Integration of Digestate-Derived Biochar into the Anaerobic Digestion Process through Circular Economic and Environmental Approaches—A Review
Abstract
1. Introduction
2. Anaerobic Digestion Description
2.1. Mechanisms of AD
- Hydrolysis (stage A): Complex materials, such as proteins, lipids, cellulose, and others, are broken down into simpler substances, including sugars, peptides, glycerol, amino acids, and fatty acids. This transformation is facilitated by exoenzymes produced by anaerobic bacteria [20]. Hydrolysis is a prerequisite for subsequent stages, as it makes the organic material more accessible to microbial degradation.
- Acetogenesis (stage D): The by-products of acidogenesis are transformed into compounds such as acetate and H2 by various pathways [22]. Homoacetogenesis is another acetogenesis mechanism in which H2 and CO2 are used to generate acetate.
- Methanogenesis (stage H): Methanogenic archaea convert acetate and hydrogen into CH4 and carbon dioxide CO2. There are two main pathways: acetotrophic methanogenenisis, which convert acetate into methane, and hydrogenotrophic methanogens, which utilize hydrogen and carbon dioxide to produce methane. Because of the slow growth rate of methanogens and their sensibility to H2 and VFA accumulation, this is one of the most critical steps of AD.
2.2. Important Factors Affecting AD
2.2.1. Feedstock (Substrate)
2.2.2. Temperature
2.2.3. pH
2.2.4. Moisture
2.2.5. Effect of Carbon to Nitrogen Ratio
2.2.6. Redox Properties Potential Effect
Mediated Electron Transfer through Hydrogen/Formate
Direct Interspecies Electron Transfer (DIET)
3. Digestate Valorization and Biochar Production in a Circular Economy
3.1. Overview of Digestate Valorization
3.2. Biochar Production from Digestate
| Digestate Source | Transformation Protocol | C(%) | H(%) | N(%) | S(%) | O(%) | SSA (m2/g) of Biochar | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Food waste | 550 °C | N.R | N.R | N.R | N.R | N.R | N.R | Soil amendment | [151] |
| Food waste | 300−700 °C | 36.7–45.4 | 0.9–4.4 | 1.9–5.36 | 0.44–0.63 | 0.3–9.14 | 0.66–26.95 | Without application | [152] |
| Food waste | 300−700 °C, 240 min | 34–45.4 | N.R | 1.9–5.36 | N.R | N.R | N.R | Soil amendment | [153] |
| Food waste | 400−800 °C, 30 min | N.R | N.R | N.R | N.R | N.R | 51.15–57.43 | Without application | [154] |
| Food waste | 500 ± 50 °C, 45 min(pilot scale) | 11.08 | 0.8 | 0.82 | 0.15 | 10.05 | 51.15–57.43 | ||
| Municipal waste | 400–450 °C | 17.1 | 0.8 | 0.9 | N.R | 1.5 | N.R | Soil amendment | [155] |
| Municipal waste | 600–650 °C | 18.5 | 0.5 | 0.6 | N.R | 0 | N.R | ||
| Municipal biowaste | 540 °C, 60 min | 72.7 | 2.2 | 7.2 | N.R | N.R | 0.51 | Without application | [156] |
| Swine manure | 500 °C, 60 min | 50.3 | 1.1 | 1.4 | 0.7 | 7 | N.R | Adsorption | [157] |
| Swine manure | 500 °C, 60 min, steam activation 800 °C, 30−60 min | 31.1–39.6 | 0.5–1 | 0.2–0.3 | N.R | 0.2−7.6 | 411–432 | ||
| Swine manure | 800 °C, 30 min | N.R | N.R | N.R | N.R | N.R | 101.9 | Without application | [158] |
| Swine manure | 550 °C, 120 min | N.R | N.R | N.R | N.R | N.R | 17.07 | Adsorption | [159] |
| Swine manure | 550 °C, 120 min HCl, NH3, Mn modification | N.R | N.R | N.R | N.R | N.R | 186.5–207 | ||
| Swine manure | 550 °C, 120 min | 53.02 | 8.88 | 4.3 | N.R | N.R | N.R | AD | [128] |
| Corn | 600 °C | 81.9–84.6 | N.R | N.R | N.R | N.R | N.R | Without application | [160] |
| Corn | 400, 600 °C | 41.3–43.8 | 0.86–1.21 | 1.58–1.91 | N.R | 3.8–3.87 | N.R | Without application | [161] |
| Cow manure/food wastes | 800 °C, 240 min | 58.99 | N.R | 1.25 | N.R | N.R | N.R | Without application | [162] |
| Corn silage, cow manure | 400–800 °C, 30 min | 76.2–88.3 | 2.8–5.19 | 2.1–3.69 | 0.72–0.77 | 5.9–14.1 | 161.6 (800 °C) | Without application | [163] |
| Corn silage, manure, and vegetable waste | 500 °C, 180 min | 55.83 | 2.03 | 1.46 | N.R | N.R | N.R | Soil amendment | [164] |
| Pig manure | 800 °C, 30 min | 21.08 | 0.45 | 1.09 | 2.33 | 10.23 | 110 | Without application | [165] |
| Pig manure | 550 °C, 120 min, MnO2 impregnation | 76.59 | N.R | N.R | N.R | 5.24 | 16.09 | Catalysis | [166] |
| Rice straw | 500 °C, 120 min | 48.2 | 2.6 | 1.71 | 0.15 | N.R | 37.53 | Adsorption | [167] |
| Rice straw | 500 °C, 120 min, CuCl2 H2O with NaBH4 | 38.68 | 2.03 | 1.29 | 0.3 | N.R | 135.35 | ||
| Agroindustrial residues, herbaceous biomass | 500 °C, 180 min | 52.1 | N.R | 1.38 | N.R | N.R | N.R | Soil amendment | [168] |
| Animal sewage, cow manure, maize, triticale silages, cereal bran | 600 °C, 10 min | 57.3 | 2 | 1.4 | 0.07 | 7.2 | 88 | Soil amendment | [169] |
| Cattle manure, maize silage | 350, 550 °C, 60 mint | 60.7–65.9 | N.R | 2.2–2.6 | 0.3–0.5 | N.R | N.R | Without application | [170] |
| Cattle manure, pig manure maize silage | 300−600 °C, 30 min | 56.3–57.2 | N.R | 2.6–2.9 | N.R | N.R | N.R | Soil amendment | [171] |
| Dairy cattle slurry, silage | 400−600 °C, 60 min | 42.9–50.6 | 1.55–2.3 | 1.88–2.3 | N.R | 45.4–52.8 | 11.3–15.3 | Adsorption | [172] |
| Dairy cattle slurry, silage | 600 °C, 60 min, urea modification | 53.1–53.5 | 1.1–1.78 | 2.4–8.99 | N.R | 35.9–43.3 | 6.8–15.1 | ||
| Groats, olive oil cake, silage of triticale, chicken manure | 600 °C, 10 min | 62.2 | 2 | 1.5 | 0.04 | 11.4 | 49 | Soil amendment | [169] |
| Herbaceous biomass, agro-industrial residues | 500 °C, 60 min | 64.34 | 2.68 | 1.78 | 0.22 | 6.38 | 23.1 | Without application | [121] |
| Maize | 550 °C | N.R | N.R | N.R | N.R | N.R | 27.5 | Adsorption | [173] |
| Sewage sludge | 550 °C | N.R | N.R | N.R | N.R | N.R | N.R | Without application | [174] |
| Sewage sludge | 300−550 °C, 15 min | N.R | N.R | N.R | N.R | N.R | N.R | AD | [175] |
| Corn straw | 700 °C, 60 min | N.R | N.R | N.R | N.R | N.R | 335 | [176] | |
| Organic fraction of municipal solid waste (OFMSW) | 300–700 °C, 120 min | N.R | N.R | N.R | N.R | N.R | N.R | [177] |
4. Biochar in AD: Investigating Properties and Outcomes
- Buffering Capacity: Several studies highlight biochar’s ability to stabilize and maintain pH levels within AD systems as a critical factor. This buffering capacity is crucial in preventing rapid pH drops resulting from VFA accumulation, thereby maintaining the activity of microorganisms responsible for methane production. Biochars with higher alkaline content, including alkali and alkaline-earth metals such as sodium, potassium, calcium, and magnesium, tend to exhibit superior buffering capacity, creating an environment conducive to efficient AD.
- Specific Surface Area: Another common factor is the specific surface area of biochar. Biochars with higher SSA provide ample attachment sites for microorganisms, promoting biofilm formation and facilitating the colonization and growth of microorganisms essential for AD. Additionally, a higher specific surface area may enable the adsorption of VFA, influencing the overall efficiency of the AD process.
4.1. Effect of Biochar pH on AD
4.2. Effect of Biochar on Electron Transfer Mechanism: Role of Redox Properties
4.3. Effect of Biochar Textural Properties on AD
4.4. Effect of Biochar on the Inhibitor Sorption
4.5. Effect of Biochar on Biogas Upgrading
5. Economic and Environmental Analysis of Biochar Utilization in AD
6. Future Prospects and Emerging Challenges
- Tailoring redox properties and EC of biochar: Developing innovative methods to tailor the redox properties and EC of biochar, potentially through the introduction of specific functional groups or improved activation processes, could further enhance its performance in AD systems.
- Microbial interactions and syntrophic partnerships: Investigating how biochar interacts with various microbial communities in AD reactors and its effects on specific syntrophic partnerships is crucial and could lead to a better understanding of the role of biochar in the evolution of microbial dynamics.
- Feedstock influence on biochar properties: Exploring and understanding how different feedstock sources for biochar production influence its properties and performance in AD could lead to feedstock-specific recommendations for optimizing AD processes.
- Understand the various functions of biochar to counter NH3 inhibition, optimize its properties for efficient NH3 removal by studying interactions with FAN/TAN, and examine the impact of its pore size and SSA on NH3 sorption during anaerobic digestion.
- Effectiveness of biochar in removing other contaminants from biogas: Investigating the ability of biochar to remove CO2 and other impurities is crucial to assess its overall potential for biogas cleanup and upgrading.
- Competitive sorption: Examining how CO2, H2S, and NH3 compete with each other for adsorbing on the sites of adsorption on biochar surfaces could help determine the efficiency of the biochar in simultaneously eliminating these contaminants. This research can identify potential modifications for enhanced selectivity and capability.
- Water vapor impact: Investigations on how the presence of water vapor in biogas impacts the sorption of CO2, H2S, and NH3 on biochar is essential.
