Geopolymer Materials from Fly Ash—A Sustainable Approach to Hazardous Waste Management
Abstract
:1. Introduction
2. Methods and Materials’ Characterisation
2.1. Leachability Analysis of Heavy Metals
2.2. Analysis of Ash and Leachate Following Water Extraction
2.3. Compressive Strength of Alkali-Activated Materials
3. Materials
4. Synthesis
5. Results and Discussion
5.1. Results of Strength Tests
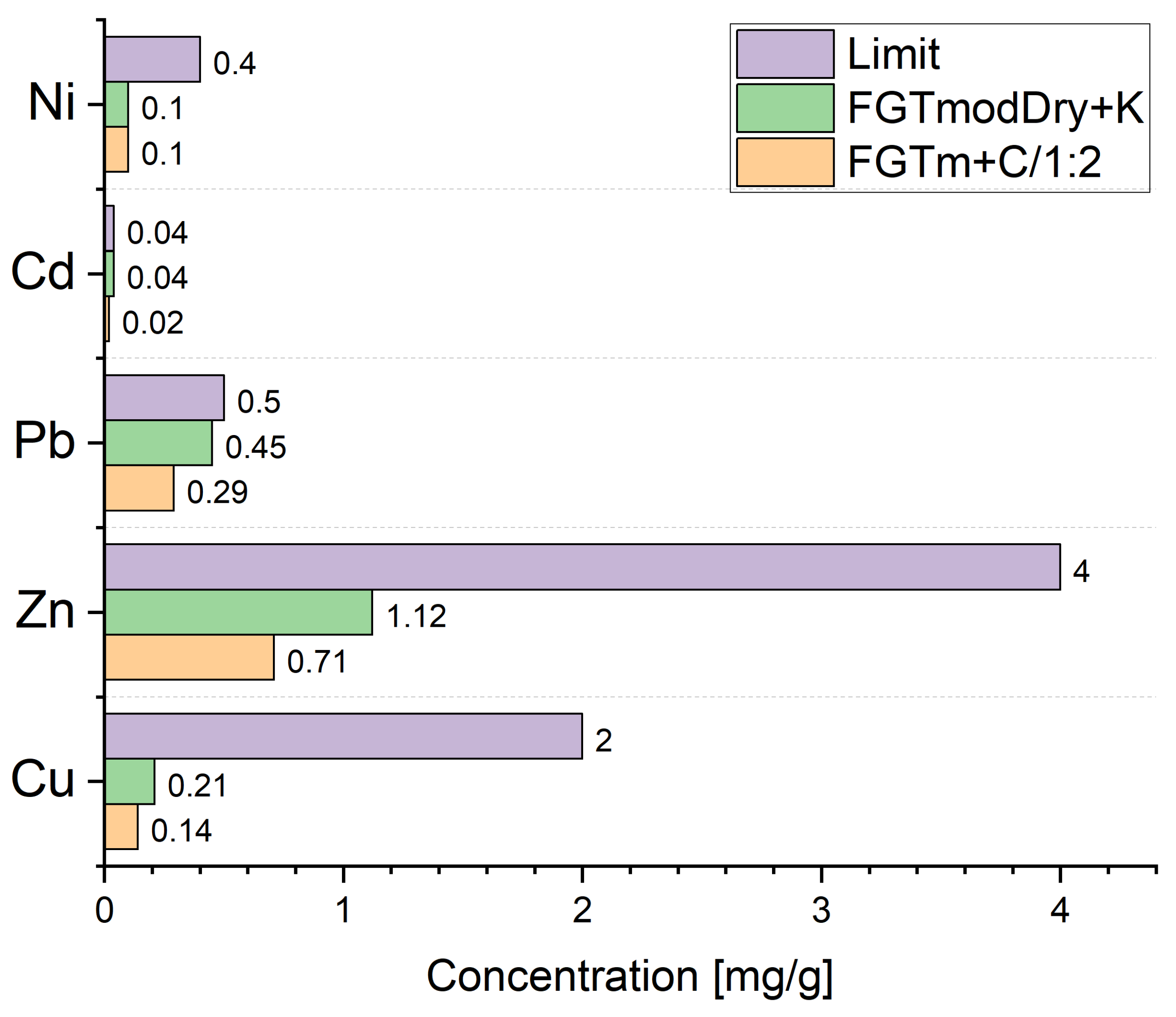
5.2. Leachability of Heavy Metals
6. Conclusions
- Geopolymer synthesis using FGT ash and coal fly ash does not yield materials with sufficient strength parameters.
