CO2-Accelerated Carbonation Modification for Recycled Coarse Aggregate with Various Original Concrete Strengths and Coarse Aggregate Sizes
Abstract
:1. Introduction
- (1)
- Carrying out accelerated carbonation tests on RCAs with various OCSs and CASs with a completely dry IMC revealed the changing rules of the physical property indexes of RCAs in terms of OCS and CAS before and after accelerated carbonation.
- (2)
- The influence of OCS and CAS on the accelerated carbonation modification of RCA was clarified, and the OCS and CAS of RCAs that had the optimal effect in terms of accelerated carbonation modification were identified.
- (3)
- By analyzing the microscopic morphology of RCA before and after accelerated carbonation, the reasons for the property enhancement of RCAs with various OCSs and CASs under optimal carbonation modification conditions were clarified.
2. Materials and Experiments
2.1. Raw Materials and Mix Proportions of Concrete for Producing the RCA
2.2. RCA Sample Acquirements
2.3. CO2-Accelerated Carbonation Experiment for RCA Samples
2.4. Test for Properties of RCA Samples
3. Results and Analysis
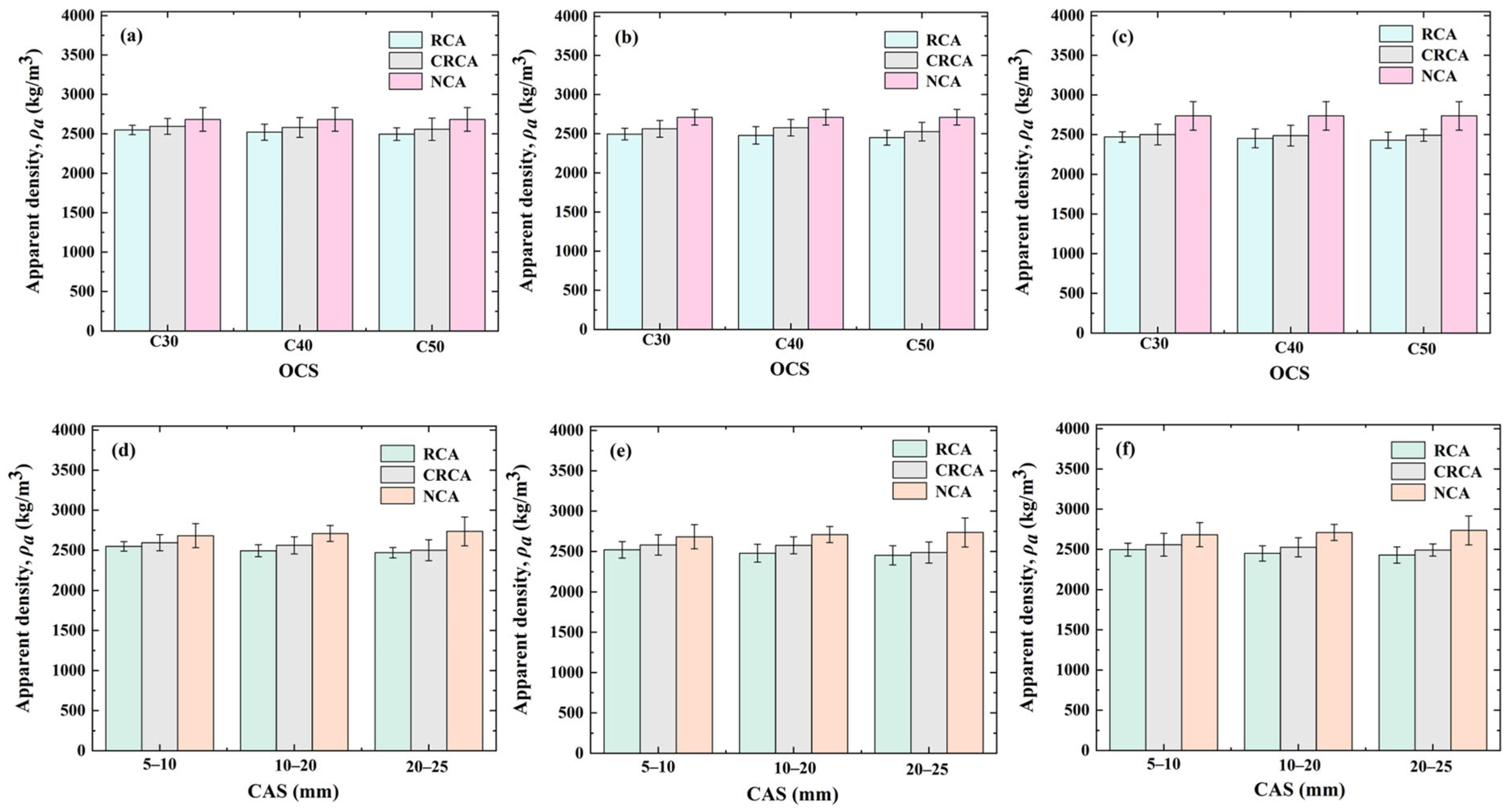
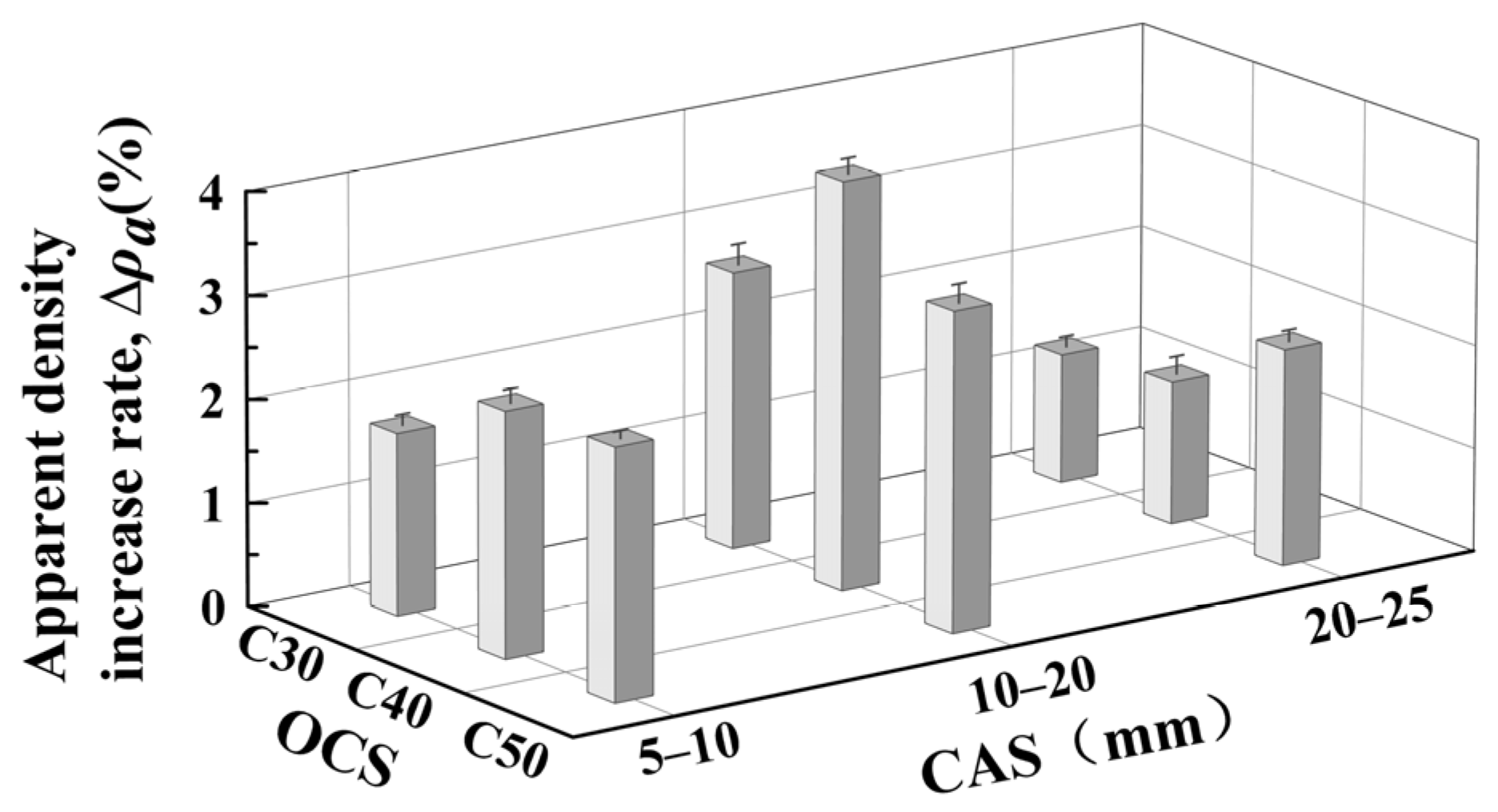
3.1. Effect of OCS and CAS on Apparent Density of CRCAs
- (1)
- Effect of OCS on CRCA apparent density
- (2)
- Effect of CAS on CRCA apparent density
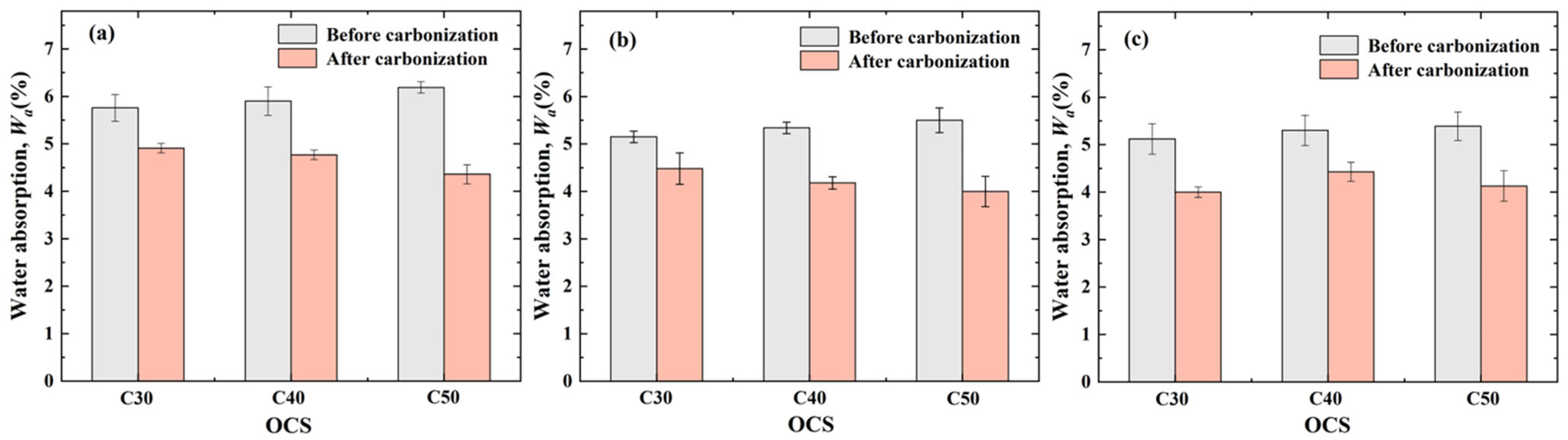
