Influence of Post-Heat Treatment on Corrosion Behaviour of Additively Manufactured CuSn10 by Laser Powder Bed Fusion
Abstract
:1. Introduction
2. Material and Methods
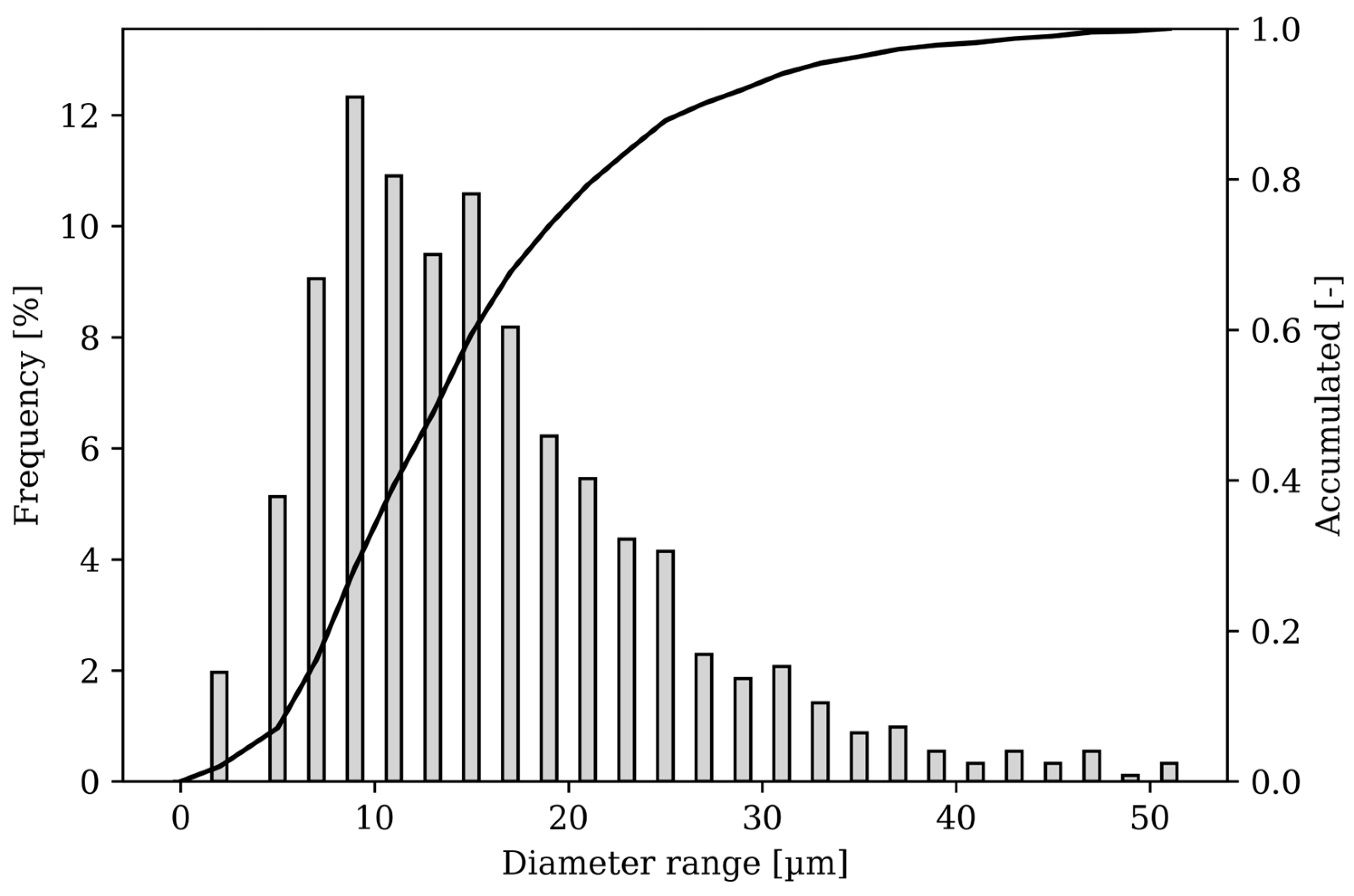
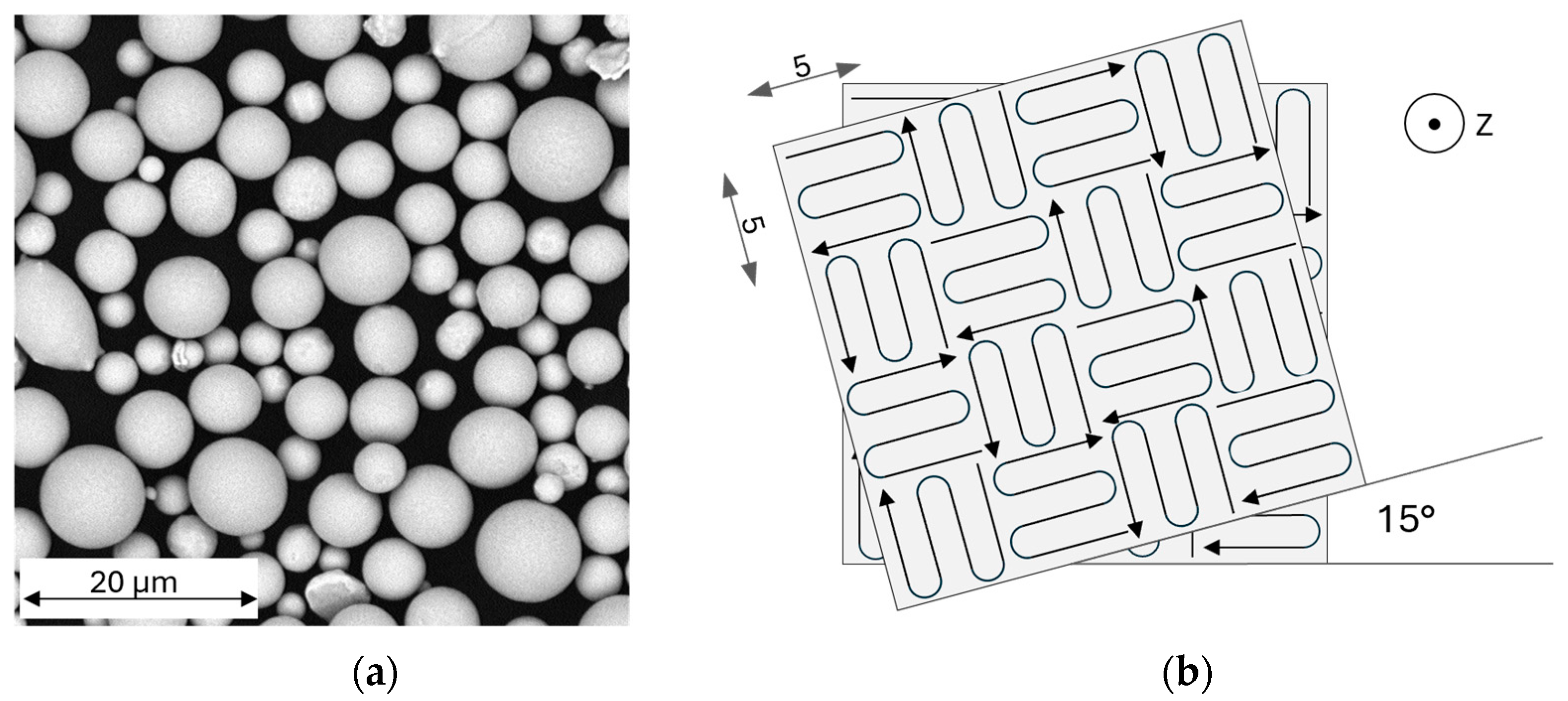
