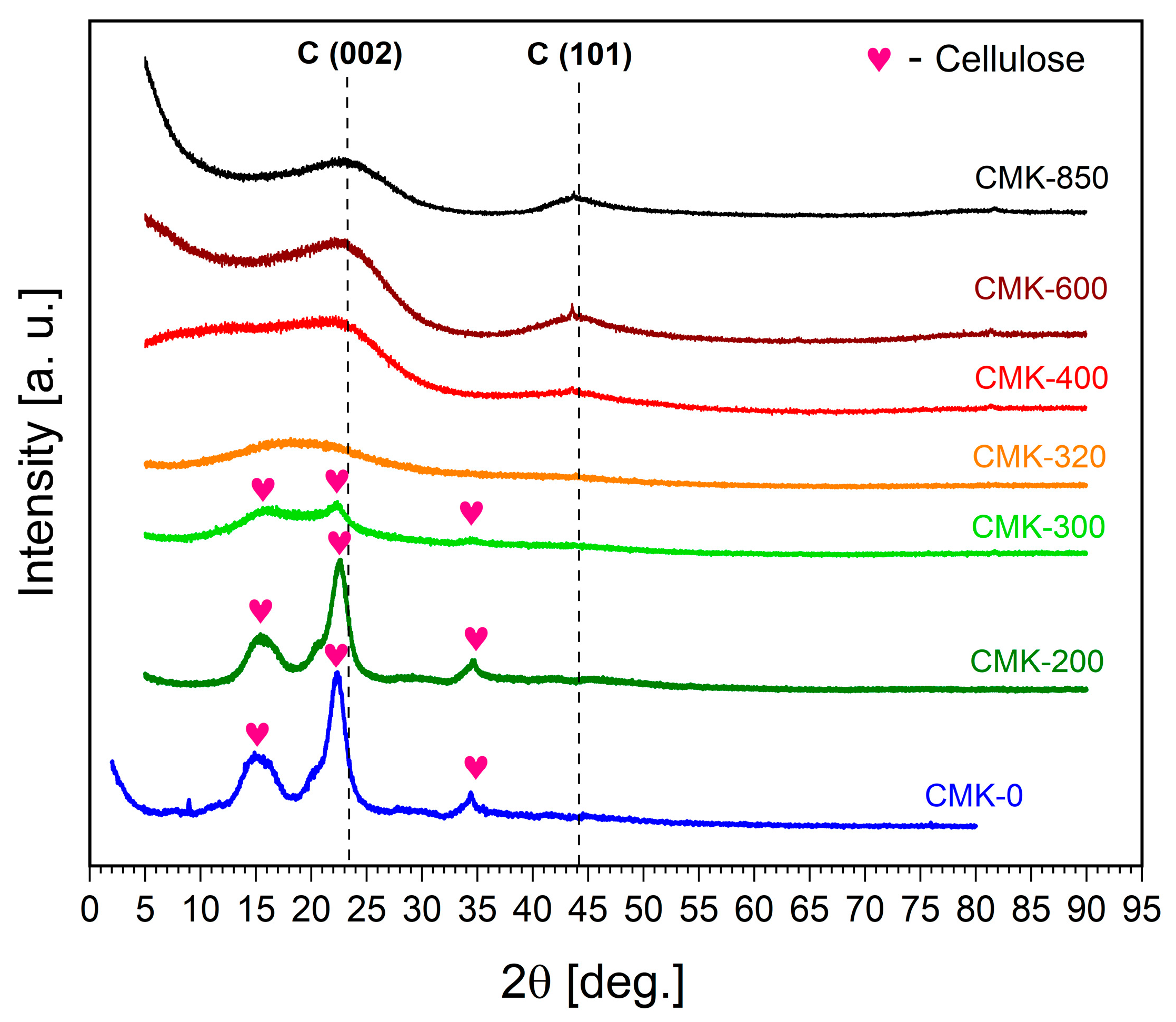
3.1. Evaluation of Biochar Physicochemical Properties
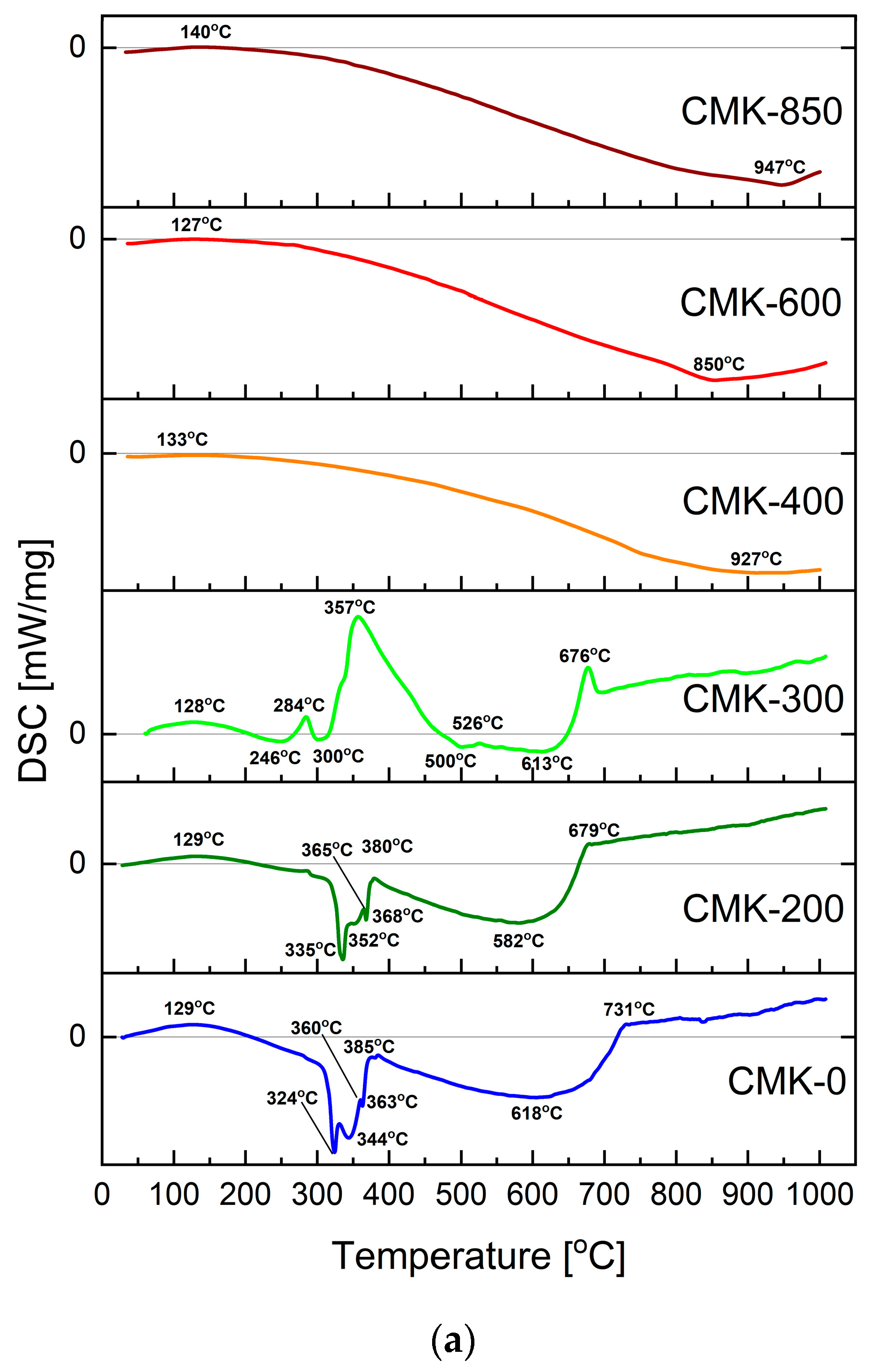
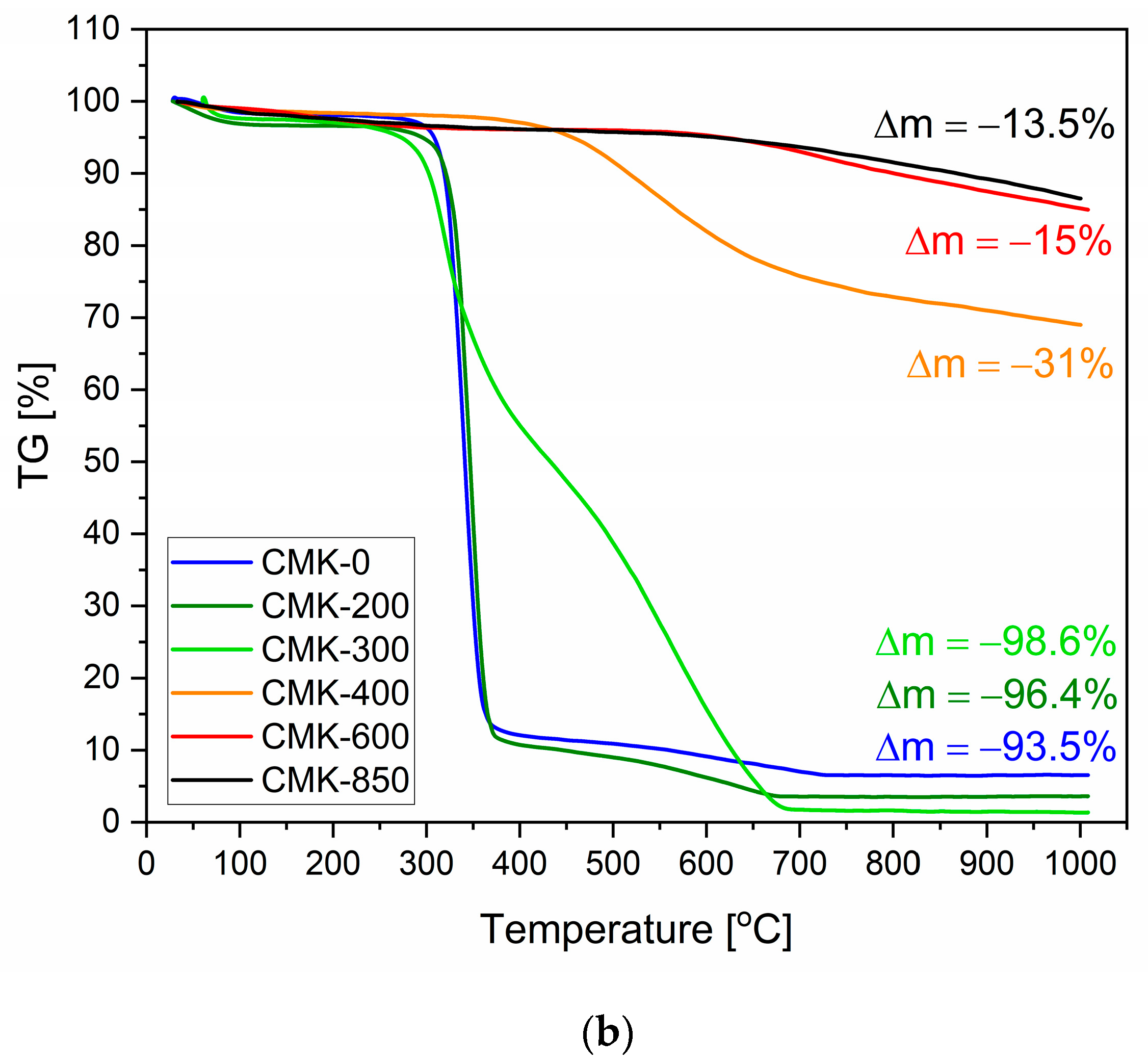
3.2. Thermochemical Conversion and Gasification Process of the Biochar Samples Studied via the Thermal Analysis Method
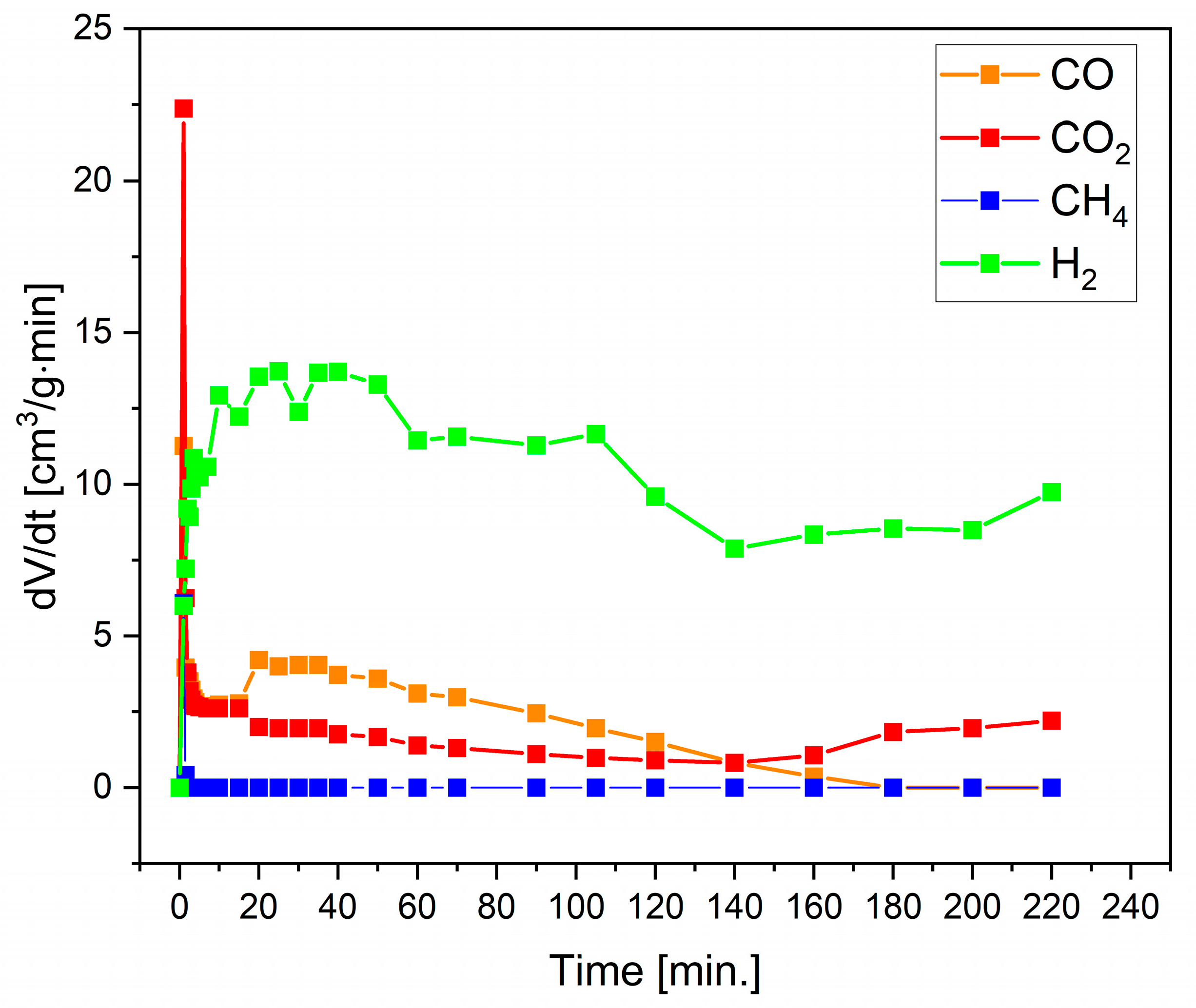
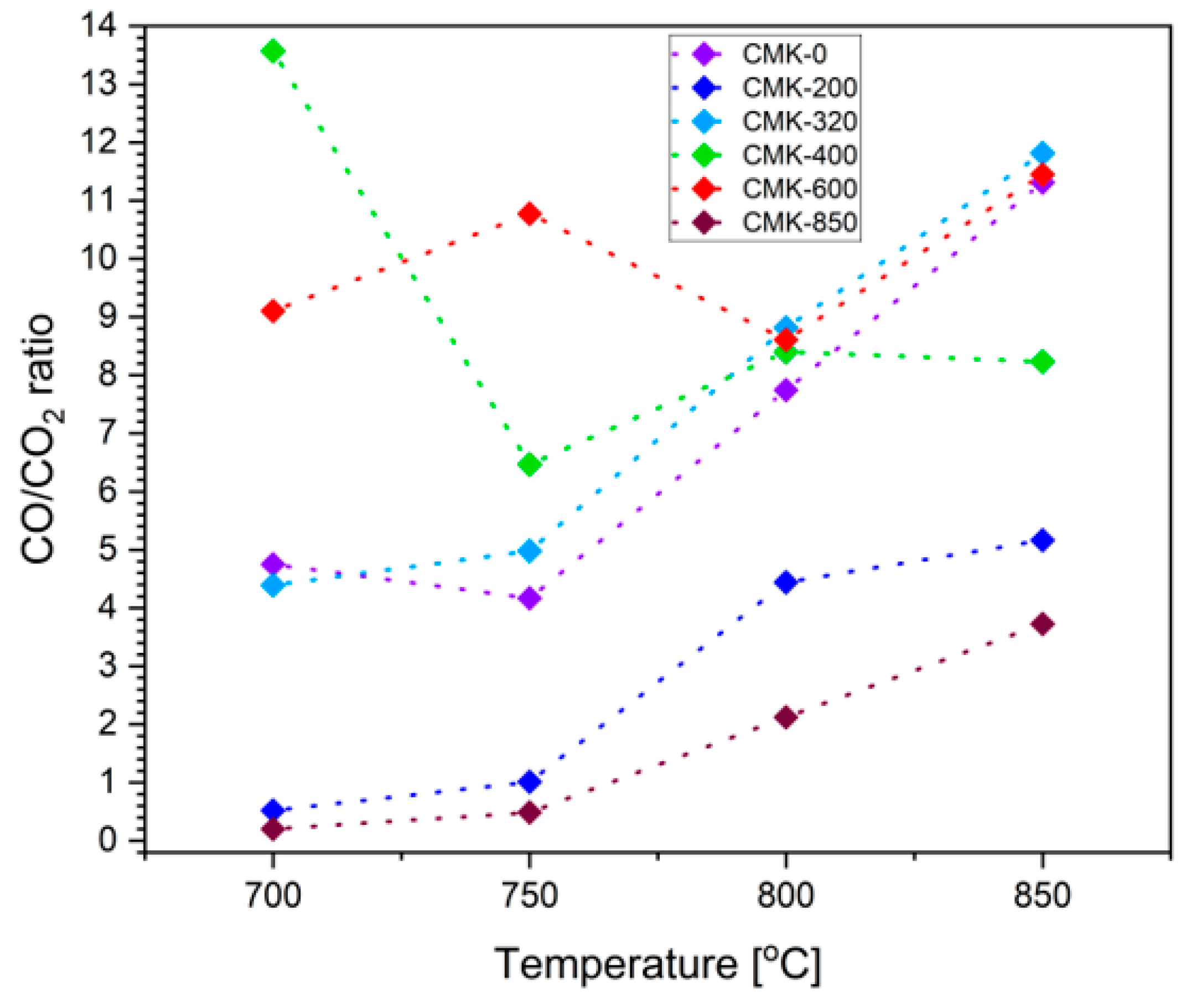
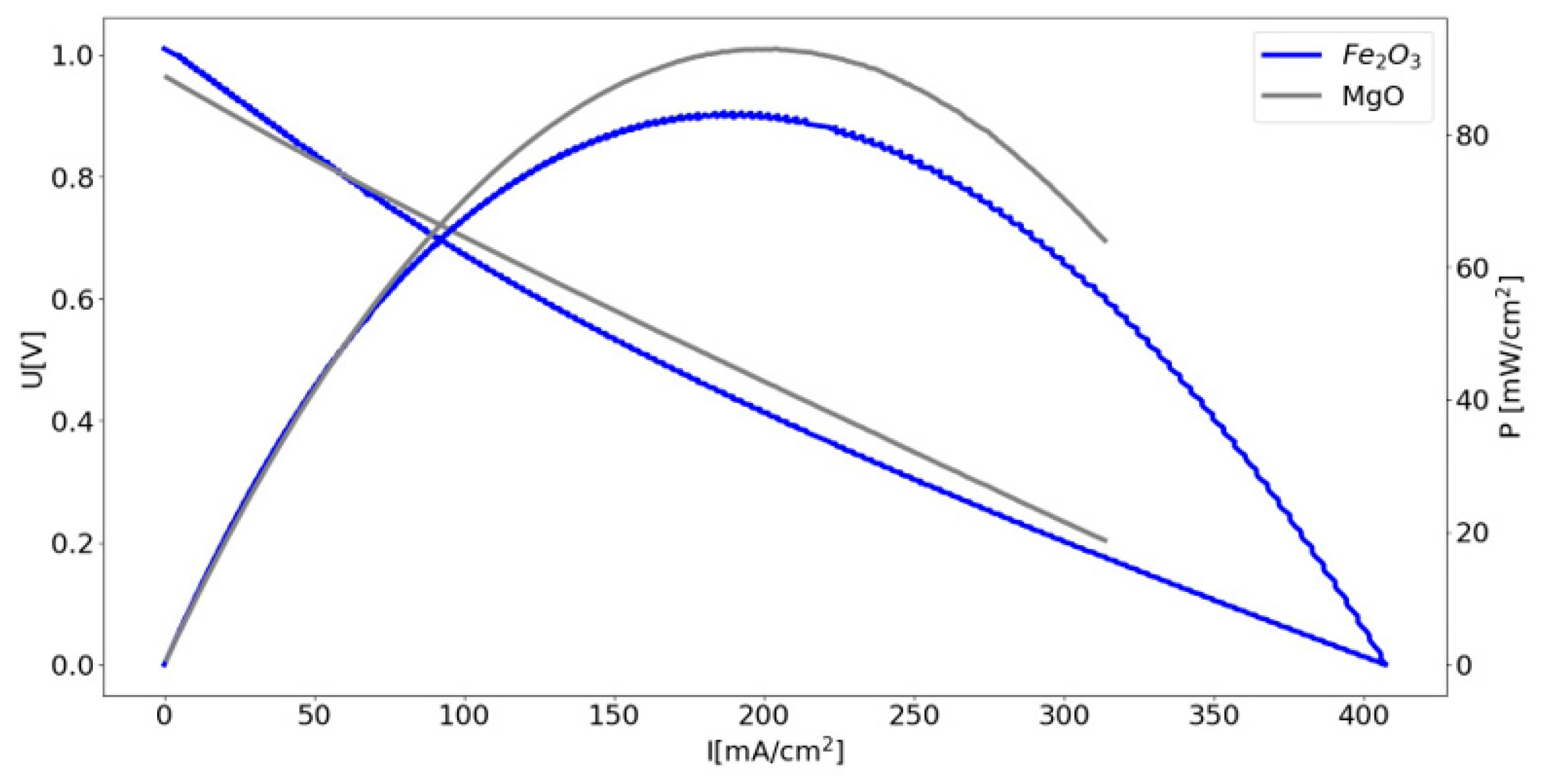
3.3. Investigation of the Biocarbon Oxidation Process Studied on the Surface of 8 mol% Y2O3 in ZrO2 (8YSZ) Oxide Electrolyte
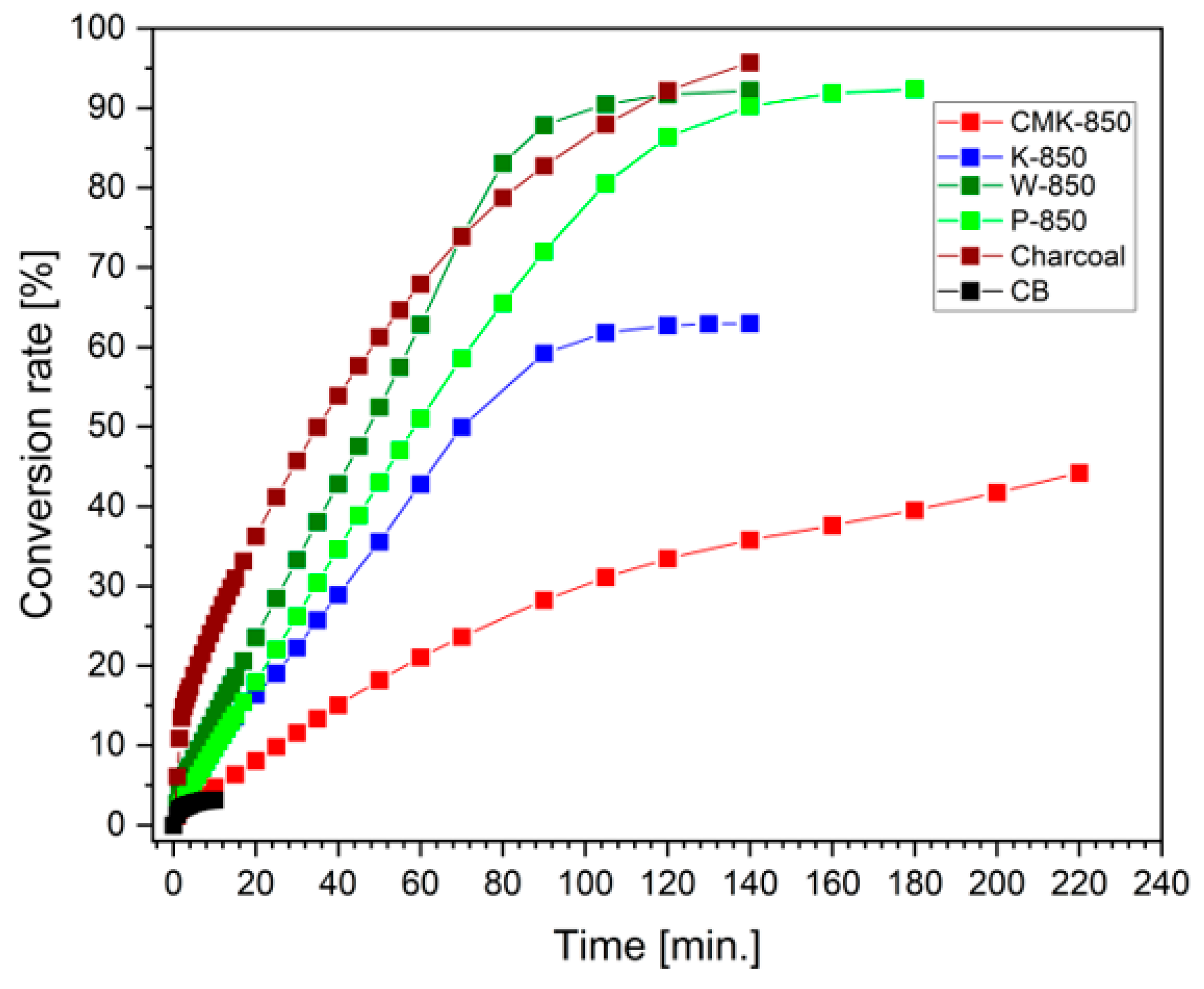
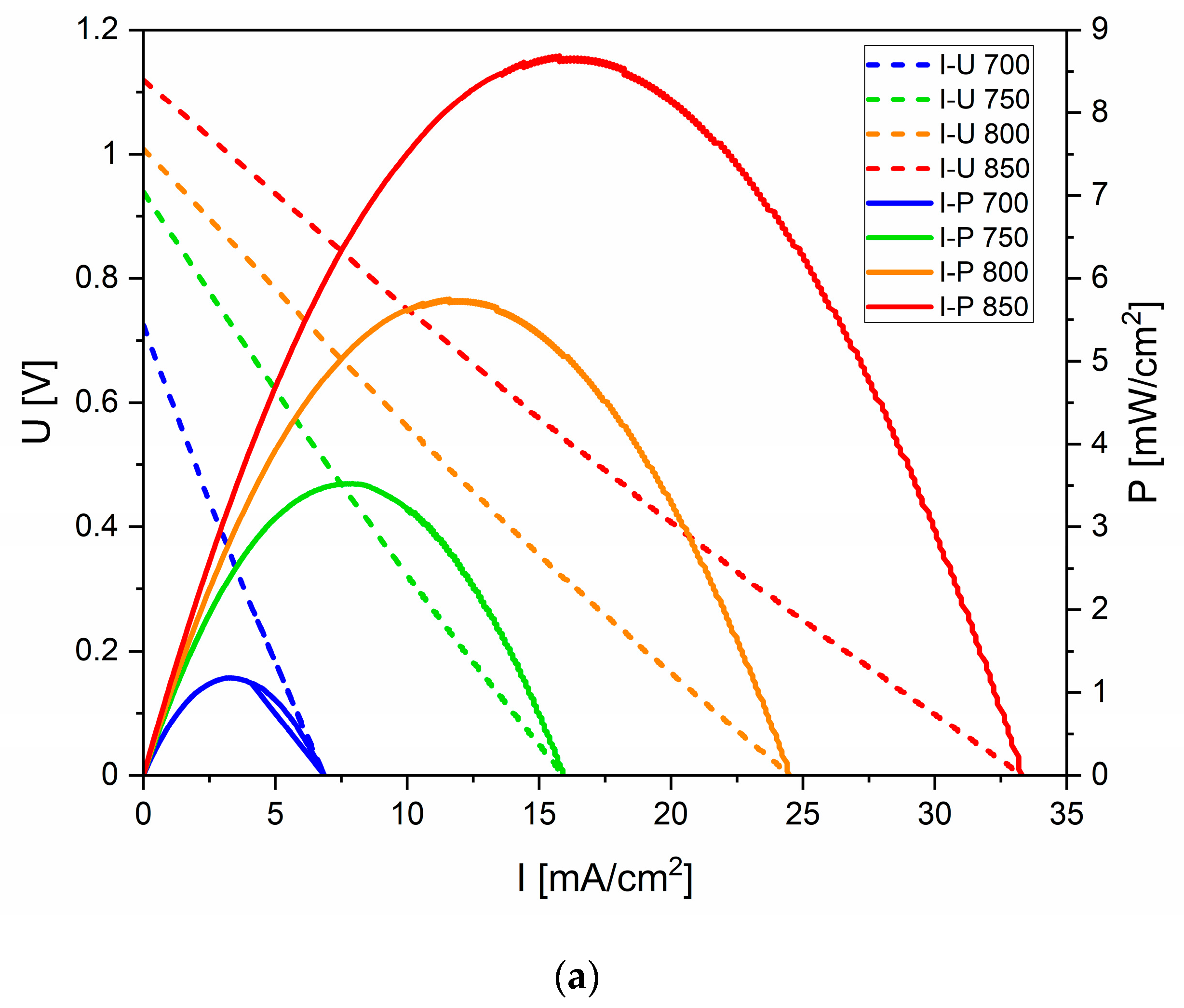
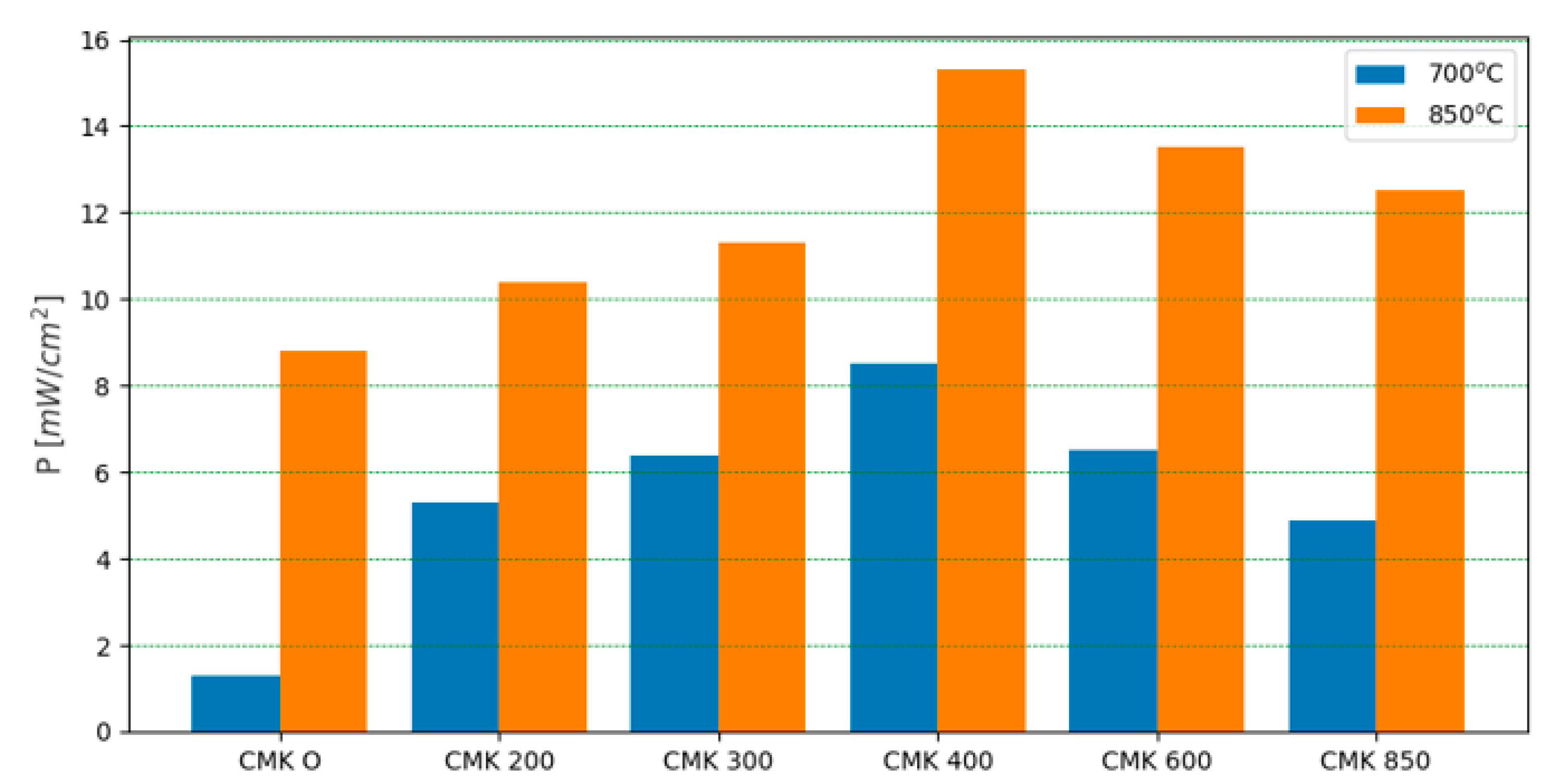
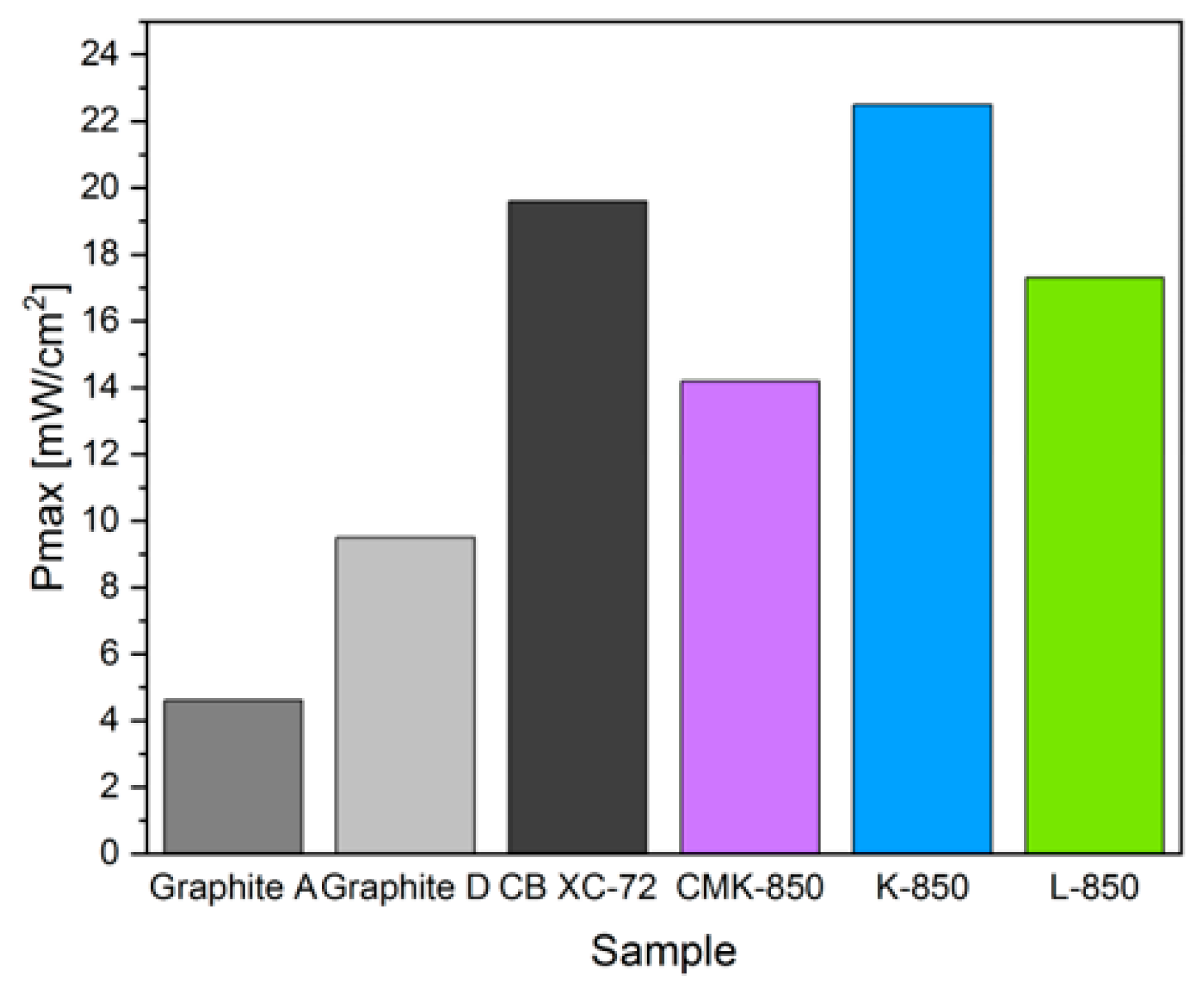
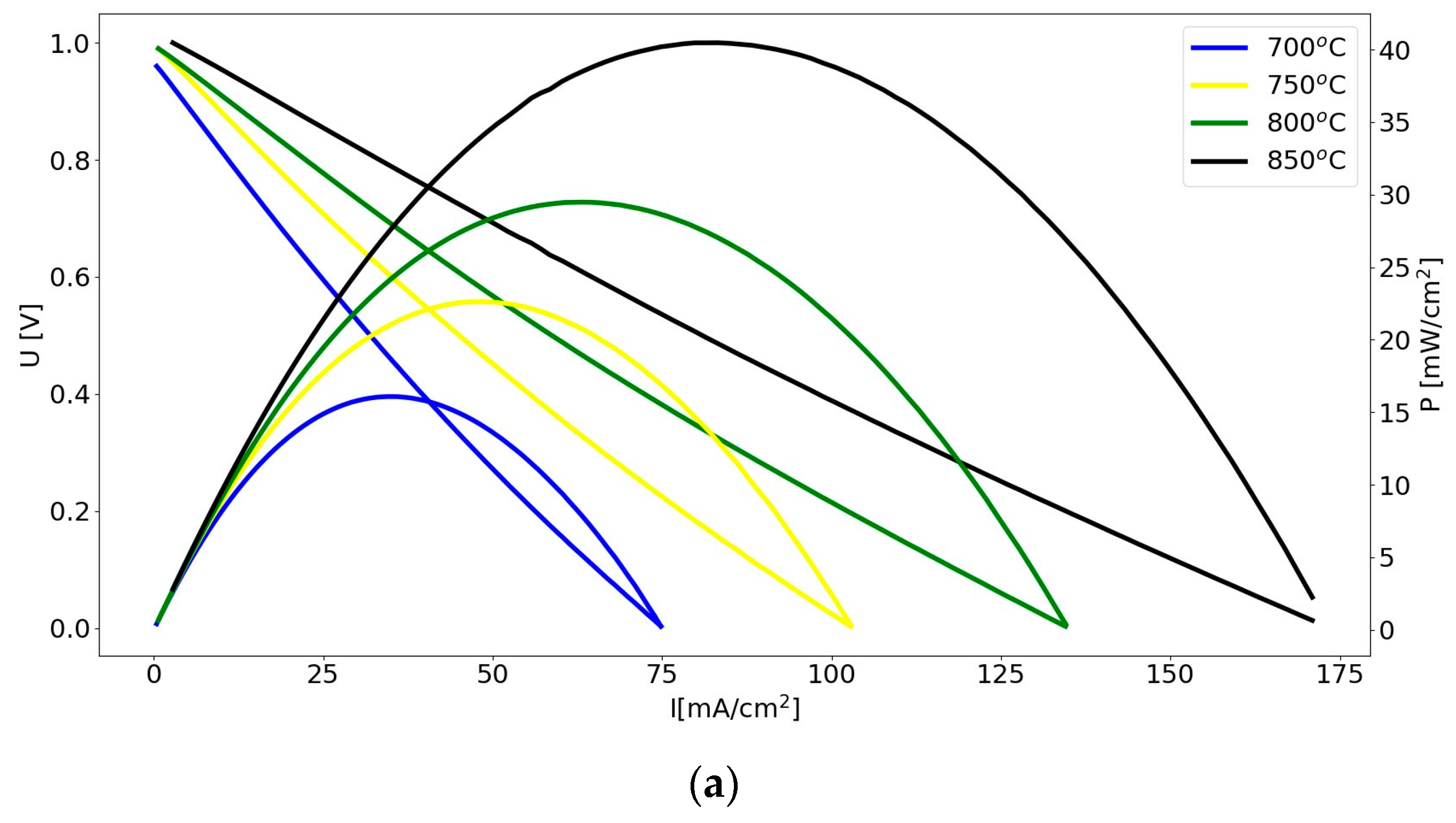
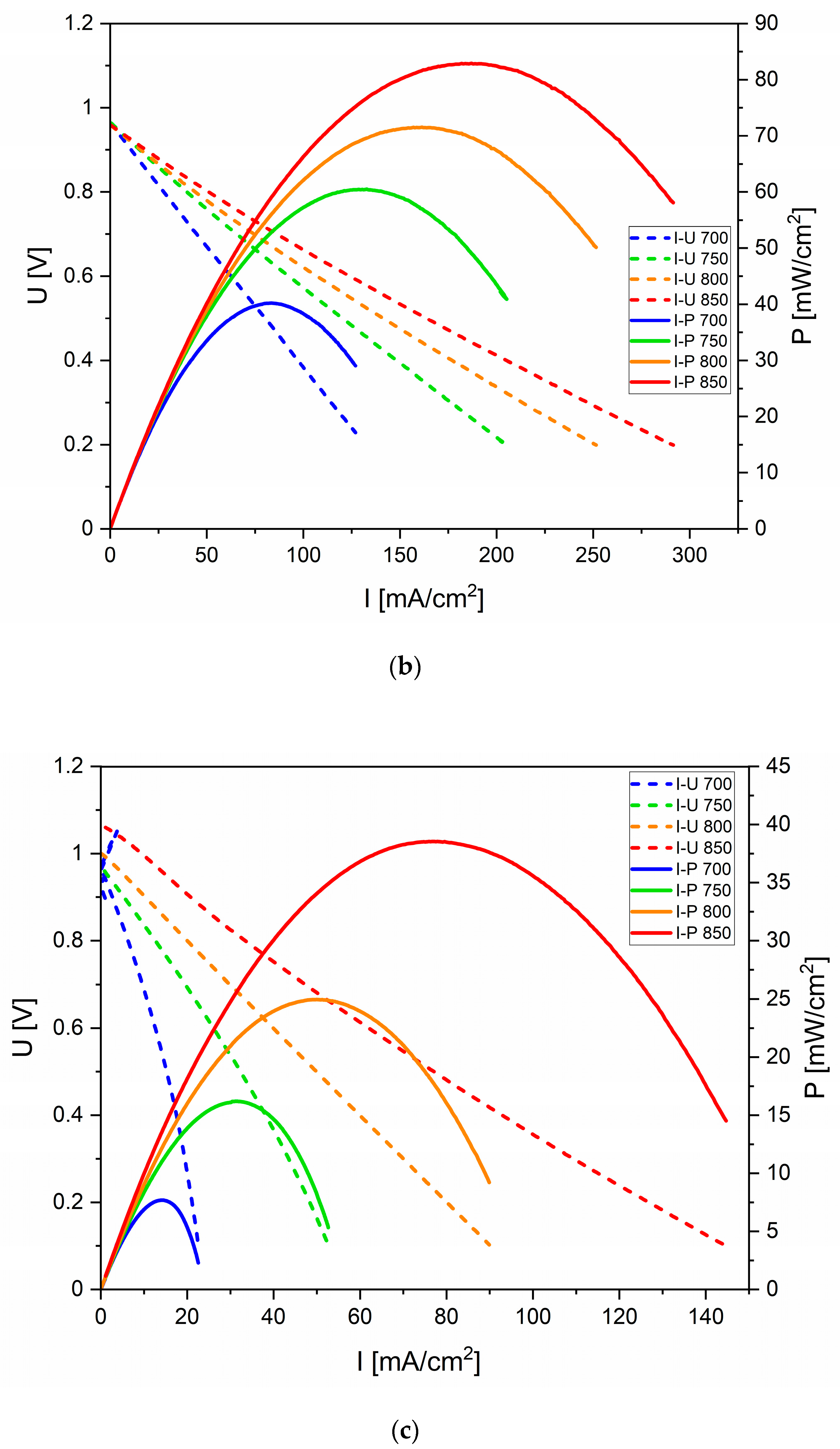
3.4. Electrochemical Performance of DC-SOFC (B) Supplied by Solid Fuels Obtained as Biochar from Cellulose Feedstock
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rady, A.C.; Giddey, S.; Badwal, S.P.S.; Ladewig, B.P.; Bhattacharya, S. Review of fuels for direct carbon fuel cells. Energy Fuels 2012, 26, 1471–1488. [Google Scholar] [CrossRef]