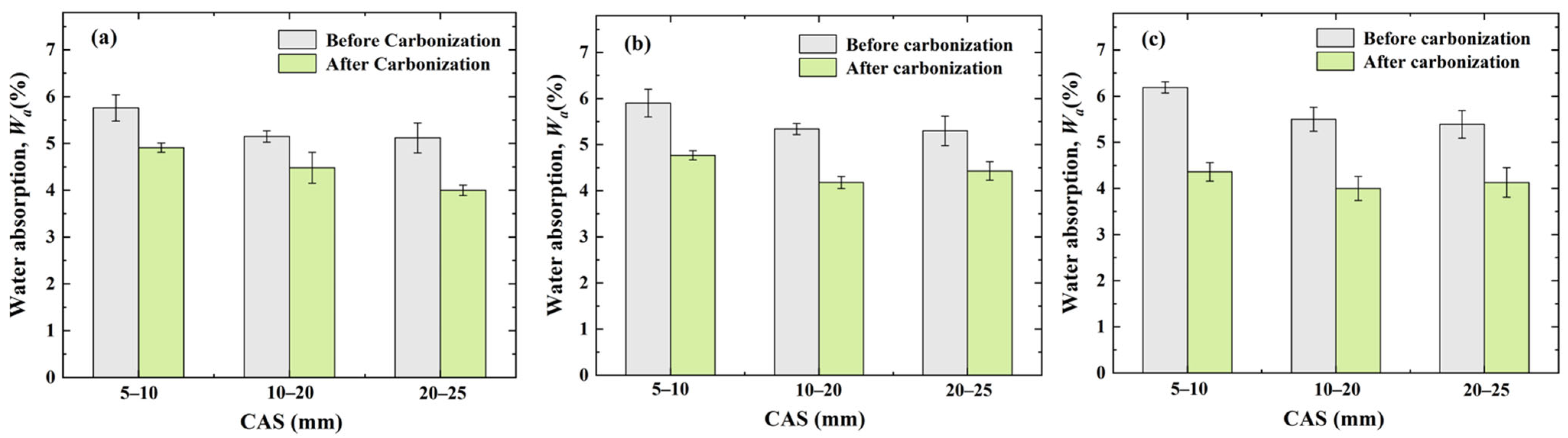
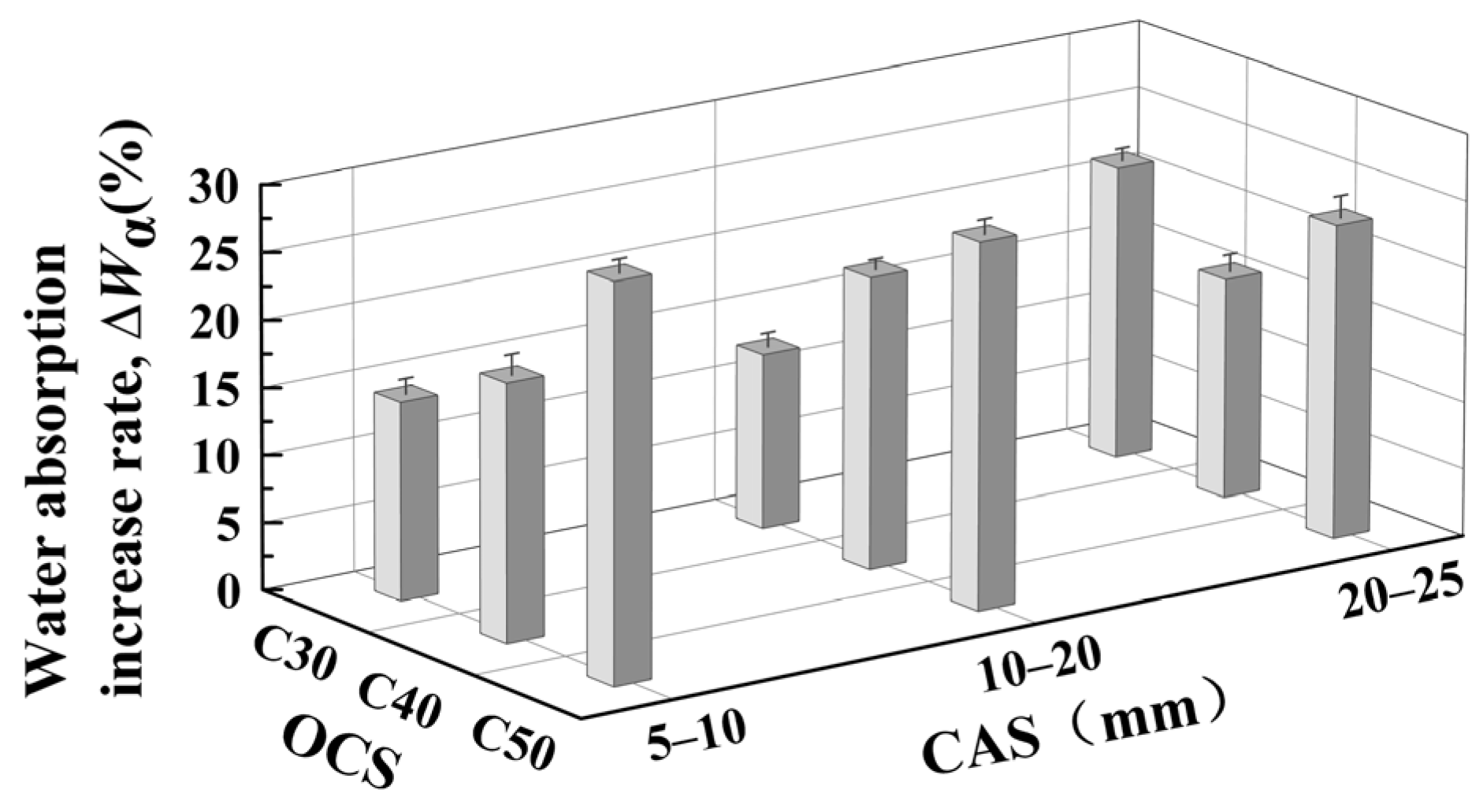
3.2. Effect of OCSs and CASs on Water Absorption of CRCAs
- (1)
- Effect of OCS on CRCA water absorption
- (2)
- Effect of CAS on CRCA water absorption
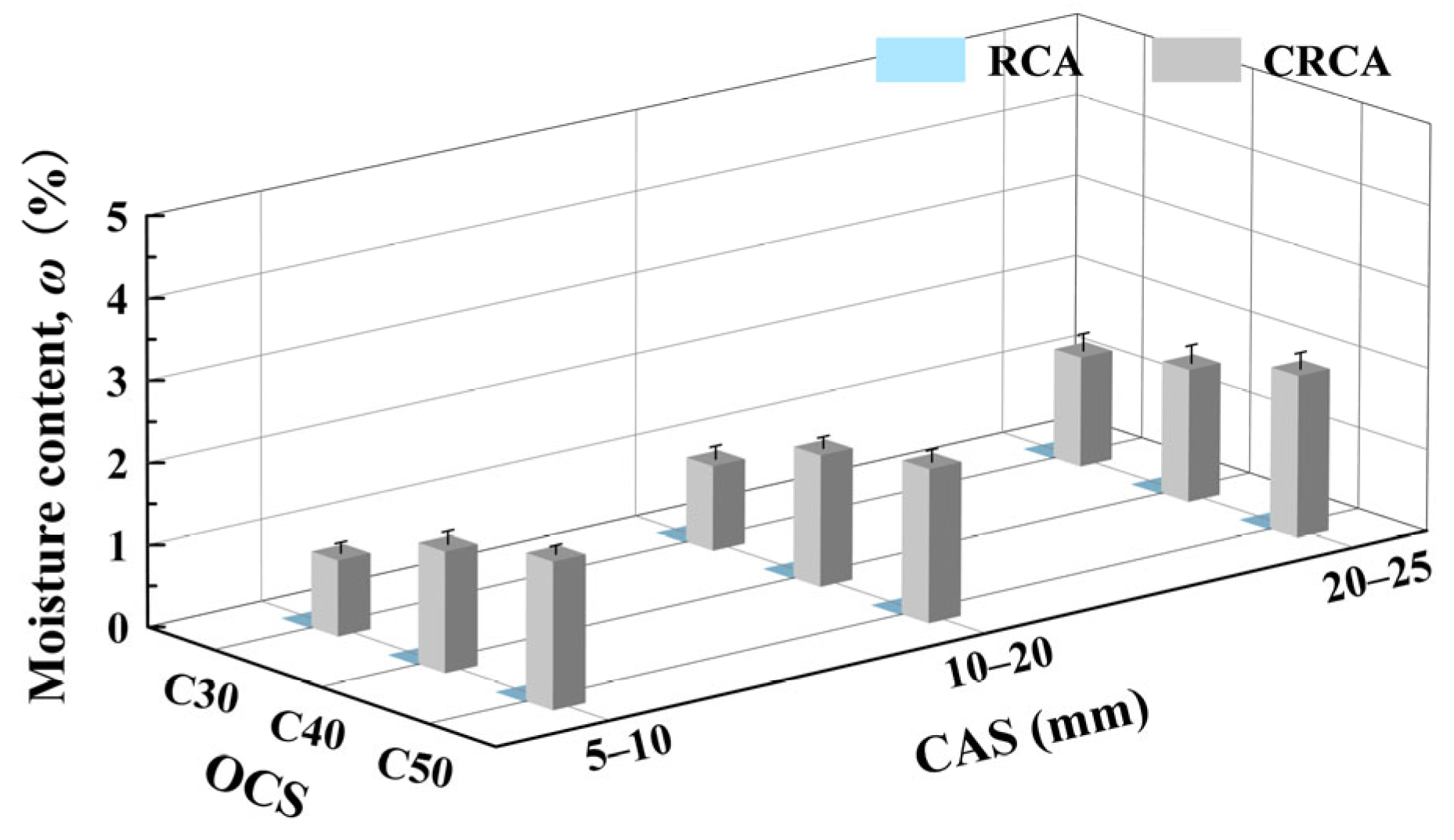
3.3. Effect of OCSs and CASs on Moisture Content of CRCAs
- (1)
- Effect of OCS on CRCA moisture content
- (2)
- Effect of CAS on CRCA moisture content
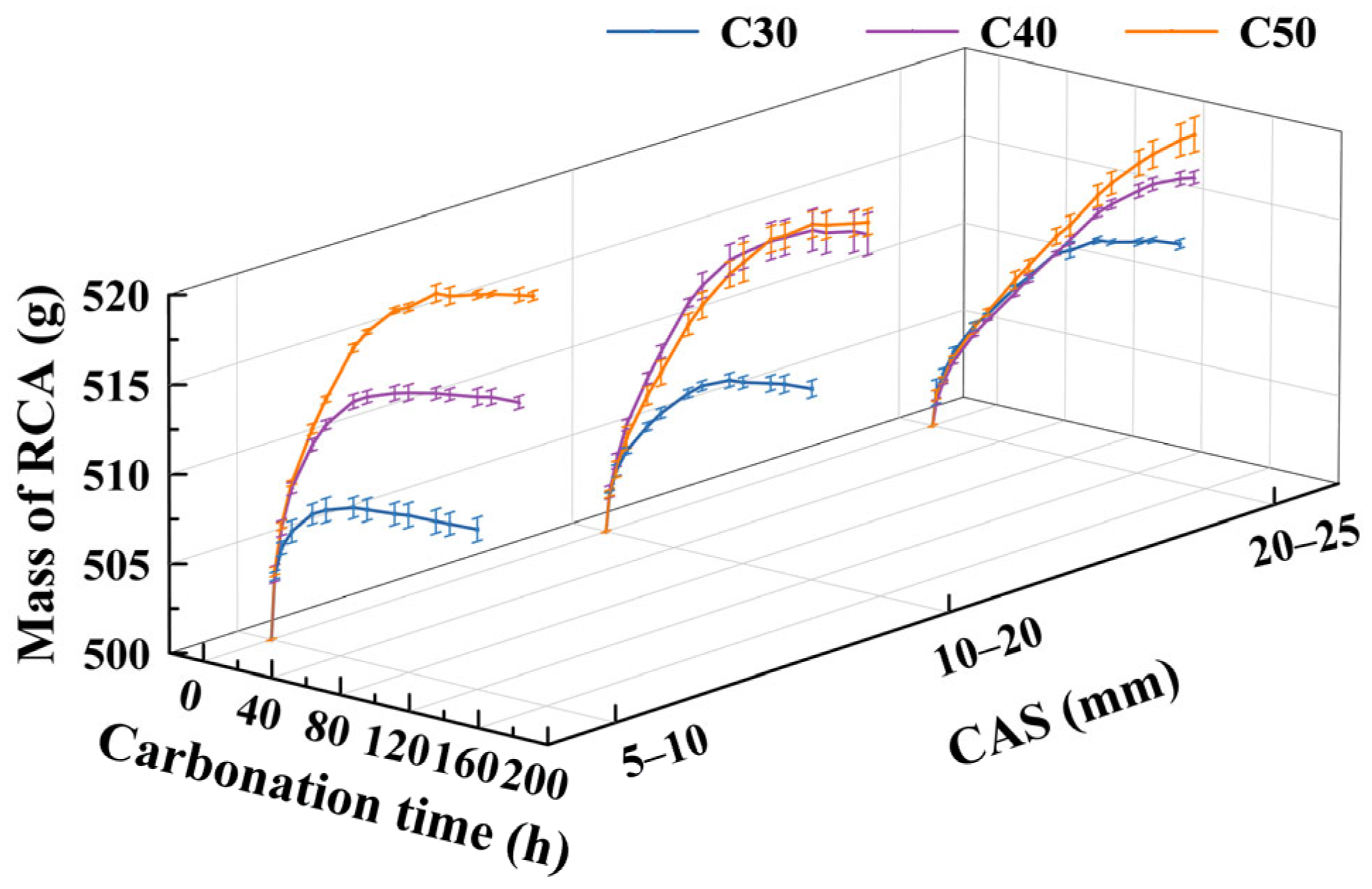