- CH4 loss: Future research should focus on understanding how methane fixes onto biochar and investigating methods to prevent or minimize this process. This will enable a greater quantity of CH4 to be maintained in purified biogas.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion | IET | interspecies electron transfer |
| BOD | biological oxygen demand | LCFA | Long-chain fatty acids |
| BC | biochar | LCA | life cycle assessment |
| BMP | biochemical methane potential | MSW | municipal solid waste |
| COD | chemical oxygen demand | MR | microwave irradiation |
| CEC | cation exchange capacity | OMSW | organic municipal solid waste |
| DIET | direct interspecies electron transfer | ORP | oxidation-reduction potential |
| DOC | dissolved organic carbon | OLR | organic loading rate |
| DM | dairy manure | SSA | porosity, specific surface area |
| EC | electrical conductivity | TEA | techno-economic assessment |
| EAC | electron-accepting capacity | TS | total solids |
| EDC | electron-donating capacity | TAN | total ammonia nitrogen |
| FAN | free ammonia nitrogen | VFA | volatile fatty acids |
| HRT | hydraulic retention time | VS | volatile solids |
| HC | hydrochar | WAS | waste activated sludge |
References
- Vlachokostas, C. Closing the Loop between Energy Production and Waste Management: A Conceptual Approach towards Sustainable Development. Sustainability 2020, 12, 5995. [Google Scholar] [CrossRef]
- Spyridi, D.; Vlachokostas, C.; Michailidou, A.V.; Sioutas, C.; Moussiopoulos, N. Strategic planning for climate change mitigation and adaptation: The case of Greece. Int. J. Clim. Chang. Strateg. Manag. 2015, 7, 272–289. [Google Scholar] [CrossRef]
- Ferreira, A.; Marques, P.; Ribeiro, B.; Assemany, P.; de Mendonça, H.V.; Barata, A.; Oliveira, A.C.; Reis, A.; Pinheiro, H.M.; Gouveia, L. Combining biotechnology with circular bioeconomy: From poultry, swine, cattle, brewery, dairy and urban wastewaters to biohydrogen. Environ. Res. 2018, 164, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Banias, G.; Achillas, C.; Vlachokostas, C.; Moussiopoulos, N.; Stefanou, M. Environmental impacts in the life cycle of olive oil: A literature review. J. Sci. Food Agric. 2017, 97, 1686–1697. [Google Scholar] [CrossRef]
- Wallace, T.; Gibbons, D.; O’Dwyer, M.; Curran, T.P. International evolution of fat, oil and grease (FOG) waste management—A review. J. Environ. Manag. 2017, 187, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.C.; Velis, C.A. Waste management–Still a global challenge in the 21st century: An evidence-based call for action. Waste Manag. Res. 2015, 33, 1049–1051. [Google Scholar] [CrossRef]
- Zupančič, M.; Možic, V.; Može, M.; Cimerman, F.; Golobič, I. Current Status and Review of Waste-to-Biogas Conversion for Selected European Countries and Worldwide. Sustainability 2022, 14, 1823. [Google Scholar] [CrossRef]
- Dobers, G.M. Acceptance of biogas plants taking into account space and place. Energy Policy 2019, 135, 110987. [Google Scholar] [CrossRef]
- Sambusiti, C.; Monlau, F.; Ficara, E.; Carrère, H.; Malpei, F. A comparison of different pre-treatments to increase methane production from two agricultural substrates. Appl. Energy 2013, 104, 62–70. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.-P.; Carrère, H. Lignocellulosic Materials into Biohydrogen and Biomethane: Impact of Structural Features and Pretreatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 260–322. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Valorization of anaerobic digestion digestate: A prospect review. Bioresour. Technol. 2021, 323, 124626. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Peng, W.; Pivato, A.; Garbo, F.; Wang, T. Effects of char from biomass gasification on carbon retention and nitrogen conversion in landfill simulation bioreactors. Environ. Sci. Pollut. Res. 2020, 27, 6401–6410. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Lü, F.; Hao, L.; Zhang, H.; Shao, L.; He, P. Digestate management for high-solid anaerobic digestion of organic wastes: A review. Bioresour. Technol. 2020, 297, 122485. [Google Scholar] [CrossRef] [PubMed]
- Golkowska, K.; Vázquez-Rowe, I.; Lebuf, V.; Accoe, F.; Koster, D. Assessing the treatment costs and the fertilizing value of the output products in digestate treatment systems. Water Sci. Technol. 2014, 69, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cui, Y.; Zhang, T.; Hu, Q.; Wah Tong, Y.; He, Y.; Dai, Y.; Wang, C.-H.; Peng, Y. Food waste treating by biochar-assisted high-solid anaerobic digestion coupled with steam gasification: Enhanced bioenergy generation and porous biochar production. Bioresour. Technol. 2021, 331, 125051. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, S.; Chen, K.; Wang, T.; Mei, M.; Li, J. Preparation of biochar from food waste digestate: Pyrolysis behavior and product properties. Bioresour. Technol. 2020, 302, 122841. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- Kumar, M.; Munoz-Arriola, F.; Furumai, H.; Chaminda, T. (Eds.) Resilience, Response, and Risk in Water Systems. In Springer Transactions in Civil and Environmental Engineering; Springer: Singapore, 2020; ISBN 978-981-15-4667-9. [Google Scholar]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.; Zhu, G. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Nitayavardhana, S.; Sawatdeenarunat, C.; Surendra, K.C.; Khanal, S.K. Biogas Production by Anaerobic Digestion: Status and Perspectives. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 763–778. [Google Scholar]
- Treu, L.; Campanaro, S.; Kougias, P.G.; Zhu, X.; Angelidaki, I. Untangling the Effect of Fatty Acid Addition at Species Level Revealed Different Transcriptional Responses of the Biogas Microbial Community Members. Environ. Sci. Technol. 2016, 50, 6079–6090. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Yang, P.; Singh, S.; Zhuang, H.; Xu, L.; Chen, W.-H.; Dolfing, J.; Li, D.; Zhang, Y.; Zeng, H.; et al. A review on the bioenergetics of anaerobic microbial metabolism close to the thermodynamic limits and its implications for digestion applications. Bioresour. Technol. 2018, 247, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameters in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Martínez, E.J.; Redondas, V.; Fierro, J.; Gómez, X.; Morán, A. Anaerobic digestion of high lipid content wastes: FOG co-digestion and milk processing fat digestion. J. Residuals Sci. Technol. 2011, 8, 53–60. [Google Scholar]
- Pereira, M.A.; Sousa, D.Z.; Mota, M.; Alves, M.M. Mineralization of LCFA associated with anaerobic sludge: Kinetics, enhancement of methanogenic activity, and effect of VFA. Biotechnol. Bioeng. 2004, 88, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Long, J.H.; Aziz, T.N.; de los Reyes, F.L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Latha, K.; Velraj, R.; Shanmugam, P.; Sivanesan, S. Mixing strategies of high solids anaerobic co-digestion using food waste with sewage sludge for enhanced biogas production. J. Clean. Prod. 2019, 210, 388–400. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, W.; Yi, H.; Qin, Y.; Wu, J.; Liu, J.; Li, Y.-Y. Biogas production by two-stage thermophilic anaerobic co-digestion of food waste and paper waste: Effect of paper waste ratio. Renew. Energy 2019, 132, 1301–1309. [Google Scholar] [CrossRef]
- Karlsson, A.; Ejlertsson, J. Addition of HCl as a means to improve biogas production from protein-rich food industry waste. Biochem. Eng. J. 2012, 61, 43–48. [Google Scholar] [CrossRef]
- Moestedt, J.; Påledal, S.; Schnürer, A.; Nordell, E. Biogas Production from Thin Stillage on an Industrial Scale—Experience and Optimisation. Energies 2013, 6, 5642–5655. [Google Scholar] [CrossRef]
- Teghammar, A. Biogas Production from Lignocelluloses: Pretreatment, Substrate Characterization, Co-Digestion, and Economic Evaluation; Chalmers Tekniska Hogskola: Göteborg, Sweden, 2013. [Google Scholar]
- Sárvári Horváth, I.; Tabatabaei, M.; Karimi, K.; Kumar, R. Recent updates on biogas production—A review. Biofuel Res. J. 2016, 3, 394–402. [Google Scholar] [CrossRef]
- M⊘ller, H.B.; Sommer, S.G.; Ahring, B.K. Biological Degradation and Greenhouse Gas Emissions during Pre-Storage of Liquid Animal Manure. J. Environ. Qual. 2004, 33, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kamperidou, V.; Terzopoulou, P. Anaerobic Digestion of Lignocellulosic Waste Materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenès, J.-P.; Carrère, H. Combination of Thermal Treatments and Anaerobic Digestion to Reduce Sewage Sludge Quantity and Improve Biogas Yield. Process Saf. Environ. Prot. 2006, 84, 280–284. [Google Scholar] [CrossRef]
- Ferrer, I.; Ponsá, S.; Vázquez, F.; Font, X. Increasing biogas production by thermal (70°C) sludge pre-treatment prior to thermophilic anaerobic digestion. Biochem. Eng. J. 2008, 42, 186–192. [Google Scholar] [CrossRef]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge. Effect of pre-treatment at elevated temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef]