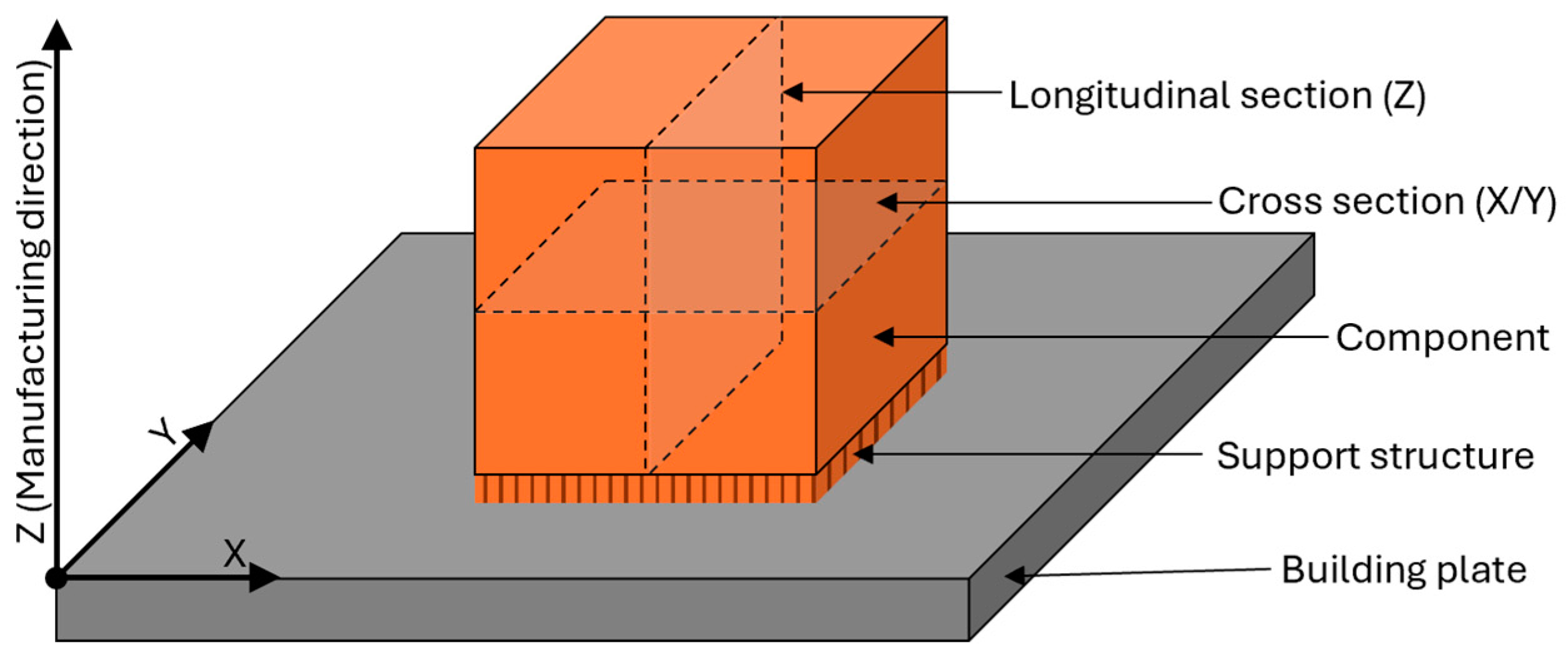
2.1. Feedstock Material, Manufacturing, Heat Treatment, and Preparation
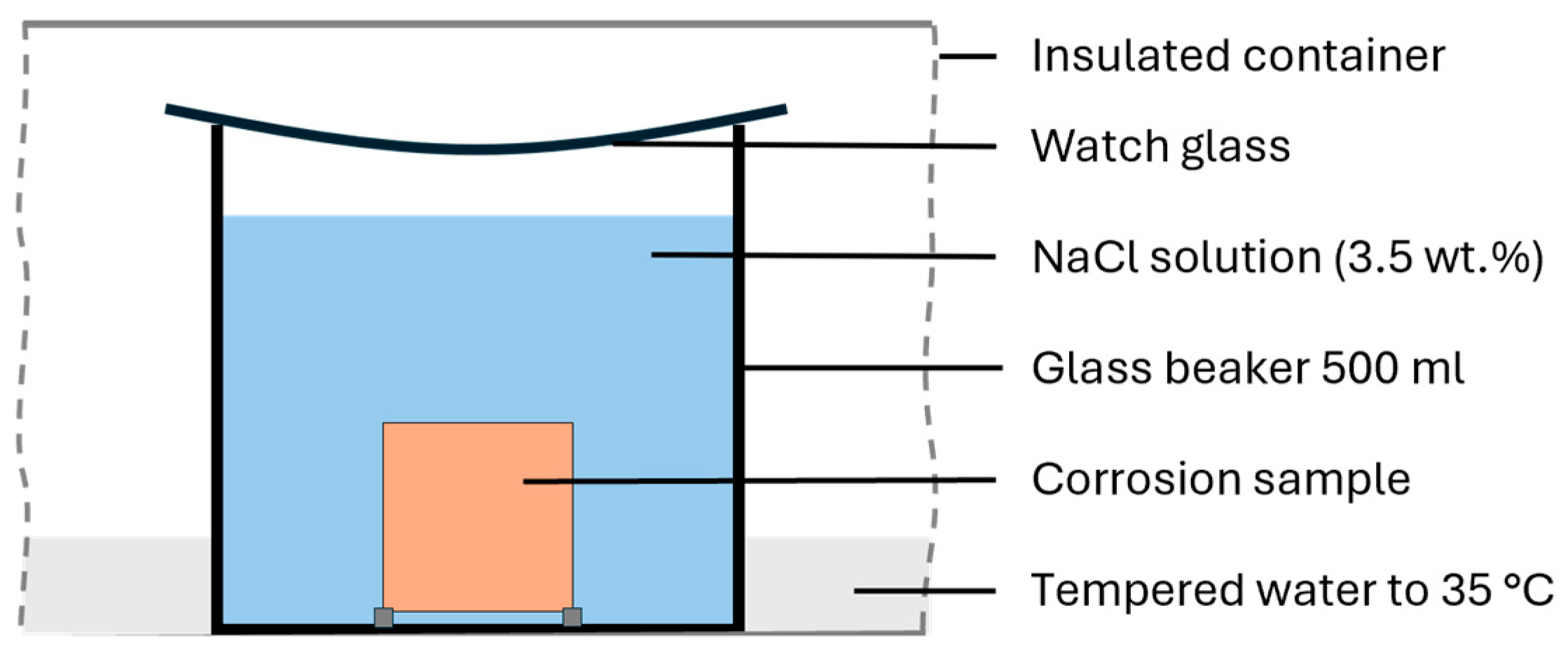
2.2. Examination Methods
- n: Number of electrons exchanged in the reaction.
