High-Temperature Oxidation and Phase Stability of AlCrCoFeNi High Entropy Alloy: Insights from In Situ HT-XRD and Thermodynamic Calculations
Abstract
1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Microstructure Analysis
2.3. Diffraction Analysis
In Situ XRD
2.4. Thermodynamic Calculations
3. Results
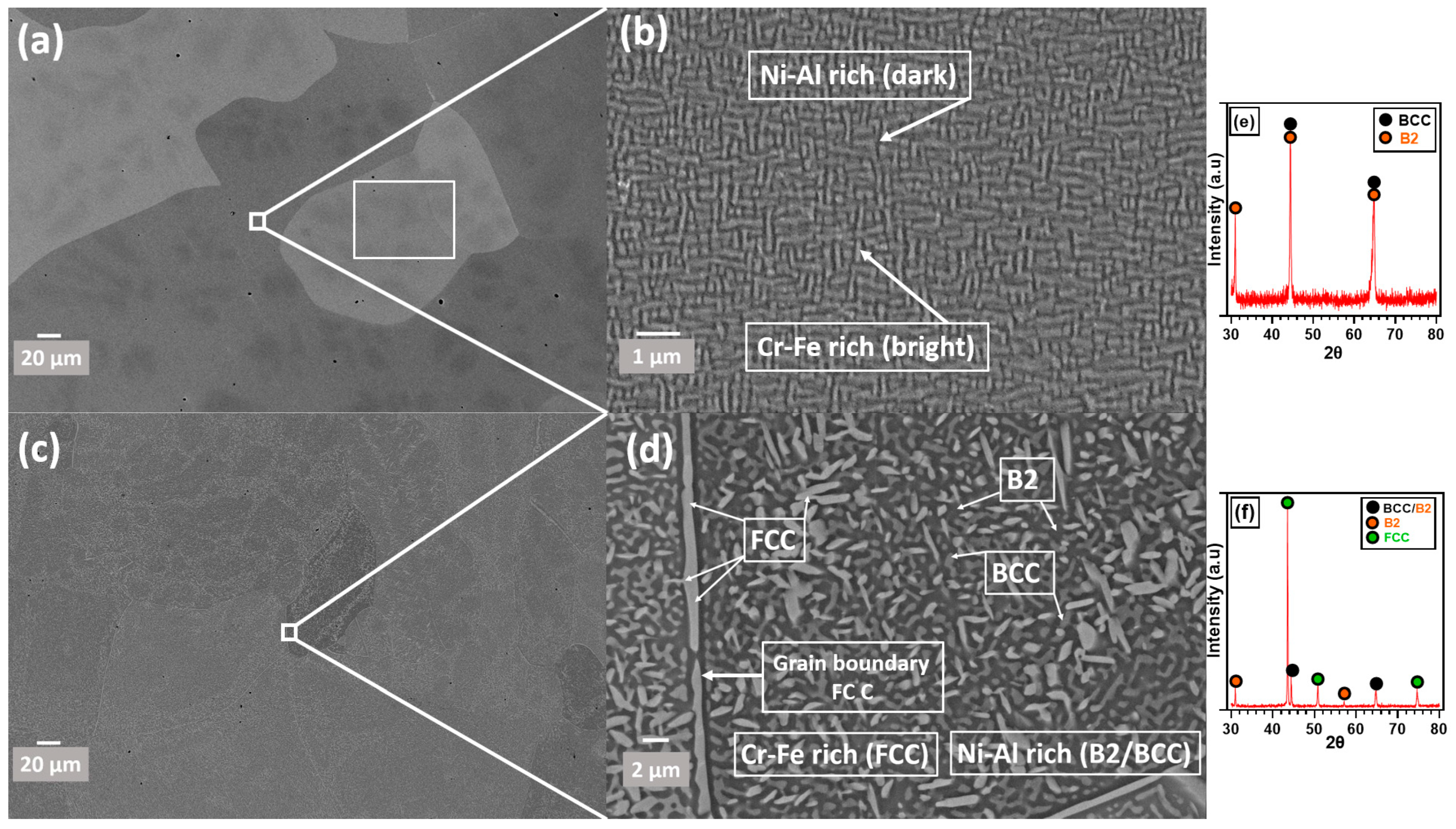
3.1. Composition and Microstructure of AlCrCoFeNi HEA
