Abstract
This is an overview of recent findings on the structural changes observed upon heating, including crystallization processes in conventional metallic glasses, bulk metallic glasses, and their corresponding supercooled liquids. This paper encapsulates the various crystallization behaviors in metallic glasses by primary, eutectic, and polymorphous mechanisms, highlighting the complexity and diversity of the nucleation and growth mechanisms involved. Mechanically induced room-temperature crystallization is also discussed.
1. Introduction
For a long time, the predominant structure type of bulk engineering metallic alloys has been crystalline. An Au-Si alloy formed an amorphous structure after rapid solidification at an exceptionally high cooling rate of 106 K/s [1]. Later, Pd-Cu-Si [2] and Pd-Ni-P [3] were found to be the best metallic glass formers, produced in bulk via flux treatment to suppress heterogeneous nucleation. In the last 15 years, advancements have enabled the production of bulk metallic glasses with dimensions of at least 1 mm in every spatial dimension, sparking widespread scientific interest and leading to specialized international conferences [4,5]. Bulk metallic glasses [6,7] have a unique supercooled liquid state that inhibits crystalline nucleation, allowing for the production of samples with thicknesses of 1 to 100 mm through various casting processes [8]. They are metastable at room temperature and undergo devitrification/crystallization upon heating [8], irradiation [9,10], or intensive mechanical impact at room temperature [11], forming nanostructures and enhancing mechanical properties. This process is now a common method for producing metallic nanomaterials with superior properties, especially magnetic properties. Some special attention is paid to nearly equiatomic compositions called high-entropy metallic glasses [12,13], some of which show a glass-to-glass transition [14,15].
Most metallic glasses are crystal nucleation-controlled while some of them contain crystal nuclei, and thus, are growth controlled. For example, Pd42.5Cu30Ni7.5P20 is a true bulk glass former containing no crystalline particles or nuclei, while Pt42.5Cu27Ni9.5P21 is a crystal growth-controlled bulk glass former containing nanoparticles around 1 nm in size of a different chemical composition [16]. These structural distinctions influence their room-temperature mechanical properties: the latter alloy is significantly more ductile in compression than the former one.
Of course, there are instances when the glass-forming ability of an alloy is insufficient and a crystal–glass composite is formed, or when heterogeneous nucleation occurs on an oxide inclusion. However, when the volume fraction of crystals is low, they typically do not affect the properties unless the oxide inclusions act as stress concentrators. This is quite common in lanthanide-based alloys with a high affinity for oxygen [17].
2. Glass Formation from Liquids
Formation of glasses takes place by rapid enough cooling of a liquid from above its liquidus temperature (Tl) to below the glass-transition temperature (Tg) when it solidifies (vitrifies) without crystallization [18]. The glass transition process is a complex phenomenon [19], and its consideration is beyond the scope of this review. Many theories describe the glass transition [20,21]. It appears as a second-order phase transformation, with continuity in material volume and entropy, but discontinuity in their derivatives [22]. However, there are reasons to suggest that the glass transition could be a first-order transition [23,24].
Among various factors influencing the glass-forming ability of a liquid alloy, such as the reduced glass-transition temperature (Trg = Tg/Tl), one can particularly mention its fragility: deviation from the Arrhenius law for the temperature dependence of the viscosity (η) [25,26]:
where η0 is a pre-exponential factor, R is the gas constant and Ea is an activation energy for viscous flow. The viscosity of an equilibrium liquid exhibits an Arrhenius temperature dependence with a constant activation energy at a moderately high temperature above TA (deviation from the Arrhenius law temperature), but Ea increases on cooling of all supercooled liquids (both strong and fragile but to varying degrees). The origin of liquid fragility is under scientific investigation. One approach indicates that the structural changes in the supercooled liquid are responsible for liquid fragility [27]. Here, deviation of the temperature dependence of viscosity of a supercooled liquid from the Arrhenius equation takes place through modification of the activation energy by structural changes in the liquid. Other authors suggest that at a lower temperature, the activation energy increases due to cooperative dynamics among atoms as the time for local structural changes becomes comparable to the phonon lifetime, leading to super-Arrhenius behavior [28]. Here one should mention that at ultrahigh temperatures, even in supercritical liquids under supercritical pressure when boiling is suppressed, the viscosity starts to rise again [29]. The electronic structure was also reported to be responsible for liquid fragility [30]. The effects of external pressure on shear viscosity were also studied [31].
3. Structural Relaxation and Phase Separation on Heating Prior to Crystallization
Metallic glasses exhibit complex relaxation phenomena upon heating. Relaxation can be divided into: (a) slow, high-temperature α-relaxation, involving the entire glass and responsible for the glass–liquid transition and vice versa [32], and (b) fast, low-temperature β-relaxation [33], in localized regions, responsible for mechanical properties at room temperature. Here, relaxation, in general, can be defined as the time-dependent response to external actions like mechanical loading at a certain temperature and can be reversible on cooling and heating. Structural relaxation involves gradual structural changes (densification) toward a more stable glass on heating, starting at the beginning of relaxation temperature (Tbr). This is irreversible below Tg, and the sample must be heated up to the supercooled liquid to restore the excess volume on subsequent cooling.
Usually, structural relaxation [34] leads to embrittlement of the glasses. However, low-temperature annealing at temperatures close to Tbr can lead to the opposite. For example, despite expectations of embrittlement, the Cu36Zr48Al8Ag8 bulk glassy samples annealed at a low temperature of 200 °C exhibited ductilization rather than embrittlement [35]. The annealing process induces structural relaxation, leading to densification and stress release, resulting in reduced hardness but increased room-temperature compressive stress and plasticity. However, high-temperature annealing near Tg led to a reduction in plasticity. Fracture surfaces exhibited dimple patterns in as-cast samples and vein patterns in annealed samples, suggesting differences in crack propagation speed and stress states. The reason for ductilization could have been phase separation, but the atomic-scale elemental mapping showed no phase separation at this temperature [35]. Phase separation in this alloy takes place at a higher temperature. Structural relaxation is mostly a topological process leading to the densification of the glassy phase, while phase separation is related to the changes in local chemistry.
Spinodal phase separation into two glassy phases was observed in the Zr-Y-Al-Ni [36] system and Zr-La-Al-Ni-Cu [37] system alloys. Phase-separated areas crystallize at different temperatures. For example, a residual globular glassy phase in the Cu35Zr45Ag20 alloy annealed at 722 K for 1 ks retained its glassy structure while the residual glassy matrix crystallized [38]. Phase separation was also observed in Zr-Cu-Fe-Al alloys on heating or deformation [39] and prevented embrittlement of the glassy samples after relaxation [40]. When the binodal phase separation mechanism is activated, then the separated particles precipitate as far from each other as possible in the glassy matrix and enable tensile ductility in the Zr-Cu-Fe-Al BMG after thermomechanical treatment [41]. In these alloys, phase separation occurred owing to repulsive interaction between Zr-Y, Zr-La, Cu-Ag, and Cu-Fe atomic pairs. Phase separation was also found in a Zr52.5Ti5Cu17.9Ni14.6Al10 glassy alloy without immiscible elements [42].
4. Crystal Nucleation and Initial Crystallization Stage
The glasses, once formed, retain their structure without crystallization for a long time. Recent results on metallic glasses stored at room temperature for at least 15 years showed that most of them retained their initial structure with only a moderate decrease in crystallization temperature (unless severely oxidized), confirming their high stability at ambient conditions [43]. The alloys with low Tg like Ce-based ones were reported to increase their stability in long-term aging [44]. However, all metallic glasses crystallize on heating.
The mystery surrounding crystal nucleation in liquids and glasses stems from several complex factors. Crystal nucleation occurs on a tiny scale and at rapid timescales, making it challenging to directly observe or capture in real-time experiments that limit our understanding of the initial stages of nucleation. Here computer simulation is a procedure that can help. Modeling and simulating nucleation processes computationally involves complexities due to the numerous atoms involved and the need to capture both short- and long-range atomic interactions accurately [45,46]. Moreover, the crystal nucleation process can be heterogeneous and homogeneous. Balancing thermodynamic barriers and kinetic stability during nucleation involves complex interactions. Due to these complexities and limitations in observation and modeling, the precise mechanisms and pathways involved in crystal nucleation in liquids and glasses remain a fascinating yet challenging area of study [47]. Thus, understanding how nucleation initiates and progresses within the context of energy landscapes and energy barriers is still an ongoing area of research [48,49].
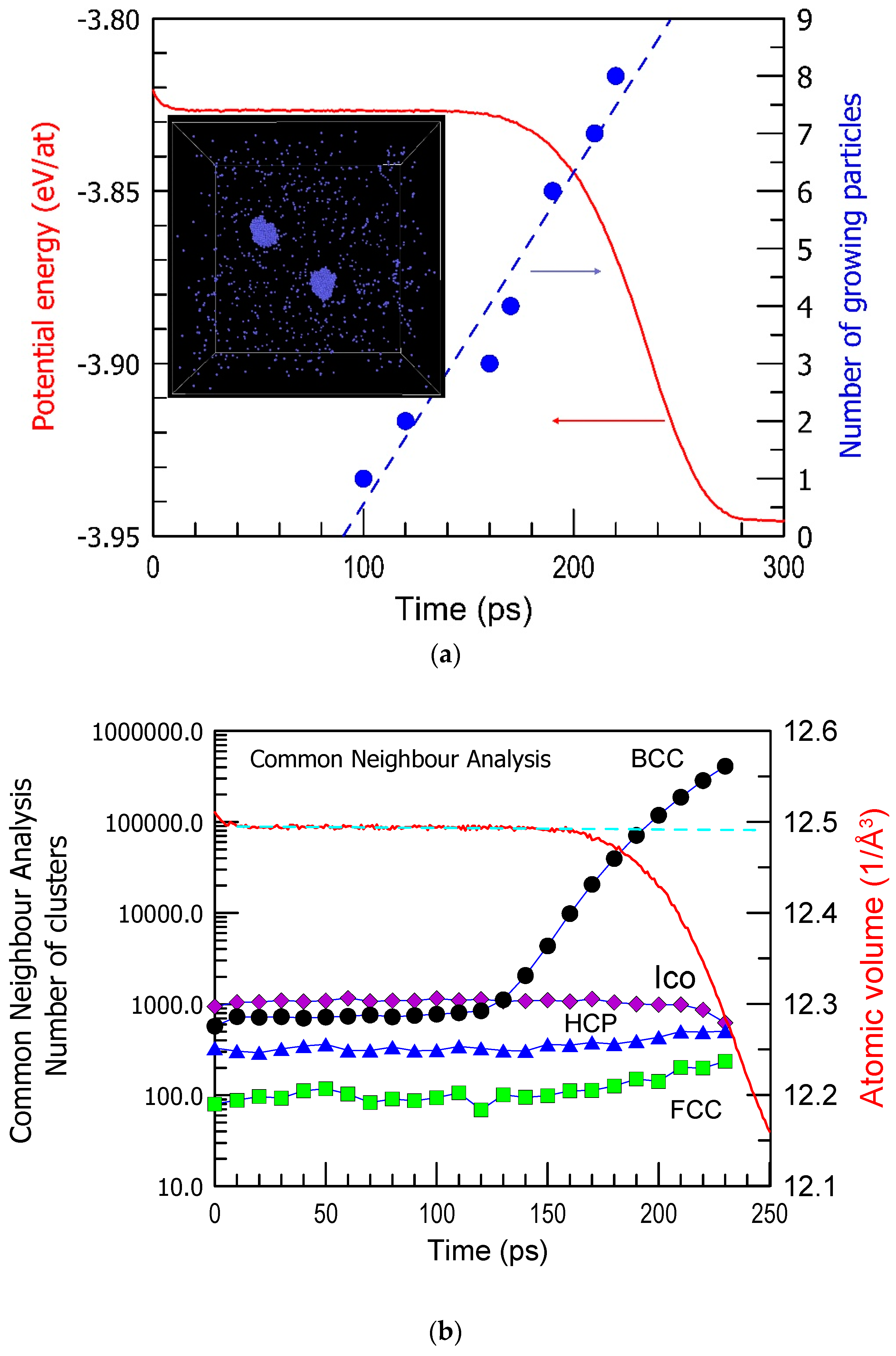
The incubation period in crystal nucleation is still an intriguing phenomenon. It refers to the time between the onset of conditions favorable for nucleation and the actual appearance of the first crystal nucleus (Figure 1a) [50]. This period is associated with a lack of observable changes, making it particularly mysterious. During the incubation period, there might be subtle, undetectable changes occurring at the atomic level, such as the formation of small clusters or structural rearrangements, which are challenging to observe directly (for example, BCC Fe in Figure 1b). The incubation period is not the only mysterious aspect of crystal nucleation, it holds significant importance in understanding the mechanisms of nucleation. Why does the system wait for a certain time with no visible changes in structure and then undergo nucleation and growth of many crystalline nuclei like in Figure 1b? What is a trigger of nucleation in various regions? Unraveling its nature remains a challenging yet essential research task in materials science and physics. It is also important for predicting the glass-forming ability of alloys, and the role of crystal–glass interfaces is emphasized [51,52]. The crystal–glass interfaces play an important role in improving the mechanical properties of metallic glasses like in situ formed composites.