- Yu, F.; Han, T.; Wang, Z.; Xie, Y.; Wu, Y.; Jin, Y.; Yang, N.; Xiao, J.; Kawi, S. Recent progress in direct carbon solid oxide fuel cell: Advanced anode catalysts, diversified carbon fuels, and heat management. Int. J. Hydrogen Energy 2021, 46, 4283–4300. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, J.; Corre, G.; Jain, S.L.; Irvine, J.T.S. Challenges in developing direct carbon fuel cells. Chem. Soc. Rev. 2017, 46, 2889–2912. [Google Scholar] [CrossRef] [PubMed]
- Skrzypkiewicz, M.; Obrębowski, S. Direct carbon, integrated gasification, and deposited carbon solid oxide fuel cells: A patent-based review of technological status. J. Power Technol. 2018, 98, 139–160. [Google Scholar]
- Dall’Osto, G.; Mombelli, D.; Pittalis, A.; Mapelli, C. Biochar and other carbonaceous materials used in steelmaking: Possibilities and synergies for power generation by direct carbon fuel cell. Biomass Bioenergy 2023, 177, 106930. [Google Scholar] [CrossRef]
- Lee, A.C.; Li, S.; Mitchell, R.E.; Gur, T.M. Conversion of solid carbonaceous fuels in a fluidized bed fuel cell. Electrochem. Solid-State Lett. 2008, 11, B20–B23. [Google Scholar] [CrossRef]