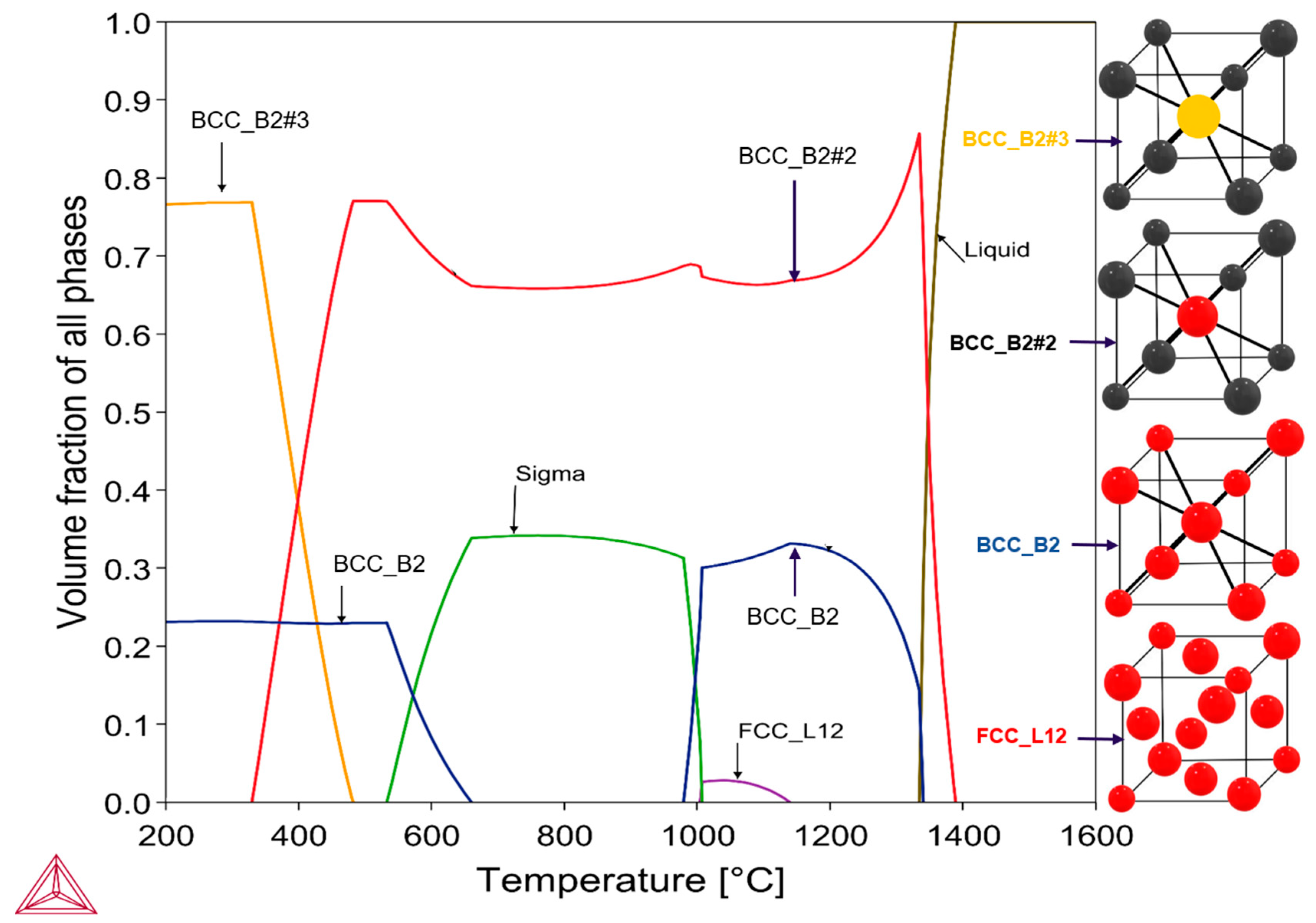
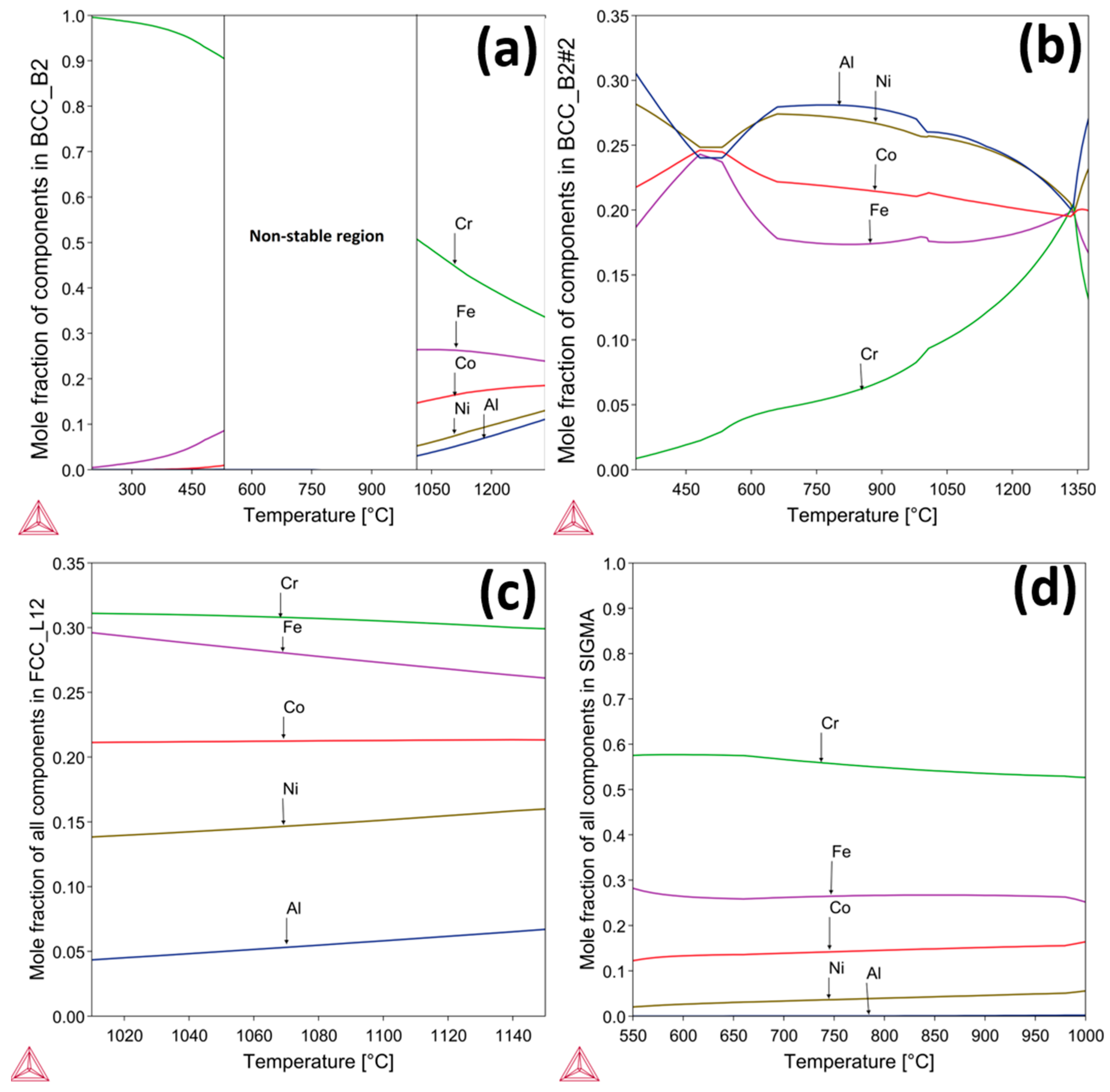
3.2. Thermodynamic Analysis of AlCrCoFeNi High Entropy Alloy
3.3. AlCrCoFeNi HEA In Situ High Temperature XRD
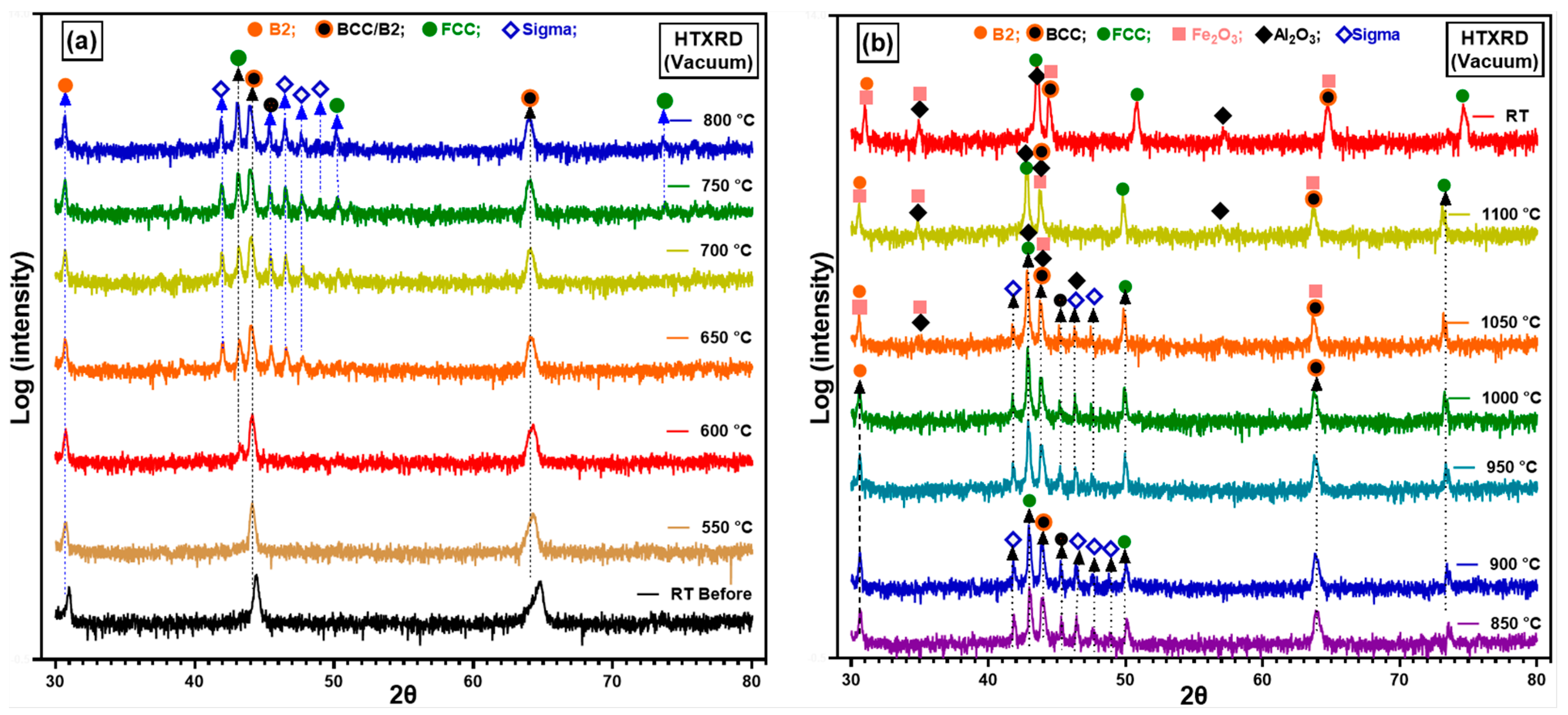
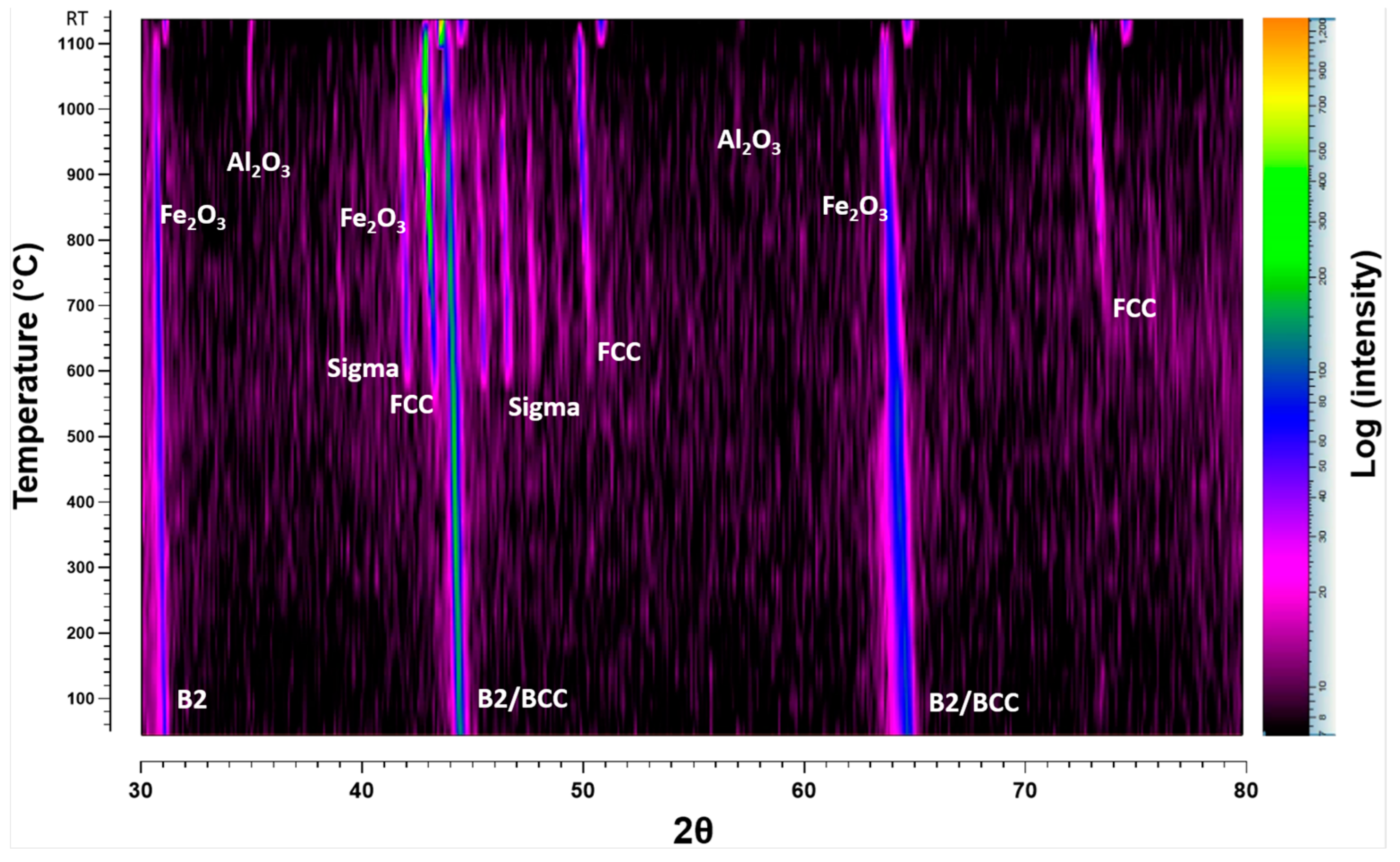
3.3.1. HTXRD in Low p(O2) Conditions
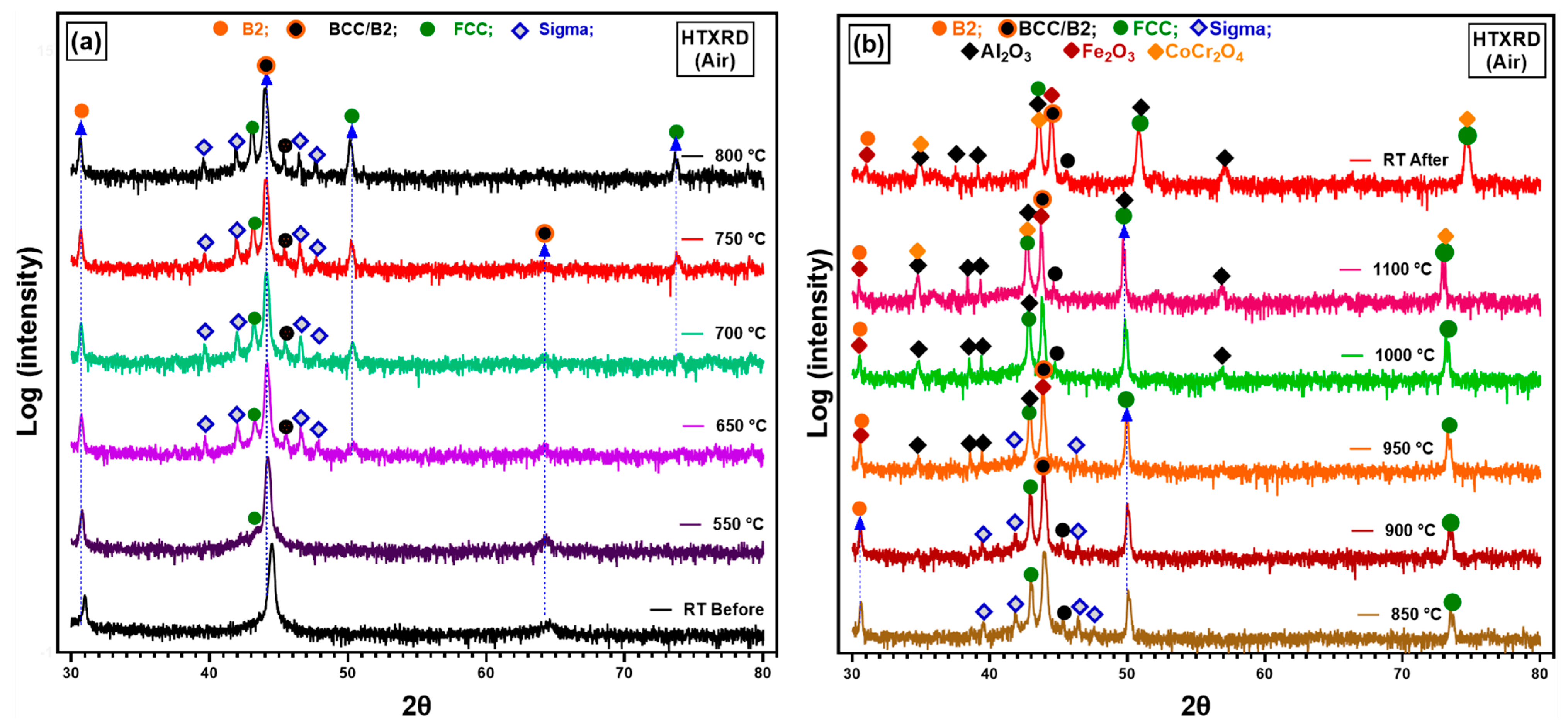
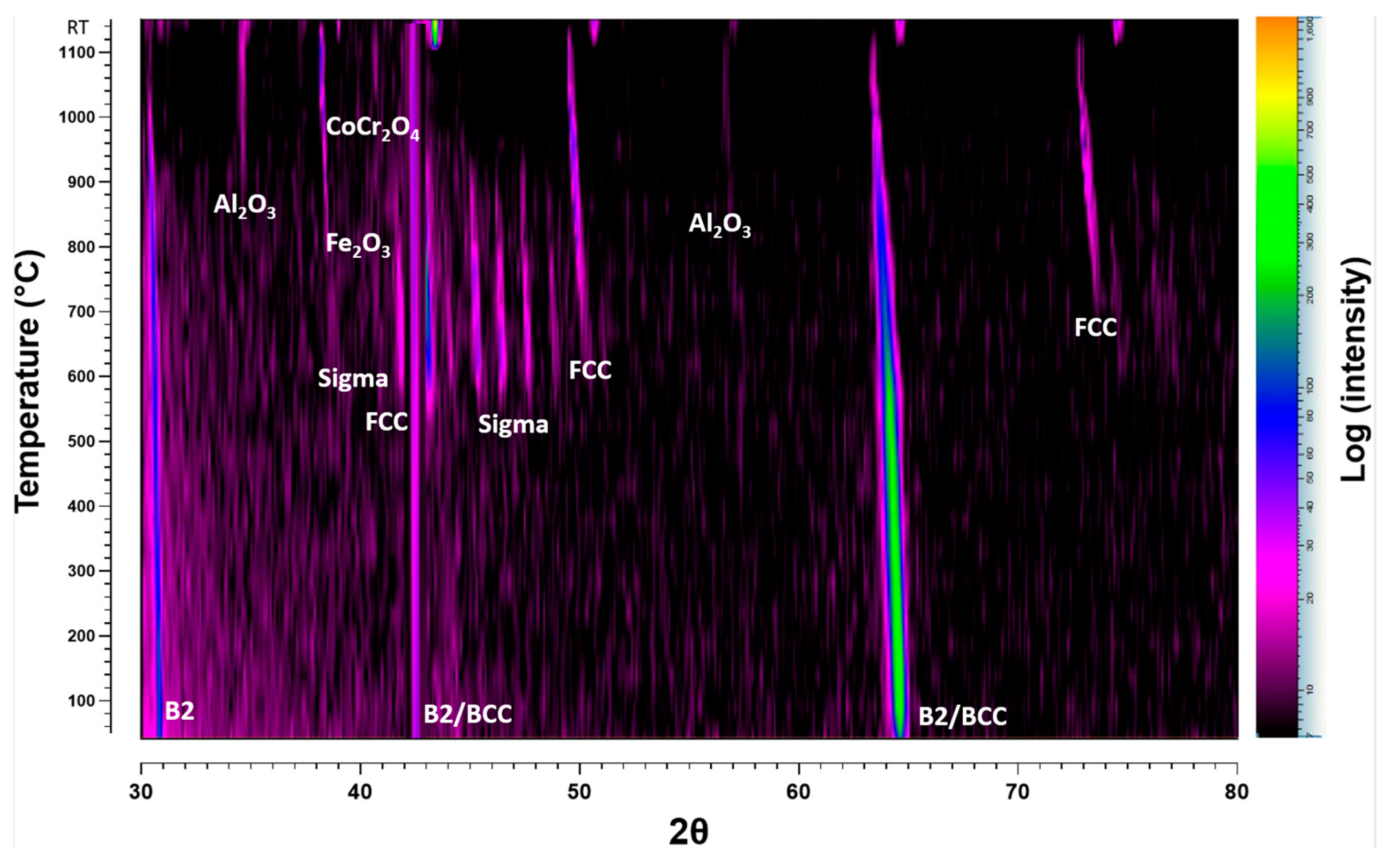
3.3.2. In Situ XRD in Atmospheric Conditions