- F: Faraday constant.
- A: Exposed surface area.
3. Results
3.1. Microstructure and Hardness
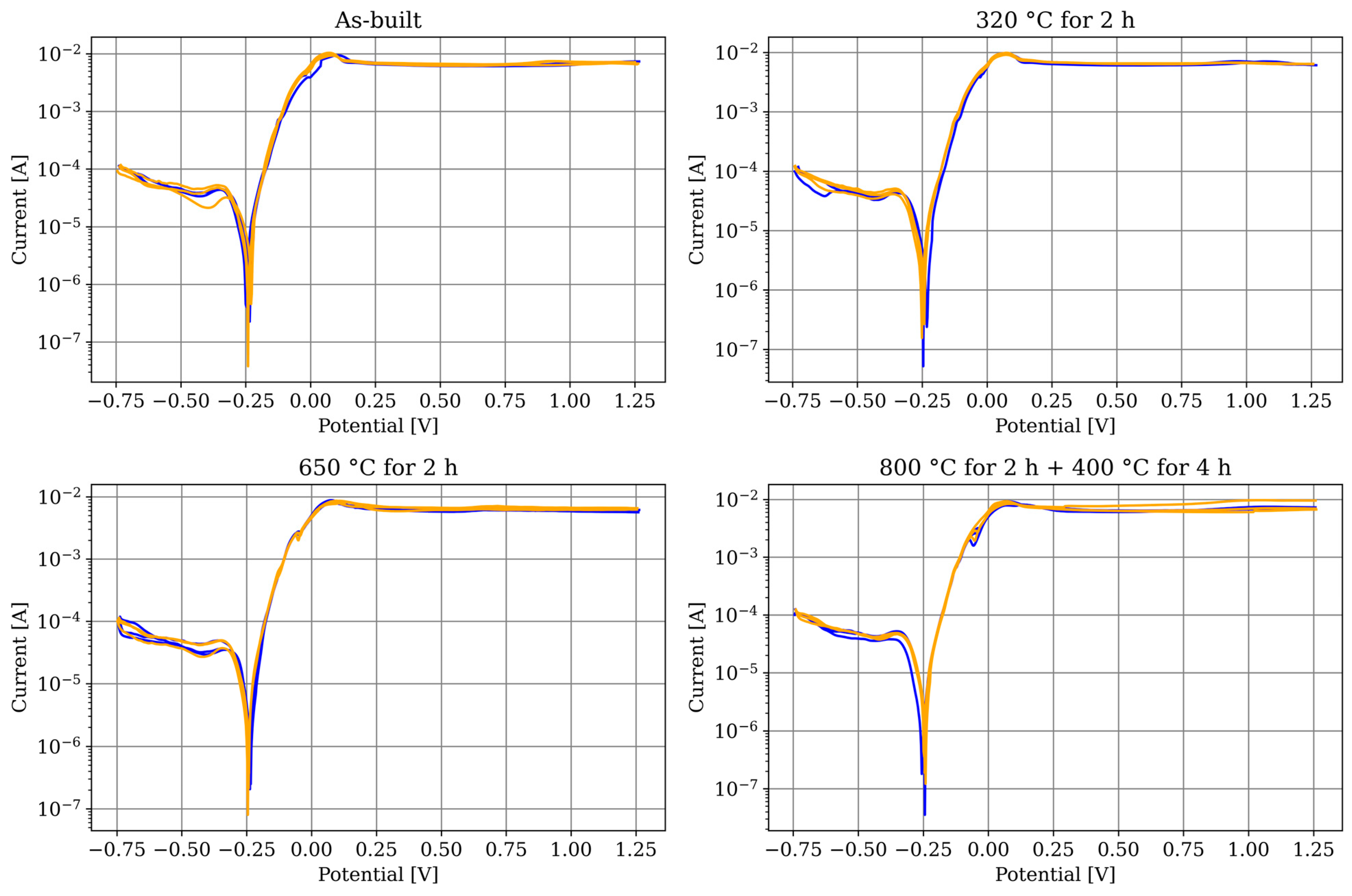
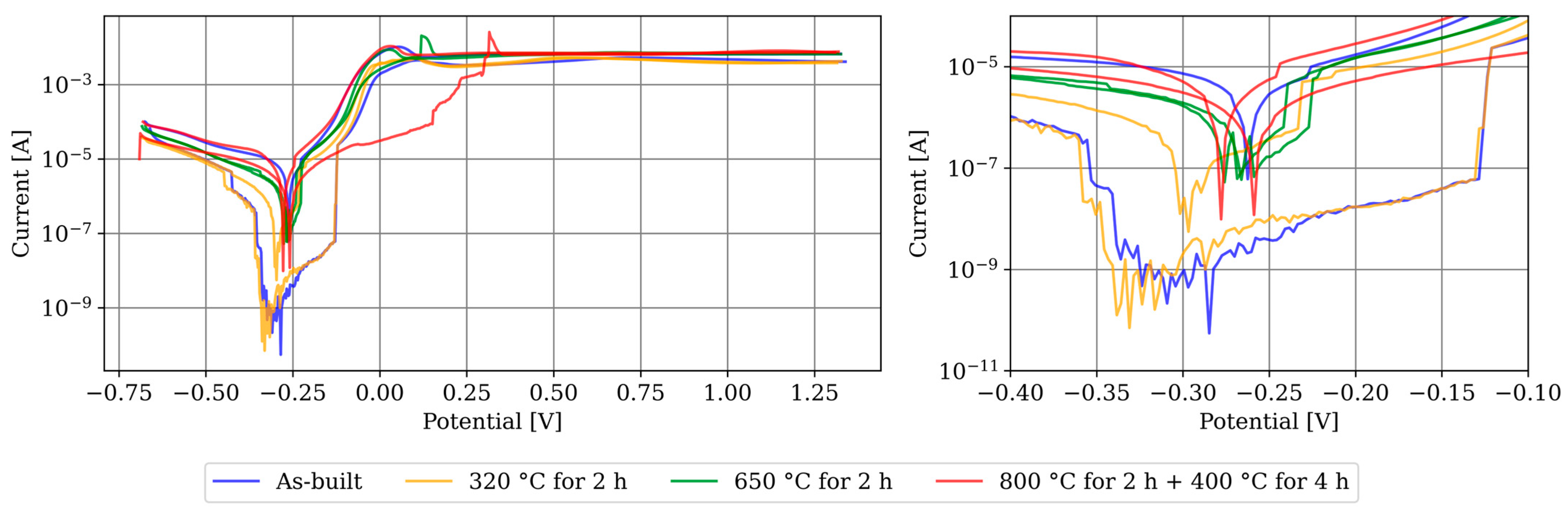
3.2. Electrochemical Corrosion Testing
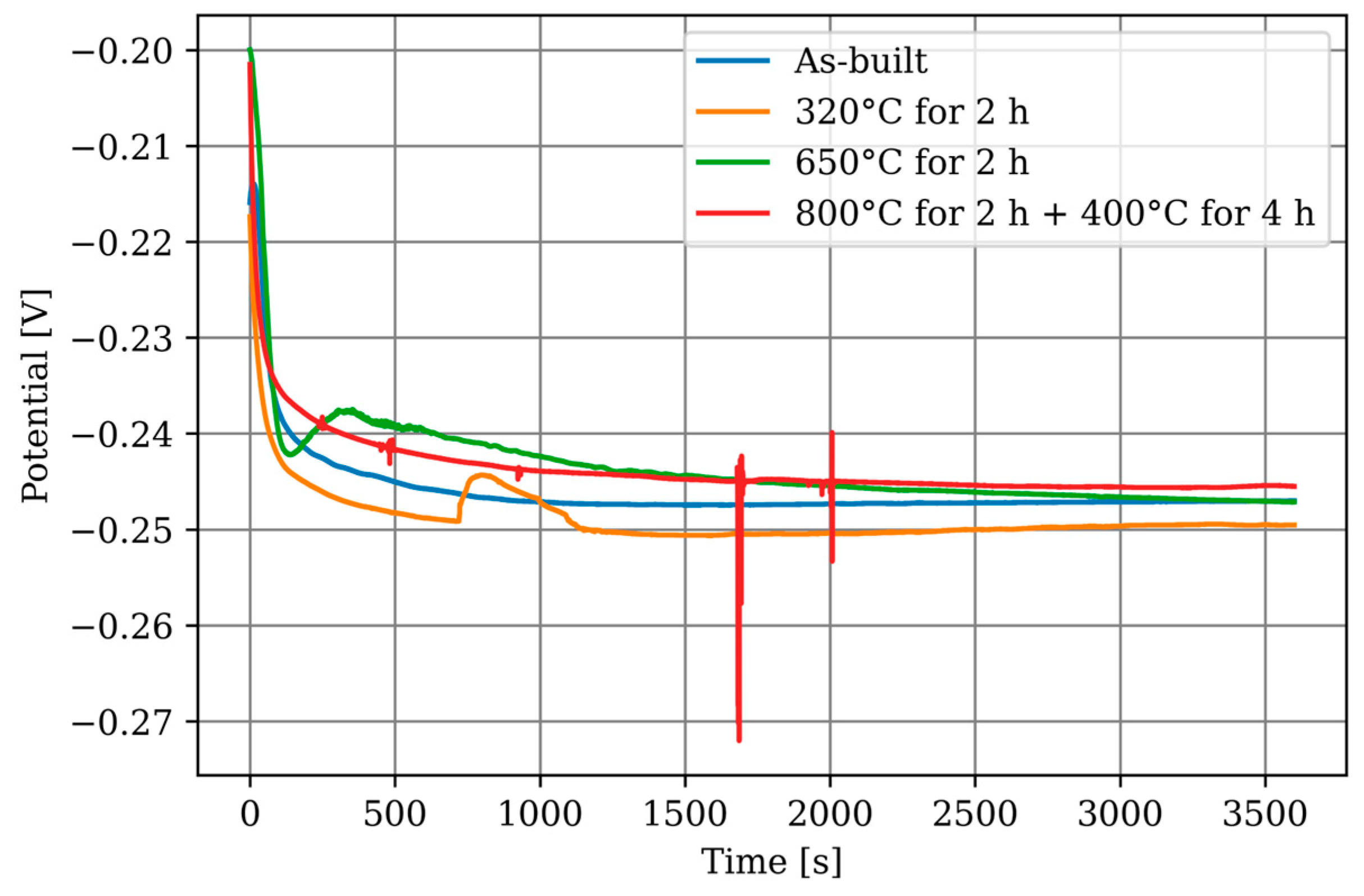
3.3. Electrochemical Corrosion Testing—Long-Time OCP
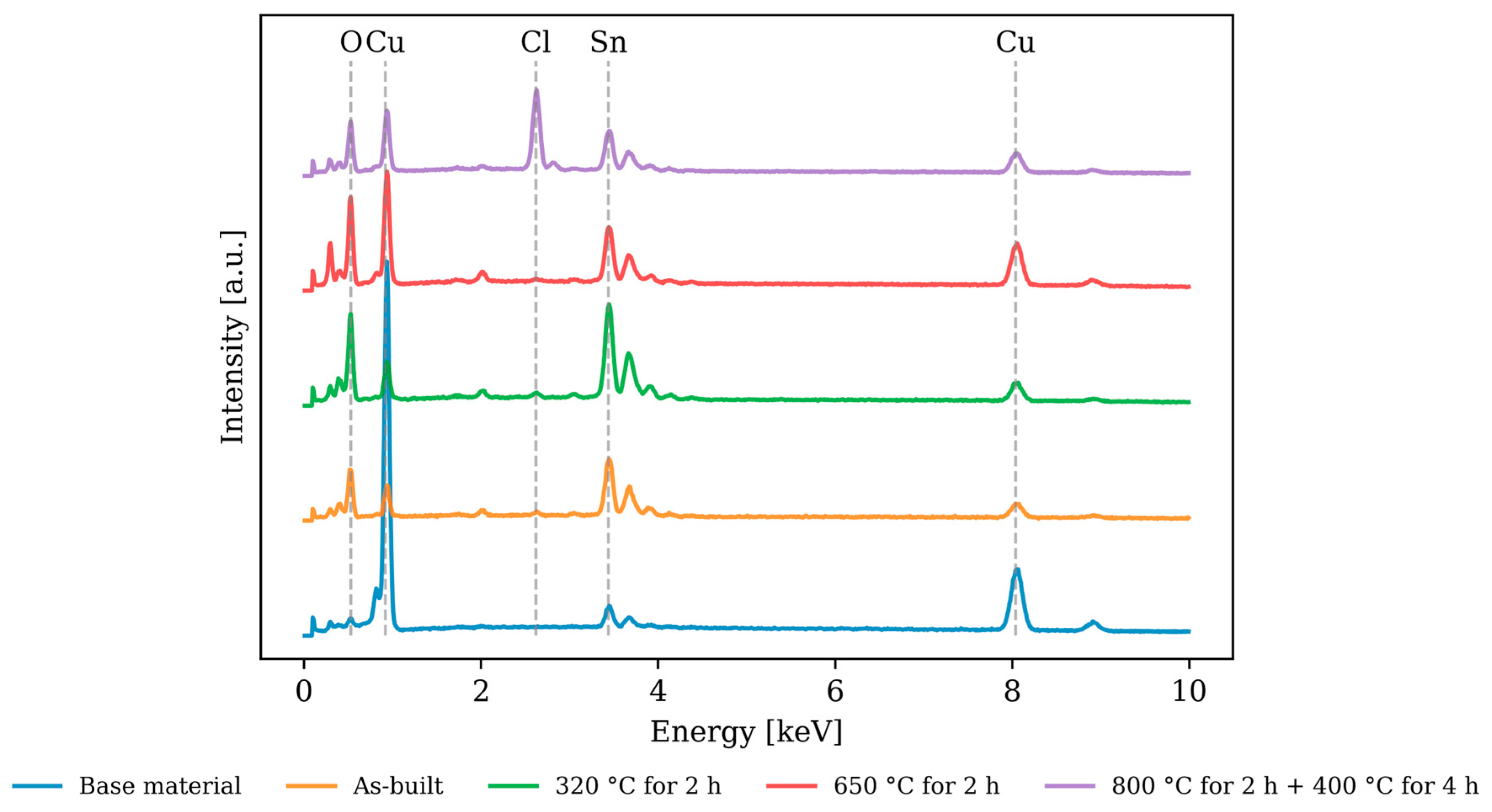
3.4. Electrochemical Corrosion Testing—Chemical Composition of the Test Surface
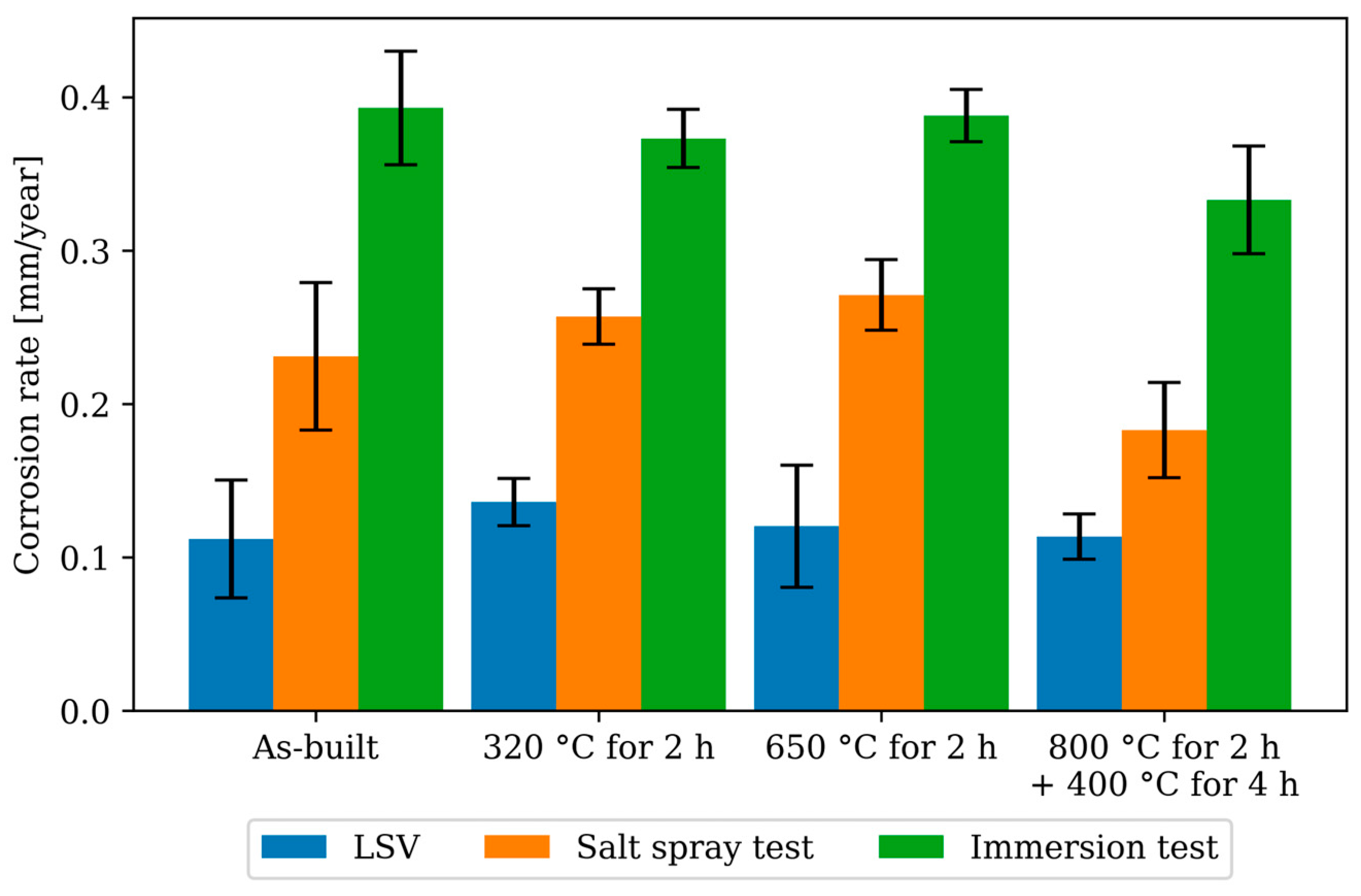
3.5. Gravimetric Corrosion Testing—Immersion and Salt Spray Test
