In Situ Growth Method for Large-Area Flexible Perovskite Nanocrystal Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of PMMA Precursors Solution
2.2.2. Preparation of Stock Solution
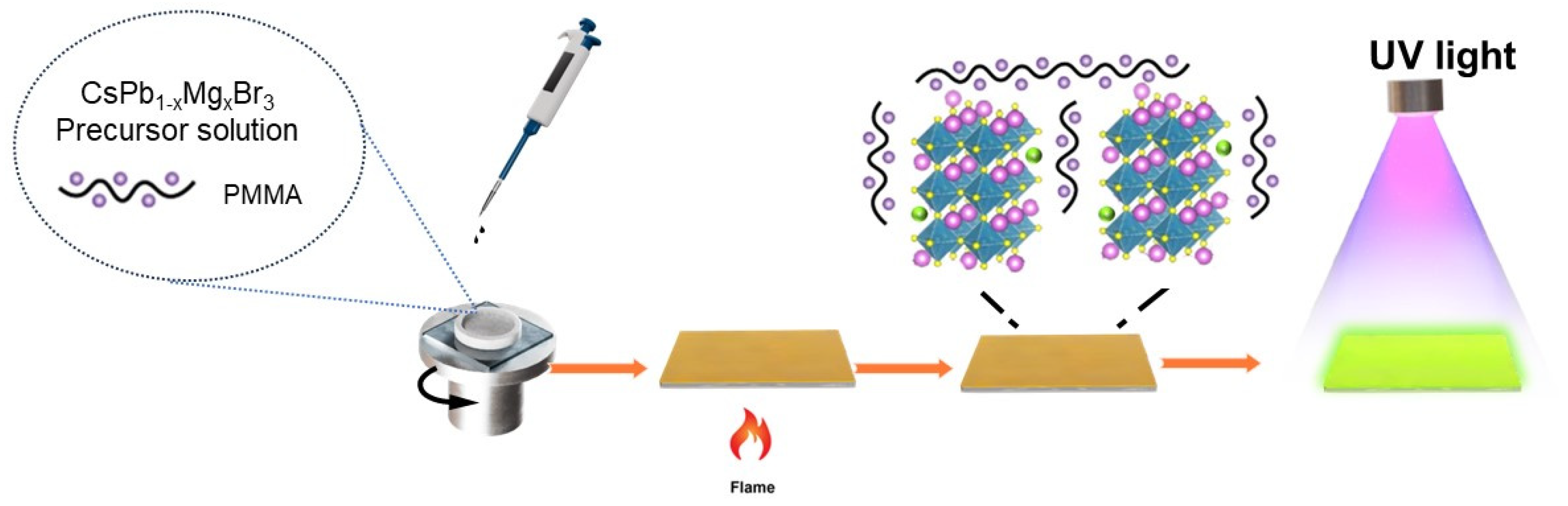
2.2.3. Synthesis of CsPb1−xMgxBr3@PMMA Films
2.3. Characterization
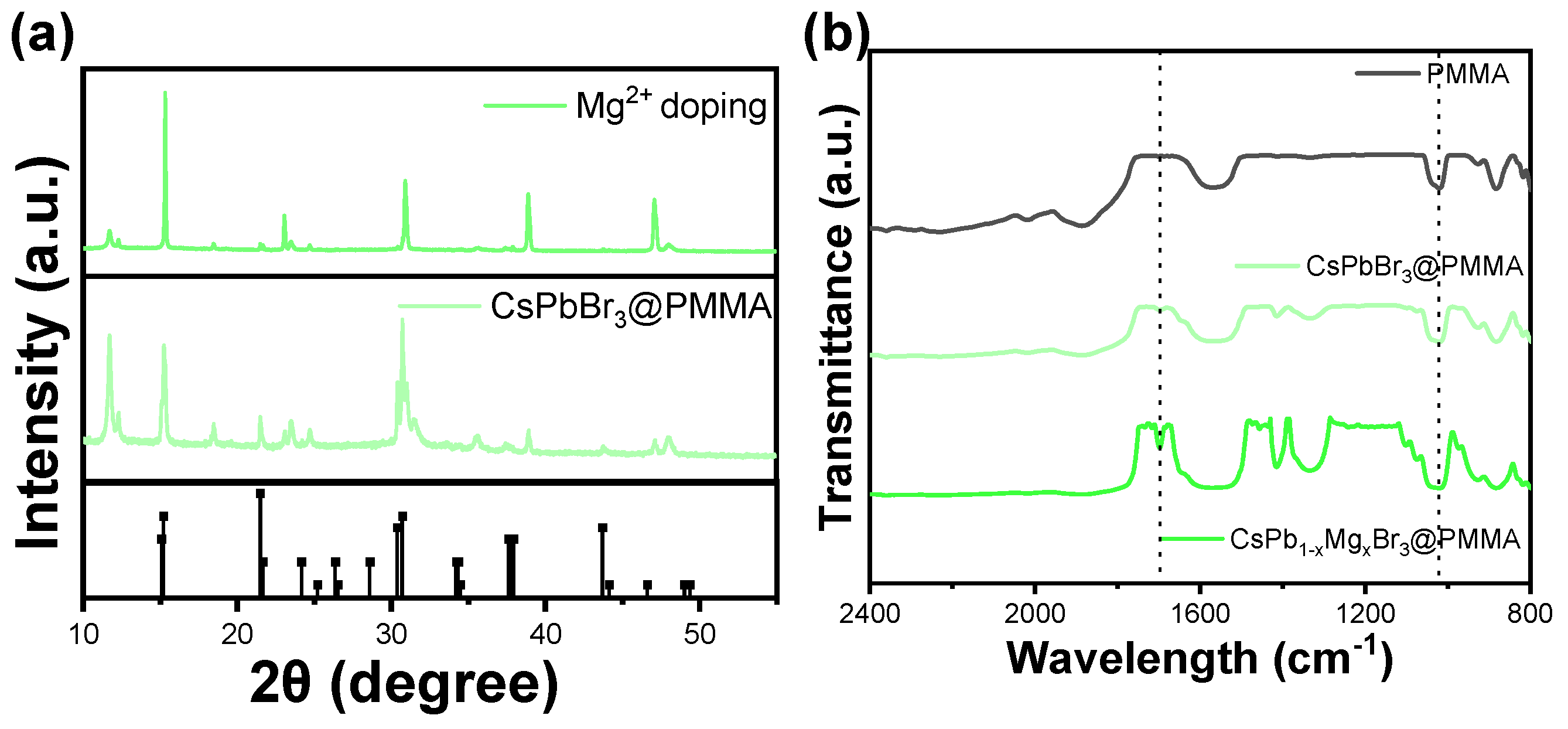
2.3.1. Characterization via X-ray Diffraction (XRD)
2.3.2. Fourier Transform Infrared (FTIR)
2.3.3. Fluorescence Spectrum Characterization
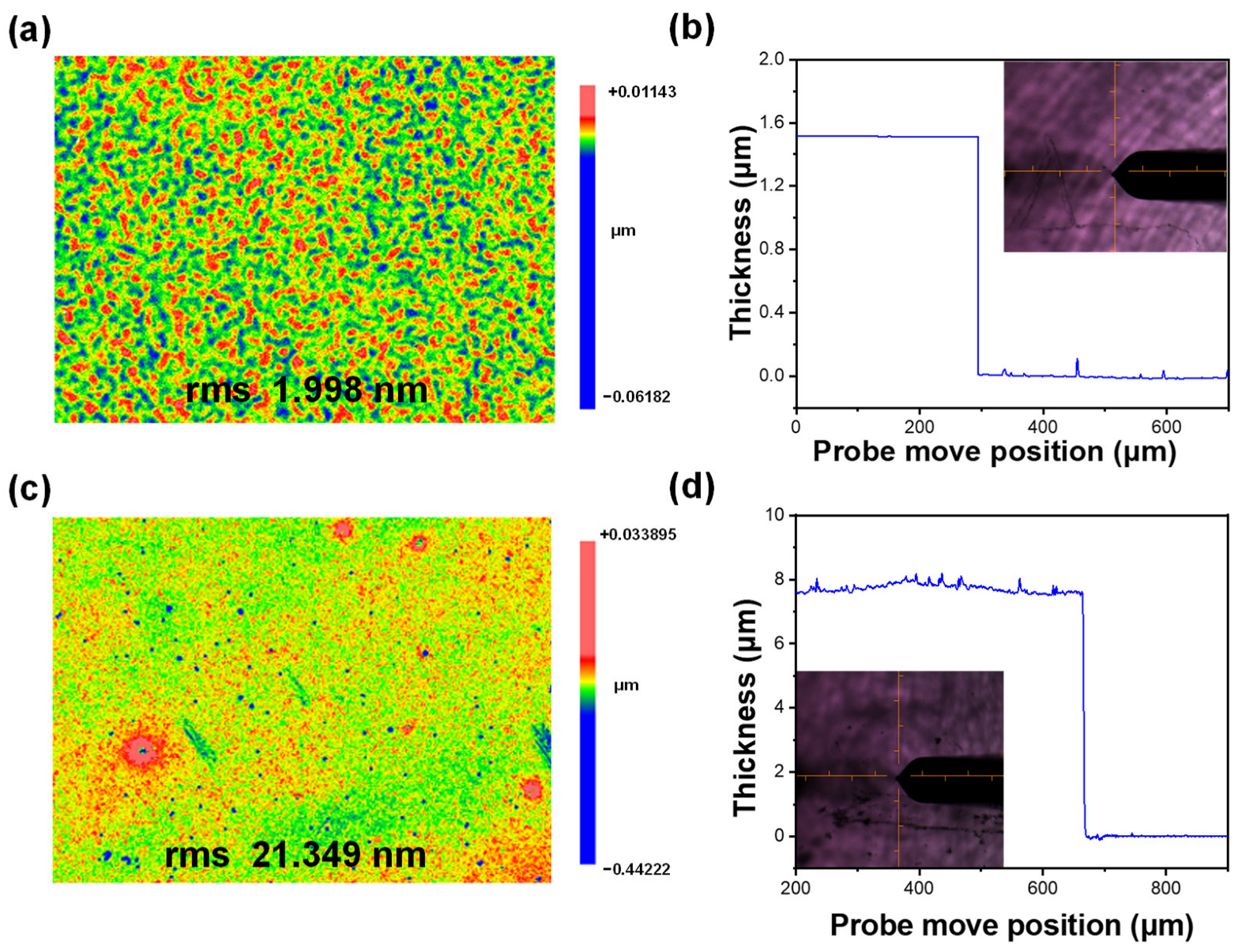
2.3.4. Thickness and Surface Roughness of the Film
2.3.5. PNC-Si Photodetector Characterization
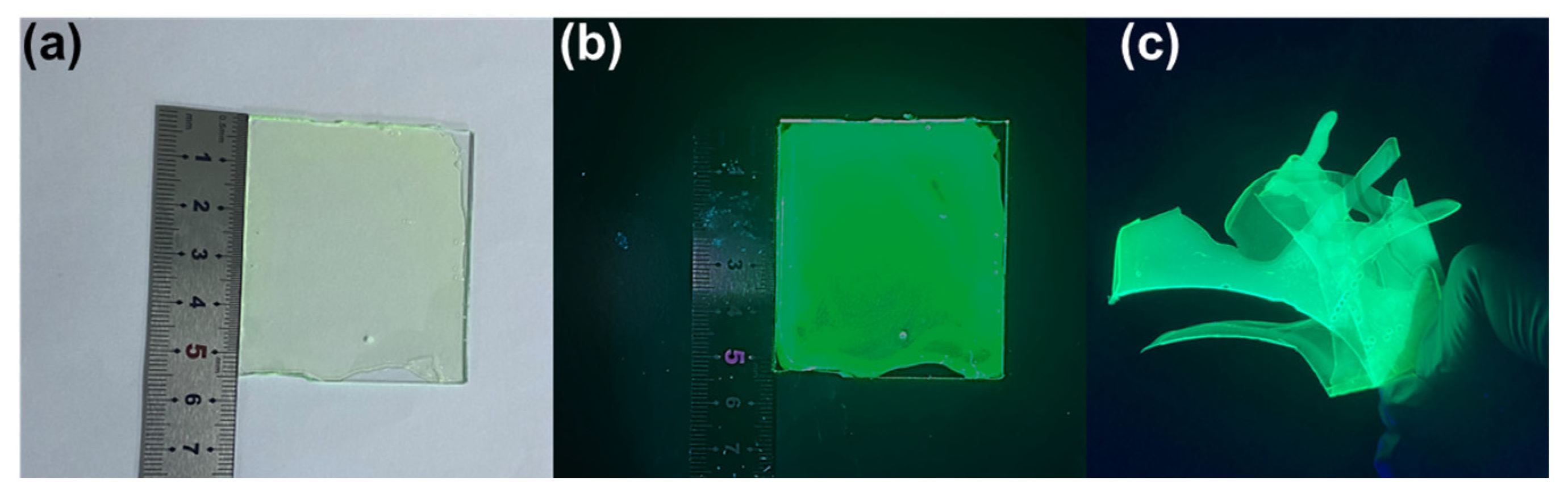
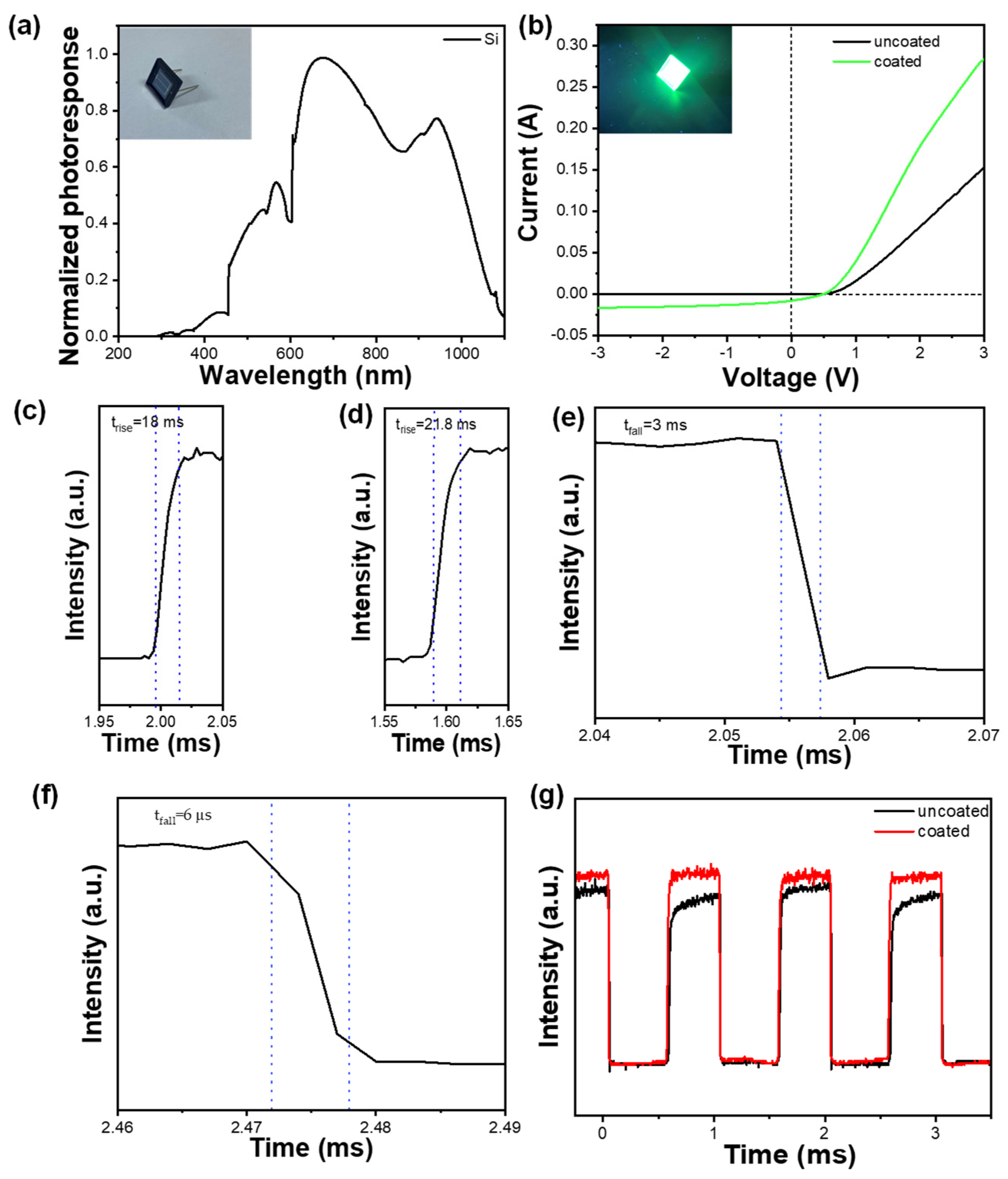
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Kim, D.H. Perovskite-based photodetectors: Materials and devices. Chem. Soc. Rev. 2017, 46, 5204–5236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, W.; Olthof, S.; Liu, S. Defects in CsPbX3 Perovskite: From Understanding to Effective Manipulation for High-Performance Solar Cells. Small Methods 2021, 5, 2100725. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, C.; Peng, X.; Li, W.; Cao, J.; Wang, Q.; Zeng, X.; Fu, X.; Yang, W. The metal doping strategy in all inorganic lead halide perovskites: Synthesis, physicochemical properties, and optoelectronic applications. Nanoscale 2021, 13, 18010–18031. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Park, J.; Lee, S. Effect of perovskite film morphology on device performance of perovskite light-emitting diodes. Nanoscale 2019, 11, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhang, Z.; Tao, J.; Gu, Y.; Feng, J.; Sun, H. Enhanced performance of perovskite light-emitting devices with improved perovskite crystallization. IEEE Photonics J. 2017, 9, 1600408. [Google Scholar] [CrossRef]
- Liu, P.; Han, N.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. High-Quality Ruddlesden–Popper Perovskite Film Formation for High-Performance Perovskite Solar Cells. Adv. Mater. 2021, 33, 2002582. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hadar, I.; Kanatzidis, M.G. Detecting Ionizing Radiation Using Halide Perovskite Semiconductors Processed through Solution and Alternative Methods. Nat. Photonics 2022, 16, 14–26. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J. Stable Perovskite Solar Cells with Efficiency Exceeding 24.8% and 0.3-V Voltage Loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.; Peng, X.; Sun, G.; Liu, X.; Liu, D.; Li, Z.; He, F.; Shen, C.; Gu, Q. Perovskite Light-Emitting Diodes with Eqe Exceeding 28% through a Synergetic Dual-Additive Strategy for Defect Passivation and Nanostructure Regulation. Adv. Mater. 2021, 33, 2103268. [Google Scholar] [CrossRef]
- Nandihalli, N. Performance analysis of photodetectors based on 2D materials and heterostructures. Crit. Rev. Solid State Mater. Sci. 2023, 1–87. [Google Scholar] [CrossRef]
- Qin, C.; Sandanayaka, A.S.; Zhao, C.; Matsushima, T.; Zhang, D.; Fujihara, T.; Adachi, C. Stable Room-Temperature Continuous Wave Lasing in Quasi-2D Perovskite Films. Nature 2020, 585, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nazeeruddin, M.K.; Choy, W.C. Perovskite Photovoltaics: The Significant Role of Ligands in Film Formation, Passivation, and Stability. Adv. Mater. 2019, 31, 1805702. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Tang, X.; Chen, M. Ionic Doping of CsPbI3 Perovskite Nanocrystals Improves Luminescence and Stability in Patterned Large-Area Light-Emitting Diodes. ACS Appl. Nano Mater. 2023, 6, 18918–18925. [Google Scholar] [CrossRef]