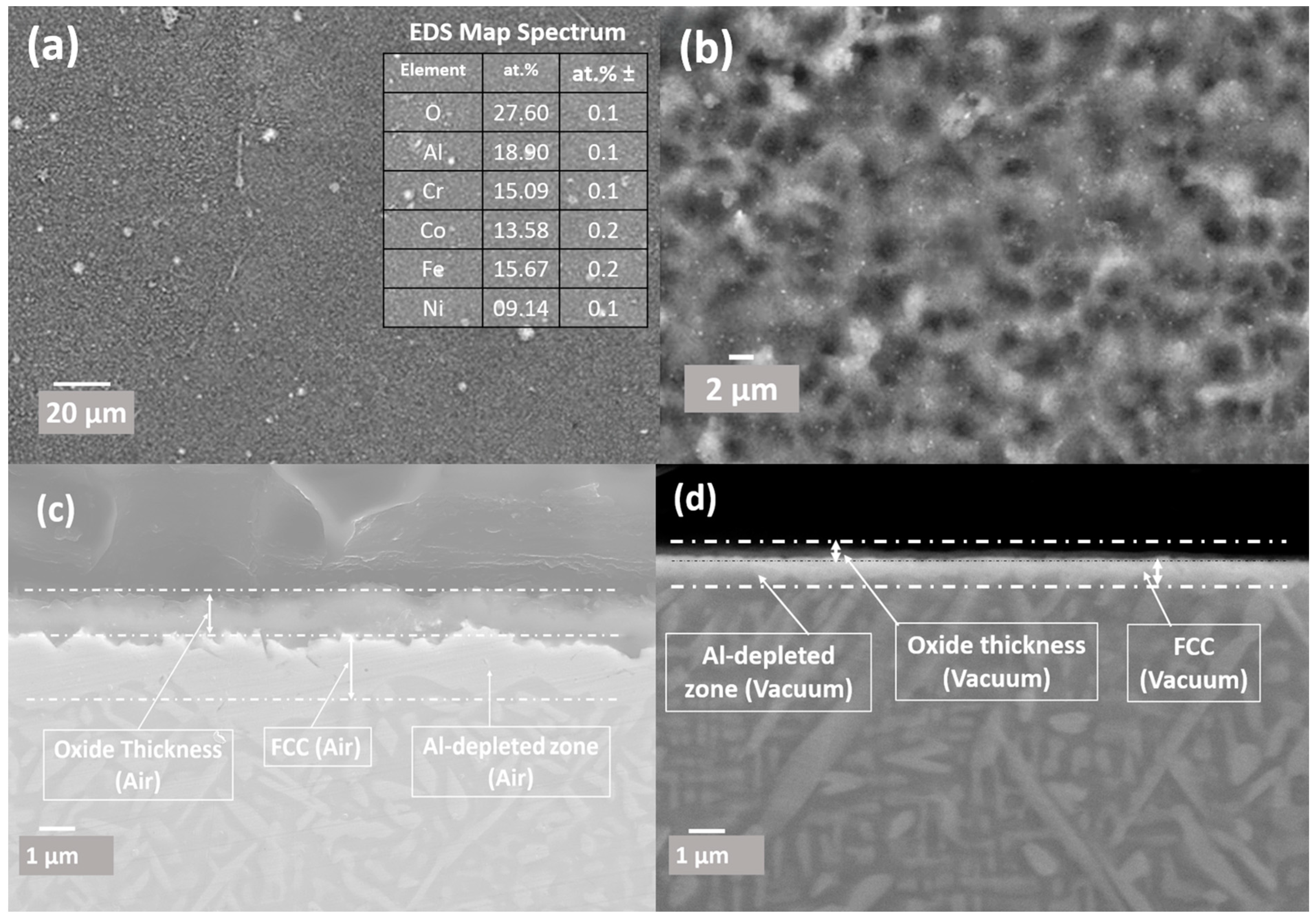
3.4. Oxide Scales Surface Analysis
3.4.1. Surface Oxides Formed in Air Conditions
3.4.2. Surface Oxidation in Vacuum Conditions
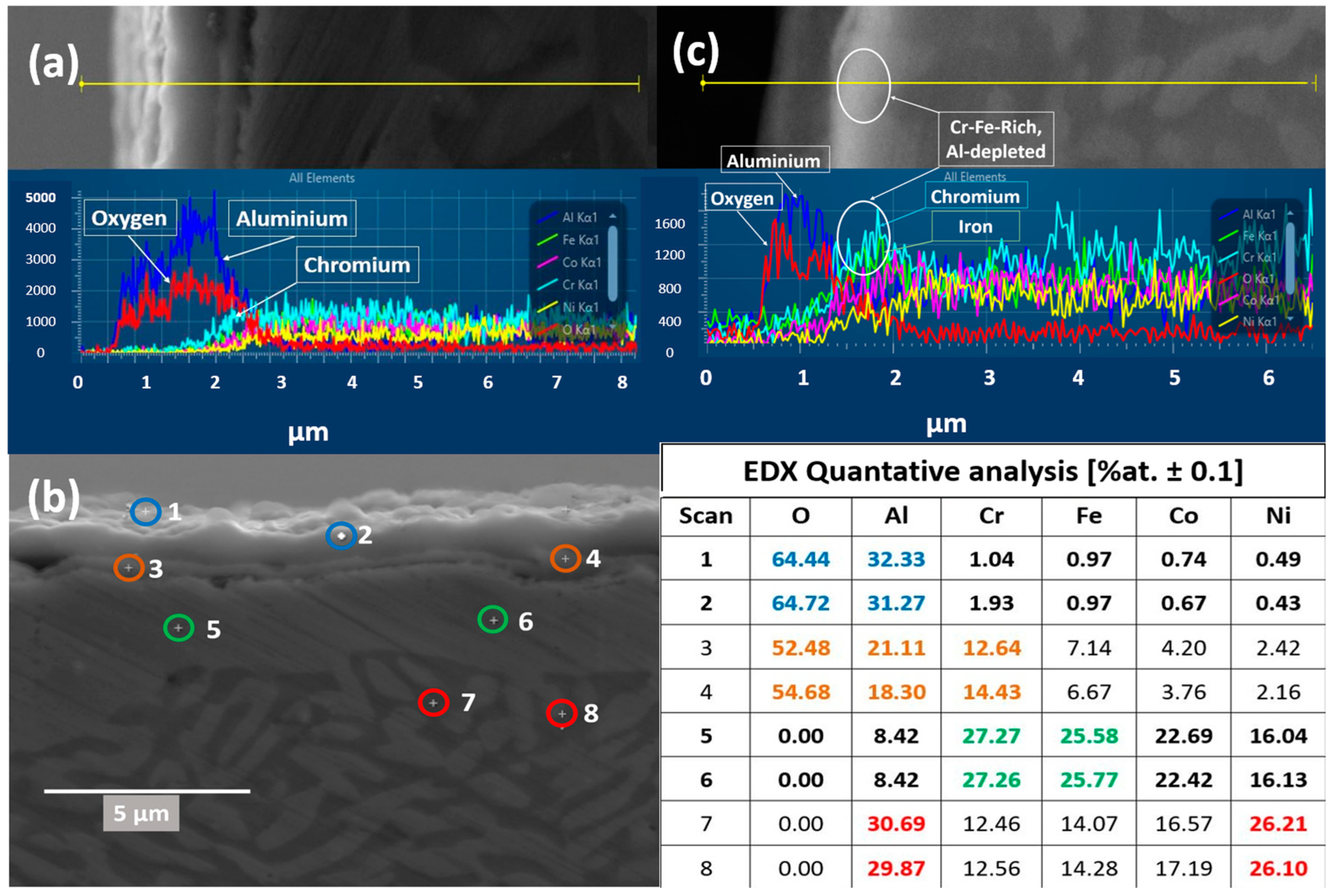
3.5. Cross-Sectional Analysis of the Oxide Scales
3.5.1. Oxides Cross-Sectional Analysis Formed in Vacuum
3.5.2. Cross-Sectional Analysis of Oxide Formed in Air
4. Discussion