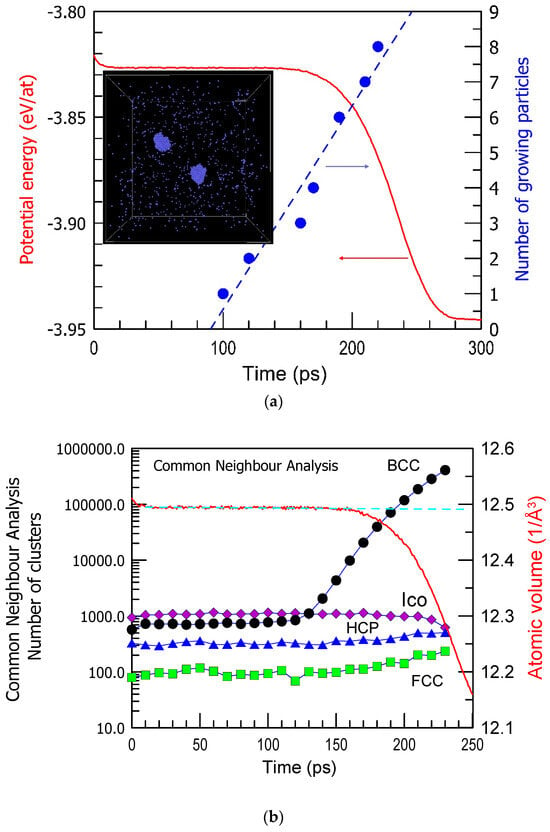
Figure 1.
(a) The number of growing BCC Fe particles and the potential energy (PE) per atom as a function of simulation time at 1100 K in a simulated cell shown in the inset (a stage at 150 ps when 2 BCC crystals are growing). The arrows indicate the corresponding axes. (b) The number of atoms belonging to FCC, BCC, HCP, and Icosahedral (Ico) clusters result from common neighbor analysis as a function of simulation time. The results are obtained from a series of computations in Ref. [50].
Crystallization of pure Cu [53,54], Ni [55], and other metals [56] was studied substantially by computer simulation and compared with the experiment [57]. The energy barriers for the crystallization of pure metals were also determined [58]. In a cell of Fe, kept at 1100 K (see Figure 1a inset), nucleation happened after the incubation period of 100 ps, and the first nucleus is observed well before the changes in the potential energy become visible. Moreover, no clear changes in the structure of a liquid are observed within the incubation period. Equilibration of a liquid at 1100 K cooled at 1013 K/s from 2500 K took about 20 ps when the potential energy of the system became stable. Yet, no structural changes in terms of the atomic clusters are seen for the next 80 ps (Figure 1b). Recent molecular dynamics studies also suggested that contrary to classical nucleation theories, liquid atoms join crystal clusters by changing their local order parameter with minimal movement and do so cooperatively rather than individually [28].
Modelling of Cu-Zr alloys showed that small additions of Zr (1–10 at.%) to Cu significantly increase incubation time and slow down crystal growth, enhancing glass formation. Detailed molecular dynamics simulations reveal that the critical cooling rate for glass formation increases substantially with Zr content, and despite low equilibrium solubility, growing FCC Cu crystals can dissolve a few atomic percent of Zr, exhibiting a concentration gradient at higher Zr contents and polymorphic growth in supersaturated alloys [59].
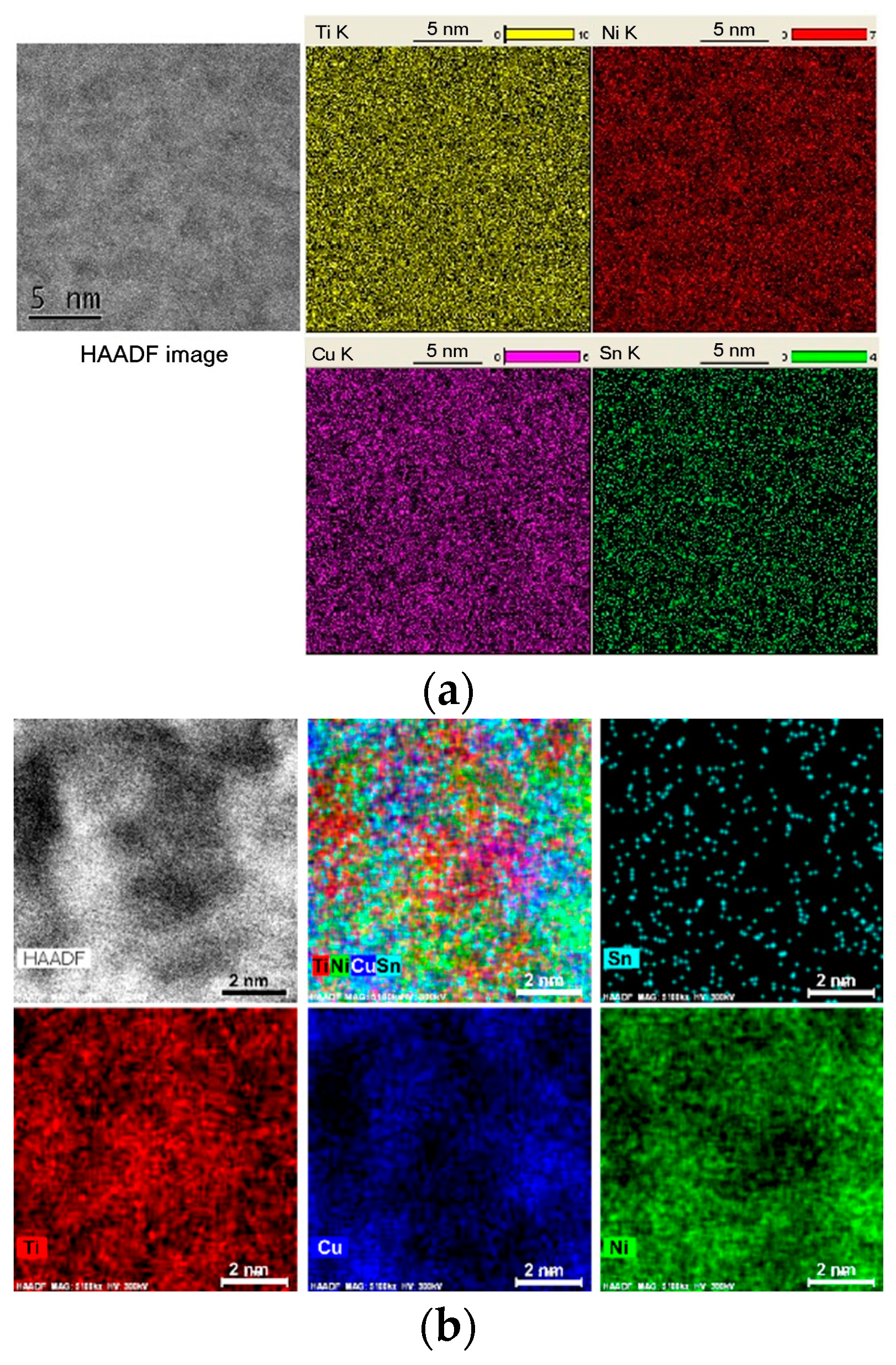
The existence of an incubation period in multicomponent metallic glasses can be explained by the time required for chemical ordering in the matrix liquid or glassy phase. The Ti50Ni23Cu22Sn5 bulk glass-forming alloy, initially homogeneously glassy, undergoes nucleation preceded by the chemical ordering of a liquid [60]. Nanoscale Ti-enriched zones and regions enriched in Ni and Cu form during the incubation period, becoming chemically ordered while remaining structurally disordered (Figure 2). This chemical ordering reduces the nucleation energy barrier for the cF96 crystalline phase, which begins to precipitate after the incubation period. The process resembles the formation of Cu-rich Guinier–Preston zones in Al-based alloys, preceding intermetallic phase formation.
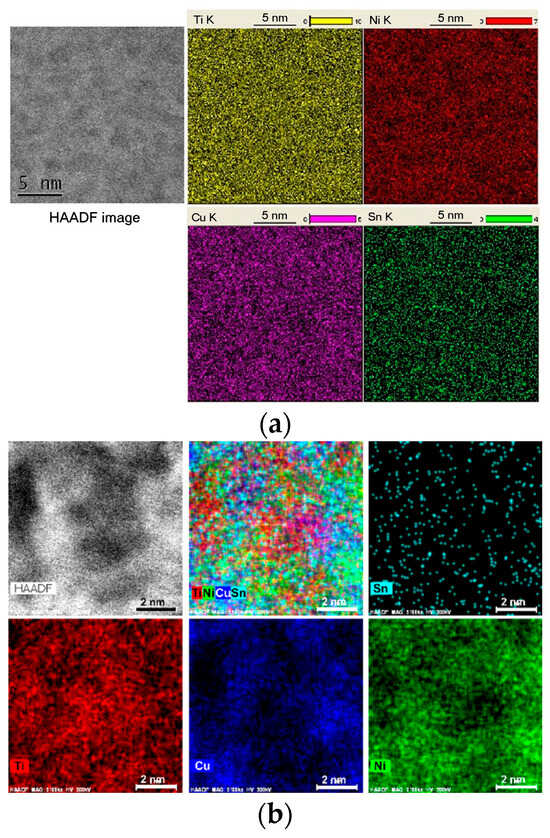
Figure 2.
High-angle annular dark-field and scanning transmission electron microscopy energy-dispersive X-ray elemental mapping (as indicated) of the as-cast Ti50Ni23Cu22Sn5 glassy alloy sample (a) and the sample annealed at 753 K for 40 s as indicated (b). Reproduced from [60] with permission of Elsevier.
5. Growth of Crystals and Kinetics of Crystallization
Crystal growth follows nucleation. The growth velocities in metallic glasses range from very low values (down to 10−1 nm/s) in the case of primary crystallization [61], up to 102 m/s in the case of pure metals [62] and chalcogenide glasses [63]. Eutectic colonies exhibit intermediate growth rate values (~100 nm/s) [64]. Fast surface-induced crystallization also takes place in some glasses [65]. A special group of metallic glassy alloys undergoes peritectic-like transformations with the participation of nanocrystalline and nanoquasicrystalline phases. This process involves the dissolution of one phase in an amorphous matrix and the release of another phase during a one-step transformation [66].