- Washing FGT ash with water produces favourable results for subsequent geopolymer synthesis, especially when the ash is dried after the washing operation.
- The materials produced met the leachability standards for the heavy metals tested in this study, qualifying them for disposal in landfills according to regulatory limits.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bandarra, B.S.; Quina, M.J. Municipal Solid Waste Incineration and Sustainable Development. In Advances in Sustainable Energy: Policy, Materials and Devices; Gao, Y., Song, W., Liu, J.L., Bashir, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 653–680. ISBN 978-3-030-74406-9. [Google Scholar]
- Council of the European Union. Council Directive 91/689/EEC of 12 December 1991 on Hazardous Waste 1991; Council of the European Union: Brussels, Belgium, 1991. [Google Scholar]
- D’Inverno, G.; Carosi, L.; Romano, G. Meeting the Challenges of the Waste Hierarchy: A Performance Evaluation of EU Countries. Ecol. Indic. 2024, 160, 111641. [Google Scholar] [CrossRef]
- Barth, E.F. An Overview of the History, Present Status, and Future Direction of Solidification/Stabilization Technologies for Hazardous Waste Treatment. J. Hazard. Mater. 1990, 24, 103–109. [Google Scholar] [CrossRef]
- Luz, C.A.; Rocha, J.C.; Cheriaf, M.; Pera, J. Use of Sulfoaluminate Cement and Bottom Ash in the Solidification/Stabilization of Galvanic Sludge. J. Hazard. Mater. 2006, 136, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.D.; Pollard, S.J.T. The Influence of Interference Effects on the Mechanical, Microstructural and Fixation Characteristics of Cement-Solidified Hazardous Waste Forms. J. Hazard. Mater. 1997, 52, 171–191. [Google Scholar] [CrossRef]
- Atanes, E.; Cuesta-García, B.; Nieto-Márquez, A.; Fernández-Martínez, F. A Mixed Separation-Immobilization Method for Soluble Salts Removal and Stabilization of Heavy Metals in Municipal Solid Waste Incineration Fly Ash. J. Environ. Manag. 2019, 240, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kuboňová, L.; Langová, Š.; Nowak, B.; Winter, F. Thermal and Hydrometallurgical Recovery Methods of Heavy Metals from Municipal Solid Waste Fly Ash. Waste Manag. 2013, 33, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Cho, D.-W.; Tsang, D.C.W.; Tong, L.; Zhou, Y.; Yang, J.; Hu, Q.; Poon, C.S. Sustainable Stabilization/Solidification of Municipal Solid Waste Incinerator Fly Ash by Incorporation of Green Materials. J. Clean. Prod. 2019, 222, 335–343. [Google Scholar] [CrossRef]
- Elomaa, H.; Seisko, S.; Lehtola, J.; Lundström, M. A Study on Selective Leaching of Heavy Metals vs. Iron from Fly Ash. J. Mater. Cycles Waste Manag. 2019, 21, 1004–1013. [Google Scholar] [CrossRef]