4. Discussion
- Classification within the field of research:
5. Conclusions
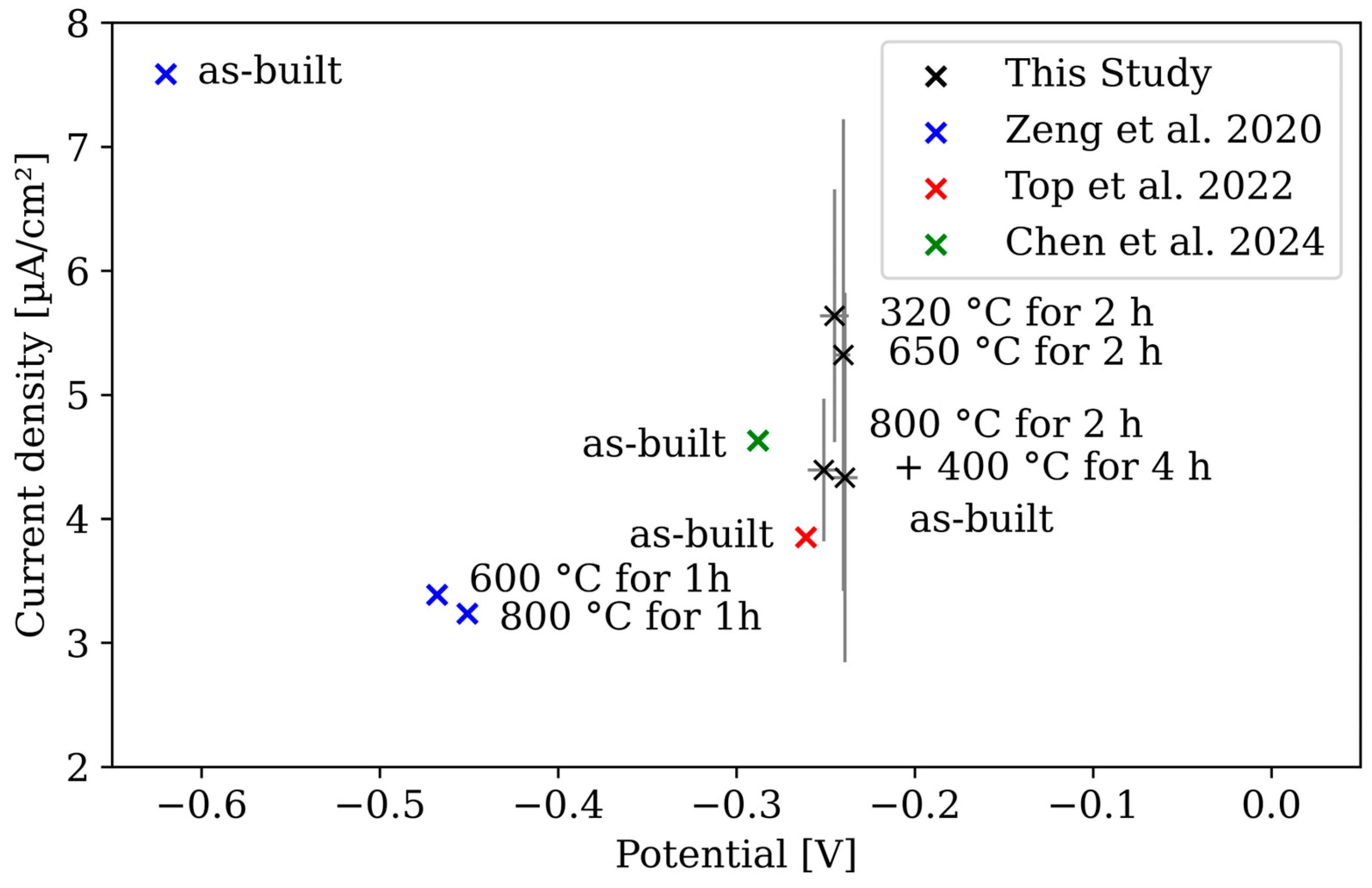
- All corrosion rates measured in this study as well as those determined in the literature are in the range of 0.1 to 0.5 mm/year, regardless of measurement method and post treatment.
- The heat treatments of 320 °C for 2 h and 650 °C for 2 h showed no effect on the corrosion rate in both accelerated and non-accelerated corrosion measurements. The heat treatment of 800 °C for 2 h followed by 400 °C for 4 h showed a tendency to improve the corrosion rate in both immersion and salt spray tests, although the specimen size (n = 5) is not sufficient to make a definitive statement.
- Parallel to the investigations in [20], no correlation could be detected between build direction and corrosion behaviour.
- The material forms a protective passive layer, which exhibits a lower open-circuit potential. The formation of this layer took approximately 30 h.
- The hardness increased from approximately 160 HV 10 with increasing heat treatment, reaching a maximum of about 190 HV 10 after 1 h at 300 °C, and then decreased to about 100 HV 10 at higher heat treatment temperatures.
- The macrostructure visible in the polished section showed no change after treatment at 320 °C for 2 h. Treatment at 650 °C for 2 h resulted in clearly visible grain growth, and the visible differences in the cross and longitudinal section were no longer discernible. Treatment at 800 °C for 2 h followed by 400 °C for 4 h led to visible tin-containing precipitates.