- Wu, Y.; Su, C.; Zhang, C.; Ran, R.; Shao, Z. A new carbon fuel cell with high power output by integrating with in situ catalytic reverse Boudouard reaction. Electrochem. Commun. 2009, 11, 1265–1268. [Google Scholar] [CrossRef]
- Hao, W.; He, X.; Mi, Y. Achieving high performance in intermediate temperature direct carbon fuel cells with renewable carbon as a fuel source. Appl. Energy 2014, 135, 174–181. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; Marco, R.D.; Bradley, J.; Dicks, A. Evaluation of raw coals as fuels for direct carbon fuel cells. J. Power Sources 2010, 195, 4051–4058. [Google Scholar] [CrossRef]
- Ju, H.K.; Eom, J.; Lee, J.K.; Choi, H.; Lim, T.H.; Song, R.H.; Lee, J. Durable power performance of a direct ash-free coal fuel cell. Electrochim. Acta 2014, 115, 511–517. [Google Scholar] [CrossRef]
- Cai, W.; Zhou, Q.; Xie, Y.; Liu, J.; Long, G.; Cheng, S.; Liu, M. A direct carbon solid oxide fuel cell operated on a plant derived biofuel with natural catalyst. Appl. Energy 2016, 179, 1232–1241. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, X.; Hao, S.; Liu, B.; Zhang, Y.; Xie, Y.; Liu, Y.; Xiao, J.; Wu, C.; Dong, P. A novel biomass modified brown coal as composite fuel for high performance direct carbon solid oxide fuel cells. Fuel 2024, 363, 131051. [Google Scholar] [CrossRef]