- Lan, T.; Meng, Y.; Ju, T.; Song, M.; Chen, Z.; Shen, P.; Du, Y.; Deng, Y.; Han, S.; Jiang, J. Manufacture of Alkali-Activated and Geopolymer Hybrid Binder (AGHB) by Municipal Waste Incineration Fly Ash Incorporating Aluminosilicate Supplementary Cementitious Materials (ASCM). Chemosphere 2022, 303, 134978. [Google Scholar] [CrossRef]
- Marieta, C.; Guerrero, A.; Leon, I. Municipal Solid Waste Incineration Fly Ash to Produce Eco-Friendly Binders for Sustainable Building Construction. Waste Manag. 2021, 120, 114–124. [Google Scholar] [CrossRef]
- Kirkelund, G.M.; Jensen, P.E. Electrodialytic Treatment of Greenlandic Municipal Solid Waste Incineration Fly Ash. Waste Manag. 2018, 80, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Chuieh, P.T. Life Cycle Assessment of Reusing Fly Ash from Municipal Solid Waste Incineration. Procedia Eng. 2015, 118, 984–991. [Google Scholar] [CrossRef]
- Gong, B.; Deng, Y.; Yang, Y.; Wang, C.; He, Y.; Sun, X.; Liu, Q.; Yang, W. Effects of Microwave-Assisted Thermal Treatment on the Fate of Heavy Metals in Municipal Solid Waste Incineration Fly Ash. Energy Fuels 2017, 31, 12446–12454. [Google Scholar] [CrossRef]
- Li, R.; Zhang, B.; Wang, Y.; Zhao, Y.; Li, F. Leaching Potential of Stabilized Fly Ash from the Incineration of Municipal Solid Waste with a New Polymer. J. Environ. Manag. 2019, 232, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Vavva, C.; Voutsas, E.; Magoulas, K. Process Development for Chemical Stabilization of Fly Ash from Municipal Solid Waste Incineration. Chem. Eng. Res. Des. 2017, 125, 57–71. [Google Scholar] [CrossRef]
- Liu, Z.; Yue, Y.; Lu, M.; Zhang, J.; Sun, F.; Huang, X.; Zhou, J.; Qian, G. Comprehension of Heavy Metal Stability in Municipal Solid Waste Incineration Fly Ash with Its Compositional Variety: A Quick Prediction Case of Leaching Potential. Waste Manag. 2019, 84, 329–339. [Google Scholar] [CrossRef] [PubMed]
- EN 12457:2002; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges. CEN/TC 292—Characterization of Waste. European Committee for Standardization: Brussels, Belgium, 2002.
- Yakubu, Y.; Zhou, J.; Ping, D.; Shu, Z.; Chen, Y. Effects of PH Dynamics on Solidification/Stabilization of Municipal Solid Waste Incineration Fly Ash. J. Environ. Manag. 2018, 207, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, L.; Zhu, Y.; Wu, J.; Lu, Z.; Lu, J. Evaluation of Heavy Metal Element Detection in Municipal Solid Waste Incineration Fly Ash Based on LIBS Sensor. Waste Manag. 2020, 102, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Baran, P.; Nazarko, M.; Włosińska, E.; Kanciruk, A.; Zarębska, K. Synthesis of Geopolymers Derived from Fly Ash with an Addition of Perlite. J. Clean. Prod. 2021, 293, 126112. [Google Scholar] [CrossRef]