- Raju, C.S.; Sutaryo, S.; Ward, A.J.; Møller, H.B. Effects of high-temperature isochoric pre-treatment on the methane yields of cattle, pig and chicken manure. Environ. Technol. 2013, 34, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Estevez, M.M.; Nielsen, H.K.; Linjordet, R.; Eijsink, V.G.H. Biogas production and saccharification of Salix pretreated at different steam explosion conditions. Bioresour. Technol. 2011, 102, 7932–7936. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Okishio, Y.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Valo, A.; Carrère, H.; Delgenès, J.P. Thermal, chemical and thermo-chemical pre-treatment of waste activated sludge for anaerobic digestion. J. Chem. Technol. Biotechnol. 2004, 79, 1197–1203. [Google Scholar] [CrossRef]
- Mladenovska, Z.; Hartmann, H.; Kvist, T.; Sales-Cruz, M.; Gani, R.; Ahring, B.K. Thermal pretreatment of the solid fraction of manure: Impact on the biogas reactor performance and microbial community. Water Sci. Technol. 2006, 53, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Devlin, D.C.; Esteves, S.R.R.; Dinsdale, R.M.; Guwy, A.J. The effect of acid pretreatment on the anaerobic digestion and dewatering of waste activated sludge. Bioresour. Technol. 2011, 102, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Vervaeren, H.; Hostyn, K.; Ghekiere, G.; Willems, B. Biological ensilage additives as pretreatment for maize to increase the biogas production. Renew. Energy 2010, 35, 2089–2093. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; de Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.J.; Tyagi, R.D.; Surampalli, R.Y. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ormaechea, P.; Castrillón, L.; Marañón, E.; Fernández-Nava, Y.; Negral, L.; Megido, L. Influence of the ultrasound pretreatment on anaerobic digestion of cattle manure, food waste and crude glycerine. Environ. Technol. 2017, 38, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.B.; de Souza Moretti, M.M.; Bezerra-Bussoli, C.; da Costa Carreira Nunes, C.; Blandino, A.; da Silva, R.; Gomes, E. Evaluation of microwave-assisted pretreatment of lignocellulosic biomass immersed in alkaline glycerol for fermentable sugars production. Bioresour. Technol. 2015, 185, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.H.; Raes, K. Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour. Technol. 2015, 192, 696–702. [Google Scholar] [CrossRef]
- Chiu, S.L.H.; Lo, I.M.C. Reviewing the anaerobic digestion and co-digestion process of food waste from the perspectives on biogas production performance and environmental impacts. Environ. Sci. Pollut. Res. 2016, 23, 24435–24450. [Google Scholar] [CrossRef] [PubMed]
- Gebreeyessus, G.; Jenicek, P. Thermophilic versus Mesophilic Anaerobic Digestion of Sewage Sludge: A Comparative Review. Bioengineering 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Steiniger, B.; Hupfauf, S.; Insam, H.; Schaum, C. Exploring Anaerobic Digestion from Mesophilic to Thermophilic Temperatures—Operational and Microbial Aspects. Fermentation 2023, 9, 798. [Google Scholar] [CrossRef]
- Boe, K. Online Monitoring and Control of the Biogas Process; Technical University of Denmark: Kongens Lyngby, Denmark, 2006. [Google Scholar]
- Hwang, M.H.; Jang, N.J.; Hyun, S.H.; Kim, I.S. Anaerobic bio-hydrogen production from ethanol fermentation: The role of pH. J. Biotechnol. 2004, 111, 297–309. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. Biomed Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, R.J.; Angelidaki, I. Effects of pH and hydraulic retention time on hydrogen production versus methanogenesis during anaerobic fermentation of organic household solid waste under extreme-thermophilic temperature (70 °C). Biotechnol. Bioeng. 2008, 100, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Sibiya, N.T.; Muzenda, E.; Tesfagiorgis, H.B. Effect of temperature and pH on the anaerobic digestion of grass silage. In Proceedings of the 6th International Conference on Green Technology, Renewable Energy and Environmental Engineering, Cape Town, South Africa, 24–25 September 2014; pp. 15–16. [Google Scholar]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Sommer, S.G.; Husted, S. A simple model of pH in slurry. J. Agric. Sci. 1995, 124, 447–453. [Google Scholar] [CrossRef]
- Georgacakis, D.; Sievers, D.M.; Iannotti, E.L. Buffer stability in manure digesters. Agric. Wastes 1982, 4, 427–441. [Google Scholar] [CrossRef]
- Kovács, E.; Wirth, R.; Maróti, G.; Bagi, Z.; Nagy, K.; Minárovits, J.; Rákhely, G.; Kovács, K.L. Augmented biogas production from protein-rich substrates and associated metagenomic changes. Bioresour. Technol. 2015, 178, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Liu, J.; Sui, Q.; Wei, Y. Biogas-pH automation control strategy for optimizing organic loading rate of anaerobic membrane bioreactor treating high COD wastewater. Bioresour. Technol. 2016, 203, 62–70. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis. Bioresour. Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Yenigun, O. Anaerobic acidogenesis of dairy wastewater: The effects of variations in hydraulic retention time with no pH control. J. Chem. Technol. Biotechnol. 2004, 79, 755–760. [Google Scholar] [CrossRef]
- Nayono, S.E.; Gallert, C.; Winter, J. Co-digestion of press water and food waste in a biowaste digester for improvement of biogas production. Bioresour. Technol. 2010, 101, 6987–6993. [Google Scholar] [CrossRef]
- Potts, L.G.; Martin, D.J. Anaerobic digestion, gasification, and pyrolysis. In Encyclopedia of Life Support; Sollars, C., Blakey, S., Eds.; Systems Waste Management Minimisation; EOLSS Publishers: Oxford, UK, 2009; pp. 194–294. [Google Scholar]
- Fernandez, J.; Perez, M.; Romero, L. Effect of substrate concentration on dry mesophilic anaerobic digestion of organic fraction of municipal solid waste (OFMSW). Bioresour. Technol. 2008, 99, 6075–6080. [Google Scholar] [CrossRef]
- Fdez-Güelfo, L.A.; Álvarez-Gallego, C.; Sales, D.; Romero García, L.I. Dry-thermophilic anaerobic digestion of organic fraction of municipal solid waste: Methane production modeling. Waste Manag. 2012, 32, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rodríguez, J.; Pérez, M.; Romero, L.I. Comparison of mesophilic and thermophilic dry anaerobic digestion of OFMSW: Kinetic analysis. Chem. Eng. J. 2013, 232, 59–64. [Google Scholar] [CrossRef]
- Hartmann, H.; Ahring, B.K. Anaerobic digestion of the organic fraction of municipal solid waste: Influence of co-digestion with manure. Water Res. 2005, 39, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Pérez, M.; Romero, L.I. Kinetics of mesophilic anaerobic digestion of the organic fraction of municipal solid waste: Influence of initial total solid concentration. Bioresour. Technol. 2010, 101, 6322–6328. [Google Scholar] [CrossRef] [PubMed]
- Lay, J.-J.; Li, Y.-Y.; Noike, T. Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res. 1997, 31, 1518–1524. [Google Scholar] [CrossRef]
- Hernández-Berriel, M.C.; Márquez-Benavides, L.; González-Pérez, D.J.; Buenrostro-Delgado, O. The effect of moisture regimes on the anaerobic degradation of municipal solid waste from Metepec (México). Waste Manag. 2008, 28, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Kondusamy, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high rate methane production–A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- Fricke, K.; Santen, H.; Wallmann, R.; Hüttner, A.; Dichtl, N. Operating problems in anaerobic digestion plants resulting from nitrogen in MSW. Waste Manag. 2007, 27, 30–43. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Zeshan; Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Heo, N.H.; Park, S.C.; Kang, H. Effects of Mixture Ratio and Hydraulic Retention Time on Single-Stage Anaerobic Co-digestion of Food Waste and Waste Activated Sludge. J. Environ. Sci. Health Part A 2004, 39, 1739–1756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Macias-Corral, M.; Samani, Z.; Hanson, A.; Smith, G.; Funk, P.; Yu, H.; Longworth, J. Anaerobic digestion of municipal solid waste and agricultural waste and the effect of co-digestion with dairy cow manure. Bioresour. Technol. 2008, 99, 8288–8293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zeng, G.; Zhang, G.; Li, Y.; Zhang, B.; Fan, M. Anaerobic co-digestion of biosolids and organic fraction of municipal solid waste by sequencing batch process. Fuel Process. Technol. 2008, 89, 485–489. [Google Scholar] [CrossRef]
- Dai, X.; Duan, N.; Dong, B.; Dai, L. High-solids anaerobic co-digestion of sewage sludge and food waste in comparison with mono digestions: Stability and performance. Waste Manag. 2013, 33, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Ponsá, S.; Gea, T.; Sánchez, A. Anaerobic co-digestion of the organic fraction of municipal solid waste with several pure organic co-substrates. Biosyst. Eng. 2011, 108, 352–360. [Google Scholar] [CrossRef]