Kinetics of the crystallization processes in metallic glasses is analyzed by Kolmogorov [67]—Johnson–Mehl [68]—Avrami [69] general exponential equation for the fraction transformed x:
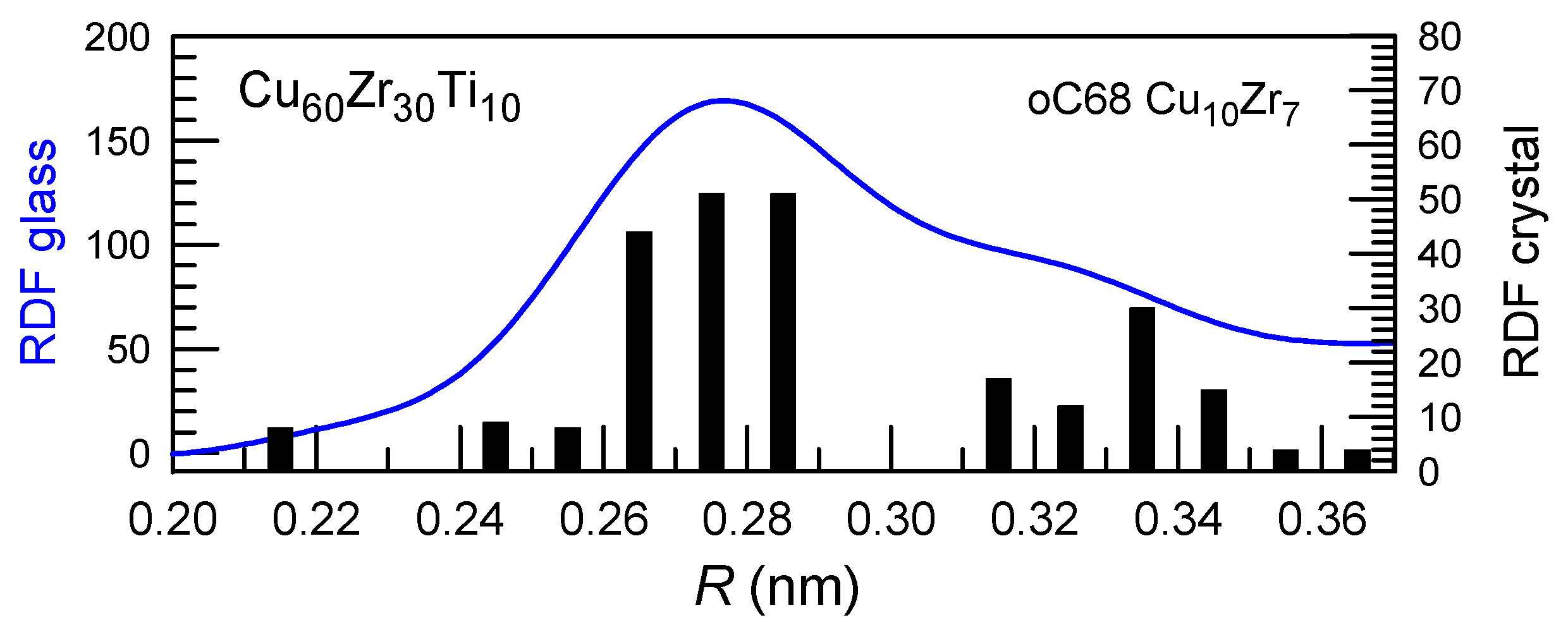
where I is the nucleation rate, u is the growth rate, and t is time. The exponent n value of about 4 corresponds to nucleation and interface-controlled growth while those close to 2.5 correspond to nucleation and diffusion-controlled growth. Usually, this law is well maintained, but one should mention that in some cases exceptionally high n exponent values are found in Cu-Zr-Al alloys like Cu50Zr45Al5 [70] and Zr45Cu49Al6/Zr46Cu46Al8 [71] with high values of n exponent in which the nucleation rate of the Cu10Zr7 phase with complex oC68 crystalline structure increases drastically with time. This crystal structure is quite close to that of some Cu-Zr-based metallic glasses (Figure 3) [72], and thus, easy nucleation with a low energy barrier could be expected.
x = 1 − exp(−(π/3)·Iu3tn)
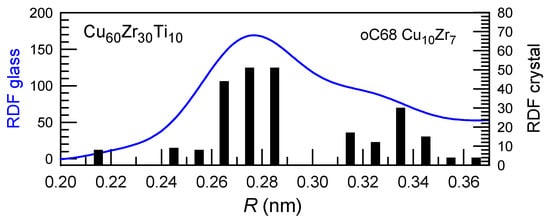
Figure 3.
Radial distribution functions (RDF) of the Cu-Zr-Ti glassy alloy in the first coordination shell (blue). Black bars show the corresponding radial distribution functions of the Cu10Zr7 intermetallic compound integrated at 0.01 nm step. Good correction is observed indicating similarities in local structures leading to a reduced energy barrier for nucleation. Reproduced from [72] with the permission of Elsevier.
The time–temperature-transformation diagrams created in the isothermal mode or under continuous heating are useful for comparison of the thermal stabilities of different glasses [73,74,75]. A comparison of the long-term thermal stabilities of different metallic glasses was performed using continuous heating transformation diagrams [76] constructed by applying a corollary from the Kissinger analysis method using the DSC data at different heating rates including ultrafast DSC [77]. Such diagrams can be recalculated from the isothermal diagrams by analogy with those for phase transformations in steels [78].
Metallic nanoglasses [79] and stabilized (ultrastable) glasses [80] have distinct crystallization mechanisms. The differences in the crystallization pathways between as-case and selectively laser-melted (SLM) metallic glasses are attributed to differences in the oxygen content. High oxygen content (~1 at%) in SLM samples reduced the thermal stability of the Zr-based BMG.
6. Fast Primary Crystallization
Nanocrystals form with higher nucleation rates and lower growth rates compared to microscale crystals, in which the nucleation rate is generally lower, and the growth rate is higher. The formation of nanocrystals often requires large undercooling. Nanocrystallization causes a high density of grains and grain boundaries due to the small size of the grains. Nanocrystallization usually occurs when the alloy is far from equilibrium, with rapid nucleation at limited atomic mobility. Once formed nanostructures are often metastable and undergo phase transformation upon heating with final transition towards microcrystalline structures. Macrocrystalline structures tend to be more stable under similar conditions.
Nanocrystals are usually formed upon primary crystallization. Glassy and partly nanocrystalline Fe-based alloys with fine Fe nanoparticles [81,82] are of high industrial importance owing to their excellent soft magnetic properties, especially at high frequencies. Their properties are constantly being improved [83]. A high crystal nucleation rate was observed in high-entropy type (Fe0.25Co0.25Ni0.25Cr0.125Mo0.125)100-xBx alloys [84].
Al-based metallic glasses exhibit the formation of primary nanoscale Al phase by diffusion-controlled growth [85,86]. They are found to contain spatial heterogeneities consisting of Al-rich regions. They contribute to an increase in the density of Al nanocrystals [87,88]. A nano-dispersed structure was obtained directly from the melt upon rapid solidification of the Al-Y-Ni-Co-Pd alloys [89]. The addition of a high amount of Y increases Tg [90]. An extremely high density of precipitates in the order of 1024 m−3 was obtained.
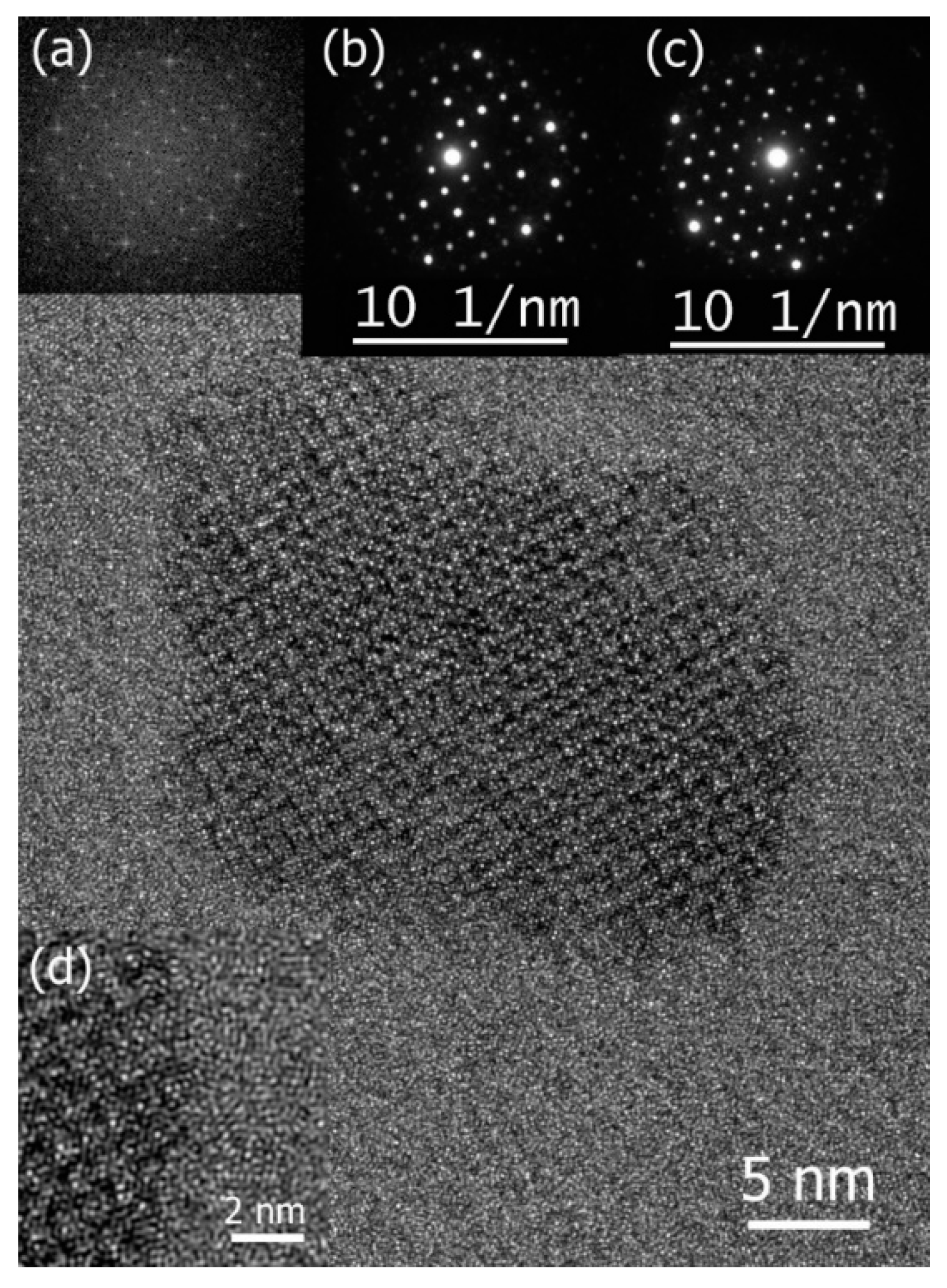
A thorough examination of the primary crystallization process within an Al-Fe-Mn-Si metallic glass [91] showed that primary crystallization occurred via homogeneous nucleation. The crystal growth rate of the alpha Al19Fe4MnSi2 phase formed in the Al68Fe10Mn4Si18 glassy alloy (Figure 4) was directly measured in TEM, allowing the estimation of the diffusion coefficient, which was compared with existing literature data. Remarkably, the diffusion of Mn, the limiting element, exhibits atomic mobility approximately three orders of magnitude faster than that in Al crystal and in the AlFe intermetallic compound. While the relatively high diffusion coefficient suggests unrealistically low viscosity, challenging the applicability of the Stokes–Einstein relationship, the absence of an endothermic signal associated with glass transition below Tx, even with Fast Differential Scanning Calorimetry (FDSC), alongside a high Trx value, suggests that Al68Fe10Mn4Si18 could be a strong glass-former that does not exhibit a glass transition upon heating below Tx. Furthermore, the significantly accelerated growth of the crystalline phase compared to estimates derived from diffusion coefficients for pure Al and the AlFe compound hints at a possible collision-limited growth regime. This accelerated growth may be facilitated by thermodynamically enhanced atomic mobility at the crystal/glass interface due to chemical potential effects.