4.1. Phase Stability and Microstructural Evaluation in AlCrCoFeNi HEA
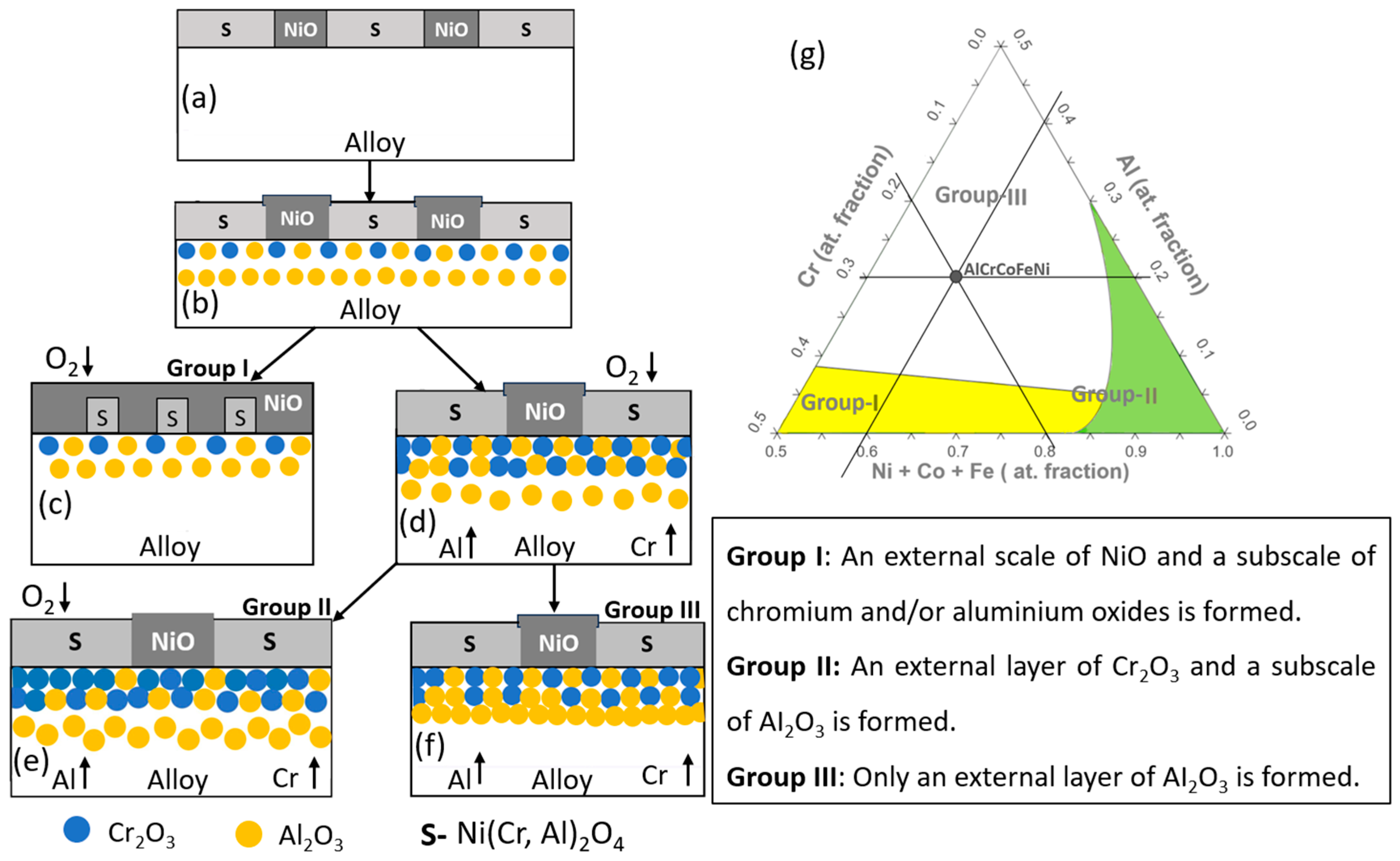
4.2. Oxidation Behaviour/Mechanism of AlCrCoFeNi HEA
4.2.1. Oxidation Behaviour in Low p(O2) Pressure
4.2.2. Oxidation Behaviour in Air Conditions
4.3. Interplay of Phase Stability and Oxidation in AlCrCoFeNi HEA
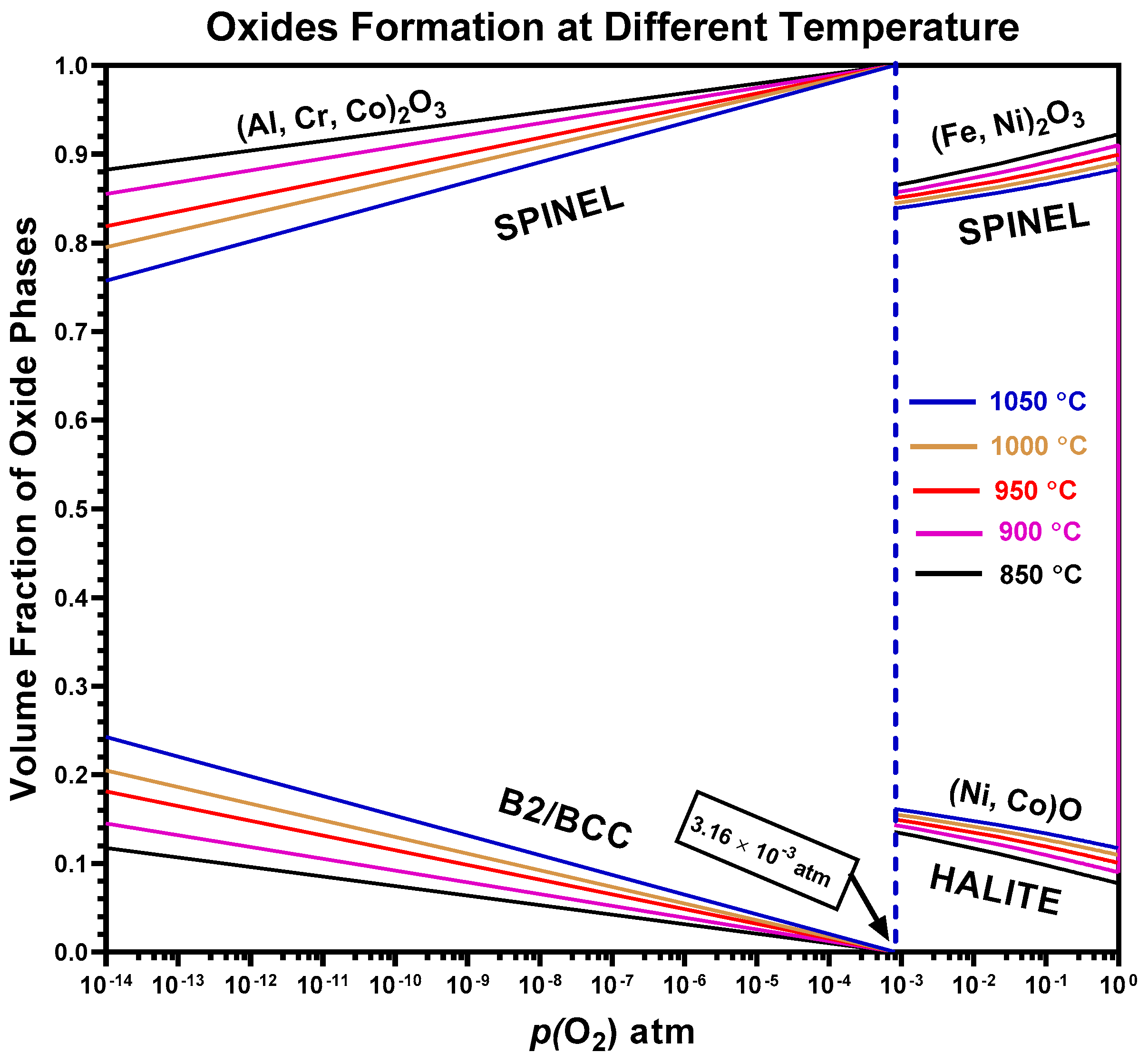
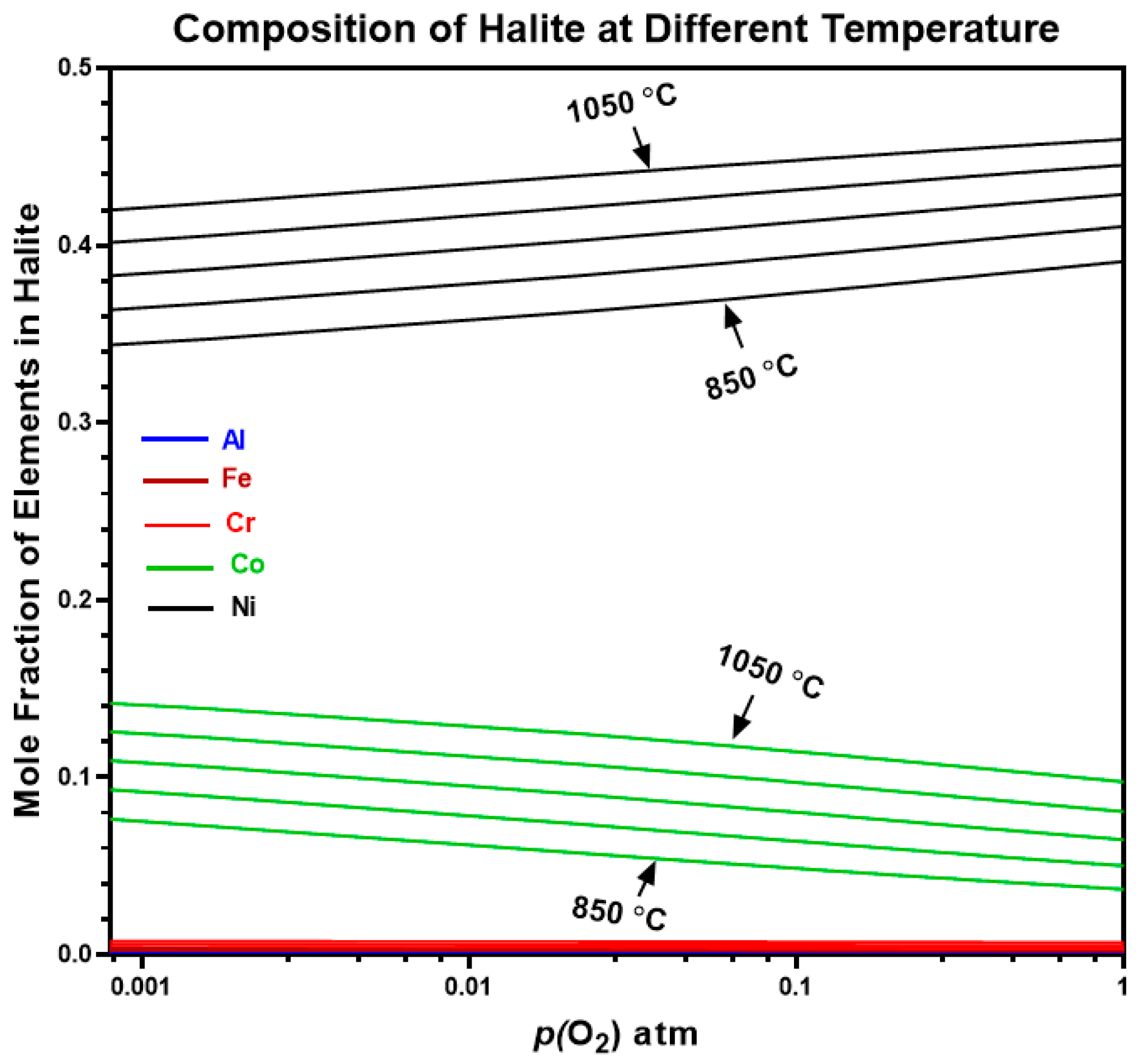
4.4. Predicted Oxides of AlCrCoFeNi HEA
5. Conclusions
- The alloy exhibits B2, BCC, Sigma and FCC, phases at various temperatures, with notable phase transitions occurring around 600 °C and 1000 °C. At intermediate temperatures, sigma phase is stable but decomposes at higher temperatures and lower temperatures as predicted by ThermoCalc.
- The creation of oxide layers of protective nature, primarily Al2O3 and Cr2O3, was observed, contributing to the alloy’s oxidation resistance. The oxide layer structure was characterised by a thick alumina top layer and a thinner chromia sublayer.
- During in situ HTXRD, the fine maze structured microstructure transformed into ultrafine micro-platelet microstructure with significant formation of FCC at expense of BCC/B2 phase. Also, it was found that thick oxide formation results in a thick layer of Al-deleted FCC phase under the oxide layer.