- The microstructure resulting from the heat treatment of 800 °C for 2 h followed by 400 °C for 4 h observed in [16] could not be reproduced in this study, which may be attributed to differences in chemical composition. Such differences may arise both in the starting material and during the manufacturing process itself. During the LPBF process, elements can evaporate from the melt, potentially altering the chemical composition. This variable is influenced by the combination of machine, material, and operator, due to different process parameters such as laser power or scanning speed, leading to variations in temperature profiles and melt pool dimensions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| α-Phase | Stable phase in copper-tin alloys (fcc) |
| β′-Phase | Metastable β’-Cu13.7Sn phase (bcc) |
| δ-Phase | Intermetallic δ-Cu41Sn11 phase (fcc) |
| α | Significance level |
| at.-% | Atom percent |
| ASTM | American Society for Testing and Materials |
| CR | Corrosion Rate |
| CuSn10 | Copper alloy with 10 wt.% tin content |
| EDX | Energy Dispersive X-ray Spectroscopy |
| Ecorr | Corrosion potential |
| F | Faraday constant |
| HV | Vickers Hardness |
| Icorr | Corrosion current |
| LPBF | Laser Powder Bed Fusion |
| LSV | Linear Sweep Voltammetry |
| NaCl | Sodium Chloride |
| OCP | Open Circuit Potential |
| OPS | Oxide Polishing Suspension |
| p-value | Probability value |
| PL | Laser power |
| PTC | Paint Test Cell |
| R2 | Coefficient of determination |
| SCE | Saturated Calomel Electrode |
| SEM: | Scanning Electron Microscopy |
| SiC | Silicon Carbide |
| Std. Dev. | Standard Deviation |
| wt.% | Weight percent |
Appendix A
| Condition | Minimum | Maximum | Median | Mean | Variance | Std. Dev. |
|---|---|---|---|---|---|---|
| As-built | 166 | 175 | 169 | 170 | 8 | 3 |
| 200 °C for 1 h | 174 | 184 | 177 | 178 | 10 | 3 |
| 300 °C for 1 h | 182 | 189 | 186 | 186 | 6 | 2 |
| 320 °C for 2 h | 147 | 151 | 150 | 150 | 2 | 1 |
| 400 °C for 1 h | 182 | 192 | 187 | 187 | 8 | 3 |
| 500 °C for 1 h | 122 | 128 | 125 | 125 | 3 | 2 |
| 600 °C for 1 h | 108 | 112 | 110 | 110 | 1 | 1 |
| 650 °C for 2 h | 99 | 109 | 105 | 104 | 9 | 3 |
| 700 °C for 1 h | 88 | 92 | 90 | 90 | 1 | 1 |
| 800 °C for 1 h | 94 | 99 | 96 | 96 | 2 | 1 |
| 800 °C for 2 h + 400 °C for 4 h | 96 | 103 | 100 | 100 | 5 | 2 |
| 900 °C for 1 h | 78 | 90 | 85 | 85 | 17 | 4 |
| 1000 °C for 1 h | 91 | 109 | 98 | 100 | 46 | 6 |
References
- Scudino, S.; Unterdörfer, C.; Prashanth, K.G.; Attar, H.; Ellendt, N.; Uhlenwinkel, V.; Eckert, J. Additive manufacturing of Cu–10Sn bronze. Mater. Lett. 2015, 156, 202–204. [Google Scholar] [CrossRef]
- Seidel, W.W. Werkstofftechnik: Werkstoffe, Eigenschaften, Prüfung, Anwendung; mit zahlreichen Tabellen, Beispielen, Übungen und Testaufgaben, 6., neu bearb. Aufl.; Hanser; Wien: München, Germany, 2005; ISBN 3-446-22900-0. [Google Scholar]
- Kupfer-Zinn- und Kupfer-Zinn-Zink-Gusslegierungen (Zinnbronzen); Informationsdruck i.25; Deutsches Kupferinstiut-Auskunfts und Beratungsstelle für die Verwendung von Kupfer und Kupferlegierungen (Eds.) Auflage: Düsseldorf, Germany, 2004; Available online: https://kupfer.de/fileadmin/user_upload/kupferinstitut.de/de/Documents/Shop/Verlag/Downloads/Werkstoffe/i015.pdf (accessed on 29 April 2024).
- Kremer, R.; Khani, S.; Appel, T.; Palkowski, H.; Foadian, F. Selective Laser Melting of CuSn10: Simulation of Mechanical Properties, Microstructure, and Residual Stresses. Materials 2022, 15, 3902. [Google Scholar] [CrossRef] [PubMed]
- Foadian, F.; Kremer, R.; Khani, S. Rapid alloying in additive manufacturing using integrated computational materials engineering. In Quality Analysis of Additively Manufactured Metals: Simulation Approaches, Processes, and Microstructure Properties; Kadkhodapour, J., Schmauder, S., Sajadi, F., Eds.; Elsevier: Amsterdam, Netherlands, 2023; pp. 583–624. ISBN 9780323886642. [Google Scholar]
- Gebhardt, A.; Kessler, J.; Schwarz, A. Produktgestaltung für die Additive Fertigung; Hanser; Ciando: München, Germany, 2019; ISBN 9783446461338. [Google Scholar]
- Kremer, R. Experimentelle und Simulative Untersuchung der Kristallstruktur und Eigenspannungen an Selektiv Lasergeschmolzenen Bauteilen aus CuSn10. Master’s Thesis, Fachhochschule Dortmund, Dortmund, Germany, 2022. [Google Scholar] [CrossRef]
- Tiberto, D.; Klotz, U.; Held, F. Einfluss der thermophysikalischen Eigenschaften auf die Verarbeitbarkeit von CuSn Legierungen durch das selektive Laserschmelzen. METALL 2017, 71, 452. Available online: https://p532423.webspaceconfig.de/fileadmin/user_upload/kupferinstitut.de/de/Documents/techUnterstuetzung/KS/Artikel/2017/452_FA_Tiberto.pdf (accessed on 29 April 2024).
- Karthik, G.M.; Haftlang, F.; Kwak, J.; Sathiyamoorthi, P.; Zargaran, A.; Kim, Y.-T.; Kim, H.S. The influence of laser powder-bed fusion microstructures on the corrosion behavior of CuSn alloy. J. Mater. Sci. 2022, 57, 17923–17934. [Google Scholar] [CrossRef]
- Mehta, A.; Le, Z.; Hyer, H.; Huynh, T.; Lu, B.; Graydon, K.; Drobner, E.J.; Park, S.H.; Sohn, Y. Microstructural characteristics and mechanical properties of additively manufactured Cu–10Sn alloys by laser powder bed fusion. Mater. Sci. Eng. A 2022, 838, 142775. [Google Scholar] [CrossRef]
- Wang, H.; Guo, L.; Nie, Z.; Lyu, Q.; Zhang, Q. Processing Technologies and Properties of Cu-10Sn Formed by Selective Laser Melting Combined with Heat Treatment. 3d Print. Addit. Manuf. 2021, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Yao, X.; Wang, D.; Wang, H.; Yan, M.; Wong, K.S. Selective Laser Melting of Cu–10Sn–0.4P: Processing, Microstructure, Properties, and Brief Comparison with Additively Manufactured Cu–10Sn. Adv. Eng. Mater. 2022, 24, 2100716. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Wang, J.; Xu, L.; Wang, D. Process Parameter Optimization on Selective Laser Melting of CuSn10 Powders Based on Response Surface Methodology. J. Phys. Conf. Ser. 2022, 2262, 012002. [Google Scholar] [CrossRef]
- Vukkum, V.B.; Gupta, R.K. Review on corrosion performance of laser powder-bed fusion printed 316L stainless steel: Effect of processing parameters, manufacturing defects, post-processing, feedstock, and microstructure. Mater. Des. 2022, 221, 110874. [Google Scholar] [CrossRef]
- Sander, G.; Tan, J.; Balan, P.; Gharbi, O.; Feenstra, D.R.; Singer, L.; Thomas, S.; Kelly, R.G.; Scully, J.R.; Birbilis, N. Corrosion of Additively Manufactured Alloys: A Review. Corrosion 2018, 74, 1318–1350. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Guo, X.; He, D.; Shao, W.; Tan, Z.; Fu, H.; Zhou, Z.; Zhang, X. Microstructure Twinning and Mechanical Properties of Laser Melted Cu-10Sn Alloy for High Strength and Plasticity. J. Mater. Eng. Perform 2022, 31, 2624–2632. [Google Scholar] [CrossRef]
- G01 Committee. Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- DIN EN ISO 14713-1:2017-08; Zinküberzüge—Leitfäden und Empfehlungen zum Schutz von Eisen-und Stahlkonstruktionen vor Korrosion—Teil_1: Allgemeine Konstruktionsgrundsätze und Korrosionsbeständigkeit (ISO 14713-1:2017). Beuth Verlag GmbH: Berlin, Germany, 2017.