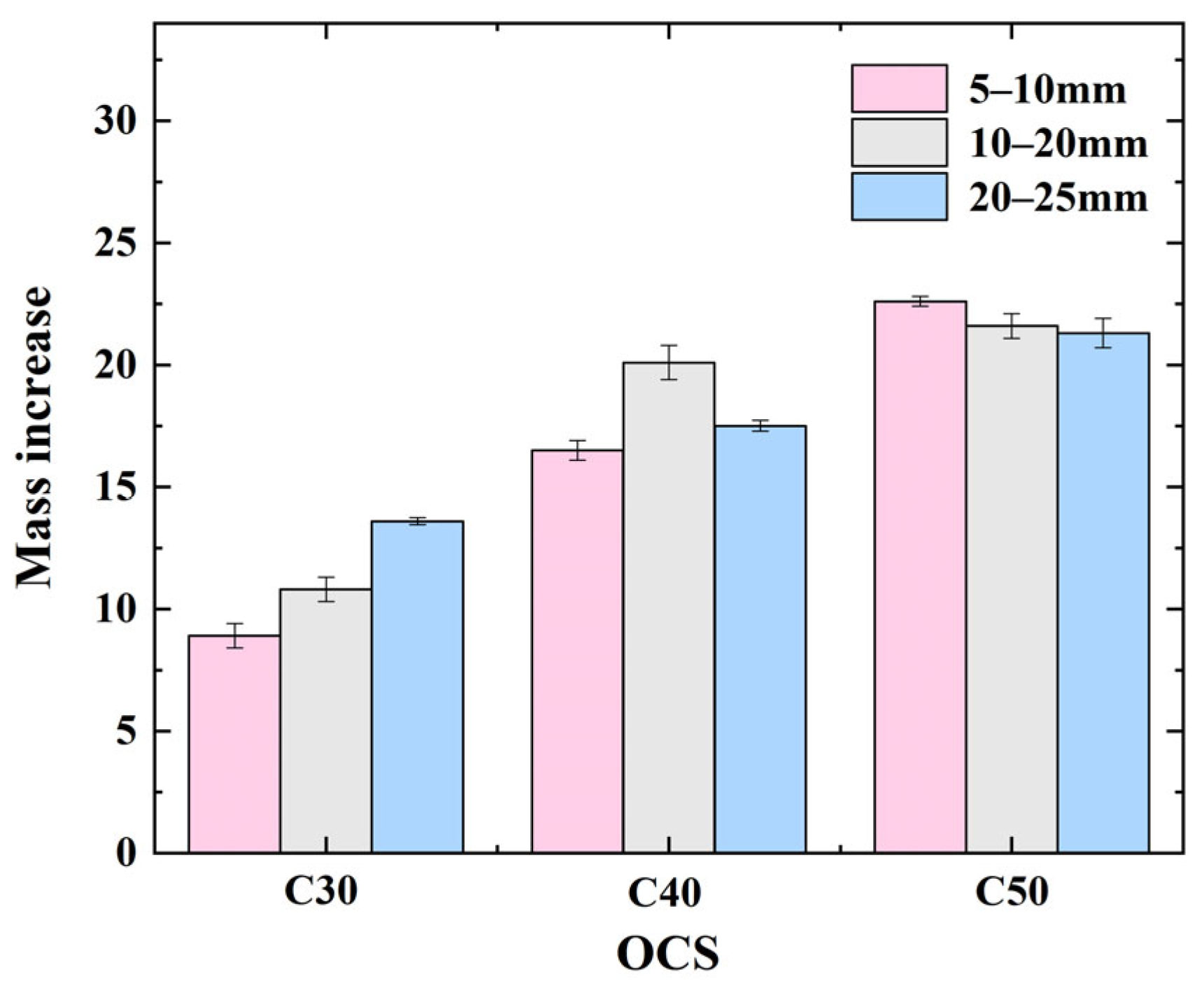
3.4. Effect of OCS and CAS on Mass Variation of CRCAs
- (1)
- Effect of OCSs on CRCA mass variation
- (2)
- Effect of CASs on CRCA mass variation
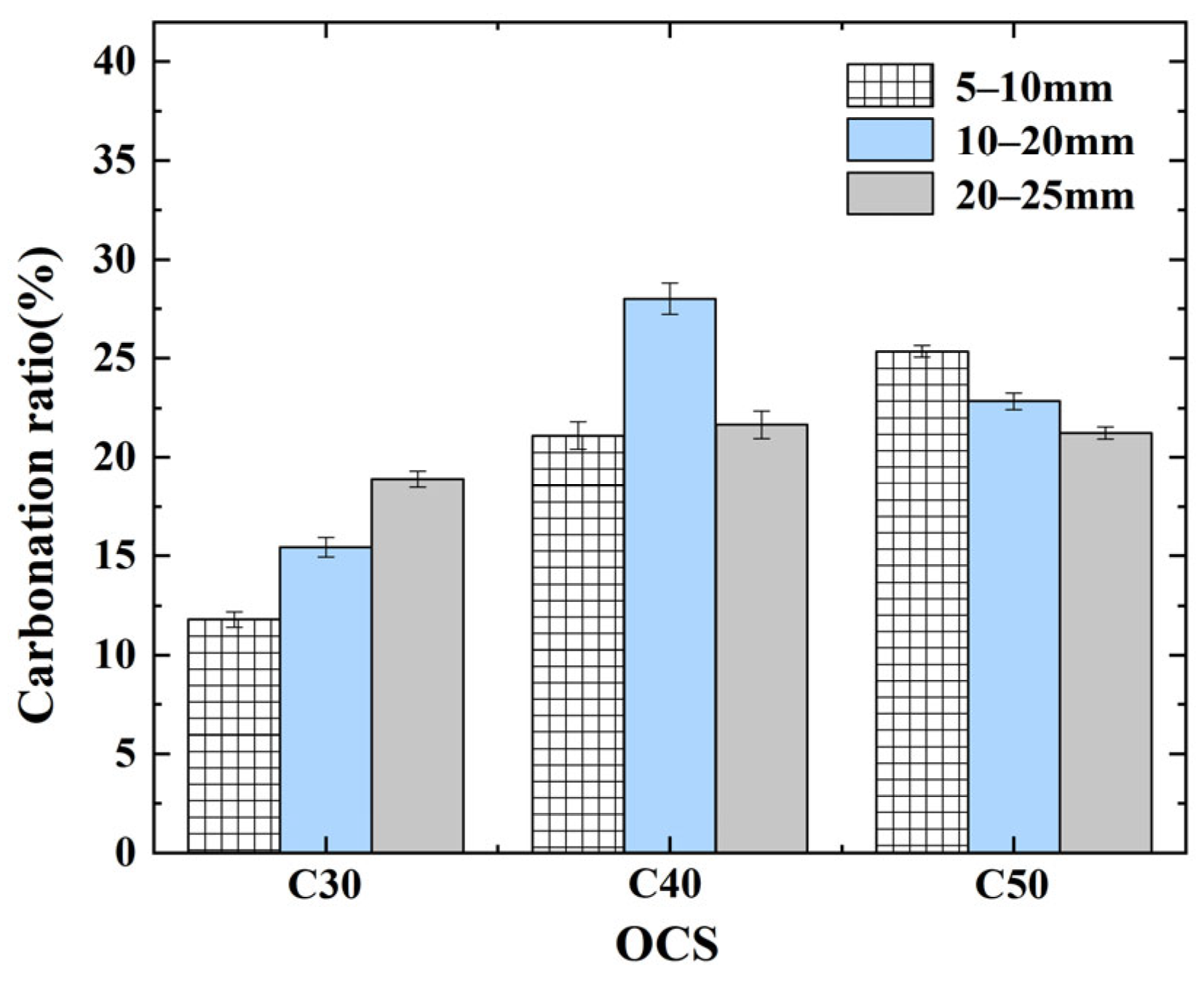
3.5. Effect of OCS and CAS on Carbonation Ratio of CRCAs
- (1)
- Effect of OCSs on CRCA carbonation ratio
- (2)
- Effect of CAS on CRCA carbonation ratio
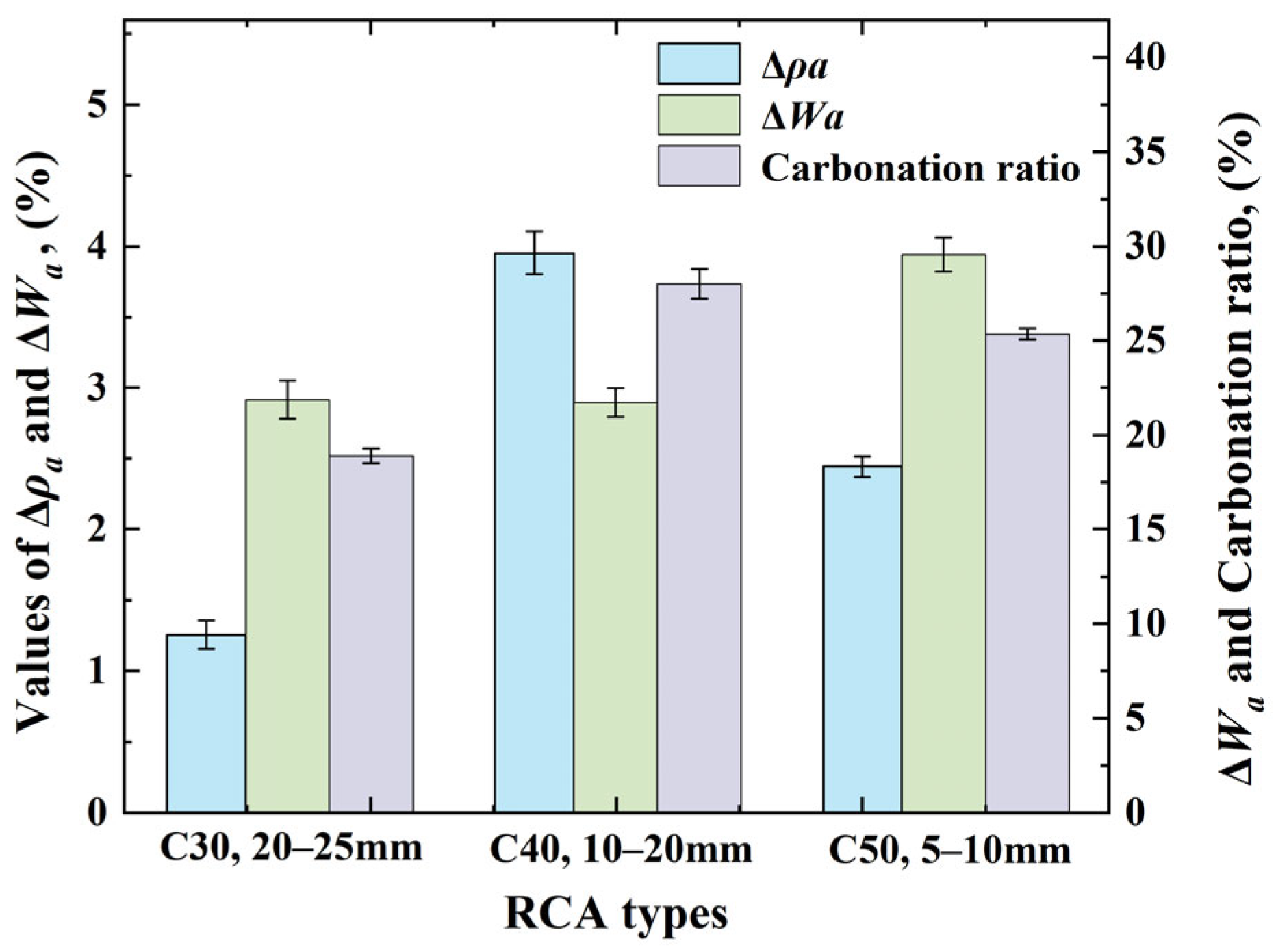
3.6. The Best Property Enhancements of CRCAs