- Jewulski, J.; Skrzypkiewicz, M.; Struzik, M.; Lubarska-Radziejewska, I. Lignite as a fuel for direct carbon fuel cell system. Int. J. Hydrogen Energy 2014, 39, 21778–21785. [Google Scholar] [CrossRef]
- Tubilla, B.C.; Sabolsky, K.; Sabolsky, M.E.; Zondlo, W.J. Investigation of pretreated switchgrass, corn stover, and hardwood fuels in direct carbon fuel cells. Int. J. Electrochem. Sci. 2016, 11, 303–321. [Google Scholar] [CrossRef]
- Han, T.; Wu, Y.; Li, L.; Xie, Z.; Xie, Y.; Zhang, J.; Meng, X.; Yu, F.; Yang, N. A high-performance direct carbon solid oxide fuel cell powered by barium-based catalyst-loaded biochar derived from sunflower seed shell. Int. J. Hydrogen Energy 2022, 47, 38747–38756. [Google Scholar] [CrossRef]
- Gu, X.; Yan, X.; Zhou, M.; Zou, G.; Fan, Z.; Liu, J. High efficiency electricity and gas cogeneration through direct carbon solid oxide fuel cell with cotton stalk biochar. Renew. Energy 2024, 226, 120471. [Google Scholar] [CrossRef]
- Cai, W.; Liu, J.; Liu, P.; Liu, Z.; Xu, H.; Chen, B.; Li, Y.; Zhou, Q.; Liu, M.; Ni, M. A direct carbon solid oxide fuel cell fueled with char from wheat straw. Int. J. Energy Res. 2019, 43, 468–2477. [Google Scholar] [CrossRef]