- Zarębska, K.; Szczurowski, J.; Gazda-Grzywacz, M.; Wróbel, W.; Bator, J.; Baran, P. Geopolymer Building Materials Based on Fly Ash in Terms of Removing SO2, CO2, and Water Vapor. Energies 2023, 16, 5188. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Baran, P.; Gazda-Grzywacz, M.; Bator, J.; Wróbel, W.; Zarębska, K. Decarbonatization of Energy Sector by CO2 Sequestration in Waste Incineration Fly Ash and Its Utilization as Raw Material for Alkali Activation. Materials 2023, 16, 6094. [Google Scholar] [CrossRef] [PubMed]
- Zarębska, K.; Zabierowski, P.; Gazda-Grzywacz, M.; Czuma, N.; Baran, P. Fly Ash-Based Geopolymers with Refractoriness Properties. Clean. Technol. Environ. Policy 2022, 24, 2161–2175. [Google Scholar] [CrossRef]
- Kaja, A.M.; Schollbach, K.; Melzer, S.; van der Laan, S.R.; Brouwers, H.J.H.; Yu, Q. Hydration of Potassium Citrate-Activated BOF Slag. Cem. Concr. Res. 2021, 140, 106291. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Properties of Alkali Activated Slag–Fly Ash Blends with Limestone Addition. Cem. Concr. Compos. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Boesch, M.E.; Vadenbo, C.; Saner, D.; Huter, C.; Hellweg, S. An LCA Model for Waste Incineration Enhanced with New Technologies for Metal Recovery and Application to the Case of Switzerland. Waste Manag. 2014, 34, 378–389. [Google Scholar] [CrossRef]
- Mizerna, K.; Król, A. Sequential Extraction of Heavy Metals in Mineral-Organic Composite. Ecol. Eng. Environ. Technol. 2018, 19, 23–29. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Guo, J.; Zhang, H.; Wang, T. Effect of Municipal Solid Waste Incineration Ash on Microstructure and Hydration Mechanism of Geopolymer Composites. Buildings 2022, 12, 723. [Google Scholar] [CrossRef]
- Łach, M.; Mierzwiński, D.; Korniejenko, K.; Mikuła, J.; Hebda, M. Geopolymers as a Material Suitable for Immobilization of Fly Ash from Municipal Waste Incineration Plants. J. Air Waste Manag. Assoc. 2018, 68, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Ran, X.; Pan, Z.; Korayem, A.H. Use of Municipal Solid Waste Incineration Fly Ash in Geopolymer Masonry Mortar Manufacturing. Materials 2022, 15, 8689. [Google Scholar] [CrossRef] [PubMed]
- Energy Recovery from the Combustion of Municipal Solid Waste (MSW). Available online: https://www.epa.gov/smm/energy-recovery-combustion-municipal-solid-waste-msw#why (accessed on 10 July 2024).
- Nevrlý, V.; Šomplák, R.; Putna, O.; Pavlas, M. Location of Mixed Municipal Waste Treatment Facilities: Cost of Reducing Greenhouse Gas Emissions. J. Clean. Prod. 2019, 239, 118003. [Google Scholar] [CrossRef]