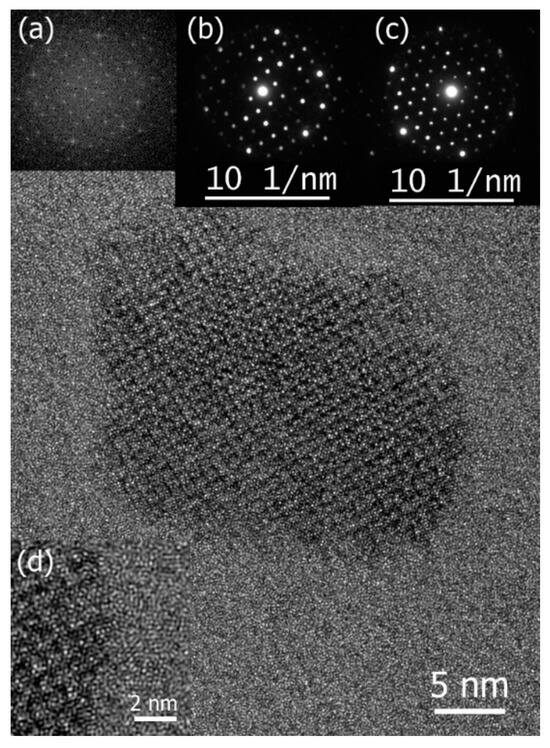
Figure 4.
High-resolution TEM image of an Al19Fe4MnSi2 nanoparticle and the insets: its fast Fourier transform (a) and two NBD patterns (b,c) were indexed according to alpha phase lattice with zone axes of [100], [100], and [111], respectively. In both cases, the reflections closest to (000) in distance are of {110} type. Strong reflections at the edge of the patterns in (b,c) are of {611} type. (d) a close-up view of the interface. Reproduced from [91] with permission of Elsevier.
The addition of rare-earth metals was found to cause nanocrystallization of Mg-Ni metallic glasses [92]. A unique primary crystallization has been observed recently in the Mg75Ni20Mm5 metallic glass [93]. The analysis revealed that despite significant solute partitioning and soft impingement of diffusion fields around neighboring crystals, the growth remains linear, suggesting interface-controlled kinetics. The crystals exhibited a constant aspect ratio during growth, defying dendritic interfacial instability typically associated with solute partitioning. Remarkably, the primary Mg2Ni crystals grow with a composition close to Mg9Ni9RE, indicating supersaturation with Ni because of partially vacant Mg sites. The constant isothermal growth rate and absence of dendritic growth are attributed to the wide range of atomic diffusivities below the glass-transition temperature. Specifically, Ni, the fastest diffusing species, preferentially partitions into the growing Mg2Ni crystals, while the immobile RE atoms remain uniformly distributed.
Very small nanoscale quasicrystals of about 10 nm in size and even below with a very high density of precipitates were formed on devitrification of the Zr-Al-Ni-Cu-Ag [94], Hf-Pd-Ni-Al [95], Hf-Al-Ni-Cu-Pd [96], and Cu-Zr-Ti-(Pd,Au) [61] alloys owing to easy nucleation of the icosahedral phase having low interfacial energy with the glassy phase [97].
7. Truly Eutectic Crystallization and Crystallization Looking like Eutectic
Many metallic glasses crystallize by the classical eutectic reaction when several crystalline phases crystallize simultaneously forming eutectic colonies as was found in Pd-Ni-P-Si (spherical colonies) [64] and Zr-Cu-Fe-Al alloys (rod-type colonies) [98] (Figure 5), for example.
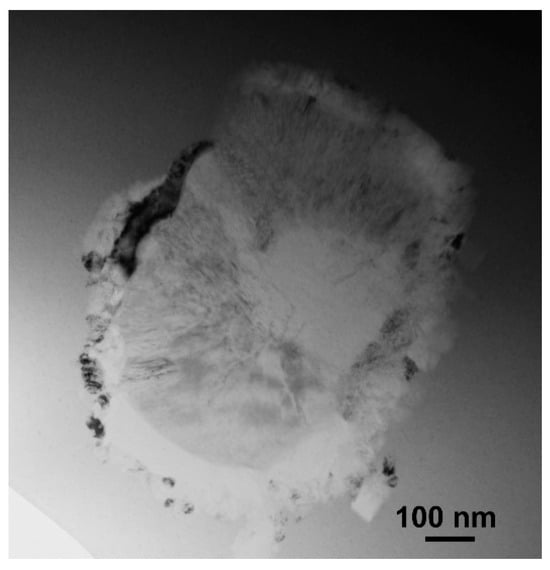
Figure 5.
Bright-field image of the eutectic colony in the annealed Zr62.5Cu22.5Al10Fe5 glassy sample, TEM.
At the same time, the detailed study of the eutectic-like reaction observed below Tg in Al85Y8Ni5Co2 (for which the phase transformation kinetics looks like the eutectic one) revealed that it is not a straightforward eutectic reaction but involves two stages: the precipitation of a primary intermetallic compound followed immediately by the crystallization of nanoscale Al around it (Figure 6) [99]. Alongside the growth of existing colonies triggered by the intermetallic compound, new needle-like intermetallic compound particles form in the glassy matrix, initiating the development and growth of new eutectic colonies (Figure 6a,b). These colonies are abnormal, often lacking boundaries between the aluminum nanoparticles and the intermetallic compound, which are separated by the glassy matrix. Despite this, the transformation statistically behaves as a single-step process with a nearly constant Avrami exponent. At higher heating rates, when the temperature quickly surpasses Tg, the crystallization process shifts to the primary crystallization of a nanoscale solid solution of Al, followed by the formation of intermetallic compounds through subsequent reactions occurring at a longer annealing time or at higher temperatures.
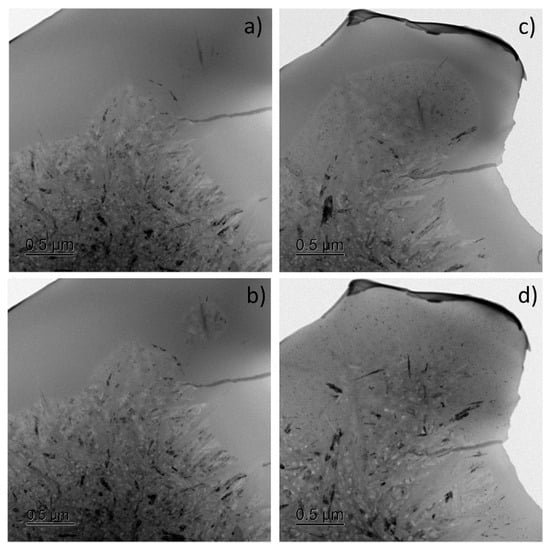
Figure 6.
Bright-field TEM images indicating growth of a single colony towards the residual liquid phase observed in Al85Y8Ni5Co2 at: (a) 603 K, (b) 608 K, (c) 613 K, and (d) 633 K. Reproduced from [99] with permission of Elsevier.
The crystallization of a Cu58Y37Sc5 glassy alloy also looks like the eutectic one at first glance [100]. However, nucleation and growth of micron-scale oI12 Cu2Y, cP2 CuY, and cP2 CuSc phases occur heterogeneously after a defined incubation period. The crystallization kinetics suggest primary crystallization with diffusion-controlled growth, rather than eutectic crystallization, highlighting the complex nature of the process. The formation of micron-scale Y-/Sc-rich particles in the as-cast state suggests a metastable phase diagram, possibly inhibiting intermetallic compound formation. Complex crystallization was observed in an Au-based bulk metallic glass [101].
8. Fast Polymorphic Crystallization
Observation of polymorphic crystallization is a rare case in metallic glasses as their composition is usually closer to eutectics [102]. Recently, reactions forming cP2/B2 phase were studied in the Ti50Ni22Cu22TM6 glassy alloys [103] close to the glass-transition temperature. XRD and SAED patterns confirm the presence of cP2/B2 TiNi solid solution phase, with minor intermetallic compounds detected in Cr- and Mn-containing alloys. The Ti50Ni22Cu22Mn6 alloy showed a high n exponent, indicating an increasing nucleation rate with time. The crystal growth rate in the Ti50Ni22Cu22Fe6 metallic glass obtained by in situ transmission electron microscopy is orders of magnitude higher than that allowed by thermal long-range diffusion estimated from viscosity. It is suggested that the atomic transfer at the atomic-scale thick glass/crystal interface may be accelerated by the crystal/glass difference in the corresponding thermodynamic and chemical potentials [104].
9. Features of Crystallization during Welding and Additive Manufacturing
Welding and joining of bulk metallic glasses are performed by friction [105], laser [106], or electron welding [107]. Additive manufacturing (AM) is performed by the sintering of powders and often leads to partial crystallization of the glassy powder [108]. For a specialized review dedicated to this topic see Ref. [109]. The ability to produce metallic glass without crystallization during selective laser melting hinges on the interaction between the laser beam and the glassy material [110]. One of the important issues here is the heat-affected zone with a partly crystalline structure formed when a subsequently applied layer anneals the underlayer. AM process is influenced by powder size and shape, laser beam power, scanning speed, and other processing parameters. A Zr52.5Cu17.9Ni14.6Al10Ti5 bulk metallic glass fabricated AM showed yield and fracture stresses slightly lower than for its as-cast counterpart. The stress drops during serrated flow were much lower and more uniform [111]. Advancements in laser technology necessitate a deeper understanding of these interactions to enhance AM processes. Systematic studies tailored to specific AM techniques and metallic glassy systems are required, along with an understanding of crystallization kinetics during laser irradiation.
10. Mechanically Induced Crystallization at Room Temperature
Mechanically induced crystallization of metallic glasses has been studied substantially and reviewed recently [112]. It is known and recently proven again that mechanical alloying, using a high-energy ball milling technique, can cause both amorphization and crystallization [113]. High-pressure torsion and multiple rolling also caused structural changes and crystallization in the metallic glassy phase [114,115]. However, possible temperature rise cannot be neglected at such an intensive deformation. Here, the emphasis is given to recent findings on room-temperature crystallization.
Quite often thermal and mechanical crystallization processes lead to different crystal structures. Heating Fe48Cr15Mo14C15B6Y2 [116] and Fe48Cr15Mo14C15B6Tm2 glassy alloys formed nanoscale particles of the χ-Fe36Cr12Mo10 phase on crystallization [117,118] at an early crystallization stage [119] and in a Fe50Cr15Mo14C15B6 alloy after partial crystallization [120]. This phase was also found in Cr- and Mo-free glassy alloys [121]. Crystallization of similar Co-based alloys was also studied [122].