- Thiele, J.H.; Zeikus, J.G. Control of Interspecies Electron Flow during Anaerobic Digestion: Significance of Formate Transfer versus Hydrogen Transfer during Syntrophic Methanogenesis in Flocs. Appl. Environ. Microbiol. 1988, 54, 20–29. [Google Scholar] [CrossRef]
- Li, C.; Fang, H.H.P. Fermentative Hydrogen Production from Wastewater and Solid Wastes by Mixed Cultures. Crit. Rev. Environ. Sci. Technol. 2007, 37, 1–39. [Google Scholar] [CrossRef]
- Logan, B.E.; Oh, S.-E.; Kim, I.S.; Van Ginkel, S. Biological Hydrogen Production Measured in Batch Anaerobic Respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, M.; Wang, G.; Chen, R.; Qiao, W.; Wang, X. Biochar assisted thermophilic co-digestion of food waste and waste activated sludge under high feedstock to seed sludge ratio in batch experiment. Bioresour. Technol. 2018, 249, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Cord-Ruwisch, R.; Lovley, D.R.; Schink, B. Growth of Geobacter sulfurreducens with Acetate in Syntrophic Cooperation with Hydrogen-Oxidizing Anaerobic Partners. Appl. Environ. Microbiol. 1998, 64, 2232–2236. [Google Scholar] [CrossRef] [PubMed]
- Boone, D.R.; Johnson, R.L.; Liu, Y. Diffusion of the Interspecies Electron Carriers H2 and Formate in Methanogenic Ecosystems and Its Implications in the Measurement of Km for H2 or Formate Uptake. Appl. Environ. Microbiol. 1989, 55, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Live wires: Direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ. Sci. 2011, 4, 4896. [Google Scholar] [CrossRef]
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 2009, 20, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- König, R.; Cuomo, M.; Pianta, E.; Buetti, A.; Mauri, F.; Tanadini, M.; Principi, P. Addition of Conductive Materials to Support Syntrophic Microorganisms in Anaerobic Digestion. Fermentation 2022, 8, 354. [Google Scholar] [CrossRef]
- Gahlot, P.; Ahmed, B.; Tiwari, S.B.; Aryal, N.; Khursheed, A.; Kazmi, A.A.; Tyagi, V.K. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application. Environ. Technol. Innov. 2020, 20, 101056. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Zhang, Y.; Lovley, D.R. Sparking Anaerobic Digestion: Promoting Direct Interspecies Electron Transfer to Enhance Methane Production. iScience 2020, 23, 101794. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A. Biology and systematics of heterokont and haptophyte algae. Am. J. Bot. 2004, 91, 1508–1522. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Saratale, R.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Dattatraya Saratale, G. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Grosser, A. The influence of decreased hydraulic retention time on the performance and stability of co-digestion of sewage sludge with grease trap sludge and organic fraction of municipal waste. J. Environ. Manag. 2017, 203, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Barbot, Y.; Al-Ghaili, H.; Benz, R. A Review on the Valorization of Macroalgal Wastes for Biomethane Production. Mar. Drugs 2016, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Q.; LI, F. Avoiding propionic acid accumulation in the anaerobic process for biohydrogen production. Biomass Bioenergy 2006, 30, 177–182. [Google Scholar] [CrossRef]
- Khanal, S.K.; Huang, J.-C. ORP-based oxygenation for sulfide control in anaerobic treatment of high-sulfate wastewater. Water Res. 2003, 37, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Ezemagu, I.G.; Ejimofor, M.I.; Menkiti, M.C.; Diyoke, C. Biofertilizer production via composting of digestate obtained from anaerobic digestion of post biocoagulation sludge blended with saw dust: Physiochemical characterization and kinetic study. Environ. Chall. 2021, 5, 100288. [Google Scholar] [CrossRef]
- Song, S.; Lim, J.W.; Lee, J.T.E.; Cheong, J.C.; Hoy, S.H.; Hu, Q.; Tan, J.K.N.; Chiam, Z.; Arora, S.; Lum, T.Q.H.; et al. Food-waste anaerobic digestate as a fertilizer: The agronomic properties of untreated digestate and biochar-filtered digestate residue. Waste Manag. 2021, 136, 143–152. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A. The valorization of the anaerobic digestate from the organic fractions of municipal solid waste: Challenges and perspectives. J. Environ. Manag. 2021, 280, 111742. [Google Scholar] [CrossRef] [PubMed]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A new nutrient source-review. Biogas 2012, 14, 295–312. [Google Scholar]
- Miliotti, E.; Casini, D.; Rosi, L.; Lotti, G.; Rizzo, A.M.; Chiaramonti, D. Lab-scale pyrolysis and hydrothermal carbonization of biomass digestate: Characterization of solid products and compliance with biochar standards. Biomass Bioenergy 2020, 139, 105593. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Pohořelý, M.; Meers, E.; Skoblia, S.; Moško, J.; Jeremiáš, M. Potential of coupling anaerobic digestion with thermochemical technologies for waste valorization. Fuel 2021, 294, 120533. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P. Characterization of biochars produced from residues from biogas production. J. Anal. Appl. Pyrolysis 2015, 115, 157–165. [Google Scholar] [CrossRef]
- Parmar, K.R.; Ross, A.B. Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X.; Cheng, Z.; Yan, B.; Dan, Z.; Ma, W. Air gasification of biogas-derived digestate in a downdraft fixed bed gasifier. Waste Manag. 2017, 69, 162–169. [Google Scholar] [CrossRef]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. The effect of augmentation of biochar and hydrochar in anaerobic digestion of a model substrate. Bioresour. Technol. 2021, 321, 124494. [Google Scholar] [CrossRef]
- Wang, R.; Lin, K.; Ren, D.; Peng, P.; Zhao, Z.; Yin, Q.; Gao, P. Energy conversion performance in co-hydrothermal carbonization of sewage sludge and pinewood sawdust coupling with anaerobic digestion of the produced wastewater. Sci. Total Environ. 2022, 803, 149964. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, C.; Duan, Y.; Wong, J.W.-C. Impact of pyrochar and hydrochar derived from digestate on the co-digestion of sewage sludge and swine manure. Bioresour. Technol. 2020, 314, 123730. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Mustafa, A.M.; Lin, H.; Xu, J.; Sheng, K. Effect of bamboo hydrochar on anaerobic digestion of fish processing waste for biogas production. Bioresour. Technol. 2019, 283, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.Y.; Tian, H.; Chen, Y.; Ni, K.; Zhang, J.; Tong, Y.W. Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresour. Technol. 2020, 314, 123751. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Shi, Z.; Ji, M.; Ren, S.; Luo, G.; Zhang, S. Microbial insights towards understanding the role of hydrochar in alleviating ammonia inhibition during anaerobic digestion. Chem. Eng. J. 2021, 419, 129541. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, H.; Li, X.; Cheng, J.J.; Wu, W. Improving methane yield from organic fraction of municipal solid waste (OFMSW) with magnetic rice-straw biochar. Bioresour. Technol. 2017, 245, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Chiappero, M.; Norouzi, O.; Hu, M.; Demichelis, F.; Berruti, F.; Di Maria, F.; Mašek, O.; Fiore, S. Review of biochar role as additive in anaerobic digestion processes. Renew. Sustain. Energy Rev. 2020, 131, 110037. [Google Scholar] [CrossRef]
- Takaya, C.A.; Parmar, K.R.; Fletcher, L.A.; Ross, A.B. Biomass-Derived Carbonaceous Adsorbents for Trapping Ammonia. Agriculture 2019, 9, 16. [Google Scholar] [CrossRef]
- Papurello, D.; Boschetti, A.; Silvestri, S.; Khomenko, I.; Biasioli, F. Real-time monitoring of removal of trace compounds with PTR-MS: Biochar experimental investigation. Renew. Energy 2018, 125, 344–355. [Google Scholar] [CrossRef]
- Meng, Z.; Hou, X.; Liu, Y.; Ma, Z.; Shen, H. Facile fabrication of iron-modified biochar as a renewable adsorbent for efficient siloxane (L2) removal. J. Environ. Chem. Eng. 2021, 9, 105799. [Google Scholar] [CrossRef]
- Gargiulo, V.; Gomis-Berenguer, A.; Giudicianni, P.; Ania, C.O.; Ragucci, R.; Alfè, M. Assessing the Potential of Biochars Prepared by Steam-Assisted Slow Pyrolysis for CO2 Adsorption and Separation. Energy Fuels 2018, 32, 10218–10227. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Deng, Y.F.; Wang, F.; Davaritouchaee, M.; Yao, Y.Q. A review on biochar-mediated anaerobic digestion with enhanced methane recovery. Renew. Sustain. Energy Rev. 2019, 115, 109373. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N.; et al. Advances and future directions of biochar characterization methods and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Wei, W.; Guo, W.; Ngo, H.H.; Mannina, G.; Wang, D.; Chen, X.; Liu, Y.; Peng, L.; Ni, B.-J. Enhanced high-quality biomethane production from anaerobic digestion of primary sludge by corn stover biochar. Bioresour. Technol. 2020, 306, 123159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.W.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Liu, Z. Comparative production of biochars from corn stalk and cow manure. Bioresour. Technol. 2019, 291, 121855. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Ren, S.; Usman, M.; Tsang, D.C.W.; O-Thong, S.; Angelidaki, I.; Zhu, X.; Zhang, S.; Luo, G. Hydrochar-Facilitated Anaerobic Digestion: Evidence for Direct Interspecies Electron Transfer Mediated through Surface Oxygen-Containing Functional Groups. Environ. Sci. Technol. 2020, 54, 5755–5766. [Google Scholar] [CrossRef] [PubMed]