- Fang, W.; Ding, Y.; Geng, J.; Liu, Z.; Huang, Y.; Yang, J.; Ma, Z.; Liu, J.; Bi, J.; Liu, M.; et al. High Potential of Coupling the Source-Separation and Incineration Promotion to Reduce Costs Based on City-Level Cost-Benefit Analysis of Municipal Solid Waste Management Strategies in China. Resour. Conserv. Recycl. 2023, 197, 107099. [Google Scholar] [CrossRef]
- PN EN ISO 11885:2009; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2009.
- PL-EN 196-1:2016; Methods of Testing Cement—Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- Chimenos, J.M.; Fernández, A.I.; Cervantes, A.; Miralles, L.; Fernández, M.A.; Espiell, F. Optimizing the APC Residue Washing Process to Minimize the Release of Chloride and Heavy Metals. Waste Manag. 2005, 25, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, Y.; Nie, Y. Investigation of Accelerated and Natural Carbonation of MSWI Fly Ash with a High Content of Ca. J. Hazard. Mater. 2010, 174, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Xiong, Z.; Tian, C.; Li, H.; Zhao, Y.; Zhang, J.; Zheng, C. Influence of Carbonation under Oxy-Fuel Combustion Flue Gas on the Leachability of Heavy Metals in MSWI Fly Ash. Waste Manag. 2017, 67, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Di Bartolomeo, E.; Polettini, A.; Pomi, R. The Effects of Accelerated Carbonation on CO2 Uptake and Metal Release from Incineration APC Residues. Waste Manag. 2009, 29, 2994–3003. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.; Hartikainen, A.; Sippula, O.; Lähde, A.; Mesceriakovas, A.; Peräniemi, S.; Jokiniemi, J. Thermal Separation of Zinc and Other Valuable Elements from Municipal Solid Waste Incineration Fly Ash. J. Clean. Prod. 2020, 253, 120014. [Google Scholar] [CrossRef]
- Sun, C.-J.; Li, M.-G.; Gau, S.-H.; Wang, Y.-H.; Jan, Y.-L. Improving the Mechanical Characteristics and Restraining Heavy Metal Evaporation from Sintered Municipal Solid Waste Incinerator Fly Ash by Wet Milling. J. Hazard. Mater. 2011, 195, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Frías Rocha, S.; Aschenbrenner, P.; Rechberger, H.; Winter, F. Heavy Metal Removal from MSW Fly Ash by Means of Chlorination and Thermal Treatment: Influence of the Chloride Type. Chem. Eng. J. 2012, 179, 178–185. [Google Scholar] [CrossRef]
- Mu, Y.; Saffarzadeh, A.; Shimaoka, T. Utilization of Waste Natural Fishbone for Heavy Metal Stabilization in Municipal Solid Waste Incineration Fly Ash. J. Clean. Prod. 2018, 172, 3111–3118. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, M.; Zhang, B.; Li, F.; Wang, H.; Zhang, H. Stabilization of Heavy Metals in MSWI Fly Ash with a Novel Dithiocarboxylate-Functionalized Polyaminoamide Dendrimer. Waste Manag. 2020, 105, 289–298. [Google Scholar] [CrossRef]