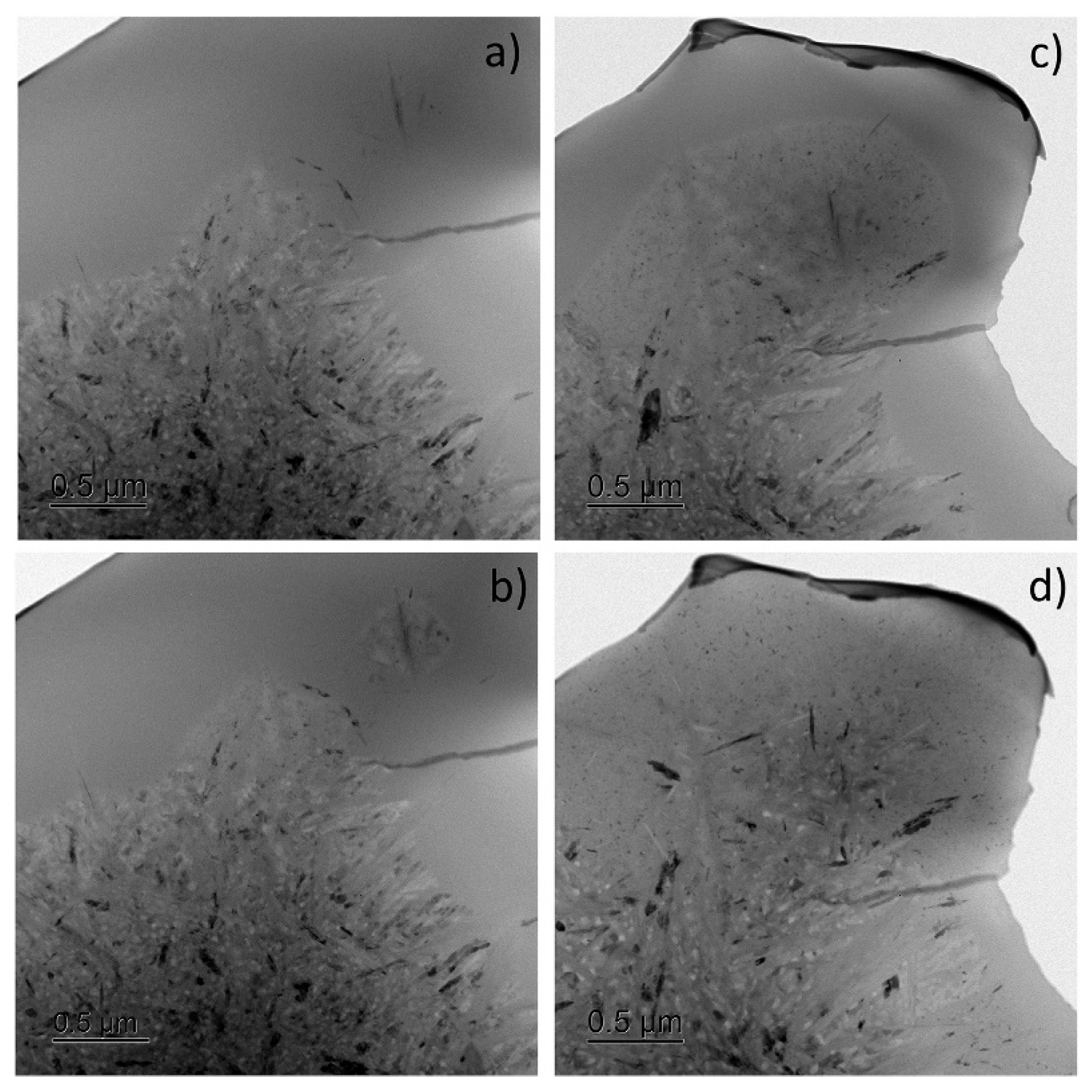
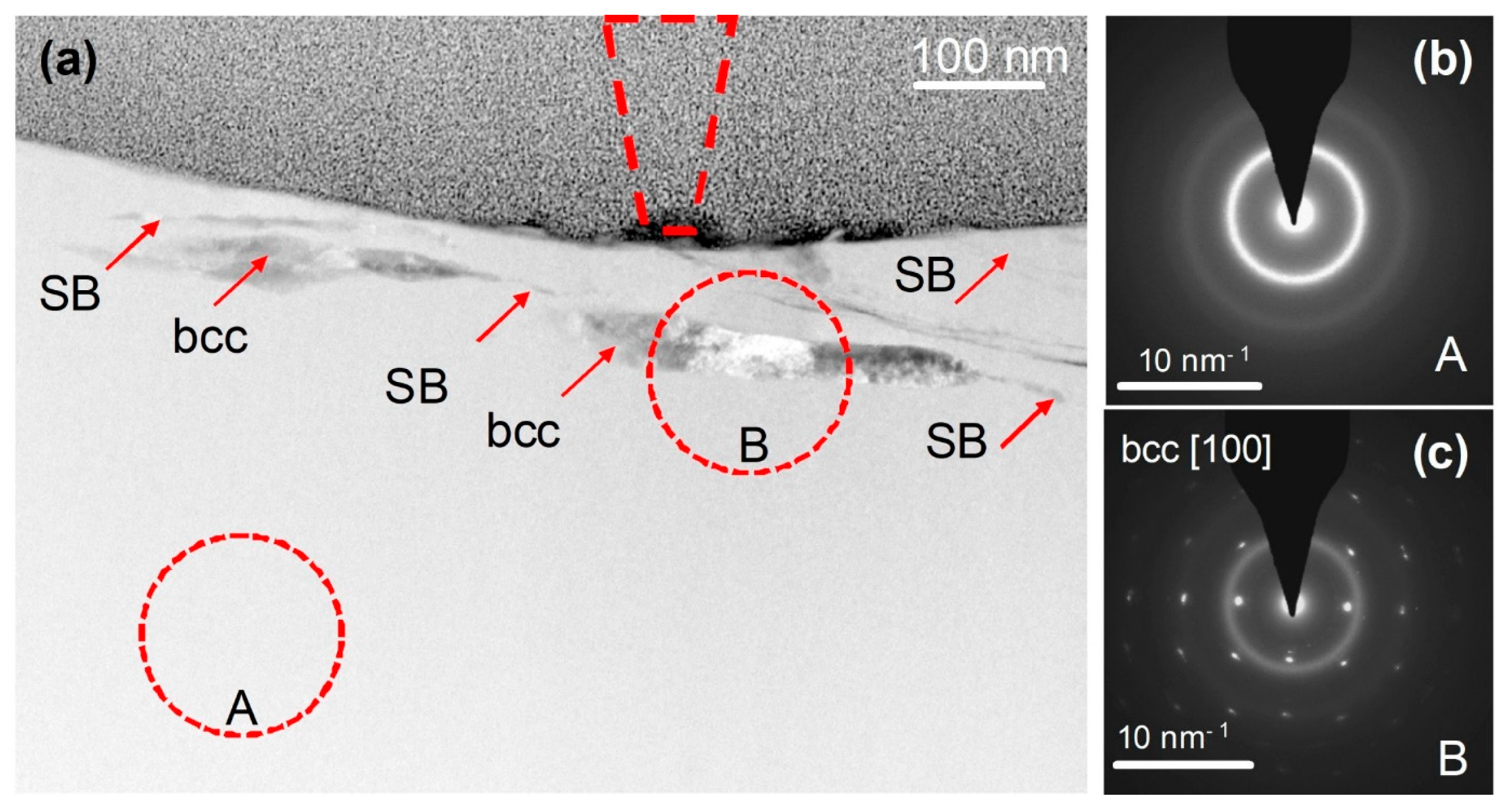
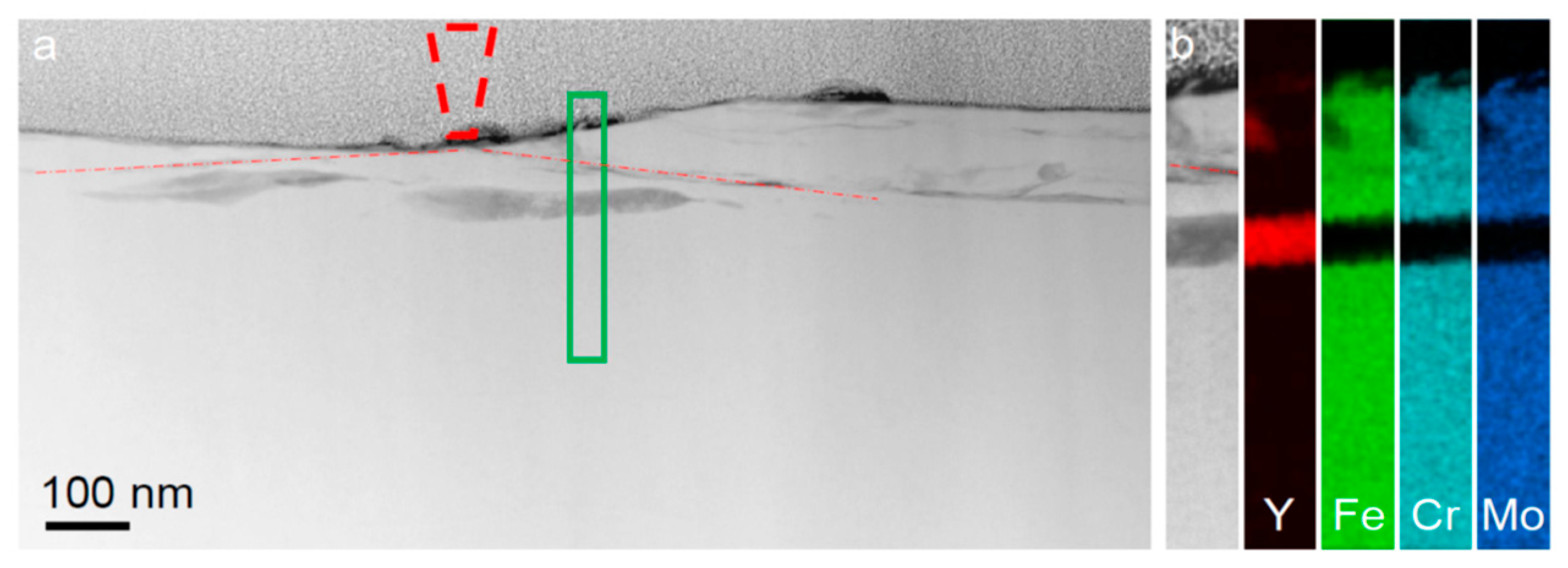
On the other hand, room-temperature wear of a Fe48Cr15Mo14C15B6Y2 bulk metallic glass, induced by a nanoscale diamond tip caused the formation of nanoscale layers of a bcc Y-based solid solution (Figure 7) with a composition of Y84Fe8Mo5Cr3 (at.%) directly beneath the wear track, at a depth of about 50 nm (Figure 8) [123]. The partly dark contrast of this crystalline phase in the HAADF image can relate to the fact that Y39 (molar volume: 19.88 cm3) having a significantly larger atomic number z = 39 than Fe26 (molar volume: 7.09 cm3) also has a much larger atomic size and thus almost three times higher atomic/molar volume. Thus, the dark contrast might have arisen from the three times lower atomic number density (ND) of Y ND = 0.030 Å−3 compared to that of Fe (ND = 0.085 Å−3).
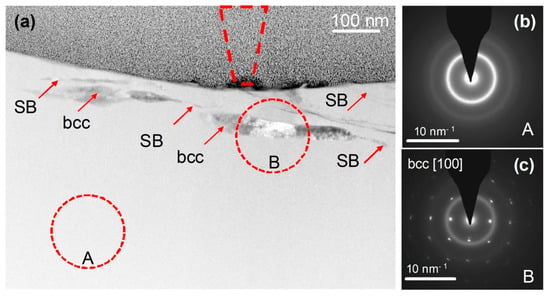
Figure 7.
Cross-sectional TEM observation of a wear track. (a) HAADF STEM image of the cross-section of the track, showing the MG matrix and within it shear bands (SBs) and bcc crystals. The position and size of the AFM tip are indicated by the dashed cone located at the center of the track, which overall is 650–750 nm wide. (b,c) are SAED patterns from regions A and B, respectively, indicating a glassy phase and a crystal induced by mechanical deformation. The A and B circles on the image show the size of the selected-area aperture used for SAED acquisition. Reproduced from [123] with permission of Springer Nature.