- Tong, X.; Hou, J.; Li, Y.; Li, H.; Wu, W.; Guo, Y.; Liu, Y.; Fu, D.; Huang, X.; Xiong, Z.; et al. Application of biochar derived from used cigarette filters in direct carbon solid oxide fuel cell. Int. J. Hydrogen Energy 2022, 47, 22972–22980. [Google Scholar] [CrossRef]
- Awogbemi, O.; Von Kallon, D.V. Application of biochar derived from crops residues for biofuel production. Fuel Commun. 2023, 15, 100088. [Google Scholar] [CrossRef]
- Nadarajah, K.; Asharp, T.; Jeganathan, Y. Biochar from waste biomass, its fundamentals, engineering aspects, and potential applications: An overview. Water Sci. Technol. 2024, 89, 1211–1239. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Eom, S.Y.; Rhie, H.Y.; Sung, M.Y.; Moon, E.C.; Choi, E.M.; Kim, J.D. Utilization of wood biomass char in a direct carbon fuel cell (DCFC) system. Appl. Energy 2013, 105, 207–216. [Google Scholar] [CrossRef]
- Jang, H.; Ocon, J.D.; Lee, S.; Lee, J.K.; Lee, J. Direct power generation from waste coffee grounds in a biomass fuel cell. J. Power Sources 2015, 296, 433–439. [Google Scholar] [CrossRef]