- Thermodynamic simulations provided valuable insights into the phase evolution and were confirmed experimentally with minor variations in temperature for different phases. Predicted spinel-type oxides as predominant oxide at high p(O2), corresponds to experimentally spinel oxide.
- The in situ HTXRD allowed real-time monitoring of phase transformations and oxide formation, highlighting the dynamic nature of the oxidation process and the significant influence of p(O2) on the oxidation behaviour.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaidya, M.; Pradeep, K.; Murty, B.; Wilde, G.; Divinski, S. Bulk tracer diffusion in CoCrFeNi and CoCrFeMnNi high entropy alloys. Acta Mater. 2018, 146, 211–224. [Google Scholar] [CrossRef]
- Arnold, K. High Temperature Oxidation Behaviour of Nickel-Base Superalloys; The University of Liverpool: Liverpool, UK, 2017. [Google Scholar]
- Akande, I.G.; Oluwole, O.; Fayomi, O.; Odunlami, O. Overview of mechanical, microstructural, oxidation properties and high-temperature applications of superalloys. Mater. Today Proc. 2021, 43, 2222–2231. [Google Scholar] [CrossRef]
- Mortazavi, N.; Geers, C.; Esmaily, M.; Babic, V.; Sattari, M.; Lindgren, K.; Malmberg, P.; Jönsson, B.; Halvarsson, M.; Svensson, J.E.; et al. Interplay of water and reactive elements in oxidation of alumina-forming alloys. Nat. Mater. 2018, 17, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Kofstad, P. High Temperature Corrosion of Metals, in Microscopy of Oxidation; CRC Press: Boca Raton, FL, USA, 2023; pp. 2–9. [Google Scholar]
- Chyrkin, A.; Mortazavi, N.; Halvarsson, M.; Grüner, D.; Quadakkers, W. Effect of thermal cycling on protective properties of alumina scale grown on thin Haynes 214 foil. Corros. Sci. 2015, 98, 688–698. [Google Scholar] [CrossRef]
- Weiser, M.; Chater, R.J.; Shollock, B.A.; Virtanen, S. Transport mechanisms during the high-temperature oxidation of ternary γ/γ′ Co-base model alloys. NPJ Mater. Degrad. 2019, 3, 33. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-entropy alloys: Potential candidates for high-temperature applications—An overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Wang, W.; Liu, B.; Lv, Y.; Yang, W.; Xu, D.; Liu, Y. A review on fundamental of high entropy alloys with promising high–temperature properties. J. Alloys Compd. 2018, 760, 15–30. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, Y.; Taylor, A.; Styles, M.; Marceau, R.; Ceguerra, A.; Gibson, M.; Liu, Z.; Fraser, H.; Birbilis, N. A lightweight single-phase AlTiVCr compositionally complex alloy. Acta Mater. 2017, 123, 115–124. [Google Scholar] [CrossRef]
- Lee, C.; Brechtl, J.; Liaw, P.K. Research on bulk-metallic glasses and high-entropy alloys in Peter K. liaw’s group and with his colleagues. Met. Mater. Trans. A 2021, 52, 2033–2093. [Google Scholar] [CrossRef]
- Kai, W.; Li, C.; Cheng, F.; Chu, K.; Huang, R.; Tsay, L.; Kai, J. The oxidation behavior of an equimolar FeCoNiCrMn high-entropy alloy at 950 °C in various oxygen-containing atmospheres. Corros. Sci. 2016, 108, 209–214. [Google Scholar] [CrossRef]
- Laplanche, G.; Volkert, U.F.; Eggeler, G.; George, E.P. Oxidation behavior of the CrMnFeCoNi high-entropy alloy. Oxid. Met. 2016, 85, 629–645. [Google Scholar] [CrossRef]
- Gorr, B.; Müller, F.; Azim, M.; Christ, H.-J.; Müller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High-temperature oxidation behavior of refractory high-entropy alloys: Effect of alloy composition. Oxid. Met. 2017, 88, 339–349. [Google Scholar] [CrossRef]
- Holcomb, G.R.; Tylczak, J.; Carney, C. Oxidation of CoCrFeMnNi high entropy alloys. JOM 2015, 67, 2326–2339. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Joo, Y.-A.; Kim, H.S.; Lee, K.-A. High temperature oxidation behavior of Cr-Mn-Fe-Co-Ni high entropy alloy. Intermetallics 2018, 98, 45–53. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Chen, Y.; Shi, J.; Wang, Z.; Wang, S.; Liu, F. Effect of Al content on high temperature oxidation resistance of AlxCoCrCuFeNi high entropy alloys (x = 0, 0.5, 1, 1.5, 2). Vacuum 2019, 169, 108837. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J. Alloys Compd. 2016, 674, 229–244. [Google Scholar] [CrossRef]
- Arshad, M.; Bano, S.; Amer, M.; Janik, V.; Hayat, Q.; Huang, Y.; Guan, D.; Bai, M. Thermodynamic Insights into the Oxidation Mechanisms of CrMnFeCoNi High-Entropy Alloy Using In Situ X-ray Diffraction. Materials 2023, 16, 5042. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, S.; Zhang, F.; Yan, X.; Xie, F.; Schmid-Fetzer, R.; Oates, W. Phase diagram calculation: Past, present and future. Prog. Mater. Sci. 2004, 49, 313–345. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Zhu, M.; Yang, T.; Shen, L. Elements loss analysis based on spectral diagnosis in laser-arc hybrid welding of aluminum alloy. Int. J. Mod. Phys. B 2017, 31, 1744036. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Mondal, S.; van Smaalen, S.; Völkl, R.; Glatzel, U.; Wanderka, N. Investigation of phases in Al23Co15Cr23Cu8Fe15Ni16 and Al8Co17Cr17Cu8Fe17Ni33 high entropy alloys and comparison with equilibrium phases predicted by Thermo-Calc. J. Alloys Compd. 2013, 552, 430–436. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Chen, S.; Cao, W. Computational thermodynamics aided high-entropy alloy design. JOM 2012, 64, 839–845. [Google Scholar] [CrossRef]
- Dolique, V.; Thomann, A.-L.; Brault, P.; Tessier, Y.; Gillon, P. Thermal stability of AlCoCrCuFeNi high entropy alloy thin films studied by in-situ XRD analysis. Surf. Coat. Technol. 2010, 204, 1989–1992. [Google Scholar] [CrossRef]
- Loeffel, K.; Anand, L.; Gasem, Z.M. On modeling the oxidation of high-temperature alloys. Acta Mater. 2013, 61, 399–424. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieslak, G.; Stygar, M.; Mroczka, K.; Berent, K.; Kulik, T.; Danielewski, M. Influence of Cu content on high temperature oxidation behavior of AlCoCrCuxFeNi high entropy alloys (x = 0; 0.5; 1). Intermetallics 2017, 84, 52–61. [Google Scholar] [CrossRef]
- Palcut, M.; Drienovský, M.; Priputen, P.; Šulhánek, P.; Stacho, P.; Gerhátová, Ž.; Gogola, P.; Krajčovič, J.; Bónová, L.; Kusý, M. Oxidation resistance of AlCoFeNiCux high entropy alloys. J. Mater. Res. Technol. 2024, 31, 1974–1990. [Google Scholar] [CrossRef]
- Butler, T.M.; Alfano, J.P.; Martens, R.L.; Weaver, M.L. High-temperature oxidation behavior of Al-Co-Cr-Ni-(Fe or Si) multicomponent high-entropy alloys. JOM 2015, 67, 246–259. [Google Scholar] [CrossRef]
- Parida, T.; Karati, A.; Guruvidyathri, K.; Murty, B.; Markandeyulu, G. Novel rare-earth and transition metal-based entropy stabilized oxides with spinel structure. Scr. Mater. 2020, 178, 513–517. [Google Scholar] [CrossRef]