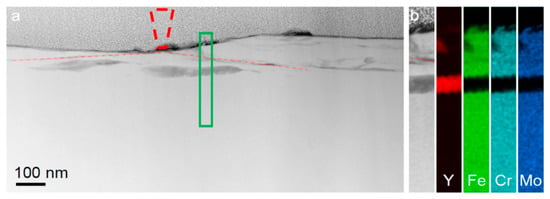
Figure 8.
Compositional changes in the Fe48Cr15Mo14C15B6Y2 bulk metallic glass underneath the wear track. (a) HAADF STEM image of the cross-section of the wear track. The position of the AFM tip is shown by the dashed cone. The red dashed–dotted lines indicate the position of the shear bands generated during the wear test. (b) HAADF image and EDX elemental mapping of the region marked by the green rectangle in (a). Reproduced from [123] with permission of Springer Nature.
This notable concentration of yttrium, characterized by its low thermal diffusivity in Fe, is attributed to a mechano-chemical pumping action in the heavily sheared regions [124] under the diamond tip. In bulk metallic glasses, larger atoms like yttrium move toward the central plane of the shear band (with high excess volume), consistent with findings in colloids and granular media. The extreme shear rate gradients in metallic glass shear bands facilitate yttrium concentration on shear planes which is enhanced by the soft Fe-Y interatomic potential increasing yttrium mobility. This mechano-chemical pumping effect is of fundamental scientific interest and suggests potential applications. Computer simulation also indicated low-temperature, stress-driven crystallization [125].
Room-temperature cyclic mechanical loading in the elastic mode also can induce structural changes in the glassy phase, altering its mechanical and thermal relaxation behavior on heating [126]. The thermal and mechanical relaxation spectra of a Zr64Cu21Fe5Al10 bulk metallic glass were examined before and after cyclic loading up to 0.4% elastic strain. This loading caused partial mechanically induced nanocrystallization after 10,000 elastic loading cycles at room temperature, affecting the relaxation behavior on subsequent heating experiments [127]. The nanocrystals being metastable dissolve in the glassy matrix on subsequent thermal relaxation. Deformation-induced crystallization reactions during bending were observed in a Pd-Cu-Ni-P metallic glass on the compressed side [128]. The changes in the crystallization kinetics under external loading have also been studied recently [129,130].
11. Summary
Crystal nucleation in liquids and glasses is a complex phenomenon. Direct observation is challenging, but computer simulations help to model these processes, revealing realistic atomic interactions. Despite some research advances, the precise mechanisms of nucleation and the role of chemical ordering in multicomponent systems continue to be a vital research area. Crystallization in metallic glasses involves diverse crystal growth velocities, from very slow primary crystallization (including nanoscale quasicrystals) to rapid polymorphic processes. Recent findings indicate that primary crystallization involves diffusion-controlled growth, while Mg-Ni-Mm glasses show interface-controlled kinetics. Eutectic crystallization forms colonies in some alloys, whereas eutectic-like reactions can involve complex multi-stage processes, as seen in Al-Y-Ni-Co alloys. Transformation kinetics are usually analyzed by the Kolmogorov–Johnson–Mehl–Avrami equation. Time–temperature–transformation diagrams are built to compare the thermal stabilities of metallic glasses. Mechanically induced crystallization occurred at room temperature, as evidenced by nanoscale particle formation in Fe-Cr-Mo-C-B-Y and Pd-Cu-Ni-P alloys and structural changes due to cyclic loading in Zr-Cu-Fe-Al glasses, demonstrating the complex nature of crystallization in metallic glasses. Crystallization mechanisms in metallic glasses need continued research to understand these processes and their implications on material properties.
Funding
This research received no external funding.
Data Availability Statement
The data will be available on request.
Conflicts of Interest
The author declares no conflict of interest.
References
- Klement, W.; Willens, R.H.; Duwez, P. Non-crystalline Structure in Solidified Gold–Silicon Alloys. Nature 1967, 187, 869–870. [Google Scholar] [CrossRef]
- Chen, H.S. Glass temperature, formation and stability of Fe, Co, Ni, Pd and Pt based glasses. Acta Metall. 1974, 22, 1505. [Google Scholar] [CrossRef]
- Kui, H.W.; Greer, A.L.; Turnbull, D. Bulk formation of a metallic glass: Pd40Ni40P20. Appl. Phys. Lett. 1982, 45, 716–717. [Google Scholar] [CrossRef]
- Molokanov, V.; Chebotnikov, V. Glass forming ability, structure and properties of Ti and Zr-intermetallic compound based alloys. Key Eng. Mater. 1990, 40–41, 319–332. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Zr–Al–Ni Amorphous Alloys with High Glass Transition Temperature and Significant Supercooled Liquid Region. Mater. Trans. JIM 1990, 31, 177–183. [Google Scholar] [CrossRef]
- Inoue, A. Nanostructured and non-crystalline materials, bulk glass-forming metallic alloys: Science and technology. Mater. Trans. JIM 1995, 36, 866. [Google Scholar] [CrossRef]
- Johnson, W.L. Bulk Glass-forming metallic alloys: Science and technology. MRS Bull. 1999, 24, 42–56. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Nagase, T.; Umakoshi, Y. Thermal crystallization and electron irradiation induced phase transformation behavior in Zr66.7Cu33.3 metallic glass. Mater. Trans. 2005, 46, 616–621. [Google Scholar] [CrossRef][Green Version]
- Ojovan, M.I. The Flow of Glasses and Glass–Liquid Transition under Electron Irradiation. Int. J. Mol. Sci. 2023, 24, 12120. [Google Scholar] [CrossRef]
- Jiang, W.H.; Pinkerton, F.E.; Atzmon, M. Effect of strain rate on the formation of nanocrystallites in an Al-based amor-phous alloy during nanoindentation. J. Appl. Phys. 2003, 93, 9287–9290. [Google Scholar] [CrossRef]
- Ding, H.Y.; Yao, K.F. High entropy Ti20Zr20Cu20Ni20Be20 bulk metallic glass. J. Non-Crystalline Solids 2013, 364, 9–12. [Google Scholar] [CrossRef]
- Bykov, V.A.; Kovalenko, D.A.; Sterkhov, E.V.; Kulikova, T.V. Crystallization kinetics of GdYScAlCo high-entropy bulk metallic glass. Chim. Techno Acta 2023, 10, 202310207. [Google Scholar] [CrossRef]
- Luan, H.; Zhang, X.; Ding, H.; Zhang, F.; Luan, J.H.; Jiao, Z.B.; Yang, Y.-C.; Bu, H.; Wang, R.; Gu, J.; et al. High-entropy induced a glass-to-glass transition in a metallic glass. Nat. Commun. 2022, 13, 2183. [Google Scholar] [CrossRef]
- Makarov, A.S. General thermodynamic approach to describe the kinetics of thermal effects in high-entropy metallic glasses. JETP Lett. 2023, 118, 182–188. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Zanaeva, E.N.; Pratama, F.R.; Wada, T.; Ito, S. Structural peculiarities of Pd-Cu-Ni-P and Pt-Cu-Ni-P metallic glasses as a reason for their significantly different room-temperature plasticity. Scr. Mater. 2023, 231, 115468. [Google Scholar] [CrossRef]
- Madge, S.V. Toughness of bulk metallic glasses. Metals 2015, 5, 1279–1305. [Google Scholar] [CrossRef]
- Chen, H.S.; Turnbull, D. Evidence of a glass–liquid transition in a gold–germanium–silicon alloy. J. Chem. Phys. 1968, 48, 2560. [Google Scholar] [CrossRef]
- Sanditov, D.S.; Ojovan, M.I. On relaxation nature of glass transition in amorphous materials. Phys. B Condens. Matter 2017, 523, 96–113. [Google Scholar] [CrossRef]
- Cohen, M.H.; Grest, G.S. Liquid-glass transition, a free-volume approach. Phys. Rev. 1979, 20, 1077. [Google Scholar] [CrossRef]
- Greer, A.L. New horizons for glass formation and stability. Nat. Mater. 2015, 14, 542–546. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Tournier, R.F. On structural rearrangements near the glass transition temperature in amorphous silica. Materials 2021, 14, 5235. [Google Scholar] [CrossRef]
- Na, J.H.; Corona, S.L.; Hoff, A.; Johnson, W.L. Observation of an apparent first-order glass transition in ultrafragile Pt–Cu–P bulk metallic glasses. Proc. Natl. Acad. Sci. USA 2020, 117, 2779–2787. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Undercooled phase behind the glass phase with superheated medium-range order above glass transition temperature. Phys. B Condens. Matter 2021, 602, 412542. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. Smithells Metals Reference Book, 8th ed.; Elsevier Butterworth-Heinemann Ltd.: Oxford, UK, 2004. [Google Scholar]
- Ojovan, M.I. Viscous flow and the viscosity of melts and glasses. Phys. Chem. Glas. Eur. J. Glass Sci. Technol. Part B 2012, 53, 143–150. [Google Scholar]
- Louzguine-Luzgin, D.V. Structural changes in metallic glass-forming liquids on cooling and subsequent vitrification in relationship with their properties. Materials 2022, 15, 7285. [Google Scholar] [CrossRef]
- Kelton, K.F. A perspective on metallic liquids and glasses. J. Appl. Phys. 2023, 134, 010902. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Louzguine-Luzgin, D.V. On crossover temperatures of viscous flow related to structural rearrangements in liquids. Materials 2024, 17, 1261. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Gao, L.; Ye, Y.-H.; Zhang, J.-X.; Zhang, T.; Bu, Q.-Z.; Yang, Q.; Zhu, Z.-W.; Wei, S.; Yu, H.-B. Fragility crossover mediated by covalent-like electronic interactions in metallic liquids. Mater. Futur. 2024, 3, 025002. [Google Scholar] [CrossRef]
- Meyer, N.; Xu, H.; Wax, J.-F. Universality of the shear viscosity of alkali metals. Phys. Rev. B 2017, 96, 094201. [Google Scholar] [CrossRef]
- Qiao, J.C.; Wang, Q.; Pelletier, J.M.; Kato, H.; Casalini, R.; Crespo, D.; Pineda, E.; Yao, Y.; Yang, Y. Structural heterogeneities and mechanical behavior of amorphous alloys. Prog. Mater. Sci. 2019, 104, 250–329. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, W.H.; Bai, H.Y.; Samwer, K. The β relaxation in metallic glasses. Natl. Sci. Rev. 2014, 1, 429–461. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yang, Y.; Qiao, J. Aging and rejuvenation during high-temperature deformation in a metallic glass. Sci. China Phys. Mech. Astron. 2022, 65, 106111. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Jiang, J. Low-temperature relaxation behavior of a bulk metallic glass leading to improvement of both strength and plasticity. Mater. Sci. Eng. A 2022, 839, 142841. [Google Scholar] [CrossRef]