- Saffari, N.; Hajabbasi, M.A.; Shirani, H.; Mosaddeghi, M.R.; Mamedov, A.I. Biochar type and pyrolysis temperature effects on soil quality indicators and structural stability. J. Environ. Manag. 2020, 261, 110190. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting Interspecies Electron Transfer with Biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.-H.; Duc Nguyen, D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Graham, M.C.; Shepherd, J.G.; Mašek, O. Suitability of marginal biomass-derived biochars for soil amendment. Sci. Total Environ. 2016, 547, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Opatokun, S.A.; Kan, T.; Al Shoaibi, A.; Srinivasakannan, C.; Strezov, V. Characterization of Food Waste and Its Digestate as Feedstock for Thermochemical Processing. Energy Fuels 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Yousef, L.F.; Strezov, V. Agronomic assessment of pyrolysed food waste digestate for sandy soil management. J. Environ. Manag. 2017, 187, 24–30. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Pan, L.; Zhu, X.; Xie, S.; Yu, G.; Wang, Y.; Pan, X.; Zhu, G.; Angelidaki, I. Treatment of digestate residues for energy recovery and biochar production: From lab to pilot-scale verification. J. Clean. Prod. 2020, 265, 121852. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Nisticò, R.; Guerretta, F.; Benzi, P.; Magnacca, G.; Mainero, D.; Montoneri, E. Thermal Conversion of Municipal Biowaste Anaerobic Digestate to Valuable Char. Resources 2019, 8, 24. [Google Scholar] [CrossRef]
- Gonsalvesh, L.; Gryglewicz, G.; Carleer, R.; Yperman, J. Valorization of swine manure into low cost activated carbons capable of Cr (VI) removal. Adv. Environ. Res. 2017, 6, 95–111. [Google Scholar]
- Hung, C.-Y.; Tsai, W.-T.; Chen, J.-W.; Lin, Y.-Q.; Chang, Y.-M. Characterization of biochar prepared from biogas digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Lin, Y.; Mbog, J.C. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Bioresour. Technol. 2018, 270, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards Biochar and Hydrochar Engineering—Influence of Process Conditions on Surface Physical and Chemical Properties, Thermal Stability, Nutrient Availability, Toxicity and Wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef]
- Rombolà, A.G.; Fabbri, D.; Meredith, W.; Snape, C.E.; Dieguez-Alonso, A. Molecular characterization of the thermally labile fraction of biochar by hydropyrolysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2016, 121, 230–239. [Google Scholar] [CrossRef]
- Rodriguez Alberto, D.; Stojak Repa, K.; Hegde, S.; Miller, C.W.; Trabold, T.A. Novel Production of Magnetite Particles via Thermochemical Processing of Digestate from Manure and Food Waste. IEEE Magn. Lett. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Garlapalli, R.K.; Wirth, B.; Reza, M.T. Pyrolysis of hydrochar from digestate: Effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Bioresour. Technol. 2016, 220, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Calamai, A.; Palchetti, E.; Masoni, A.; Marini, L.; Chiaramonti, D.; Dibari, C.; Brilli, L. The Influence of Biochar and Solid Digestate on Rose-Scented Geranium (Pelargonium graveolens L’Hér.) Productivity and Essential Oil Quality. Agronomy 2019, 9, 260. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Fang, Y.-Y.; Cheng, P.-H.; Lin, Y.-Q. Characterization of mesoporous biochar produced from biogas digestate implemented in an anaerobic process of large-scale hog farm. Biomass Convers. Biorefinery 2018, 8, 945–951. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, D.; Zeng, W.; Yang, J. Removal of refractory organics from piggery bio-treatment effluent by the catalytic ozonation process with piggery biogas residue biochar as the catalyst. Sci. Total Environ. 2020, 734, 139448. [Google Scholar] [CrossRef]
- Fu, D.; Chen, Z.; Xia, D.; Shen, L.; Wang, Y.; Li, Q. A novel solid digestate-derived biochar-Cu NP composite activating H2O2 system for simultaneous adsorption and degradation of tetracycline. Environ. Pollut. 2017, 221, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Calamai, A.; Chiaramonti, D.; Casini, D.; Masoni, A.; Palchetti, E. Short-Term Effects of Organic Amendments on Soil Properties and Maize (Zea maize L.) Growth. Agriculture 2020, 10, 158. [Google Scholar] [CrossRef]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Piccoli, I.; Torreggiani, A.; Pituello, C.; Pisi, A.; Morari, F.; Francioso, O. Automated image analysis and hyperspectral imagery with enhanced dark field microscopy applied to biochars produced at different temperatures. Waste Manag. 2020, 105, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Gusiatin, Z.M.; Kurkowski, R.; Brym, S.; Wiśniewski, D. Properties of biochars from conventional and alternative feedstocks and their suitability for metal immobilization in industrial soil. Environ. Sci. Pollut. Res. 2016, 23, 21249–21261. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, S.; Quan, C.; Gao, N.; Johnston, C.; Wu, C. One-pot synthesis of digestate-derived biochar for carbon dioxide capture. Fuel 2020, 279, 118525. [Google Scholar] [CrossRef]
- Ngigi, A.N.; Ok, Y.S.; Thiele-Bruhn, S. Biochar-mediated sorption of antibiotics in pig manure. J. Hazard. Mater. 2019, 364, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, E.; Buss, W.; Edo, M.; Mašek, O.; Jansson, S. Influence of pyrolysis temperature and production unit on formation of selected PAHs, oxy-PAHs, N-PACs, PCDDs, and PCDFs in biochar—A screening study. Environ. Sci. Pollut. Res. 2018, 25, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Rene, E.R.; Dupont, C.; Wongrod, S.; van Hullebusch, E.D. Anaerobic Digestion of Fruit Waste Mixed with Sewage Sludge Digestate Biochar: Influence on Biomethane Production. Front. Energy Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Yang, H.-J.; Yang, Z.-M.; Xu, X.-H.; Guo, R.-B. Increasing the methane production rate of hydrogenotrophic methanogens using biochar as a biocarrier. Bioresour. Technol. 2020, 302, 122829. [Google Scholar] [CrossRef]
- Alghashm, S.; Song, L.; Liu, L.; Ouyang, C.; Zhou, J.L.; Li, X. Improvement of Biogas Production Using Biochar from Digestate at Different Pyrolysis Temperatures during OFMSW Anaerobic Digestion. Sustainability 2023, 15, 11917. [Google Scholar] [CrossRef]
- González, J.; Sánchez, M.; Gómez, X. Enhancing Anaerobic Digestion: The Effect of Carbon Conductive Materials. C 2018, 4, 59. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent achievements in enhancing anaerobic digestion with carbon- based functional materials. Bioresour. Technol. 2018, 266, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an Exceptional Bioresource for Energy, Agronomy, Carbon Sequestration, Activated Carbon and Specialty Materials. Waste Biomass Valoriz. 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Pecchi, M.; Baratieri, M. Coupling anaerobic digestion with gasification, pyrolysis or hydrothermal carbonization: A review. Renew. Sustain. Energy Rev. 2019, 105, 462–475. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of biochar application for enhanced anaerobic digestion: A review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Sawdust-Derived Biochar Much Mitigates VFAs Accumulation and Improves Microbial Activities to Enhance Methane Production in Thermophilic Anaerobic Digestion. ACS Sustain. Chem. Eng. 2019, 7, 2141–2150. [Google Scholar] [CrossRef]
- Su, C.; Zhao, L.; Liao, L.; Qin, J.; Lu, Y.; Deng, Q.; Chen, M.; Huang, Z. Application of biochar in a CIC reactor to relieve ammonia nitrogen stress and promote microbial community during food waste treatment. J. Clean. Prod. 2019, 209, 353–362. [Google Scholar] [CrossRef]
- Codignole Luz, F.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V. Biochar characteristics and early applications in anaerobic digestion-a review. J. Environ. Chem. Eng. 2018, 6, 2892–2909. [Google Scholar] [CrossRef]
- Salman, C.A.; Schwede, S.; Thorin, E.; Yan, J. Enhancing biomethane production by integrating pyrolysis and anaerobic digestion processes. Appl. Energy 2017, 204, 1074–1083. [Google Scholar] [CrossRef]