- Das, S.; De, A.; Samanta, A. Ambient condition Mg2+ doping producing highly luminescent green-and violet-emitting perovskite nanocrystals with reduced toxicity and enhanced stability. J. Phys. Chem. Lett. 2020, 11, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Yang, Y.; Gong, X. Recent Advancements and Challenges for Low-Toxicity Perovskite Materials. ACS Appl. Mater. Interfaces 2020, 12, 26776–26811. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, S.; Xing, K.; Ning, M.; Zeng, R.; Wang, Y.; Zhao, J. Mg2+-Assisted Passivation of Defects in CsPbI3 Perovskite Nanocrystals for High-Efficiency Photoluminescence. J. Phys. Chem. Lett. 2021, 12, 11090–11097. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. Zn(II)-Doped Cesium Lead Halide Perovskite Nanocrystals with High Quantum Yield and Wide Color Tunability for Color-Conversion Light-Emitting Displays. ACS Appl. Nano Mater. 2020, 3, 7621–7632. [Google Scholar] [CrossRef]
- Roy, B.; Mahato, S.; Bose, S.; Ghorai, A.; Srivastava, S.K.; Das, N.C.; Ray, S.K. Cu-Doping Induced Phase Transformation in CsPbI3 Nanocrystals with Enhanced Structural Stability and Photoluminescence Quantum Yield. Chem. Mater. 2023, 35, 1601–1609. [Google Scholar]