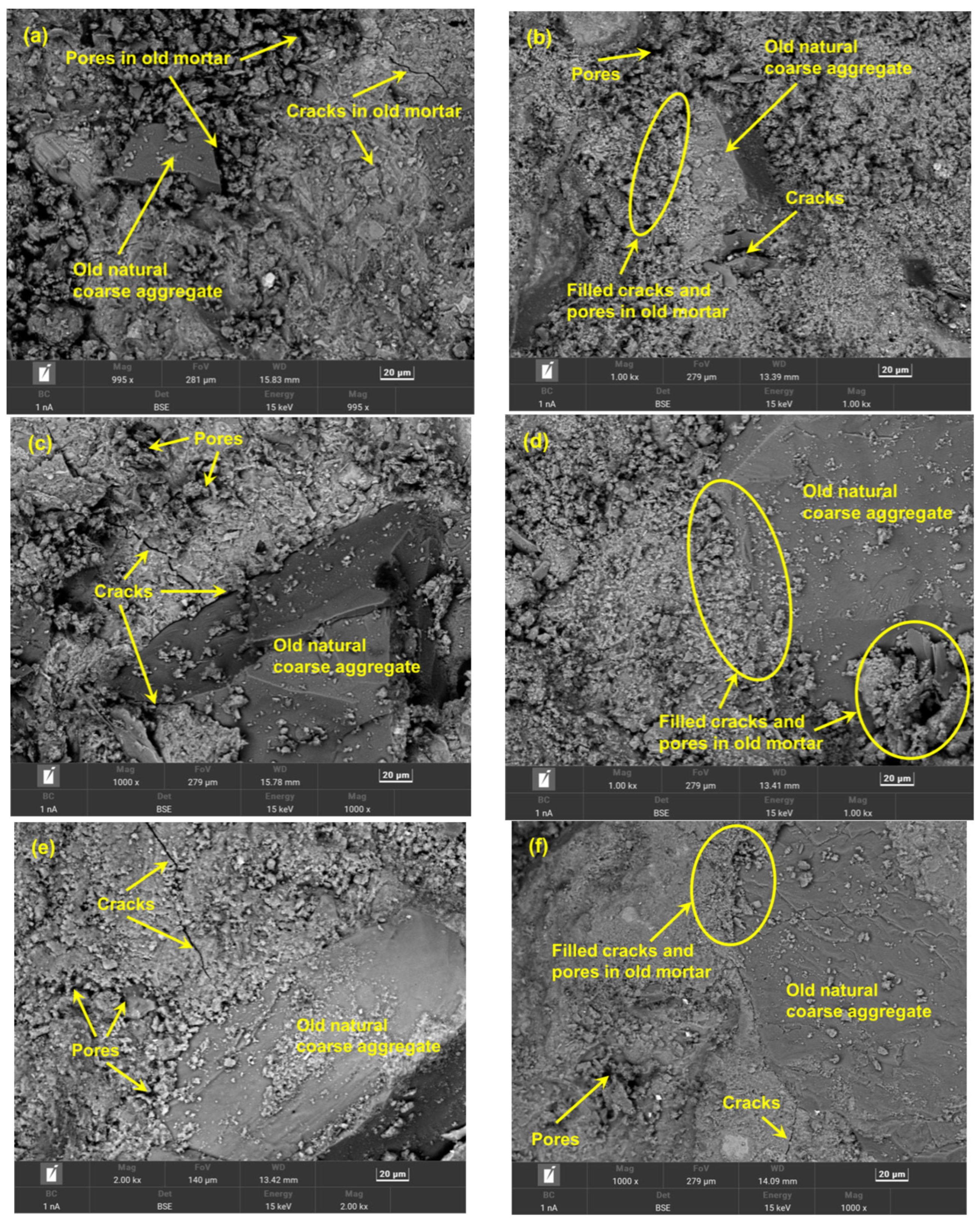
3.7. Microstructure (SEM Analysis) of the RCAs before and after Carbonation
4. Conclusions
- (1)
- The CRCAs obtained via the CO2-accelerated carbonization of RCAs with various OCSs and CASs were improved to varying degrees in terms of apparent density, water absorption, quality change, and carbonization ratio. This indicates that the CO2-based accelerated carbonization method can improve the performance of RCAs.