- Linville, J.L.; Shen, Y.; Ignacio-de Leon, P.A.; Schoene, R.P.; Urgun-Demirtas, M. In-situ biogas upgrading during anaerobic digestion of food waste amended with walnut shell biochar at bench scale. Waste Manag. Res. 2017, 35, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, F.; Song, Z.; Wang, J.; Li, Y.; Pan, Y.; Sheng, T.; Li, L. Feasibility of dry anaerobic digestion of beer lees for methane production and biochar enhanced performance at mesophilic and thermophilic temperature. Bioresour. Technol. 2019, 276, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Forrester, S.; Koval, J.; Urgun-Demirtas, M. Yearlong semi-continuous operation of thermophilic two-stage anaerobic digesters amended with biochar for enhanced biomethane production. J. Clean. Prod. 2017, 167, 863–874. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef]
- Wang, D.; Ai, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song, C. Improving anaerobic digestion of easy-acidification substrates by promoting buffering capacity using biochar derived from vermicompost. Bioresour. Technol. 2017, 227, 286–296. [Google Scholar] [CrossRef]
- Di Maria, F.; Sordi, A.; Cirulli, G.; Micale, C. Amount of energy recoverable from an existing sludge digester with the co-digestion with fruit and vegetable waste at reduced retention time. Appl. Energy 2015, 150, 9–14. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Shen, Y.; Yuan, R.; Zhu, N.; Yuan, H.; Lou, Z. Sludge-based biochar-assisted thermophilic anaerobic digestion of waste-activated sludge in microbial electrolysis cell for methane production. Bioresour. Technol. 2019, 284, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Choi, Y.-K.; Kan, E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Finnerty, G.L.; Camargo-Valero, M.A.; Ross, A.B. Valorisation of macroalgae via the integration of hydrothermal carbonisation and anaerobic digestion. Bioresour. Technol. 2020, 312, 123539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, J.; Yang, Q.; Sun, J.; Peng, C.; Chen, F.; Xu, Q.; Wang, S.; Wang, D.; Li, X.; et al. Evaluating the potential impact of hydrochar on the production of short-chain fatty acid from sludge anaerobic digestion. Bioresour. Technol. 2017, 246, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Chacón, F.J.; Sánchez-Monedero, M.A.; Lezama, L.; Cayuela, M.L. Enhancing biochar redox properties through feedstock selection, metal preloading and post-pyrolysis treatments. Chem. Eng. J. 2020, 395, 125100. [Google Scholar] [CrossRef]
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; Sun, Y.; Yu, I.K.M.; Tsang, D.C.W.; Hou, D.; Gupta, J.; Bhaskar, T.; Pandey, A. Critical Review on Biochar-Supported Catalysts for Pollutant Degradation and Sustainable Biorefinery. Adv. Sustain. Syst. 2020, 4, 1900149. [Google Scholar] [CrossRef]
- Joseph, S.; Husson, O.; Graber, E.; van Zwieten, L.; Taherymoosavi, S.; Thomas, T.; Nielsen, S.; Ye, J.; Pan, G.; Chia, C.; et al. The Electrochemical Properties of Biochars and How They Affect Soil Redox Properties and Processes. Agronomy 2015, 5, 322–340. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Anca-Couce, A.; Frišták, V.; Moreno-Jiménez, E.; Bacher, M.; Bucheli, T.D.; Cimò, G.; Conte, P.; Hagemann, N.; Haller, A.; et al. Designing biochar properties through the blending of biomass feedstock with metals: Impact on oxyanions adsorption behavior. Chemosphere 2019, 214, 743–753. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982. [Google Scholar] [CrossRef]
- Rotaru, A.-E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Link between capacity for current production and syntrophic growth in Geobacter species. Front. Microbiol. 2015, 6, 744. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef]
- Lovley, D.R. Happy together: Microbial communities that hook up to swap electrons. ISME J. 2017, 11, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Gao, W.; Wang, G.; Dzakpasu, M.; Li, Y.-Y.; Chen, R. New insights into the mechanisms underlying biochar-assisted sustained high-efficient co-digestion: Reducing thermodynamic constraints and enhancing extracellular electron transfer flux. Sci. Total Environ. 2022, 811, 151416. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Gao, X.; Chen, H.; Xu, X.; Zhu, L. Role of biochar in the granulation of anaerobic sludge and improvement of electron transfer characteristics. Bioresour. Technol. 2018, 268, 28–35. [Google Scholar] [CrossRef]
- Luz, F.C.; Cordiner, S.; Manni, A.; Mulone, V.; Rocco, V.; Braglia, R.; Canini, A. Ampelodesmos mauritanicus pyrolysis biochar in anaerobic digestion process: Evaluation of the biogas yield. Energy 2018, 161, 663–669. [Google Scholar] [CrossRef]
- Martins, G.; Salvador, A.F.; Pereira, L.; Alves, M.M. Methane Production and Conductive Materials: A Critical Review. Environ. Sci. Technol. 2018, 52, 10241–10253. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef]
- Cruz Viggi, C.; Simonetti, S.; Palma, E.; Pagliaccia, P.; Braguglia, C.; Fazi, S.; Baronti, S.; Navarra, M.A.; Pettiti, I.; Koch, C.; et al. Enhancing methane production from food waste fermentate using biochar: The added value of electrochemical testing in pre-selecting the most effective type of biochar. Biotechnol. Biofuels 2017, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Bolan, N.; Prévoteau, A.; Vithanage, M.; Biswas, J.K.; Ok, Y.S.; Wang, H. Applications of biochar in redox-mediated reactions. Bioresour. Technol. 2017, 246, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015; ISBN 1134489536. [Google Scholar]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review. Environ. Sci. Pollut. Res. 2017, 24, 26297–26309. [Google Scholar] [CrossRef] [PubMed]
- Trigo, C.; Cox, L.; Spokas, K. Influence of pyrolysis temperature and hardwood species on resulting biochar properties and their effect on azimsulfuron sorption as compared to other sorbents. Sci. Total Environ. 2016, 566–567, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar] [CrossRef]
- Lü, F.; Luo, C.; Shao, L.; He, P. Biochar alleviates combined stress of ammonium and acids by firstly enriching Methanosaeta and then Methanosarcina. Water Res. 2016, 90, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Bowen, T.L.; Boone, D.R. The Methanogenic Bacteria. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 165–207. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Ciccoli, R.; Sperandei, M.; Petrazzuolo, F.; Broglia, M.; Chiarini, L.; Correnti, A.; Farneti, A.; Pignatelli, V.; Tabacchioni, S. Anaerobic digestion of the above ground biomass of Jerusalem Artichoke in a pilot plant: Impact of the preservation method on the biogas yield and microbial community. Biomass Bioenergy 2018, 108, 190–197. [Google Scholar] [CrossRef]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef]
- Lü, F.; Hua, Z.; Shao, L.; He, P. Loop bioenergy production and carbon sequestration of polymeric waste by integrating biochemical and thermochemical conversion processes: A conceptual framework and recent advances. Renew. Energy 2018, 124, 202–211. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kipkemoi Kirui, W.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Wu, S.; Wandera, S.M.; Guo, L.; Dong, R. Evaluation of ammonium adsorption in biochar-fixed beds for treatment of anaerobically digested swine slurry: Experimental optimization and modeling. Sci. Total Environ. 2016, 563–564, 1095–1104. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Li, T.; Zhao, F.; He, Z.; Zhao, H.; Yang, X.; Wang, H.; Zhao, J.; Rafiq, M.T. Sorption of ammonium and phosphate from aqueous solution by biochar derived from phytoremediation plants. J. Zhejiang Univ. Sci. B 2013, 14, 1152–1161. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Mahmood, I.B. Recovery of NH4+ by corn cob produced biochars and its potential application as soil conditioner. Front. Environ. Sci. Eng. 2014, 8, 825–834. [Google Scholar] [CrossRef]
- Kanjanarong, J.; Giri, B.S.; Jaisi, D.P.; Oliveira, F.R.; Boonsawang, P.; Chaiprapat, S.; Singh, R.S.; Balakrishna, A.; Khanal, S.K. Removal of hydrogen sulfide generated during anaerobic treatment of sulfate-laden wastewater using biochar: Evaluation of efficiency and mechanisms. Bioresour. Technol. 2017, 234, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Lin, C.S.K.; Ji, X.; Rainey, T.J. Returning biochar to fields: A review. Appl. Soil Ecol. 2017, 116, 1–11. [Google Scholar] [CrossRef]