- J01 Committee. Guide for Laboratory Immersion Corrosion Testing of Metals; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- Zeng, C.; Zhang, B.; Hemmasian Ettefagh, A.; Wen, H.; Yao, H.; Meng, W.J.; Guo, S. Mechanical, thermal, and corrosion properties of Cu-10Sn alloy prepared by laser-powder-bed-fusion additive manufacturing. Addit. Manuf. 2020, 35, 101411. [Google Scholar] [CrossRef]
- Kupferverband. CuSn10-C: Material Data Sheet. Available online: https://kupfer.de/wp-content/uploads/2019/11/CuSn10-C.pdf (accessed on 9 July 2024).
- Sicius, H. Handbuch der Chemischen Elemente; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-662-55938-3. [Google Scholar]
- Metrohm, A.G. Determining the Corrosion Rate with INTELLO—Tafel Analysis and More. Available online: https://www.metrohm.com/en_gb/applications/application-notes/autolab-applikationen-anautolab/an-cor-019.html (accessed on 29 April 2024).
- Top, M.N.; Ozbey, S.; Soruşbay, B.; Gülsoy, H. Investigation of Microstructural, Mechanical and Corrosion Properties of Cu-10Sn Bronze Parts Produced by Selective Laser Melting. Türk. Doğa Ve Fen Derg. 2022, 11, 76–81. [Google Scholar] [CrossRef]
- Schmitz Metallographie GmbH. CC483K|CuSn12|C90800 Bronze. Available online: https://www.schmitz-metallographie.de/gefuge/cc483k-cusn12-c90800-bronze/ (accessed on 29 February 2024).
- DECHEMA. DECHEMA Corrosion Handbook: Corrosive and Chemical Resistance of Materials. Available online: https://ac.dechema.de/dwt+.html (accessed on 17 May 2024).
- Kupferinstitut, D.; Alliance, C. Beständigkeitstabellen von Kupferwerkstoffen in Verschiedenen Medien. Available online: https://q9j8s3a5.rocketcdn.me/wp-content/uploads/2020/02/DKI_Bestaendigkeitstabellen_DE.pdf (accessed on 17 May 2024).
- Chen, J.; Zhang, M.; Zhao, D.; Bi, G.; Bai, Y.; Xiao, Y.; Wang, D. The impact of interfacial characteristics on the interfacial properties of 316 L/CuSn10 multi-material manufactured by laser powder bed fusion. Mater. Charact. 2024, 211, 113862. [Google Scholar] [CrossRef]






| Parameter | Value | Unit |
|---|---|---|
| Laser power PL | 100 | W |
| Scan speed v | 300 | mm/s |
| Slice thickness t | 0.015 | mm |
| Hatch spacing h | 0.06 | mm |
| Scan strategy | Islands 5 × 5, 15° rotation | |
| Inert gas | Nitrogen | |
| Open Circuit Potential | Ecorr [V] | Icorr [µA/cm2] | Corrosion Rate (CR) | |||
|---|---|---|---|---|---|---|
| OCP [V] | p-Value | CR [mm/Year] | p-Value | |||
| As-built | −0.243 ± 0.005 | −0.239 ± 0.007 | 4.332 ± 1.490 | 0.112 ± 0.039 | ||
| 320 °C for 2 h | −0.244 ± 0.004 | 0.185 | −0.245 ± 0.008 | 5.637 ± 1.018 | 0.136 ± 0.015 | 0.959 |
| 650 °C for 2 h | −0.246 ± 0.004 | 0.930 | −0.240 ± 0.004 | 5.320 ± 1.902 | 0.120 ± 0.040 | 0.262 |
| 800 °C for 2 h + 400 °C for 4 h | −0.246 ± 0.001 | 0.413 | −0.251 ± 0.009 | 4.392 ± 0.575 | 0.113 ± 0.015 | 0.717 |
| Cu [at.-%] | Sn [at.-%] | O [at.-%] | Cl [at.-%] | P [at.-%] | |
|---|---|---|---|---|---|
| Starting material | 79.6 | 6.4 | 13.6 | 0.1 | 0.4 |
| As-built | 15.1 | 13.6 | 69.7 | 0.6 | 1.0 |
| 320 °C for 2 h | 12.1 | 13.5 | 73.0 | 0.4 | 0.9 |
| 650 °C for 2 h | 29.9 | 8.9 | 59.4 | 0.3 | 1.5 |
| 800 °C for 2 h + 400 °C for 4 h | 19.6 | 9.2 | 58.0 | 12.6 | 0.6 |
| Corrosion Rate (CR) | Salt Spray Test | Immersion Test | ||
|---|---|---|---|---|
| CR [mm/Year] | p-Value | CR [mm/Year] | p-Value | |
| As-built | 0.231 ± 0.048 | 0.393 ± 0.037 | ||
| 320 °C for 2 h | 0.257 ± 0.018 | 0.325 | 0.373 ± 0.019 | 0.374 |
| 650 °C for 2 h | 0.271 ± 0.023 | 0.169 | 0.388 ± 0.017 | 0.820 |
| 800 °C for 2 h + 400 °C for 4 h | 0.183 ± 0.031 | 0.132 | 0.333 ± 0.035 * | 0.0450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremer, R.; Etzkorn, J.; Khani, S.; Appel, T.; Buhl, J.; Palkowski, H. Influence of Post-Heat Treatment on Corrosion Behaviour of Additively Manufactured CuSn10 by Laser Powder Bed Fusion. Materials 2024, 17, 3525. https://doi.org/10.3390/ma17143525
Kremer R, Etzkorn J, Khani S, Appel T, Buhl J, Palkowski H. Influence of Post-Heat Treatment on Corrosion Behaviour of Additively Manufactured CuSn10 by Laser Powder Bed Fusion. Materials. 2024; 17(14):3525. https://doi.org/10.3390/ma17143525
Chicago/Turabian StyleKremer, Robert, Johannes Etzkorn, Somayeh Khani, Tamara Appel, Johannes Buhl, and Heinz Palkowski. 2024. "Influence of Post-Heat Treatment on Corrosion Behaviour of Additively Manufactured CuSn10 by Laser Powder Bed Fusion" Materials 17, no. 14: 3525. https://doi.org/10.3390/ma17143525