- Inoue, A.; Chen, S.; Masumoto, T. Zr-Y base amorphous alloys with two glass transitions and two supercooled liquid regions. Mater. Sci. Eng. A 1994, 179–180, 346–350. [Google Scholar] [CrossRef]
- Kündig, A.; Ohnuma, M.; Ping, D.; Ohkubo, T.; Hono, K. In situ formed two-phase metallic glass with surface fractal microstructure. Acta Mater. 2004, 52, 2441. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Xie, G.; Zhang, W.; Inoue, A. Devitrification behavior and glass-forming ability of Cu–Zr–Ag alloys. Mater. Sci. Eng. A 2007, 465, 146–152. [Google Scholar] [CrossRef]
- Bazlov, A.I.; Parkhomenko, M.S.; Tabachkova, N.Y.; Igrevskaya, A.G.; Zanaeva, E.N.; Mamzurina, O.I.; Medvedeva, S.V.; Bazlova, T.A.; Louzguine-Luzgin, D.V. Formation of a phase separated structure in the Zr–Cu–Fe–Al alloys by thermo-mechanical processing. Intermetallics 2021, 35, 107224. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Jiang, J.; Bazlov, A.I.; Zolotorevzky, V.S.; Mao, H.; Ivanov, Y.P.; Greer, A.L. Phase separation process preventing thermal embrittlement of a Zr-Cu-Fe-Al bulk metallic glass. Scr. Mater. 2019, 167, 31–36. [Google Scholar] [CrossRef]
- Bazlov, A.I.; Igrevskaya, A.G.; Tabachkova, N.Y.; Chen, C.; Cheverikin, V.V.; Pozdniakov, A.V.; Jiang, J.; Louzguine-Luzgin, D.V. Thermo-mechanical processing of a Zr62.5Cu22.5Fe5Al10 glassy alloy as a way to obtain tensile ductility. J. Alloys Compd. 2021, 853, 157138. [Google Scholar] [CrossRef]
- Pekarskaya, E.; Löffler, J.F.; Johnson, W.L. Microstructural studies of crystallization of a Zr-based bulk metallic glass. Acta Mater. 2003, 51, 4045–4057. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Jiang, J. On Long-Term Stability of Metallic Glasses. Metals 2019, 9, 1076. [Google Scholar] [CrossRef]
- Zhao, Y.; Shang, B.; Zhang, B.; Tong, X.; Ke, H.; Bai, H.; Wang, W.H. Ultrastable metallic glass by room temperature aging. Sci. Adv. 2022, 8, 3623. [Google Scholar] [CrossRef] [PubMed]
- Kelton, K.F.; Greer, A.L. Nucleation in condensed matter applications in materials and biology. Pergamon Mater. Ser. 2010, 15, 1–743. [Google Scholar]
- Schmelzer, J.W.P.; Abyzov, A.S.; Baidakov, V.G. Time of formation of the first supercritical nucleus, time-lag, and the steady-state nucleation rate. Int. J. Appl. Glas. Sci. 2016, 8, 48–60. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Yang, Y.; Kim, D.S.; Yuan, A.; Tian, X.; Ophus, C.; Sun, F.; Schmid, A.; Nathanson, M.; et al. Observing crystal nucleation in four dimensions using atomic electron tomography. Nature 2019, 570, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer JW, P.; Tropin, T.V. Theory of crystal nucleation of glass-forming liquids: Some new developments. Int. J. Appl. Glass Sci. 2022, 13, 171–198. [Google Scholar] [CrossRef]
- Gasser, U.; Weeks, E.R.; Schofield, A.; Pusey, P.N.; Weitz, D.A. Real-space imaging of nucleation and growth in colloidal crystallization. Science 2001, 292, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Louzguine-Luzgin, D.V.; Bazlov, A.I. Crystallization of FCC and BCC liquid metals studied by molecular dynamics simulation. Metals 2020, 10, 1532. [Google Scholar] [CrossRef]
- Hu, Y.C.; Tanaka, H. Physical origin of glass formation from multicomponent systems. Sci. Adv. 2020, 6, eabd2928. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Tipeev, A.O. Effect of planar interfaces on nucleation in melting and crystallization. Entropy 2022, 24, 1029. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Q.; An, Q.; Xie, Y.; Sun, Z.H.; Luo, S.-N. Homogeneous nucleation and growth of melt in copper. J. Chem. Phys. 2007, 127, 164503. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.E.L.; Cai, Y.; Wu, H.A.; Luo, S.N. Crystallization in supercooled liquid Cu: Homogeneous nucleation and growth. J. Chem. Phys. 2015, 142, 064704. [Google Scholar]
- Louzguine-Luzgin, D.V.; Miyama, M.; Nishio, K.; Tsarkov, A.A.; Greer, A.L. Vitrification and nanocrystallization of pure liquid Ni studied using molecular-dynamics simulation. J. Chem. Phys. 2019, 151, 124502. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazy, Y.; Averback, R. Kinetic stages in the crystallization of deeply undercooled body-centered-cubic and face-centered-cubic metals. Acta Mater. 2010, 58, 524–530. [Google Scholar] [CrossRef]
- Wilde, G.; Sebright, J.L.; Perepezko, J.H. Bulk liquid undercooling and nucleation in gold. Acta Mater. 2006, 54, 4759–4769. [Google Scholar] [CrossRef]
- Bokeloh, J.; Wilde, G.; Rozas, R.; Benjamin, R.; Horbach, J. Nucleation barriers for the liquid-to-crystal transition in simple metals: Experiment vs. simulation. Eur. Phys. J. Spéc. Top. 2014, 223, 511–526. [Google Scholar] [CrossRef]
- Lu, A.K.A.; Louzguine-Luzgin, D.V. Crystal nucleation and growth processes in Cu-rich glass-forming Cu–Zr alloys. J. Chem. Phys. 2022, 157, 014506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, C.L.; Ketov, S.V.; Akagi, K.; Tsarkov, A.A.; Ikuhara, Y.; Louzguine-Luzgin, D.V. Local chemical ordering within the incubation period as a trigger for nanocrystallization of a highly supercooled Ti-based liquid. Mater. Des. 2018, 156, 504–513. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Nanoparticles with icosahedral symmetry in Cu-based bulk glass former induced by Pd addition. Scripta Mater. 2003, 48, 1325–1329. [Google Scholar] [CrossRef]
- Orava, J.; Greer, A.L.; Gholipour, B.; Hewak, D.W.; Smith, C.E. Characterization of supercooled liquid Ge2Sb2Te5 and its crystallization by ultrafast-heating calorimetry. Nat. Mater. 2012, 11, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Zalden, P.; von Hoegen, A.; Landreman, P.; Wuttig, M.; Lindenberg, A.M. How supercooled liquid phase-change materials crystallize: Snapshots after femtosecond optical excitation. Chem. Mater. 2015, 27, 5641–5646. [Google Scholar] [CrossRef]
- Chen, N.; Gu, L.; Xie, G.Q.; Louzguine-Luzgin, D.V.A.R.; Yavari, A.; Vaughan, G.; Imhoff, S.; Perepezko, J.; Abe, T.; Inoue, A. Flux-induced structural modification and phase transformations in a Pd40Ni40Si4P16 bulk-glassy alloy. Acta Mater. 2010, 58, 5886–5897. [Google Scholar] [CrossRef]
- Cao, C.R.; Lu, Y.M.; Bai, H.Y.; Wang, W.H. High surface mobility and fast surface enhanced crystallization of metallic glass. Appl. Phys. Lett. 2015, 107, 141606. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Kaloshkin, S.D.; Inoue, A. Peritectic-like reactions involving glassy phase. Rev. Adv. Mater. Sci. 2008, 18, 653–659. [Google Scholar]
- Kolmogorov, A.N. A statistical theory for the recrystallisation of metals. Isz. Akad. Nauk. USSR Ser. Matem. 1937, 3, 355–359. [Google Scholar]
- Johnson, M.W.A.; Mehl, K.F. Reaction kinetics in processes of nucleation and growth. Trans. Trans. Am. Inst. Min. Metall. Eng. 1939, 135, 416–442. [Google Scholar]
- Avrami, M. Granulation, phase change, and microstructure kinetics of phase change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Xie, G.; Zhang, W.; Inoue, A. Influence of Al and Ag on the devitrification behavior of a Cu-Zr glassy alloy. Mater. Trans. 2007, 48, 2128–2132. [Google Scholar] [CrossRef]
- Lan, S.; Wu, Z.D.; Wei, X.Y.; Zhou, J.; Lu, Z.P.; Neuefeind, J.; Wang, X.-L. Structure origin of a transition of classic-to-avalanche nucleation in Zr-Cu-Al bulk metallic glasses. Acta Mater. 2018, 149, 108–118. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Yavari, A.R.; Vaughan, G.; Inoue, A. Clustered crystalline structures as glassy phase approximants. Intermetallics 2009, 17, 477–480. [Google Scholar] [CrossRef]
- Nishiyama, N.; Inoue, A. Supercooling investigation and critical cooling rate for glass formation in Pd–Cu–Ni–P alloy. Acta Mater. 1999, 47, 1487–1495. [Google Scholar] [CrossRef]
- Löffler, J.F.; Schroers, J.; Johnson, W.L. Time–temperature–transformation diagram and microstructures of bulk glass forming Pd40Cu30Ni10P20. Appl. Phys. Lett. 2000, 77, 681–683. [Google Scholar] [CrossRef]
- Janlewing, R.; Köster, U. Nucleation in crystallization of Zr–Cu–Ni–Al metallic glasses. Mater. Sci. Eng. A 2001, 304–306, 833–838. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Inoue, A. Comparison of the long-term thermal stability of various metallic glasses under continuous heating. Scr. Mater. 2002, 47, 887–891. [Google Scholar] [CrossRef]
- Duan, T.; Kim, W.; Gao, M.; Perepezko, J.H. Crystallization of an undercooled Zn-based glass forming alloy. J. Non Crystalline Solids 2024, 627, 122823. [Google Scholar] [CrossRef]
- Grange, R.A.; Kiefer, J.M. Transformation of austenite on continuous cooling and its relation to transformation at constant temperature. Trans. ASM 1941, 29, 85–144. [Google Scholar]
- Wang, J.; Chen, N.; Liu, P.; Wang, Z.; Louzguine-Luzgin, D.; Chen, M.; Perepezko, J. The ultrastable kinetic behavior of an Au-based nanoglass. Acta Mater. 2014, 79, 30–36. [Google Scholar] [CrossRef]
- Luo, P.C.; Cao, R.; Zhu, F.; Lv, Y.M.; Liu, Y.H.; Wen, P.; Bai, H.Y.; Vaughan, G.; di Michiel, M.; Ruta, B.; et al. Ul-trastable metallic glasses formed on cold substrates. Nat. Commun. 2018, 9, 1389. [Google Scholar] [CrossRef]
- Yoshizawa, H.; Yamauchi, K.; Yamane, T.; Sugihara, H. Common mode choke cores using the new Fe-based alloys composed of ultrafine grain structure. J. Appl. Phys. 1988, 64, 6047–6049. [Google Scholar] [CrossRef]
- Suzuki, K.; Kataoka, N.; Inoue, A.; Makino, A.; Masumoto, T. High saturation magnetization and soft magnetic properties of bcc fe–zr–b alloys with ultrafine grain structure. Mater. Trans. JIM 1990, 31, 743–746. [Google Scholar] [CrossRef]
- Milkova, D.; Bazlov, A.; Zanaeva, E.; Churyumov, A.; Strochko, I.; Ubyivovk, E.; Inoue, A. (Fe-Ni)-based glassy alloy containing Nb and Cu with excellent soft magnetic properties. J. Non Crystalline Solids 2023, 609, 122234. [Google Scholar] [CrossRef]
- Wang, F.; Inoue, A.; Kong, F.L.; Han, Y.; Zhu, S.L.; Shalaan, E.; Al-Marouki, F. Formation, thermal stability and mechanical properties of high entropy (Fe,Co,Ni,Cr,Mo)-B amorphous alloys. J. Alloys Compd. 2018, 732, 637–645. [Google Scholar] [CrossRef]