- Cheng, Q.; de los Reyes, F.L.; Call, D.F. Amending anaerobic bioreactors with pyrogenic carbonaceous materials: The influence of material properties on methane generation. Environ. Sci. Water Res. Technol. 2018, 4, 1794–1806. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Nam, H.; Kar Sajib, S. Effect of bio-char on methane generation from glucose and aqueous phase of algae liquefaction using mixed anaerobic cultures. Biomass Bioenergy 2018, 108, 479–486. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste–Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Browne, J.; Nizami, A.-S.; Thamsiriroj, T.; Murphy, J.D. Assessing the cost of biofuel production with increasing penetration of the transport fuel market: A case study of gaseous biomethane in Ireland. Renew. Sustain. Energy Rev. 2011, 15, 4537–4547. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Miltner, M.; Makaruk, A.; Harasek, M. Review on available biogas upgrading technologies and innovations towards advanced solutions. J. Clean. Prod. 2017, 161, 1329–1337. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition. J. CO2 Util. 2018, 26, 281–293. [Google Scholar] [CrossRef]
- Saha, D.; Kienbaum, M.J. Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: A critical review. Microporous Mesoporous Mater. 2019, 287, 29–55. [Google Scholar] [CrossRef]
- Zhou, K.; Chaemchuen, S.; Verpoort, F. Alternative materials in technologies for Biogas upgrading via CO2 capture. Renew. Sustain. Energy Rev. 2017, 79, 1414–1441. [Google Scholar] [CrossRef]
- Sahota, S.; Vijay, V.K.; Subbarao, P.M.V.; Chandra, R.; Ghosh, P.; Shah, G.; Kapoor, R.; Vijay, V.; Koutu, V.; Thakur, I.S. Characterization of leaf waste based biochar for cost effective hydrogen sulphide removal from biogas. Bioresour. Technol. 2018, 250, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation-A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.-H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Smith, M.W.; Zhao, Q.; Garcia-Perez, T.; Frear, C.; Garcia-Perez, M. Charcoal from anaerobically digested dairy fiber for removal of hydrogen sulfide within biogas. Waste Manag. 2018, 76, 374–382. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.P.; Wen, C.; Zhang, L. An enhanced approach for biochar preparation using fluidized bed and its application for H2S removal. Chem. Eng. Process. Process Intensif. 2016, 104, 1–12. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Sun, T. Comparison of sewage sludge- and pig manure-derived biochars for hydrogen sulfide removal. Chemosphere 2014, 111, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, G.; Zhang, L.; Sun, Z. Preparation of high performance H2S removal biochar by direct fluidized bed carbonization using potato peel waste. Process Saf. Environ. Prot. 2017, 107, 281–288. [Google Scholar] [CrossRef]
- Shang, G.; Shen, G.; Liu, L.; Chen, Q.; Xu, Z. Kinetics and mechanisms of hydrogen sulfide adsorption by biochars. Bioresour. Technol. 2013, 133, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A.; Lee, S.; Mohamad Nor, N.; Mohamed, A.; Al-Wabel, M.; Lee, S.; Ok, Y. Biochars as Potential Adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Creamer, A.E.; Gao, B. Carbon-Based Adsorbents for Postcombustion CO2 Capture: A Critical Review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059. [Google Scholar] [CrossRef]
- Xu, X.; Kan, Y.; Zhao, L.; Cao, X. Chemical transformation of CO2 during its capture by waste biomass derived biochars. Environ. Pollut. 2016, 213, 533–540. [Google Scholar] [CrossRef]
- Jung, S.; Park, Y.-K.; Kwon, E.E. Strategic use of biochar for CO2 capture and sequestration. J. CO2 Util. 2019, 32, 128–139. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. A review on common adsorbents for acid gases removal: Focus on biochar. Renew. Sustain. Energy Rev. 2018, 81, 1705–1720. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Mukumba, P.; Chikukwa, E.S.; Makaka, G. Biogas Upgrading Approaches with Special Focus on Siloxane Removal—A Review. Energies 2020, 13, 6088. [Google Scholar] [CrossRef]
- Cabrera-Codony, A.; Montes-Morán, M.A.; Sánchez-Polo, M.; Martín, M.J.; Gonzalez-Olmos, R. Biogas Upgrading: Optimal Activated Carbon Properties for Siloxane Removal. Environ. Sci. Technol. 2014, 48, 7187–7195. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhang, Y.; Hu, D.; Fan, J.; Zeng, G. A review on removal of siloxanes from biogas: With a special focus on volatile methylsiloxanes. Environ. Sci. Pollut. Res. 2018, 25, 30847–30862. [Google Scholar] [CrossRef] [PubMed]
- Gislon, P.; Galli, S.; Monteleone, G. Siloxanes removal from biogas by high surface area adsorbents. Waste Manag. 2013, 33, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Papurello, D.; Gandiglio, M.; Kafashan, J.; Lanzini, A. Biogas Purification: A Comparison of Adsorption Performance in D4 Siloxane Removal between Commercial Activated Carbons and Waste Wood-Derived Char Using Isotherm Equations. Processes 2019, 7, 774. [Google Scholar] [CrossRef]
- Research and Markets. Biochar Market Research Report by Raw Material, Feedstock, Technology, Application, and Region-Global Forecast to 2026-Cumulative Impact of COVID-19; Research and Markets: Dublin, Ireland, 2022. [Google Scholar]
- Transparency Market Research. Biochar Market-Global Industry Report, 2021–2031; Transparency Market Research: Pune, India, 2021. [Google Scholar]
- Mohammadi, A.; Sandberg, M.; Eskandari, S.; Granström, K. Life cycle assessment of combination of anaerobic digestion and pyrolysis: Focusing on different options for biogas use. Adv. Geosci. 2019, 49, 57–66. [Google Scholar] [CrossRef]
- Wang, J.; Okopi, S.I.; Ma, H.; Wang, M.; Chen, R.; Tian, W.; Xu, F. Life cycle assessment of the integration of anaerobic digestion and pyrolysis for treatment of municipal solid waste. Bioresour. Technol. 2021, 338, 125486. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; He, Y.; Zhang, C.; Liu, X.; Chen, C.; Liu, G. Anaerobic co-digestion of chicken manure and corn stover in batch and continuously stirred tank reactor (CSTR). Bioresour. Technol. 2014, 156, 342–347. [Google Scholar] [CrossRef]
- Lin, L.; Shah, A.; Keener, H.; Li, Y. Techno-economic analyses of solid-state anaerobic digestion and composting of yard trimmings. Waste Manag. 2019, 85, 405–416. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Zhang, Y.; Luo, W.; Li, G. Anaerobic digestion of different agricultural wastes: A techno-economic assessment. Bioresour. Technol. 2020, 315, 123836. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, W.G.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Techno-economic assessment of bioenergy and fertilizer production by anaerobic digestion of brewer’s spent grains in a biorefinery concept. J. Clean. Prod. 2021, 297, 126600. [Google Scholar] [CrossRef]
- Haeldermans, T.; Campion, L.; Kuppens, T.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. A comparative techno-economic assessment of biochar production from different residue streams using conventional and microwave pyrolysis. Bioresour. Technol. 2020, 318, 124083. [Google Scholar] [CrossRef] [PubMed]
- Nematian, M.; Keske, C.; Ng’ombe, J.N. A techno-economic analysis of biochar production and the bioeconomy for orchard biomass. Waste Manag. 2021, 135, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Al Yahya, S.; Iqbal, T.; Omar, M.M.; Ahmad, M. Techno-Economic Analysis of Fast Pyrolysis of Date Palm Waste for Adoption in Saudi Arabia. Energies 2021, 14, 6048. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Li, W.; Dang, Q.; Brown, R.C.; Laird, D.; Wright, M.M. The impacts of biomass properties on pyrolysis yields, economic and environmental performance of the pyrolysis-bioenergy-biochar platform to carbon negative energy. Bioresour. Technol. 2017, 241, 959–968. [Google Scholar] [CrossRef]
- Ji, C.; Cheng, K.; Nayak, D.; Pan, G. Environmental and economic assessment of crop residue competitive utilization for biochar, briquette fuel and combined heat and power generation. J. Clean. Prod. 2018, 192, 916–923. [Google Scholar] [CrossRef]
- Harsono, S.S.; Grundman, P.; Lau, L.H.; Hansen, A.; Salleh, M.A.M.; Meyer-Aurich, A.; Idris, A.; Ghazi, T.I.M. Energy balances, greenhouse gas emissions and economics of biochar production from palm oil empty fruit bunches. Resour. Conserv. Recycl. 2013, 77, 108–115. [Google Scholar] [CrossRef]
- Sahoo, K.; Bilek, E.; Bergman, R.; Mani, S. Techno-economic analysis of producing solid biofuels and biochar from forest residues using portable systems. Appl. Energy 2019, 235, 578–590. [Google Scholar] [CrossRef]
- ICIS Analytics-ICIS Explore. Available online: https://www.icis.com/explore/services/analytics/?intcmp=mega-menu-explore-services-analytics&redirect=english (accessed on 1 July 2023).
- Monlau, F.; Sambusiti, C.; Antoniou, N.; Barakat, A.; Zabaniotou, A. A new concept for enhancing energy recovery from agricultural residues by coupling anaerobic digestion and pyrolysis process. Appl. Energy 2015, 148, 32–38. [Google Scholar] [CrossRef]
- Antoniou, N.; Monlau, F.; Sambusiti, C.; Ficara, E.; Barakat, A.; Zabaniotou, A. Contribution to Circular Economy options of mixed agricultural wastes management: Coupling anaerobic digestion with gasification for enhanced energy and material recovery. J. Clean. Prod. 2019, 209, 505–514. [Google Scholar] [CrossRef]