- Chang, P.; Zhai, Y.; Wu, N.; Zhang, H.; Zhu, Q.-Q.; Wang, L. Fluorescence properties of potassium ions doped CsPbCl3: Mn perovskite quantum dots. Chin. J. Liq. Cryst. Disp. 2021, 36, 1352–1361. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.X.; Zhang, J.F.; Liu, H.Y.; Chen, G.; Li, K.; Hong, M.Q.; Zuo, W.B.; Wang, C.N.; Yang, W.; et al. Improving the Mn2+ emission and stability of CsPb(Cl/Br)3 nanocrystals by Ni2+ doping in ambient air. J. Mater. Sci. 2021, 56, 7494–7507. [Google Scholar] [CrossRef]
- Jin, M.; Zhai, X.; Huang, Y.; Zhang, M.; Ma, T.; Zeng, Z.; Fu, H.; Yin, L.; Zhang, Y.; Du, Y. Biocompatible Silica-Coated Europium-Doped CsPbBr3 Nanoparticles with Luminescence in Water for Zebrafish Bioimaging. Small 2024, 20, 2310238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.; Liu, G.; Ma, Y.; Gong, L.; Guan, R.; Cui, X.; Yan, J.; Zhao, J.; Yang, J. Pressure-Engineered Optical and Charge Transport Properties of Mn2+/Cu2+ Codoped CsPbCl3 Perovskite Nanocrystals via Structural Progression. ACS Appl. Mater. Interfaces 2020, 12, 48225–48236. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; An, S.X.; Li, Y.; Orooji, S.; Singh, R.; Schackmar, F.; Laufer, F.; Jin, Q.; Feeney, T.; Diercks, A. Triple-Junction Perovskite Perovskite-Silicon Solar Cells with Power Conversion Efficiency of 24.4%. Energy Environ. Sci. 2024, 17, 2800–2814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cao, H.; Xue, C.; Zhang, H.; Qin, M.; Wang, J.; Wen, K.; Fu, Z.; Jiang, T.; Xu, L. Unveiling the Additive-Assisted Oriented Growth of Perovskite Crystallite for High Performance Light-Emitting Diodes. Nat. Commun. 2021, 12, 5081. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hao, S.; Ouyang, D.; Liu, S.; Zhang, N.; Zeng, Z.; Zhang, Y.; Spanopoulos, I.; Wolverton, C.; Li, Y. Universal Vapor-Phase Synthesis of Large-Scale Ultrathin Perovskites with Superior Stability for Photodetectors and Image Sensors. Adv. Funct. Mater. 2024, 34, 2313163. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhao, K.; Yang, Z.; Feng, J.; Zhang, X.; Wang, K.; Meng, L.; Ye, H.; Liu, M. A 1300 mm2 Ultrahigh-Performance Digital Imaging Assembly Using High-Quality Perovskite Single Crystals. Adv. Mater. 2018, 30, 1707314. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Shi, F.; Tian, Y.; Zhang, W.; Xie, L.; Guo, S.; Song, C.; Tie, G. Ultra-Smooth Polishing of Single-Crystal Silicon Carbide by Pulsed-Ion-Beam Sputtering of Quantum-Dot Sacrificial Layers. Materials 2024, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Tu, Y.; Jiao, C.; Li, W.; Zhou, P.; Cheng, J.; Yang, G.; Shao, T.; Ye, J.; Li, G.; et al. Green N1 additive modified perovskite precursor enables effective manufacturing of large-area solar cell modules with high efficiency and stability. Chem. Eng. J. 2024, 480, 148133. [Google Scholar] [CrossRef]
- Xu, L.; Wu, D.; Lv, W.; Xiang, Y.; Liu, Y.; Tao, Y.; Yin, J.; Qian, M.; Li, P.; Zhang, L.; et al. Resonance-Mediated Dynamic Modulation of Perovskite Crystallization for Efficient and Stable Solar Cells. Adv. Mater. 2022, 34, 2107111. [Google Scholar] [CrossRef]
- Tian, L.; Wen, F.; Zhang, W.; Zhang, H.; Yu, H.; Lin, P.; Liu, X.; Zhou, S.; Zhou, X.; Jiang, Y.; et al. Rising from the Ashes: Gaseous Therapy for Robust and Large-Area Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 49648–49658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, S.; Jiang, J.; Sun, Q.; Liu, J.; Ou, D.; Zhao, J.; Yang, W.; Fu, H.; Zheng, J. Aqueous strategy