- Shen, Y.; Perepezko, J.H. Al-based amorphous alloys: Glass-forming ability, crystallization behavior and effects of minor al-loying additions. J. Alloys Compd. 2017, 707, 3–11. [Google Scholar] [CrossRef]
- Abrosimova, G.; Matveev, D.; Pershina, E.; Aronin, A. Effect of treatment conditions on parameters of nanocrystalline structure in Al-based alloys. Mater. Lett. 2016, 183, 131–134. [Google Scholar] [CrossRef]
- Duan, T.; Shen, Y.; Imhoff, S.D.; Voyles, P.M.; Perepezko, J.H. Nucleation kinetics model for primary crystallization in Al-Y-Fe metallic glass. J. Chem. Phys. 2023, 158, 064504. [Google Scholar] [CrossRef] [PubMed]
- Abrosimova, G.; Aronin, A. On decomposition of amorphous phase in metallic glasses. Rev. Adv. Mater. Sci. 2017, 50, 55–61. [Google Scholar]
- Louzguine-Luzgin, D.V.; Inoue, A. Structure and transformation behaviour of a rapidly solidified Al–Y–Ni–Co–Pd alloy. J. Alloy Compd. 2005, 399, 78–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Jing, Z.; Chen, C.; Liang, X. Effects of rare-earth element Y content on the microstructure and properties of quinary Al–Ni–Zr–Co–Y high entropy metallic glasses. Intermetallics 2024, 165, 108164. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Pratama, F.R. In-situ studies of primary nucleation and growth of the cubic Al19Fe4MnSi2 phase in an Al–Fe–Mn–Si metallic glass. Intermetallics 2024, 164, 108120. [Google Scholar] [CrossRef]
- Tan, M.; Wang, J.; Wu, Y.; Jin, C.; Sun, Y.; Song, L.; Zhang, Y.; Wang, J.; Huo, J.; Gao, M. Accelerated crystallization kinetics and grain refinement in Mg-Ni-Y metallic glass via multiple rare earth elements doping. J. Alloys Compd. 2024, 999, 175080. [Google Scholar] [CrossRef]
- Ivanov Yu, P.; Semin, V.O.; Lu, Z.; Jiang, J.; Greer, A.L.; Louzguine-Luzgin, D.V. Long-range-diffusion-assisted but inter-face-controlled crystallization of a Mg-Ni-Mm glass below its glass-transition temperature. J. Alloys Compd. 2022, 909, 164732. [Google Scholar] [CrossRef]
- Chen, M.W.; Zhang, T.; Inoue, A.; Sakai, A.; Sakurai, T. Quasicrystals in a partially devitrified Zr65Al7.5Ni10Cu12.5Ag5 bulk metallic glass. Appl. Phys. Lett. 1999, 75, 1697–1699. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Ko, M.S.; Inoue, A. Nanoquasicrystalline phase produced by devitrification of Hf-Pd-Ni-Al metallic glass. Appl. Phys. Lett. 2000, 76, 3424–3426. [Google Scholar] [CrossRef]
- Li, C.; Saida, J.; Matsushita, M.; Inoue, A. Precipitation of icosahedral quasicrystalline phase in Hf65Al7.5Ni10Cu12.5Pd5 metallic glass. Appl. Phys. Lett. 2000, 77, 528–530. [Google Scholar] [CrossRef]
- Kelton, K.F.; Lee, G.W.; Gangopadhyay, A.K.; Hyers, R.W.; Rathz, T.J.; Rogers, J.R.; Robinson, M.B.; Robinson, D.S. First X-ray scattering studies on electrostatically levitated metallic liquids: Demonstrated influence of local icosahedral order on the nucleation barrier. Phys. Rev. Lett. 2003, 90, 195504. [Google Scholar] [CrossRef] [PubMed]
- Louzguine-Luzgin, D.V.; Xie, G.; Zhang, Q.; Inoue, A. Effect of Fe on the glass-forming ability, structure and devitrification behavior of Zr-Cu-Al bulk glass-forming alloys. Philos. Mag. 2010, 90, 1955–1968. [Google Scholar] [CrossRef]
- Bazlov, A.; Tabachkova, N.Y.; Zolotorevsky, V.; Louzguine-Luzgin, D. Unusual crystallization of Al85Y8Ni5Co2 metallic glass observed in situ in TEM at different heating rates. Intermetallics 2018, 94, 192–199. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Ivanov, Y.P.; Greer, A.L. Separate primary crystallization of three crystalline phases in a nearly eutectic Cu58Y37Sc5 metallic glass on heating and deformation. J. Alloys Compd. 2023, 960, 170618. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Meylan, C.M.; Panagiotopoulos, N.T.; Georgarakis, K.; Greer, A.L. In-situ TEM study of the crystallization se-quence in a gold-based metallic glass. Acta Mater. 2020, 196, 52–60. [Google Scholar] [CrossRef]
- Köster, U.; Schünemann, U.; Stephenson, G.; Brauer, S.; Sutton, M. Polymorphic Crystallization of Metal-Metalloid-Glasses above the Glass Transition Temperature. MRS Proc. 1990, 205, 233–238. [Google Scholar] [CrossRef]
- Semin, V.; Jiang, J.; Polkin, V.; Saito, M.; Ikuhara, Y.; Louzguine-Luzgin, D.V. Crystallization of Ti-Ni-Cu-(Cr, Fe, Mn) metallic glasses. J. Alloys Compd. 2021, 876, 160185. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Ivanov, Y.P.; Semin, V.; Nohira, N.; Hosoda, H.; Greer, A.L. On polymorphic crystal growth in a Ti-Ni-Cu-Fe system metallic glass at the glass-transition temperature. Scr. Mater. 2024, 242, 115927. [Google Scholar] [CrossRef]
- Ji, Y.S.; Fujii, H.; Sun, Y.; Maeda, M.; Nakata, K.; Kimura, H.; Inoue, A.; Nogi, K. Friction Stir Welding of Zr55Cu30Ni5Al10 Bulk Metallic Glass. Mater. Trans. 2009, 50, 1300–1303. [Google Scholar] [CrossRef]
- Jun, H.-J.; Lee, K.S. Microstructural evolution of laser-welded Ti-based bulk metallic glass. Results Mater. 2023, 20, 100484. [Google Scholar] [CrossRef]
- Maeda, M.; Takahashi, Y. Welding of metallic glasses. Mater. Jpn. 2011, 50, 439–445. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, P.; Tan, J.; Ma, S.; Wu, D. Influence of the microstructure on mechanical properties of SLM additive man-ufacturing Fe-based bulk metallic glasses. J. Alloys Compd. 2022, 894, 162525. [Google Scholar] [CrossRef]
- Liu, H.S.; Jiang, Q.; Huo, J.T.; Zhang, Y.; Yang, W.M. Crystallization in additive manufacturing of metallic glasses: A review. Addit. Manuf. 2020, 36, 101568. [Google Scholar] [CrossRef]
- Pauly, S.; Schricker, C.; Scudino, S.; Deng, L.; Kühn, U. Processing a glass-forming Zr-based alloy by selective laser melting. Mater. Des. 2017, 135, 133–141. [Google Scholar] [CrossRef]
- Deng, L.; Kosiba, K.; Limbach, R.; Wondraczek, L.; Kühn, U.; Pauly, S. Plastic deformation of a Zr-based bulk metallic glass fabricated by selective laser melting. J. Mater. Sci. Technol. 2021, 60, 139–146. [Google Scholar] [CrossRef]
- Suryanarayana, C. In situ mechanical crystallization of amorphous alloys. J. Alloys Compd. 2023, 961, 171032. [Google Scholar] [CrossRef]
- Urban, P.; Fernández, F.T.; Louvier, R.M.A.; Lopez, R.A.; Cuevas, F.G. Mechanical crystallization of amorphous Ti50Al30Ni20 alloy prepared by mechanical alloying. Mater. Sci. Forum 2022, 1059, 3–8. [Google Scholar] [CrossRef]
- Abrosimova, G.; Gunderov, D.; Postnova, E.; Aronin, A. Changes in the structure of amorphous alloys under deformation by high-pressure torsion and multiple rolling. Materials 2023, 16, 1321. [Google Scholar] [CrossRef] [PubMed]
- Gunderov, D.; Astanin, V.; Churakova, A.; Sitdikov, V.; Ubyivovk, E.; Islamov, A.; Wang, J.T. Influence of high-pressure torsion and accumulative high-pressure torsion on microstructure and properties of Zr-based bulk metallic glass Vit105. Metals 2020, 10, 1433. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Bazlov, A.I.; Ketov, S.V.; Greer, A.L.; Inoue, A. Crystal growth limitation as a critical factor for formation of Fe-based bulk metallic glasses. Acta Mater. 2015, 82, 396–402. [Google Scholar] [CrossRef]
- Duarte, M.; Kostka, A.; Crespo, D.; Renner, F.; Dehm, G. Kinetics and crystallization path of a Fe-based metallic glass alloy. Acta Mater. 2017, 127, 341–350. [Google Scholar] [CrossRef]
- Paul, T.; Loganathan, A.; Agarwal, A.; Harimkar, S.P. Kinetics of isochronal crystallization in a Fe-based amorphous alloy. J. Alloys Compd. 2018, 753, 679–687. [Google Scholar] [CrossRef]
- Hirata, A.; Hirotsu, Y.; Amiya, K.; Inoue, A. Crystallization process and glass stability of an Fe48Cr15Mo14C15B6Tm2 bulk metallic glass. Phys. Rev. B 2008, 78, 144205. [Google Scholar] [CrossRef]
- Duarte, M.J.; Kostka, A.; Jimenez, J.A.; Choi, P.; Klemm, J.; Crespo, D.; Raabe, D.; Renner, F.U. Crystallization, phase evolution and corrosion of Fe-based metallic glasses: An atomic-scale structural and chemical characterization study. Acta Mater. 2014, 71, 20–30. [Google Scholar] [CrossRef]
- Lyasotsky, I.; Dyakonova, N.; Dyakonov, D. Metastable primary precipitation phases in multicomponent glass forming Fe-base alloys with metalloids. J. Alloys Compd. 2014, 586, 20–23. [Google Scholar] [CrossRef]
- Abrosimova, G.; Chirkova, V.; Volkov, N.; Straumal, B.; Aronin, A. The effect of a coating on the crystallization of multi-component Co-based amorphous alloys. Coatings 2024, 14, 116. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Trifonov, A.S.; Ivanov, Y.P.; Lu, A.K.A.; Lubenchenko, A.V.; Greer, A.L. Shear-induced chemical segregation in a Fe-based bulk metallic glass at room temperature. Sci. Rep. 2021, 11, 136502. [Google Scholar] [CrossRef] [PubMed]
- Aronin, A.S.; Volkov, N.A.; Pershina, E.A. Shear bands in amorphous alloys and their role in the formation of nanocrystals. J. Surf. Investig. 2024, 18, 27–33. [Google Scholar] [CrossRef]
- Mao, Y.; Li, J.; Lo, Y.-C.; Qian, X.; Ma, E. Stress-driven crystallization via shear-diffusion transformations in a metallic glass at very low temperatures. Phys. Rev. B 2015, 91, 214103. [Google Scholar] [CrossRef]
- Liang, S.Y.; Zhang, L.T.; Wang, Y.J.; Wang, B.; Pelletier, J.M.; Qiao, J.C. A model on the coupling between cyclic fatigue and microstructure evolution in a metallic glass. Int. J. Fatigue 2024, 187, 108446. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.; Zadorozhnyy, M.; Ketov, S.; Jiang, J.; Golovin, I.; Aronin, A. Influence of cyclic loading on the structure and double-stage structure relaxation behavior of a Zr-Cu-Fe-Al metallic glass. Mater. Sci. Eng. A 2019, 742, 526–531. [Google Scholar] [CrossRef]
- Ammari, C.; Yousfi, M.A.; Hajlaoui, K.; Georgarakis, K. Investigation on the mechanically induced nanocrystallization in me-tallic glasses. J. Alloys Compd. 2021, 859, 157864. [Google Scholar] [CrossRef]
- Shang, B.; Jakse, N.; Guan, P.; Wang, W.; Barrat, J.-L. Influence of oscillatory shear on nucleation in metallic glasses: A mo-lecular dynamics study. Acta Mater. 2023, 246, 118668. [Google Scholar] [CrossRef]
- Mitrofanov, Y.P.; Peterlechner, M.; Binkowski, I.; Divinski, S.V.; Wilde, G. The impact of elastic and plastic strain on relaxation and crystallization of Pd–Ni–P-based bulk metallic glasses. Acta Mater. 2015, 90, 318–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).