- Zhang, L.; Lim, E.Y.; Loh, K.-C.; Ok, Y.S.; Lee, J.T.E.; Shen, Y.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Biochar enhanced thermophilic anaerobic digestion of food waste: Focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Convers. Manag. 2020, 209, 112654. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Xiang, S.; Huang, Z.; Ruan, R.; Liu, Y. Nutrient removal from digested swine wastewater by combining ammonia stripping with struvite precipitation. Environ. Sci. Pollut. Res. 2019, 26, 6725–6734. [Google Scholar] [CrossRef]
- Cao, Z.; Jung, D.; Olszewski, M.P.; Arauzo, P.J.; Kruse, A. Hydrothermal carbonization of biogas digestate: Effect of digestate origin and process conditions. Waste Manag. 2019, 100, 138–150. [Google Scholar] [CrossRef]
- Zhao, X.; Becker, G.C.; Faweya, N.; Rodriguez Correa, C.; Yang, S.; Xie, X.; Kruse, A. Fertilizer and activated carbon production by hydrothermal carbonization of digestate. Biomass Convers. Biorefinery 2018, 8, 423–436. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Niedzwiecki, L.; Sieradzka, M.; Mlonka-Mędrala, A.; Baranowski, M.; Serafin-Tkaczuk, M.; Magdziarz, A. Hydrothermal carbonization of agricultural and municipal solid waste digestates – Structure and energetic properties of the solid products. Fuel 2020, 275, 117837. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Urbanowska, A.; Yang, W.; Brem, G.; Magdziarz, A.; Seruga, P.; Niedzwiecki, L.; Pozarlik, A.; Mlonka-Mędrala, A.; Kabsch-Korbutowicz, M.; et al. Industrial Process Description for the Recovery of Agricultural Water from Digestate. J. Energy Resour. Technol. 2020, 142, 070917. [Google Scholar] [CrossRef]
- Urbanowska, A.; Kabsch-Korbutowicz, M.; Wnukowski, M.; Seruga, P.; Baranowski, M.; Pawlak-Kruczek, H.; Serafin-Tkaczuk, M.; Krochmalny, K.; Niedzwiecki, L. Treatment of Liquid By-Products of Hydrothermal Carbonization (HTC) of Agricultural Digestate Using Membrane Separation. Energies 2020, 13, 262. [Google Scholar] [CrossRef]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 29–36. [Google Scholar] [CrossRef]
- Zhang, B.; Joseph, G.; Wang, L.; Li, X.; Shahbazi, A. Thermophilic anaerobic digestion of cattail and hydrothermal carbonization of the digestate for co-production of biomethane and hydrochar. J. Environ. Sci. Heal. Part A 2020, 55, 230–238. [Google Scholar] [CrossRef]
- Zhang, T.; He, X.; Deng, Y.; Tsang, D.C.W.; Jiang, R.; Becker, G.C.; Kruse, A. Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. Sci. Total Environ. 2020, 698, 134240. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Shaheen, S.M.; Zhao, Q.; Liu, X.; Rinklebe, J.; Ren, H. Ammonium nitrogen recovery from digestate by hydrothermal pretreatment followed by activated hydrochar sorption. Chem. Eng. J. 2020, 379, 122254. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Xia, C. Multi-Complementary Model for Long-Term Tracking. Sensors 2018, 18, 527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Fan, H.-X.; Dai, X.-H.; Yuan, S.-J. Digested sludge-derived three-dimensional hierarchical porous carbon for high-performance supercapacitor electrode. R. Soc. Open Sci. 2018, 5, 172456. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Z.; Cao, L.; Ma, L.; You, S.; Li, W. Co-gasification of digestate and lignite in a downdraft fixed bed gasifier: Effect of temperature. Energy Convers. Manag. 2020, 213, 112798. [Google Scholar] [CrossRef]
- Pecchi, M.; Patuzzi, F.; Benedetti, V.; Di Maggio, R.; Baratieri, M. Thermodynamics of hydrothermal carbonization: Assessment of the heat release profile and process enthalpy change. Fuel Process. Technol. 2020, 197, 106206. [Google Scholar] [CrossRef]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Illi, L.; Oechsner, H.; Kruse, A. Valorization of maize silage digestate from two-stage anaerobic digestion by hydrothermal carbonization. Energy Convers. Manag. 2020, 222, 113218. [Google Scholar] [CrossRef]
- Barbanera, M.; Pelosi, C.; Taddei, A.R.; Cotana, F. Optimization of bio-oil production from solid digestate by microwave-assisted liquefaction. Energy Convers. Manag. 2018, 171, 1263–1272. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Mohedano, A.F.; Rodríguez, J.J.; de la Rubia, M.A. Energy and phosphorous recovery through hydrothermal carbonization of digested sewage sludge. Waste Manag. 2020, 105, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; He, P.; Wang, Y.; Shao, L.; Lü, F. Effects and optimization of the use of biochar in anaerobic digestion of food wastes. Waste Manag. Res. J. a Sustain. Circ. Econ. 2016, 34, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sunyoto, N.M.S.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef] [PubMed]
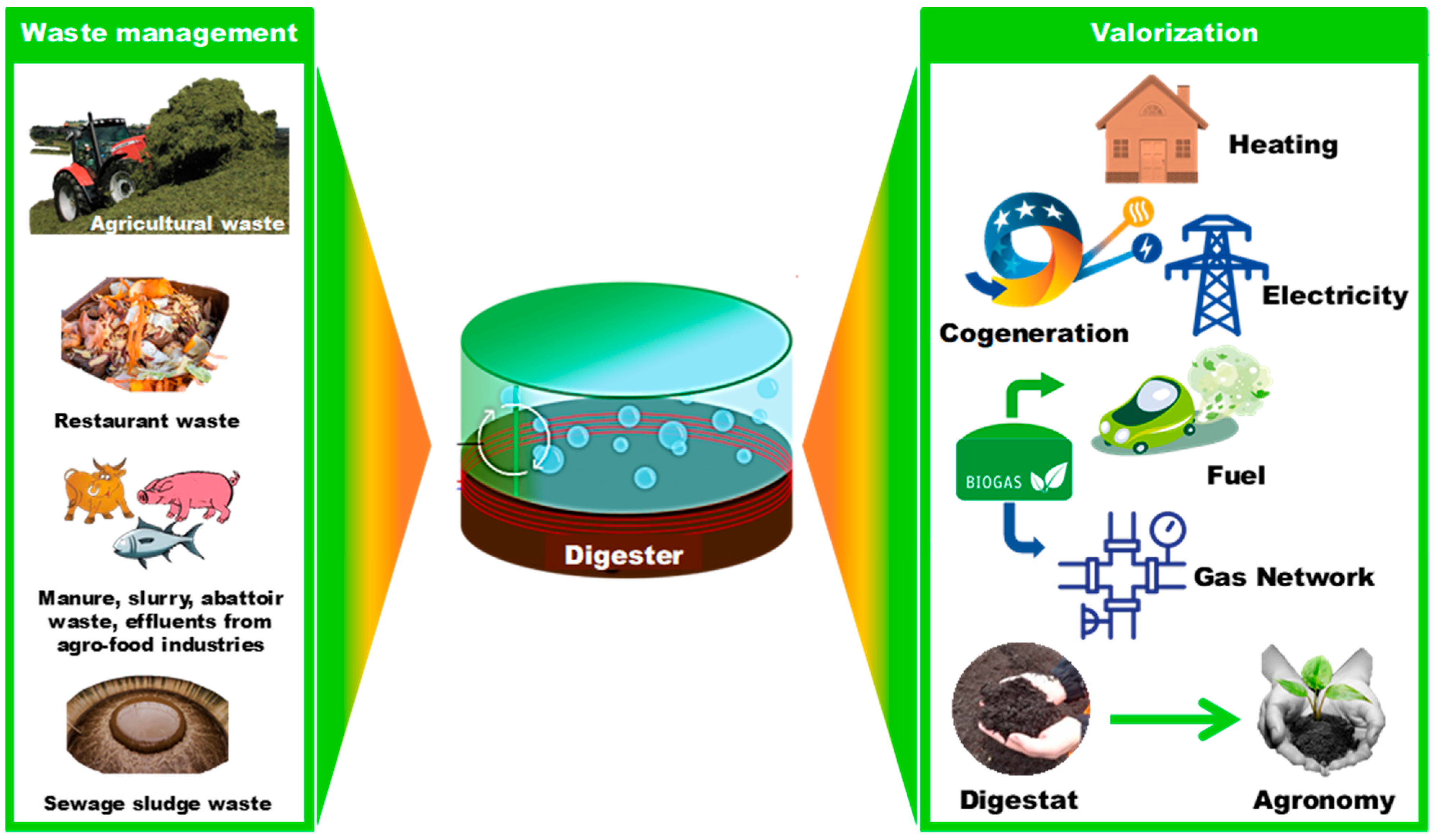
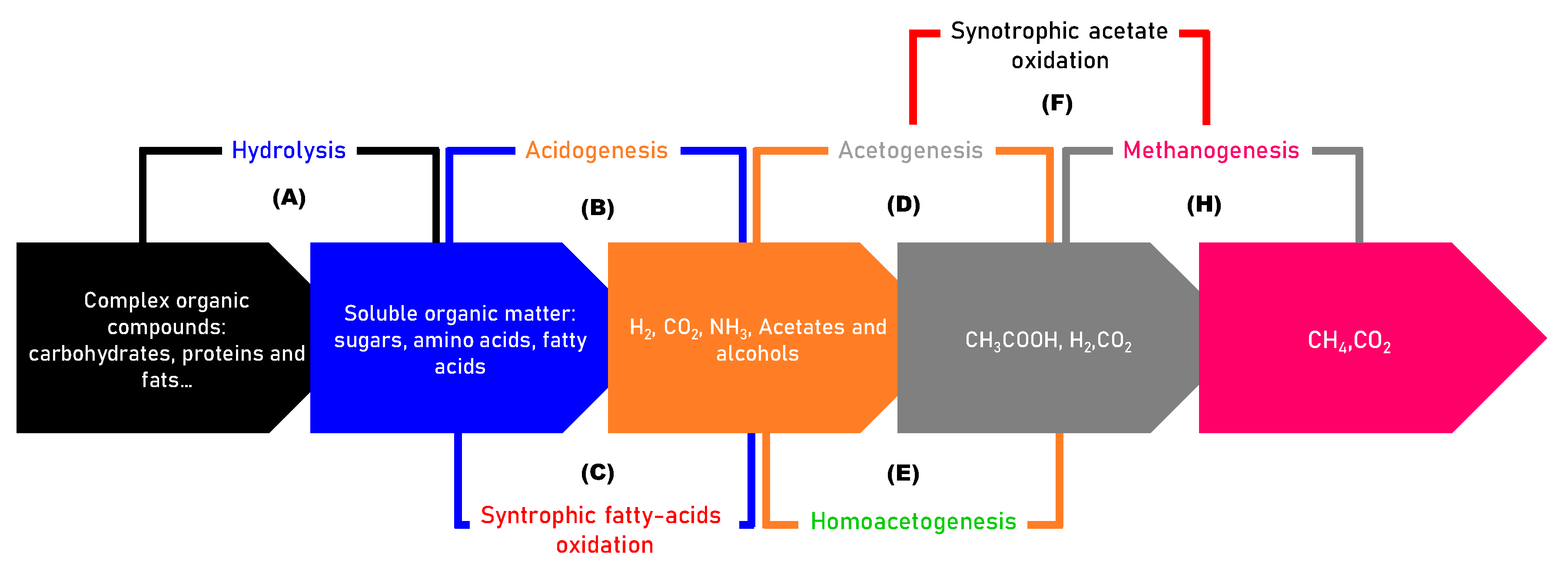
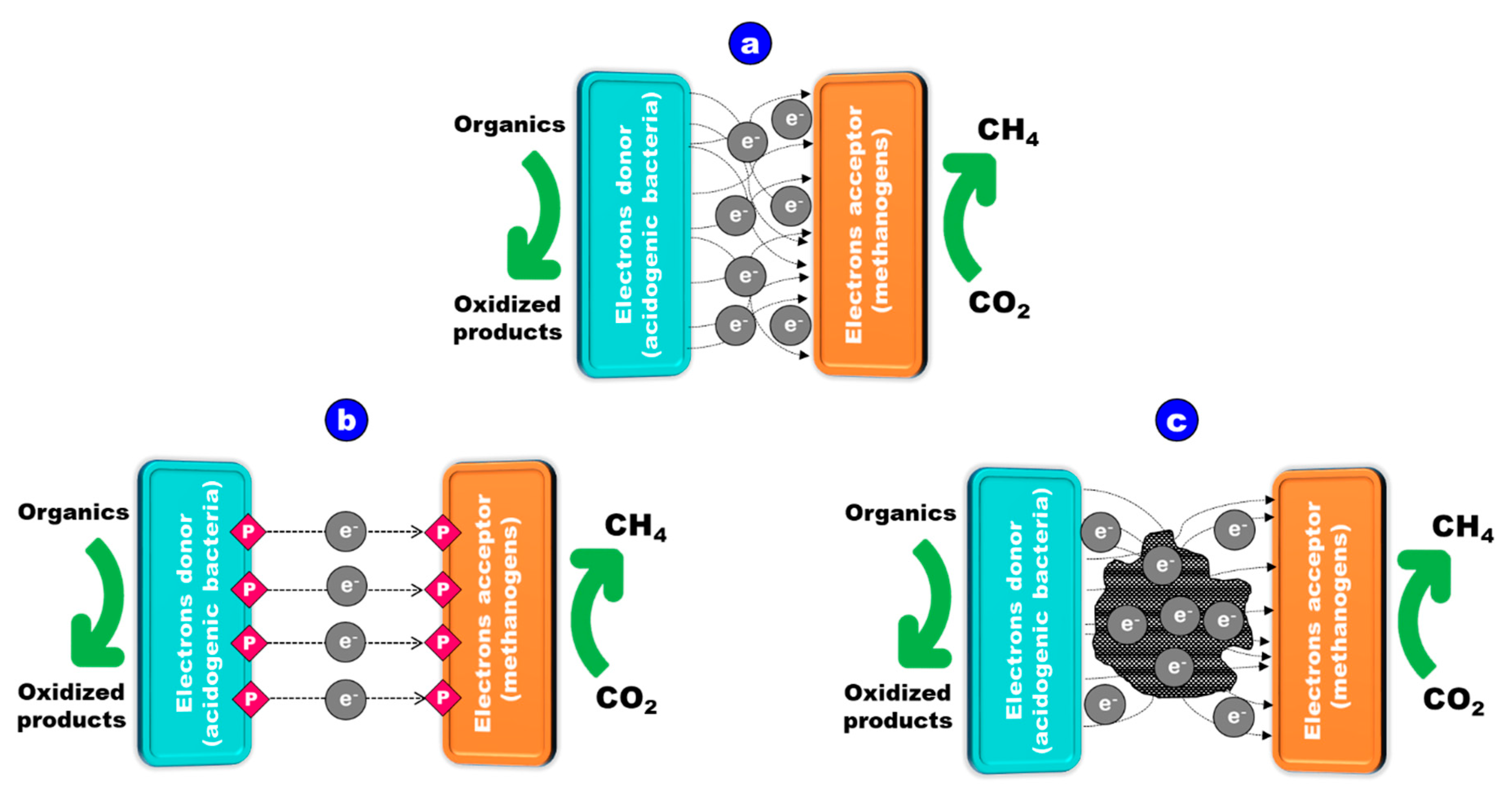
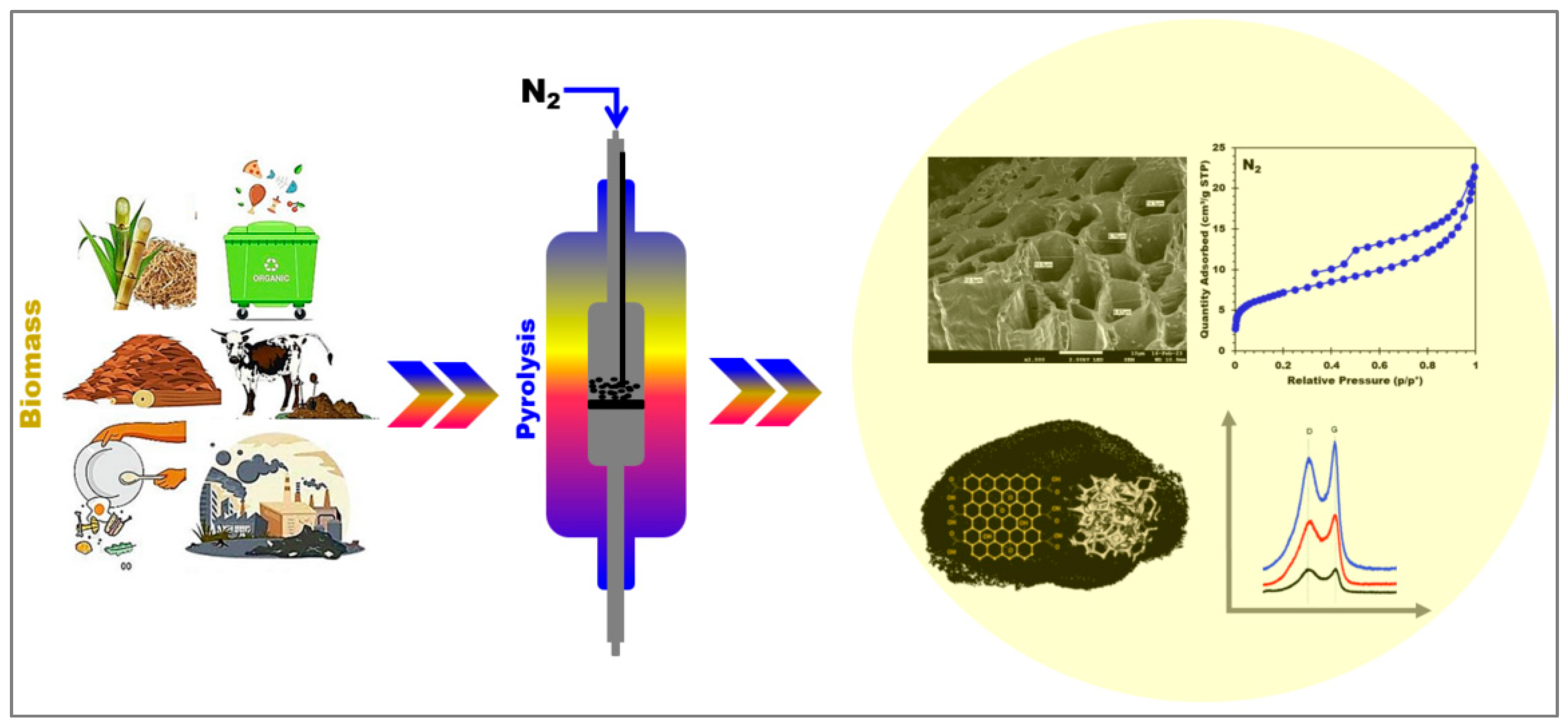
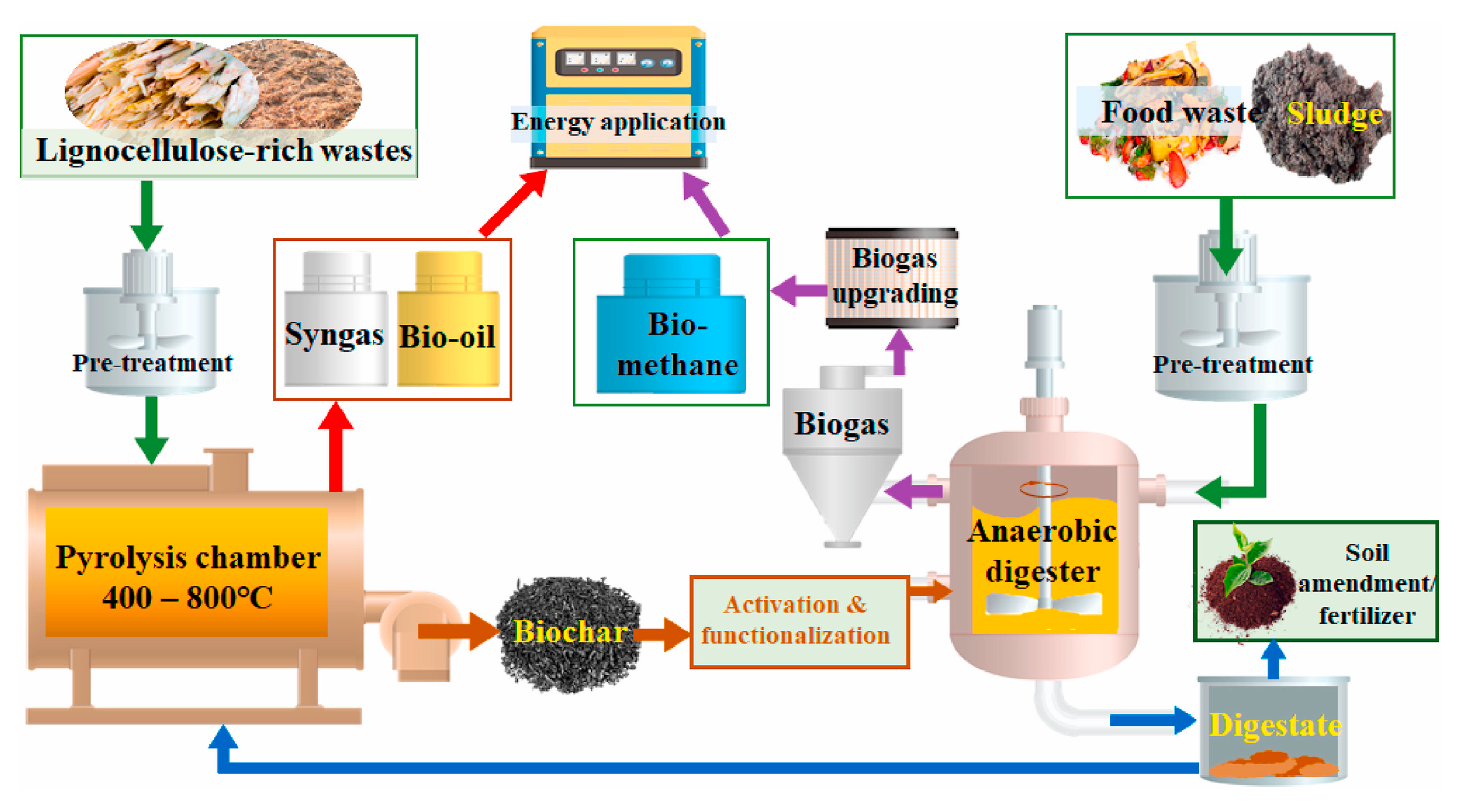
| Microbial Groups | Substrates | Products | Examples |
|---|---|---|---|
| Hydrolytic/fermentative bacteria | Cellulose, hemicelluloses | Cellobiose, hexoses, pentoses, acetic acid, ethanol, CO2 | Phylum Firmicutes and Bacteroidetes.Genus Acetivibrio, Bacteroides, Clostridium, Ruminococcus, Thermotoga |
| Protein | Peptides, amino acids | Phylum Proteobacteria | |
| Syntrophs | Propionate, butyrate, | Acetate, H2, CO2 | Syntrophomonas, Smithella, Synergistes |
| Syntrophic acetate-oxidizing bacteria | Acetate | H2, CO2 | Clostridium ultunense, Thermotoga lettingae, Thermacetogenium phaeum |
| Homoacetogens | H2, CO2 | Acetate | Acetobacterium |
| Acetotrophic methanogens | Acetate | CH4, CO2 | Methanosaeta, Methanosarcina |
| Hydrogenotrophic methanogens | H2, CO2 | CH4 | Methanosarcina, Methanobacteriales, Methanospirillum, Methanoculleus |
| Mesophilic | Thermophilic | |
|---|---|---|
| Benefits | Works with tough bacteria that can tolerate more environmental changes. The system is more reliable and straightforward to maintain. Bacteria [61]: phyla Firmicutes, Proteobacteria, Bacteriodetes and Chloroflexi. Lower energy costs. | The needed retention time is decreased as the temperature rises. Bacteria [61]: Firmicutes, Proteobacteria, Chloroflexi and Actinobacteria. Increased pathogen elimination. |
| Drawbacks | Increased retention time. Decreased biogas production. | Because the microbial population is less diversified, the process is less steady; the system is more difficult to maintain. Extra energy input is required for heating. |
| AD | Substrates | Dry Weight Ratio | C/N Ratio | CH4 Yield (LCH4/gVS) | Refs. |
|---|---|---|---|---|---|
| Thermophilic batch | OMSW:Sludge | 2:1 | 14.19 | 0.14 | [88] |
| Two-stage: thermophilic-mesophilic | OMSW:Sludge | 2:1 | 14.19 | 0.18 | |
| Single-stage: mesophilic | Food waste:Sludge | 1:9 | 5.97 | 0.18 | [89] |
| 3:7 | 6.99 | 0.21 | |||
| 1:1 | 8.9 | 0.32 | |||
| 7:3 | 11 | 0.33 | |||
| 9:1 | 14.7 | 0.34 | |||
| Mesophilic batch | Food waste:Cattle manure | 2:1 | 15.8 | 0.38 | [90] |
| Two-phase | OMSW:Cow manure | 10:1 | 20 | 0.10 | [91] |
| Mesophilic batch | OMSW:Sludge | 1:34 | 17.68 | 0.15 | [92] |
| 1:19 | 20.55 | 0.20 | |||
| Singles stage: mesophilic | Food waste:Sludge | 1:2.4 | 7.1 | 0.30 | [93] |
| 1:0.9 | 10.2 | 0.35 | |||
| 1:0.4 | 11.4 | 0.40 | |||
| Mesophilic | OMSW | 14.1 | 0.38 | [94] | |
| OMSW:Vegetable oil | 5:1 | 0.69 | |||
| OMSW:Animal fat | 5:1 | 0.50 | |||
| OMSW:Cellulose | 5:1 | 0.25 | |||
| OMSW:Protein | 5:1 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zbair, M.; Limousy, L.; Drané, M.; Richard, C.; Juge, M.; Aemig, Q.; Trably, E.; Escudié, R.; Peyrelasse, C.; Bennici, S. Integration of Digestate-Derived Biochar into the Anaerobic Digestion Process through Circular Economic and Environmental Approaches—A Review. Materials 2024, 17, 3527. https://doi.org/10.3390/ma17143527
Zbair M, Limousy L, Drané M, Richard C, Juge M, Aemig Q, Trably E, Escudié R, Peyrelasse C, Bennici S. Integration of Digestate-Derived Biochar into the Anaerobic Digestion Process through Circular Economic and Environmental Approaches—A Review. Materials. 2024; 17(14):3527. https://doi.org/10.3390/ma17143527
Chicago/Turabian StyleZbair, Mohamed, Lionel Limousy, Méghane Drané, Charlotte Richard, Marine Juge, Quentin Aemig, Eric Trably, Renaud Escudié, Christine Peyrelasse, and Simona Bennici. 2024. "Integration of Digestate-Derived Biochar into the Anaerobic Digestion Process through Circular Economic and Environmental Approaches—A Review" Materials 17, no. 14: 3527. https://doi.org/10.3390/ma17143527
APA StyleZbair, M., Limousy, L., Drané, M., Richard, C., Juge, M., Aemig, Q., Trably, E., Escudié, R., Peyrelasse, C., & Bennici, S. (2024). Integration of Digestate-Derived Biochar into the Anaerobic Digestion Process through Circular Economic and Environmental Approaches—A Review. Materials, 17(14), 3527. https://doi.org/10.3390/ma17143527