for controllable in-situ growth of high-quality perovskite nanocrystals@polymer films toward white light-emitting diodes. Chem. Eng. J. 2023, 462, 142330. [Google Scholar] [CrossRef]
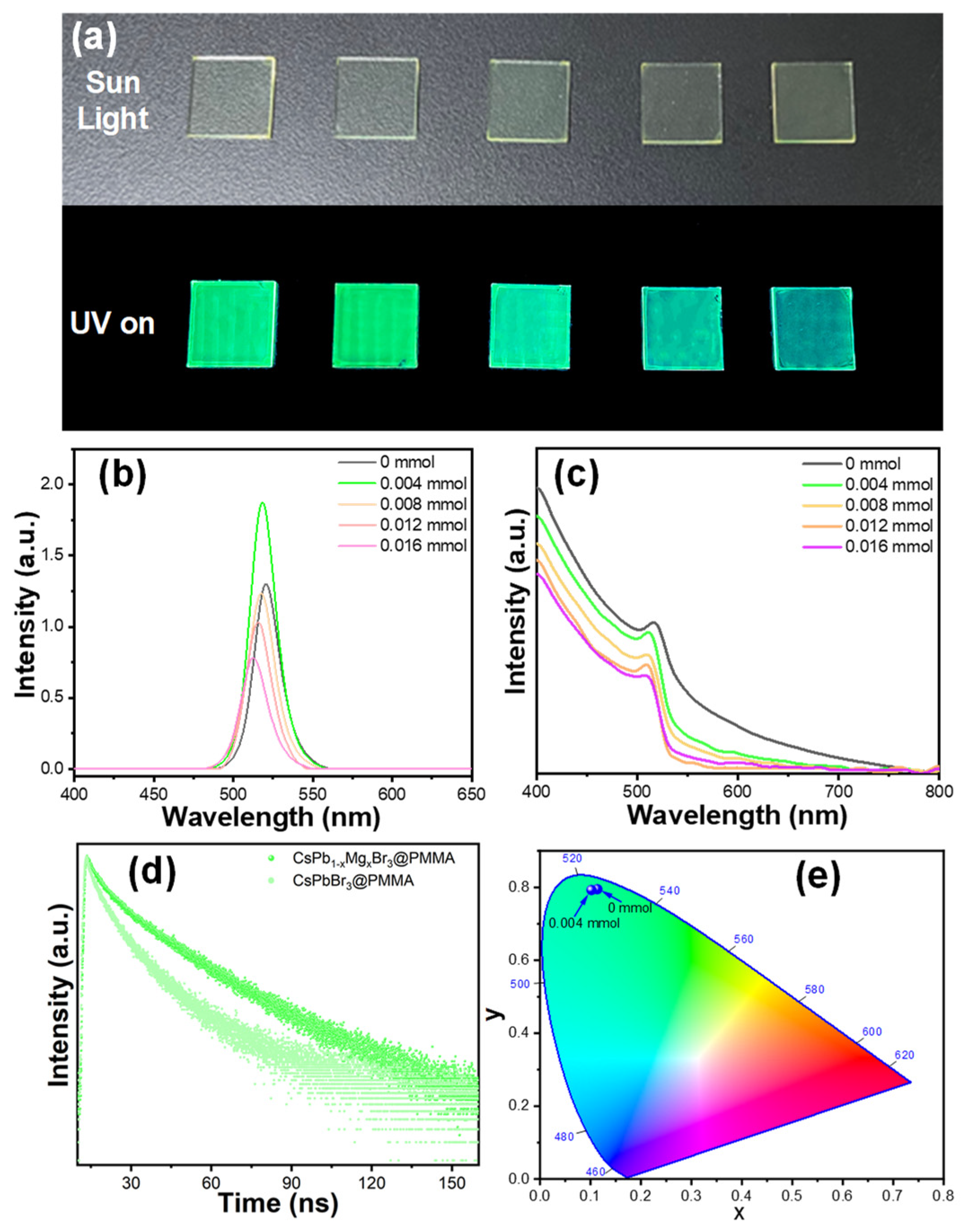

| Sample | A1 | τ1 (ns) | A2 | τ2 (ns) | τavg (ns) | x2 |
|---|---|---|---|---|---|---|
| CsPbBr3@PMMA | 0.30 | 5.24 | 0.76 | 18.92 | 17.57 | 0.99 |
| CsPb1−XMgxBr3@PMMA | 0.24 | 5.16 | 0.83 | 33.54 | 32.22 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Xu, B.; Zhao, X.; Lv, H.; Qiao, D.; Peng, X.; Shi, F.; Chen, M.; Hao, Q. In Situ Growth Method for Large-Area Flexible Perovskite Nanocrystal Films. Materials 2024, 17, 3550. https://doi.org/10.3390/ma17143550
Zhou X, Xu B, Zhao X, Lv H, Qiao D, Peng X, Shi F, Chen M, Hao Q. In Situ Growth Method for Large-Area Flexible Perovskite Nanocrystal Films. Materials. 2024; 17(14):3550. https://doi.org/10.3390/ma17143550
Chicago/Turabian StyleZhou, Xingting, Bin Xu, Xue Zhao, Hongyu Lv, Dongyang Qiao, Xing Peng, Feng Shi, Menglu Chen, and Qun Hao. 2024. "In Situ Growth Method for Large-Area Flexible Perovskite Nanocrystal Films" Materials 17, no. 14: 3550. https://doi.org/10.3390/ma17143550