- Jung, C.H.; Matsuto, T.; Tanaka, N.; Okada, T. Metal Distribution in Incineration Residues of Municipal Solid Waste (MSW) in Japan. Waste Manag. 2004, 24, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cheng, Y.; He, D.; Yang, E.-H. Review of Leaching Behavior of Municipal Solid Waste Incineration (MSWI) Ash. Sci. Total Environ. 2019, 668, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Richardson, I.G.; Cabrera, J.G. The Nature of C-S-H in Model Slag-Cements. Cem. Concr. Compos. 2000, 22, 259–266. [Google Scholar] [CrossRef]
- Phair, J.W.; van Deventer, J.S.J. Characterization of Fly-Ash-Based Geopolymeric Binders Activated with Sodium Aluminate. Ind. Eng. Chem. Res. 2002, 41, 4242–4251. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovics, M. Geopolymer: Room-Temperature Ceramic Matrix for Composites. Ceram. Eng. Sci. Proc. 1988, 9, 835–842. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Calcium Wastes as an Additive for a Low Calcium Fly Ash Geopolymer. Sci. Rep. 2023, 13, 16351. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The Coexistence of Geopolymeric Gel and Calcium Silicate Hydrate at the Early Stage of Alkaline Activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lorenzen, L. The Potential Use of Geopolymeric Materials to Immobilise Toxic Metals: Part I. Theory and Applications. Min. Eng. 1997, 10, 659–669. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J. Effect of Silicate Activator PH on the Leaching and Material Characteristics of Waste-Based Inorganic Polymers. Min. Eng. 2001, 14, 289–304. [Google Scholar] [CrossRef]
- van Deventer, J.S.J.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction Mechanisms in the Geopolymeric Conversion of Inorganic Waste to Useful Products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef]
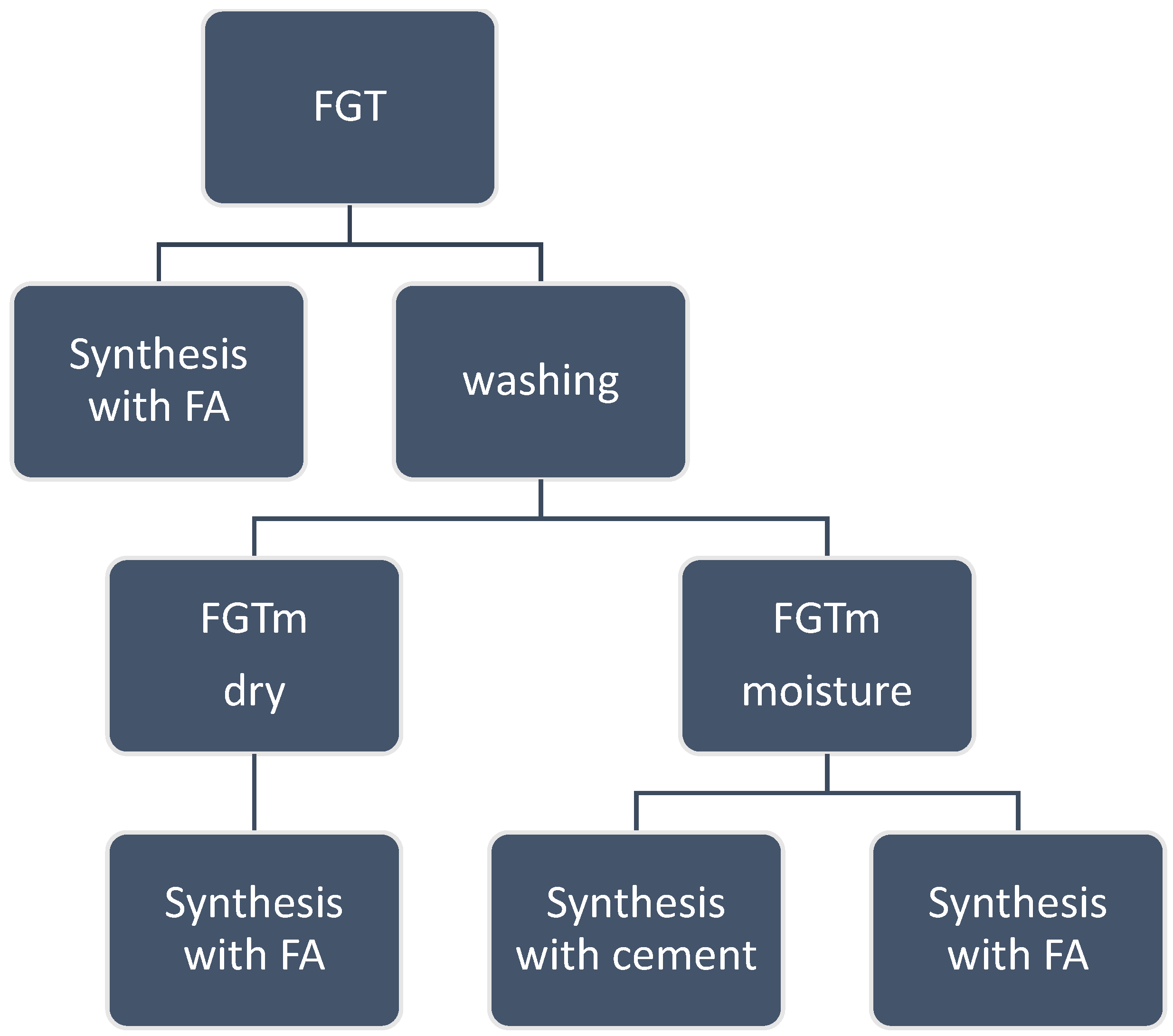



| Sample Symbol | FGT | FGTm | FA | Cem | Sodium Silicate | Sodium Hydroxide 10 mol/dm3 | Water |
|---|---|---|---|---|---|---|---|
| cm3 g | cm3 g | cm3 g | cm3 g | g | g | g | |
| FGT+B | 100 60 | 200 170 | 80 | 40 | 100 | ||
| FGT+K | 100 60 | 200 170 | 80 | 40 | 100 | ||
| FGT+L | 100 60 | 200 230 | 80 | 40 | 100 | ||
| FGTm+B | 100 150 | 200 170 | 80 | 40 | |||
| FGTm+K | 100 150 | 200 170 | 80 | 40 | |||
| FGTm+L | 100 150 | 200 230 | 80 | 40 | |||
| FGTm+C(2:1) | 100 150 | 200 250 | 120 | 60 | |||
| FGTm+C(1:2) | 200 300 | 100 125 | 100 | 50 | |||
| FGTmDRY+B | 95 (Dry) 100 | 200 170 | 80 | 40 | |||
| FGTmDRY+K | 95 (Dry) 100 | 200 170 | 80 | 40 | |||
| FGTmDRY+L | 95 (Dry) 100 | 200 230 | 80 | 40 |