- Rady, A.C.; Giddy, S.; Kulkarni, A.; Badwal, S.P.S.; Bhattacharya, S.P.S. Degradation Mechanism in a Direct Carbon Fuel Cell Operated with Demineralised Brown Coal. Electrochim. Acta 2014, 143, 278–290. [Google Scholar] [CrossRef]
- Kacprzak, A.; Włodarczyk, R. Utilization of organic waste in a direct carbon fuel cell for sustainable electricity generation. Energies 2023, 16, 7359. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhou, M.; Cai, W.; Zhou, Q.; Zhang, Y.; Wang, W.; Liu, M.; Liu, J. A comparative investigation on direct carbon solid oxide fuel cells operated with fuels of biochar derived from wheat straw, corncob, and bagasse. Biomass Bioenergy 2019, 121, 56–63. [Google Scholar] [CrossRef]
- Dudek, M.; Adamczyk, B.; Grzywacz, P.; Lach, R.; Sitarz, M.; Leśniak, M.; Gajek, M.; Mech, K.; Wilk, M.; Rapacz-Kmita, A.; et al. The utilisation of solid fuels derived from waste pistachio shells in direct carbon solid oxide fuel cells. Materials 2021, 14, 6755. [Google Scholar] [CrossRef] [PubMed]
- Naumkin, A.V.; Kraut-Vass, A.; Powell, C.J.; Gaarenstroom, S.W. NIST Standard Reference Database 20, Version 4.1; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2012.