- Guruvidyathri, K.; Vaidya, M.; Murty, B. Challenges in design and development of high entropy alloys: A thermodynamic and kinetic perspective. Scr. Mater. 2020, 188, 37–43. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Chen, S.; Zhu, J.; Cao, W.; Kattner, U. An understanding of high entropy alloys from phase diagram calculations. Calphad 2014, 45, 1–10. [Google Scholar] [CrossRef]
- Rogachev, A.; Kovalev, D.; Kochetov, N.; Shchukin, A.; Vadchenko, S. Evolution of crystal structure in high-entropy AlCoCrFeNi alloy: An in situ high-temperature X-ray diffraction study. J. Alloys Compd. 2021, 861, 158562. [Google Scholar] [CrossRef]
- Reynolds, C.R.; Herl, Z.; Ley, N.A.; Choudhuri, D.; Lloyd, J.T.; Young, M.L. Comparing CALPHAD predictions with high energy synchrotron radiation X-ray diffraction measurements during in situ annealing of Al0.3CoCrFeNi high entropy alloy. Materialia 2020, 12, 100784. [Google Scholar] [CrossRef]
- Strumza, E.; Hayun, S. Comprehensive study of phase transitions in equiatomic AlCoCrFeNi high-entropy alloy. J. Alloys Compd. 2021, 856, 158220. [Google Scholar] [CrossRef]
- Luo, S.; Vitos, L.; Zhao, C.; Su, Y.; Wang, Z. Predicting phase formation in complex concentrated alloys from first-principles. Comput. Mater. Sci. 2021, 186, 110021. [Google Scholar] [CrossRef]
- Panda, J.P.; Arya, P.; Guruvidyathri, K.; Ravikirana; Murty, B.S. Studies on kinetics of BCC to FCC phase transformation in AlCoCrFeNi equiatomic high entropy alloy. Met. Mater. Trans. A 2021, 52, 1679–1688. [Google Scholar] [CrossRef]
- Tatami, J. Phase Equilibrium and Phase Diagram. In Materials Chemistry of Ceramics; Springer: Singapore, 2019; pp. 23–43. [Google Scholar]
- Munitz, A.; Salhov, S.; Hayun, S.; Frage, N. Heat treatment impacts the micro-structure and mechanical properties of AlCoCrFeNi high entropy alloy. J. Alloys Compd. 2016, 683, 221–230. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Yeh, J.-W. Phases, microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloys at elevated temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Yang, T.; Xia, S.; Liu, S.; Wang, C.; Liu, S.; Fang, Y.; Zhang, Y.; Xue, J.; Yan, S.; Wang, Y. Precipitation behavior of AlxCoCrFeNi high entropy alloys under ion irradiation. Sci. Rep. 2016, 6, 32146. [Google Scholar] [CrossRef]
- Ng, C.; Guo, S.; Luan, J.; Shi, S.; Liu, C. Entropy-driven phase stability and slow diffusion kinetics in an Al0.5CoCrCuFeNi high entropy alloy. Intermetallics 2012, 31, 165–172. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Influence of annealing on the microstructures and oxidation behaviors of Al8(CoCrFeNi)92, Al15(CoCrFeNi)85, and Al30(CoCrFeNi)70 high-entropy alloys. Metals 2016, 6, 222. [Google Scholar] [CrossRef]
- Giggins, C.; Pettit, F.S. Oxidation of Ni-Cr-Al alloys between 1000 and 1200 C. J. Electrochem. Soc. 1971, 118, 1782. [Google Scholar] [CrossRef]
- Sato, I.; Takaki, M.; Arima, T.; Furuya, H.; Idemitsu, K.; Inagaki, Y.; Momoda, M.; Namekawa, T. Oxidation behavior of modified SUS316 (PNC316) stainless steel under low oxygen partial pressure. J. Nucl. Mater. 2002, 304, 21–28. [Google Scholar] [CrossRef]
- Duval, A.; Miserque, F.; Tabarant, M.; Nogier, J.-P.; Gédéon, A. Influence of the oxygen partial pressure on the oxidation of Inconel 617 alloy at high temperature. Oxid. Met. 2010, 74, 215–238. [Google Scholar] [CrossRef]
- Song, S.S.S.; Placido, F.P.F. Investigation on initial oxidation kinetics of Al, Ni, and Hf metal film surfaces. Chin. Opt. Lett. 2010, 8, 87–90. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Yan, S.; Yu, G.; Chen, J.; Yin, F. High temperature oxidation behavior of atmosphere plasma sprayed AlCoCrFeNi high-entropy alloy coatings. Mater. Chem. Phys. 2022, 282, 125939. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, S.; Jin, Y.; Xu, L.; Xu, X.; Yin, C.; Jia, Y. High-temperature oxidation behaviours of AlCoCrFeNi high-entropy alloy at 1073–1273 K. Oxid. Met. 2020, 94, 265–281. [Google Scholar] [CrossRef]
- Society, E. Journal of the Electrochemical Society; Electrochemical Society: Honolulu, HI, USA, 2009. [Google Scholar]
- Lu, J.; Zhang, H.; Chen, Y.; Zhao, X.; Guo, F.; Xiao, P. Effect of microstructure of a NiCoCrAlY coating fabricated by high-velocity air fuel on the isothermal oxidation. Corros. Sci. 2019, 159, 108126. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhao, C.; Zhang, H.; Luo, L.; Xu, B.; Zhao, X.; Guo, F.; Xiao, P. Significantly improving the oxidation and spallation resistance of a MCrAlY alloy by controlling the distribution of yttrium. Corros. Sci. 2019, 153, 178–190. [Google Scholar] [CrossRef]
- Veselkov, S.; Samoilova, O.; Shaburova, N.; Trofimov, E. High-temperature oxidation of high-entropic alloys: A review. Materials 2021, 14, 2595. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kivy, M.B.; Zaeem, M.A. Competition between formation of Al2O3 and Cr2O3 in oxidation of Al0.3CoCrCuFeNi high entropy alloy: A first-principles study. Scr. Mater. 2019, 168, 139–143. [Google Scholar] [CrossRef]



| Temperature | 1000 (°C) | |
| Pressure | 101,325 (Pa) | |
| System Size | 1.0 (Mole) | |
| Composition (at.%) | Al | 20.0 |
| Ni | 20.0 | |
| Co | 20.0 | |
| Fe | 20.0 | |
| Cr | 20.0 | |
| O | 05.0 | |
| O2 Activity | Min | 1.0 × 10−14 |
| Max | 1.0 | |
| Steps | 100 | |
| Composition (at%) | Co | Cr | Al | Fe | Ni |
|---|---|---|---|---|---|
| Nominal | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Measured (EDS) | 20.72 ± 0.2 | 20.95 ± 0.2 | 17.26 ± 0.1 | 20.72 ± 0.2 | 20.35 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, M.; Bano, S.; Amer, M.; Janik, V.; Hayat, Q.; Bai, M. High-Temperature Oxidation and Phase Stability of AlCrCoFeNi High Entropy Alloy: Insights from In Situ HT-XRD and Thermodynamic Calculations. Materials 2024, 17, 3579. https://doi.org/10.3390/ma17143579
Arshad M, Bano S, Amer M, Janik V, Hayat Q, Bai M. High-Temperature Oxidation and Phase Stability of AlCrCoFeNi High Entropy Alloy: Insights from In Situ HT-XRD and Thermodynamic Calculations. Materials. 2024; 17(14):3579. https://doi.org/10.3390/ma17143579
Chicago/Turabian StyleArshad, Muhammad, Saira Bano, Mohamed Amer, Vit Janik, Qamar Hayat, and Mingwen Bai. 2024. "High-Temperature Oxidation and Phase Stability of AlCrCoFeNi High Entropy Alloy: Insights from In Situ HT-XRD and Thermodynamic Calculations" Materials 17, no. 14: 3579. https://doi.org/10.3390/ma17143579
APA StyleArshad, M., Bano, S., Amer, M., Janik, V., Hayat, Q., & Bai, M. (2024). High-Temperature Oxidation and Phase Stability of AlCrCoFeNi High Entropy Alloy: Insights from In Situ HT-XRD and Thermodynamic Calculations. Materials, 17(14), 3579. https://doi.org/10.3390/ma17143579