| Compound | B Fly Ash | K Fly Ash | L Fly Ash |
|---|---|---|---|
| Concentration, % | |||
| Na2O | 0.93 | 3.75 | 2.30 |
| MgO | 0.85 | 0.72 | 1.77 |
| Al2O3 | 13.15 | 17.62 | 15.71 |
| SiO2 | 37.22 | 47.50 | 38.45 |
| P2O5 | 0.45 | 1.05 | 0.74 |
| SO3 | 2.93 | 0.55 | 1.04 |
| K2O | 0.21 | 2.23 | 2.77 |
| CaO | 15.34 | 1.95 | 3.58 |
| TiO2 | 1.59 | 1.32 | 1.00 |
| V2O5 | 0.04 | - | 0.04 |
| Cr2O3 | 0.02 | 0.03 | 0.05 |
| MnO | 0.02 | - | 0.17 |
| Fe2O3 | 4.99 | 5.45 | 10.09 |
| NiO | 0.01 | 0.03 | 0.02 |
| CuO | 0.01 | - | 0.09 |
| ZnO | 0.01 | 0.06 | 0.99 |
| BaO | 0.02 | 0.06 | 0.09 |
| Elements | References | ||||
|---|---|---|---|---|---|
| Pb | Cd | Zn | Cu | Ni | |
| 1350 | 129 | 15,600 | 707 | 100 | [42] |
| 5090 | 246 | 10,800 | 1270 | N/A | [43] |
| 4500 | 350 | 19,000 | 890 | 94 | [8] |
| 4600 | 500 | 22,000 | 98 | 61 | [44] |
| 1850 | 181 | 9322 | 500 | 38 | [45] |
| 1330 | 314 | 13,200 | 718 | N/A | [13] |
| 2075 | 255 | 7787 | 545 | N/A | [46] |
| Sample Symbol | Comprehensive Strength [MPa] | Comprehensive Strength [MPa] |
|---|---|---|
| After 7 Days | After 28 Days | |
| FGT+B | 0.58 | 0.58 |
| FGT+K | 0.36 | 0.37 |
| FGT+L | 0.30 | 0.35 |
| FGTm+B | 6.10 | 3.15 |
| FGTm+K | 2.96 | 1.24 |
| FGTm+L | 3.16 | 1.93 |
| FGTm+C(2:1) | 1.60 | 2.01 |
| FGTm+C(1:2) | 8.51 | 11.12 |
| FGTmDRY+B | 7.88 | 12.20 |
| FGTmDRY+K | 17.70 | 17.93 |
| FGTmDRY+L | 11.79 | 12.90 |
| Compound | Concentration | Result |
|---|---|---|
| Sb | mg/kg | 40.0 |
| Cr | mg/kg | 3.80 |
| Zn | mg/kg | 1220 |
| Al | mg/kg | 1080 |
| Cd | mg/kg | 11.2 |
| Si | mg/kg | 248 |
| Cu | mg/kg | 28.1 |
| Ni | mg/kg | <1.0 |
| Pb | mg/kg | 234 |
| K | mg/kg | 72,800 |
| Na | mg/kg | 157,000 |
| Ca | mg/kg | 157,000 |
| Fe | mg/kg | 170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarębska, K.; Szczurowski, J.; Muszyńska, J.; Baran, P. Geopolymer Materials from Fly Ash—A Sustainable Approach to Hazardous Waste Management. Materials 2024, 17, 3515. https://doi.org/10.3390/ma17143515
Zarębska K, Szczurowski J, Muszyńska J, Baran P. Geopolymer Materials from Fly Ash—A Sustainable Approach to Hazardous Waste Management. Materials. 2024; 17(14):3515. https://doi.org/10.3390/ma17143515
Chicago/Turabian StyleZarębska, Katarzyna, Jakub Szczurowski, Joanna Muszyńska, and Paweł Baran. 2024. "Geopolymer Materials from Fly Ash—A Sustainable Approach to Hazardous Waste Management" Materials 17, no. 14: 3515. https://doi.org/10.3390/ma17143515
APA StyleZarębska, K., Szczurowski, J., Muszyńska, J., & Baran, P. (2024). Geopolymer Materials from Fly Ash—A Sustainable Approach to Hazardous Waste Management. Materials, 17(14), 3515. https://doi.org/10.3390/ma17143515