- Śpiewak, K.; Czerski, G.; Porada, S. Effect of K, Na and Ca-based catalysts on the steam gasification reactions of coal. Part I: Type and amount of one-component catalysts. Chem. Eng. Sci. 2021, 229, 116024. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Porada, S. Effect of K, Na and Ca-based catalysts on the steam gasification reactions of coal. Part II: Composition and amount of multi-component catalysts. Chem. Eng. Sci. 2021, 229, 116023. [Google Scholar] [CrossRef]
- Dudek, M.; Adamczyk, B.; Sitarz, M.; Śliwa, M.; Lach, R.; Skrzypkiewicz, M.; Raźniak, A.; Ziąbka, M.; Zuwała, J.; Grzywacz, P. The usefulness of walnut shells as waste biomass fuels in direct carbon solid oxide fuel cells. Biomass Bioenergy 2018, 119, 144–154. [Google Scholar] [CrossRef]
- Dudek, M.; Tomczyk, P.; Juda, K.L.; Tomov, R.; Glowacki, B.A.; Batty, S.; Risby, P.; Socha, R. Comparison of the performances of DCFC fuelled with the product of methane RF plasma reforming and carbon black. Int. J. Electrochem. Sci. 2012, 7, 6704–6721. [Google Scholar] [CrossRef]
- Dudek, M.; Tomczyk, P. Composite fuel for direct carbon fuel cell. Catal. Today 2010, 176, 388–392. [Google Scholar] [CrossRef]
- Hackett, G.A.; Zondlo, W.; Svensson, R. Evaluation of carbon materials for use in a direct carbon fuel cell. J. Power Sources 2008, 168, 111–118. [Google Scholar] [CrossRef]
- Jain, S.L.; Lakeman, J.B.; Pointon, K.D.; Marshall, R.; Irvine, J.T. Electrochemical performance of a hybrid direct carbon fuel cell powered by pyrolysed MDF. Energy Environ. Sci. 2009, 2, 687–693. [Google Scholar] [CrossRef]
- Bitia, S.; Mccueb, A.; Dionisia, D.; Graçaa, I.; Martín, C.F. Sustainable microcrystalline cellulose-based activated carbons for a greener carbon capture at post-combustion conditions. Int. J. Greenh. Gas Control 2023, 125, 103876. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Wang, Q.; Allred, D.D.; González-Hernández, J. Low-frequency feature in the first-order Raman spectrum of amorphous carbon. Phys. Rev. B 1993, 47, 6119–6121. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, M.; Czosnek, C.; Jeleń, P.; Odziomek, M.; Olejniczak, Z.; Kozanecki, M.; Janik, J.F. SiOC glasses produced by the aerosol-assisted vapor synthesis method. Spectrochim. Acta Part A 2013, 112, 440–445. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Fan, M.; Shao, Y.; Sun, Y.; Zhang, L.; Zhang, S.; Huang, Y.; Li, B.; Wang, S.; et al. Investigation of property of biochar in staged pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2023, 172, 105999. [Google Scholar] [CrossRef]
- Orrego-Restrepo, E.; Ordonez-Loza, J.; Chejne, F. Novel methodology for evaluation of cellulose pyrolysis kinetics implementing infrared spectroscopy in situ. J. Anal. Appl. Pyrolysis 2022, 166, 105589. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic XPS Spectra, Volume 1—The Elements and Native Oxides; XPS International, LLC: Mountain View, CA, USA, 1999. [Google Scholar]
- Rouxhet, P.G.; Genet, M.J. XPS analysis of bio-organic systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Leroy, V.; Cancellieri, D.; Leoni, E. Thermal degradation of lignocellulosic fuels: DSC and TGA studies. Thermochim. Acta 2006, 451, 131–138. [Google Scholar] [CrossRef]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.K.B.S.; Nataraj, M.; Tayappa, M.; Vyas, Y.; Mishra, R.K.; Acharya, B. Production of biochar from waste biomass using slow pyrolysis: Studies of the effect of pyrolysis temperature and holding time on biochar yield and properties. Mater. Sci. Energy Technol. 2024, 7, 318–334. [Google Scholar] [CrossRef]
- Han, T.; Xie, Y.; Li, L.; Wu, Y.; Yu, F.; Wang, M.; Zhang, J.; Li, G.; Yang, N. Enhanced electrochemical performance of direct carbon solid oxide fuel cells by MgO-catalyzed carbon gasification: Experimental and DFT simulation studies. Ceram. Int. 2024, 50, 16435–16442. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, J.; Wang, X.; Wu, J.; Chen, Y.; Chen, X.; Li, D.; Sang, W.; Xu, S.; Qu, G. Development of multiple alkali metals doped La-Ni based perovskites for CO2 gasification of biochar to produce CO rich syngas. J. Energy Inst. 2024, 114, 101637. [Google Scholar] [CrossRef]
- Desclaux, P.; Schirmer, H.C.; Woiton, M.; Stern, E.; Rzepka, M. Influence of the carbon/anode interaction on direct carbon conversion in a SOFC. Int. J. Electrochem. Sci. 2013, 8, 9125–9132. [Google Scholar] [CrossRef]
- Yu, X.; Shi, Y.; Wang, H.; Cai, N.; Li, C.; Tomov, R.I.; Hanna, J.; Glowacki, B.A.; Ghoniem, A.F. Experimental characterization and elementary reaction modeling of solid oxide electrolyte direct carbon fuel cell. J. Power 2013, 243, 159–171. [Google Scholar] [CrossRef]
- Cooper, J.F.; Selman, J.R. Analysis of the carbon anode in direct carbon conversion fuel cells. Int. J. Hydrogen Energy 2012, 37, 19319–19328. [Google Scholar] [CrossRef]
- Nürnberger, S.; Bußar, R.; Desclaux, P.; Franke, B.; Rzepka, M.; Stimming, U. Direct carbon conversion in a SOFC-system with a non-porous anode. Energy Environ. Sci. 2010, 3, 150–153. [Google Scholar] [CrossRef]