- (2)
- Based on the degree of improvement in the performance indexes, such as apparent density, water absorption, mass change, and carbonation ratio, before and after the accelerated carbonation of RCA specimens, the OCSs and CASs with the optimal effect of RCA carbonation modification were clarified as follows: C30 and 20–25 mm, C40 and 10–20 mm, and C50 and 5–10 mm, respectively.
- (3)
- Through the comparative analysis of the macroscopic properties and microscopic morphology of RCAs with optimal carbonation modification effects, it was finally determined that, when the OCS is C40 and the CAS is 10–20 mm, the performance improvement of RCAs is higher than that of C30 and 20–25 mm and C50 and 5–10 mm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, T.M.; Nascimento, C.F.G.; Teixeira, I.A.R.; Lima, K.P.B.A.; Fernandes, I.V.; Oliveira, M.C.B.M.; Monteiro, E.C.B.; Neto, A.A.M.; Delgado, J.M.P.Q. Enhancing the physical performance of concrete containing construction and demolition waste against the effects of accelerated carbonation and chloride ingression. J. Build. Pathol. Rehabil. 2024, 9, 84. [Google Scholar] [CrossRef]
- Lima, C.; Caggiano, A.; Faella, C.; Martinelli, E.; Pepe, M.; Realfonzo, R. Physical properties and mechanical behaviour of concrete made with recycled aggregates and fly ash. Constr. Build. Mater. 2013, 47, 47547–47559. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A.; Zhang, Z.; Dai, J.-G.; Liu, K.; Chu, Y.; Guan, Y.; Sun, D. Enhancing the performance of alkali-activated material based coral concrete through microbubble aeration clean technology. Compos. Part B Eng. 2023, 252, 110519. [Google Scholar] [CrossRef]
- Lu, S.; Xia, W.; Bai, E.; Ling, L.; Du, Y. Interfacial modification: The dynamic compression properties and enhancement mechanism of concrete added with micro-nano hierarchical carbon-based fiber. Compos. Part B Eng. 2022, 247, 110340. [Google Scholar] [CrossRef]
- Qin, J.; Dai, F.; Ma, H.; Dai, X.; Li, Z.; Jia, X.; Qian, J. Development and characterization of magnesium phosphate cement based ultra-high performance concrete. Compos. Part B Eng. 2022, 234, 109694. [Google Scholar] [CrossRef]
- Saravanan, S.S.; Jagadeesh, P. Effect of manufactured sand on the durability characteristics of concrete. Carbon Sci. Technol. 2016, 8, 70–81. [Google Scholar]
- Li, H.; Guo, Q.; Wang, J.; Zhang, K. A review on interfacial structure and durability of recycled concrete. Mater. Her. 2020, 34, 13050–13057. [Google Scholar]
- Jonny, N. Smart materials and technologies for sustainable concrete construction. Dev. Built Environ. 2023, 15, 100177. [Google Scholar]
- Stochino, F.; Alibeigibeni, A.; Milia, A.; Zucca, M.; Pani, L.; Simoncelli, M. Environmental and Economic Analysis of Using Recycled Concrete Aggregates in Composite Steel-Concrete Slabs. In Computational Science and Its Applications—ICCSA 2023 Workshops. ICCSA 2023; Gervasi, O., Murgante, B., Rocha, A.M.A.C., Garau, C., Scorza, F., Karaca, Y., Torre, C.M., Eds.; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2023; Volume 14110. [Google Scholar] [CrossRef]
- Ouyang, K.; Liu, J.; Liu, S.; Song, B.; Guo, H.; Li, G.; Shi, C. Influence of pre-treatment methods for recycled concrete aggregate on the performance of recycled concrete: A review. Resour. Conserv. Recycl. 2023, 188, 106717. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Wang, Q.; Shi, X.; Fu, L.; Zhang, G.; Luan, C.; Abomohra, A.E.-F. Accelerated carbonation treatment of recycled concrete aggregates using flue gas: A comparative study towards performance improvement. J. CO2 Util. 2021, 43, 101362. [Google Scholar] [CrossRef]
- Khedmati, M.; Kim, Y.-R.; Turner, J.A. Investigation of the interphase between recycled aggregates and cementitious binding materials using integrated microstructural-nanomechanical-chemical characterization. Compos. Part B Eng. 2019, 158, 218–229. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon Dioxide Uptake by Cement-Based Materials: A Spanish Case Study. Appl. Sci. 2020, 10, 339. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon Dioxide Uptake by Mortars and Concretes Made with Portuguese Cements. Appl. Sci. 2020, 10, 646. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, B.; Liang, C.; Xiao, J.; He, J. Characterization of CO2 reinforced recycled aggregates and its effect on the performance of recycled concrete. J. Civ. Environ. Eng. (Chin. Engl.) 2021, 43, 95–102. [Google Scholar]
- Gao, Y.; Jiang, Y.; Tao, Y.; Shen, P.; Poon, C.S. Accelerated carbonation of recycled concrete aggregate in semi-wet environments: A promising technique for CO2 utilization. Cem. Concr. Res. 2024, 180, 107486. [Google Scholar] [CrossRef]
- Zhu, P.; Li, H.; Liu, H.; Yan, X.; Wang, X.; Chen, C. Effect of CO2 Curing on the Physical Properties of Recycled Coarse Aggregate with Different Attached Mortar Contents. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2022, 37, 905–911. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Chung, C.W.; Wang, S.; Lee, M. Accelerated Carbonation of Recycled Aggregates Using the Pressurized Supercritical Carbon Dioxide Sparging Process. Minerals 2020, 10, 486. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, K.; Wang, S.; Wang, L.; Yang, W.; Qian, C. Preparation Technology and Properties of Carbon-Reinforced Recycled Aggregate Concrete and Mortar. Sci. Adv. Mater. 2022, 14, 1692–1701. [Google Scholar] [CrossRef]
- Ni, S.; Liu, H.; Li, Q.; Quan, H.; Gheibi, M.; Fathollahi-Fard, A.M.; Tian, G. Assessment of the engineering properties, carbon dioxide emission and economic of biomass recycled aggregate concrete: A novel approach for building green concretes. J. Clean. Prod. 2022, 365, 132780. [Google Scholar] [CrossRef]
- Prentice, D.P.; AlShareedah, O.; Sarkar, M.; Arabit, J.; Mehdipour, I.; Afzal, S.; Luo, J.; Abdullah, F.; Yun, S.; Christofides, P.D.; et al. Process modeling guides operational variables that affect CO2 utilization during the accelerated carbonation of concrete. AIChE J. 2024, 70, e18387. [Google Scholar] [CrossRef]
- Russo, N.; Lollini, F. Effect of carbonated recycled coarse aggregates on the mechanical and durability properties of concrete. J. Build. Eng. 2022, 51, 104290. [Google Scholar] [CrossRef]
- Toshifumi, K.; Yasuhiro, K. Carbon Dioxide Uptake in Demolished and Crushed Concrete. J. Adv. Concr. Technol. 2011, 9, 115–124. [Google Scholar]
- Thiery, M.; Dangla, P.; Belin, P.; Habert, G.; Roussel, N. Carbonation kinetics of a bed of recycled concrete aggregates: A laboratory study on model materials. Cem. Concr. Res. 2013, 46, 50–65. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Jiang, H.; Ju, X.; Guan, L.; Liu, H.; Chen, S. Synergistic Effects of Environmental Relative Humidity and Initial Water Content of Recycled Concrete Aggregate on the Improvement in Properties via Carbonation Reactions. Materials 2023, 16, 5251. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Wu, L.; Liu, M.; Jiang, H.; Zhang, W.; Guan, L.; Chen, X.; Fan, X. Influence of the Original Concrete Strength and Initial Moisture Condition on the Properties Improvement of Recycled Coarse Aggregate via Accelerated Carbonation Reactions. Materials 2024, 17, 706. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J. Effect of curing parameters on CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 71, 122–130. [Google Scholar] [CrossRef]
- Mu, X.; Xiao, E.; Xu, J. Analysis about the Properties Impact of the Recycled Aggregate which were Caused by Different Produced Methods and Original Concrete. Appl. Mech. Mater. 2013, 2746, 1081–1084. [Google Scholar]
- Pan, G.; Zhan, M.; Fu, M.; Wang, Y.; Lu, X. Effect of CO2 curing on demolition recycled fine aggregates enhanced by calcium hydroxide pre-soaking. Constr. Build. Mater. 2017, 154, 810–818. [Google Scholar] [CrossRef]
- Etxeberria, M.; Vázquez, E.; Marí, A.; Barra, M. Influence of amount of recycled coarse aggregates and production process on properties of recycled aggregate concrete. Cem. Concr. Res. 2007, 37, 735–742. [Google Scholar] [CrossRef]
- Hyvert, N.; Sellier, A.; Duprat, F.; Rougeau, P.; Francisco, P. Dependency of C–S–H carbonation rate on CO2 pressure to explain transition from accelerated tests to natural carbonation. Cem. Concr. Res. 2010, 40, 1582–1589. [Google Scholar] [CrossRef]
- Wu, L.; Guan, L.; Yang, X.; Li, T.; Hu, H.; Yuan, X. Effect of carbonated recycled aggregate on mechanical strength and durability performance of port recycled concrete. Waterw. Port 2022, 43, 370–376. [Google Scholar]
- Zhan, B.; Poon, C.S.; Liu, Q.; Kou, S.; Shi, C. Experimental study on CO2 curing for enhancement of recycled aggregate properties. Constr. Build. Mater. 2014, 67, 3–7. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Development of a new generation of eco-friendly concrete blocks by accelerated mineral carbonation. J. Clean. Prod. 2016, 133, 1235–1241. [Google Scholar] [CrossRef]
- Fang, X.; Xuan, D.; Poon, C.S. Empirical modelling of CO2 uptake by recycled concrete aggregates under accelerated carbonation conditions. Mater. Struct. 2017, 50, 1–13. [Google Scholar] [CrossRef]
- JTS 202-2011; Specifications for Concrete Construction of Port and Waterway Engineering. Chinese Ministry of Communications: Beijing, China, 2011. (In Chinese)
- JTS 151-2011; Design Code for Concrete Structures of Port and Waterway Engineering. Chinese Ministry of Communications: Beijing, China, 2011. (In Chinese)
- Yang, S.; Gu, M.; Lin, H.; Gong, Y. Property Improvement of Recycled Coarse Aggregate by Accelerated Carbonation Treatment under Different Curing Conditions. Sustainability 2023, 15, 4908. [Google Scholar] [CrossRef]
- GB/T 14685-2011; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Pebbles and Gravel for Construction. China Standard Press: Beijing, China, 2011.
- Song, Y.; Dong, M.; Wang, Z.; Qian, X.; Yan, D.; Shen, S.; Zhang, L.; Sun, G.; Lai, J.; Ruan, S. Effects of red mud on workability and mechanical properties of autoclaved aerated concrete (AAC). J. Build. Eng. 2022, 61, 105238. [Google Scholar] [CrossRef]


| Strength Grade | w/c | Components of Raw Materials (kg/m3) | |||
|---|---|---|---|---|---|
| Water | Cement | Coarse Aggregate | Fine Aggregate | ||
| C50 | 0.4 | 195 | 488 | 1134 | 584 |
| C40 | 0.5 | 195 | 390 | 1198 | 617 |
| C30 | 0.6 | 195 | 325 | 1241 | 639 |
| Specimen Properties | Original Concrete Strength, OCS (MPa) | Coarse Aggregate Size, CAS (mm) |
|---|---|---|
| SP-30-10 | C30 | 5–10 |
| SP-30-20 | 10–20 | |
| SP-30-25 | 20–25 | |
| SP-40-10 | C40 | 5–10 |
| SP-40-20 | 10–20 | |
| SP-40-25 | 20–25 | |
| SP-50-10 | C50 | 5–10 |
| SP-50-20 | 10–20 | |
| SP-50-25 | 20–25 |
| Types of CA | OCS (MPa) | CAS (mm) | ρa (kg/m3) | Wa (%) |
|---|---|---|---|---|
| NCA | — | 5–10 | 2682 | 0.34 |
| 10–20 | 2710 | 0.33 | ||
| 20–25 | 2736 | 0.31 | ||
| RCA | C30 | 5–10 | 2549 | 5.76 |
| 10–20 | 2495 | 5.15 | ||
| 20–25 | 2470 | 5.12 | ||
| C40 | 5–10 | 2521 | 5.90 | |
| 10–20 | 2479 | 5.34 | ||
| 20–25 | 2453 | 5.30 | ||
| C50 | 5–10 | 2497 | 6.19 | |
| 10–20 | 2450 | 5.50 | ||
| 20–25 | 2430 | 5.39 |
| OCS | Δρa (%) | ΔWa (%) | Mass Variation (g) | Carbonation Ratio (%) |
|---|---|---|---|---|
| C30 | 10–20 mm | 20–25 mm | 20–25 mm | 20–25 mm |
| C40 | 10–20 mm | 10–20 mm | 10–20 mm | 10–20 mm |
| C50 | 10–20 mm | 5–10 mm | 5–10 mm | 5–10 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, W.; Fan, X.; Jiang, X. CO2-Accelerated Carbonation Modification for Recycled Coarse Aggregate with Various Original Concrete Strengths and Coarse Aggregate Sizes. Materials 2024, 17, 3567. https://doi.org/10.3390/ma17143567
Qin W, Fan X, Jiang X. CO2-Accelerated Carbonation Modification for Recycled Coarse Aggregate with Various Original Concrete Strengths and Coarse Aggregate Sizes. Materials. 2024; 17(14):3567. https://doi.org/10.3390/ma17143567
Chicago/Turabian StyleQin, Wei, Xinhui Fan, and Xiaohui Jiang. 2024. "CO2-Accelerated Carbonation Modification for Recycled Coarse Aggregate with Various Original Concrete Strengths and Coarse Aggregate Sizes" Materials 17, no. 14: 3567. https://doi.org/10.3390/ma17143567