- Gür, T.M.; Huggins, R.A. Direct electrochemical conversion of carbon to electrical energy in a high temperature fuel cell. J. Electrochem. Soc. 1992, 139, L95–L97. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Cai, N. Mechanism for carbon direct electrochemical reactions in a solid oxide electrolyte direct carbon fuel cell. J. Power Sources 2011, 196, 754–763. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A.; Munnings, C. A comprehensive review of direct carbon fuel cell technology. Prog. Energy Combust. Sci. 2012, 38, 360–399. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Y.; Liu, J. A verification of the reaction mechanism of direct carbon solid oxide fuel cells. J. Solid State Electrochem. 2013, 17, 121–127. [Google Scholar] [CrossRef]
- Dudek, M.; Sitarz, M.; Tomczyk, P. Effect of structural properties of carbon-based fuels on efficiency of direct carbon fuel cells. J. Solid State Electrochem. 2014, 18, 3023–3032. [Google Scholar] [CrossRef]
- Cooper, J. Direct conversion of coal derived carbon in fuel cells. In Recent Trends in Fuel Cell Science and Technology; Basu, S., Ed.; Anamaya Publishers: New Delhi, India, 2007; pp. 110–131. [Google Scholar]
- Weber, A.; Ivers-Tiffe, E. Materials and concepts for solid oxide fuel cells (SOFCs) in stationary and mobile application. J. Power Sources 2004, 127, 273–283. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Hitomi, T. Biomass solid oxide fuel cell using solid weed waste as fuel. Electrochim. Acta 2021, 388, 138681. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ihara, M. Reaction mechanism of solid carbon fuel in rechargeable direct carbon SOFCs with methane for charging. J. Electrochem. Soc. 2008, 155, B58. [Google Scholar] [CrossRef]
- Gür, T. Mechanistic modes for solid carbon conversion in high temperature fuel cells. J. Electrochem. Soc. 2010, 157, B751–B759. [Google Scholar] [CrossRef]
- Li, L.; Han, T.; Wu, X.; Cheng, J.; Yao, P.; Yu, F.; Zhang, J.; Zeng, W.; Yang, N.; Li, Y. Innovative application of tomato straw biochar in direct carbon solid oxide fuel cells for power generation. Catal. Today 2024, 430, 114518. [Google Scholar] [CrossRef]
- Chun, D.D.; Ni, D.; Simson, A. The effect of inherent inorganics and CO2 co-pyrolysis on biochar production from biowastes and their gasification reactivity. Biomass Bioenergy 2022, 158, 106361. [Google Scholar] [CrossRef]
- Jiang, C.; Irvine, J.T.S. Catalysis and oxidation of carbon in a hybrid direct carbon fuel cell. J. Power Sources 2011, 196, 7318–7322. [Google Scholar] [CrossRef]







| Sample Name | Pyrolysis Temperature [°C] | Sample Name | Pyrolysis Temperature [°C] |
|---|---|---|---|
| CMK-0 | None | CMK-320 | 320 |
| CMK-200 | 200 | CMK-400 | 400 |
| CMK-250 | 250 | CMK-600 | 600 |
| CMK-300 | 300 | CMK-850 | 850 |
| Sample | C | H | S |
|---|---|---|---|
| CKM-0 | 41.6 | 9.77 | 0.00 |
| CKM-200 | 42.1 | 9.30 | 0.00 |
| CKM-250 | 51.4 | 8.74 | 0.00 |
| CKM-300 | 55.7 | 4.73 | 0.00 |
| CKM-320 | 62.0 | 4.06 | 0.00 |
| CKM-400 | 75.5 | 3.34 | 0.00 |
| CKM-600 | 83.9 | 1.83 | 0.00 |
| CKM-850 | 90.1 | 1.43 | 0.01 |
| Object | Parameter | Value |
|---|---|---|
| 8YSZ sample (A1) | Total electrical conductivity σ (S/cm) at 850 °C | 4.1 × 10−2 (S/cm) |
| Energy activation (eV) in the temperature range of 500–850 °C | 0.91 eV | |
| 8YSZ sample (A2) | Total electrical conductivity σ (S/cm) at 850 °C | 8.6 × 10−2 (S/cm) |
| eV in the temperature range of 500–850 °C | 0.85 eV | |
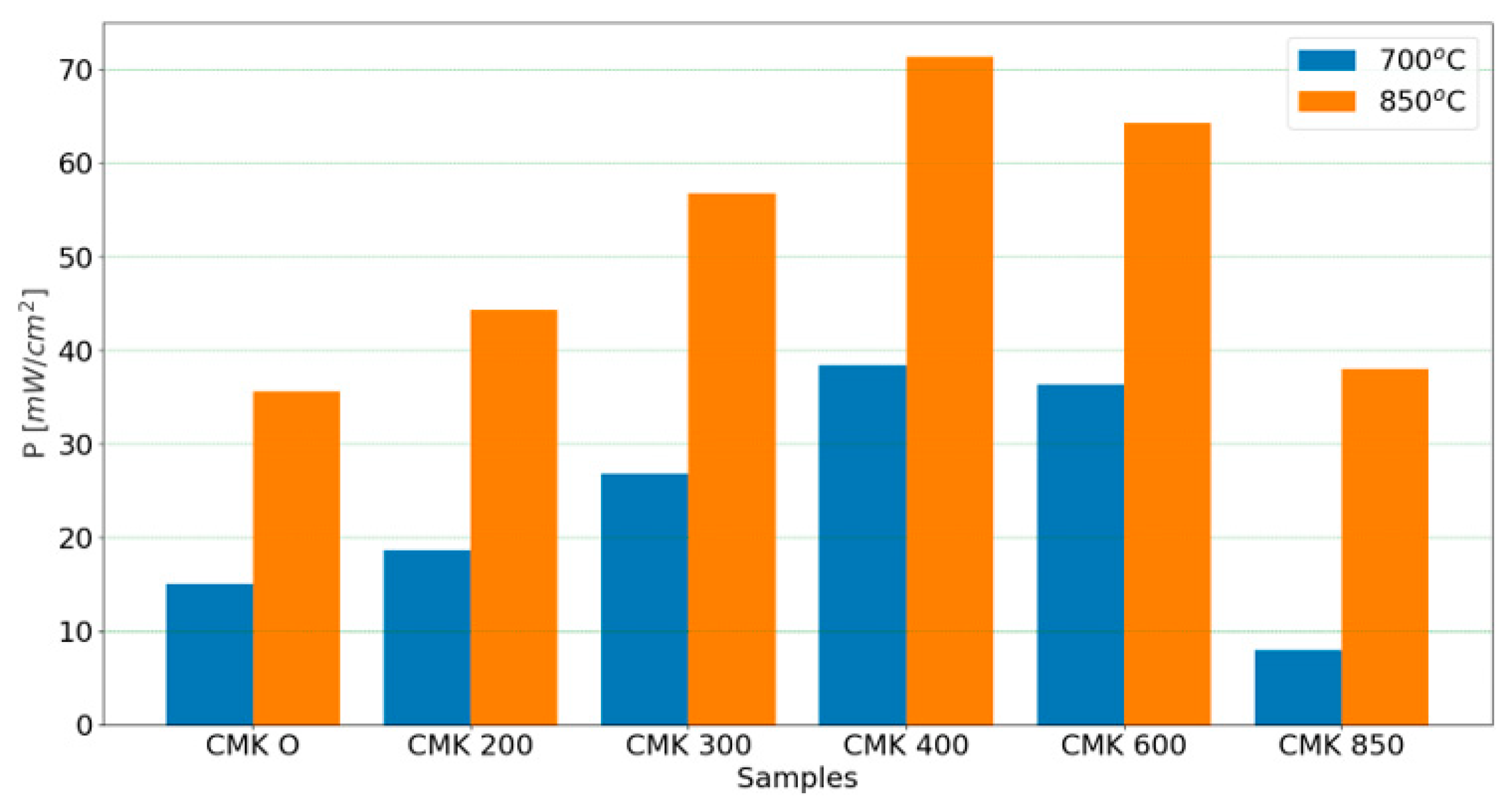
| DC-SOFC (A1) | Electrical power output of CMK-400 fuel at 850 °C | 14.3 mW/cm2 |
| DC-SOFC (A2) | 21.6 mW/cm2 | |
| DC-SOFC (A1) | Electrical power output of CMK-850 fuel at 850 °C | 12.1 mW/cm2 |
| DC-SOFC (A2) | 16.8 mW/cm2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, B.; Dudek, M.; Zych, A.; Gajek, M.; Sitarz, M.; Ziąbka, M.; Dudek, P.; Grzywacz, P.; Witkowska, M.; Kowalska, J.; et al. Investigating the Effects of the Physicochemical Properties of Cellulose-Derived Biocarbon on Direct Carbon Solid Oxide Fuel Cell Performance. Materials 2024, 17, 3503. https://doi.org/10.3390/ma17143503
Adamczyk B, Dudek M, Zych A, Gajek M, Sitarz M, Ziąbka M, Dudek P, Grzywacz P, Witkowska M, Kowalska J, et al. Investigating the Effects of the Physicochemical Properties of Cellulose-Derived Biocarbon on Direct Carbon Solid Oxide Fuel Cell Performance. Materials. 2024; 17(14):3503. https://doi.org/10.3390/ma17143503
Chicago/Turabian StyleAdamczyk, Bartosz, Magdalena Dudek, Anita Zych, Marcin Gajek, Maciej Sitarz, Magdalena Ziąbka, Piotr Dudek, Przemysław Grzywacz, Małgorzata Witkowska, Joanna Kowalska, and et al. 2024. "Investigating the Effects of the Physicochemical Properties of Cellulose-Derived Biocarbon on Direct Carbon Solid Oxide Fuel Cell Performance" Materials 17, no. 14: 3503. https://doi.org/10.3390/ma17143503







