Insights into Cis-Amide-Modified Carbon Nanotubes for Selective Purification of CH4 and H2 from Gas Mixtures: A Comparative DFT Study
Abstract
:1. Introduction
2. Models and Computational Details
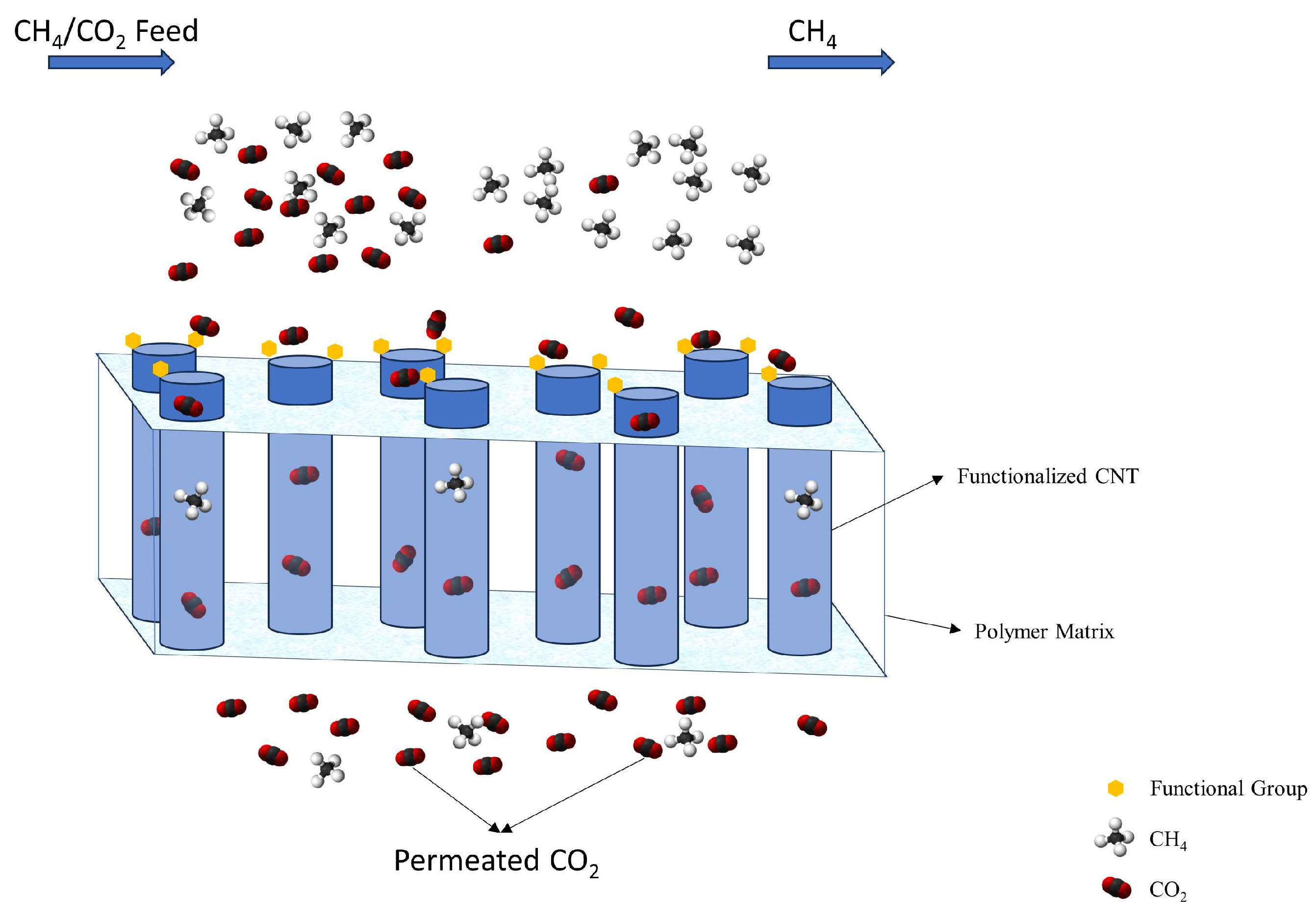
3. Results and Discussion
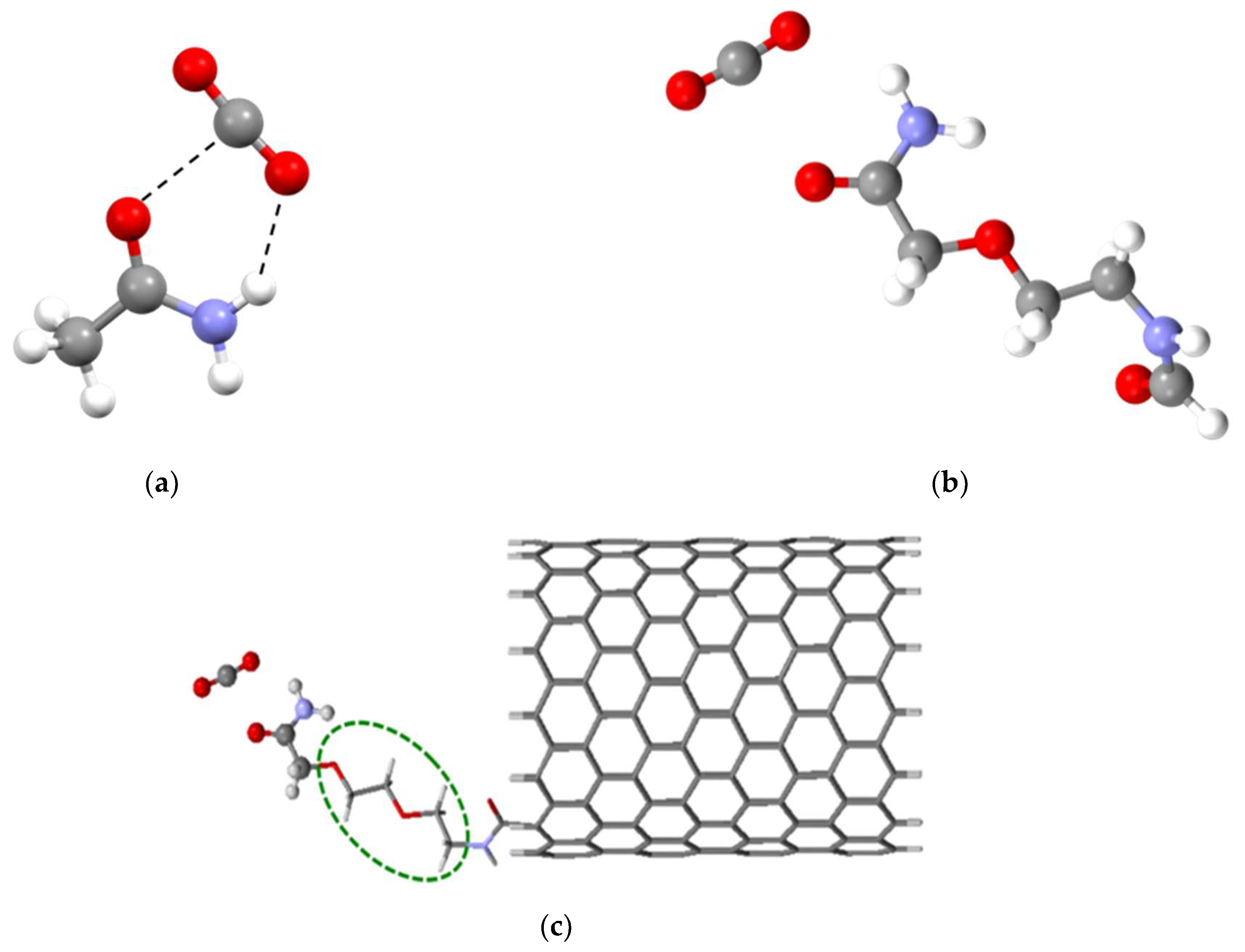
3.1. Design of the Functional Group
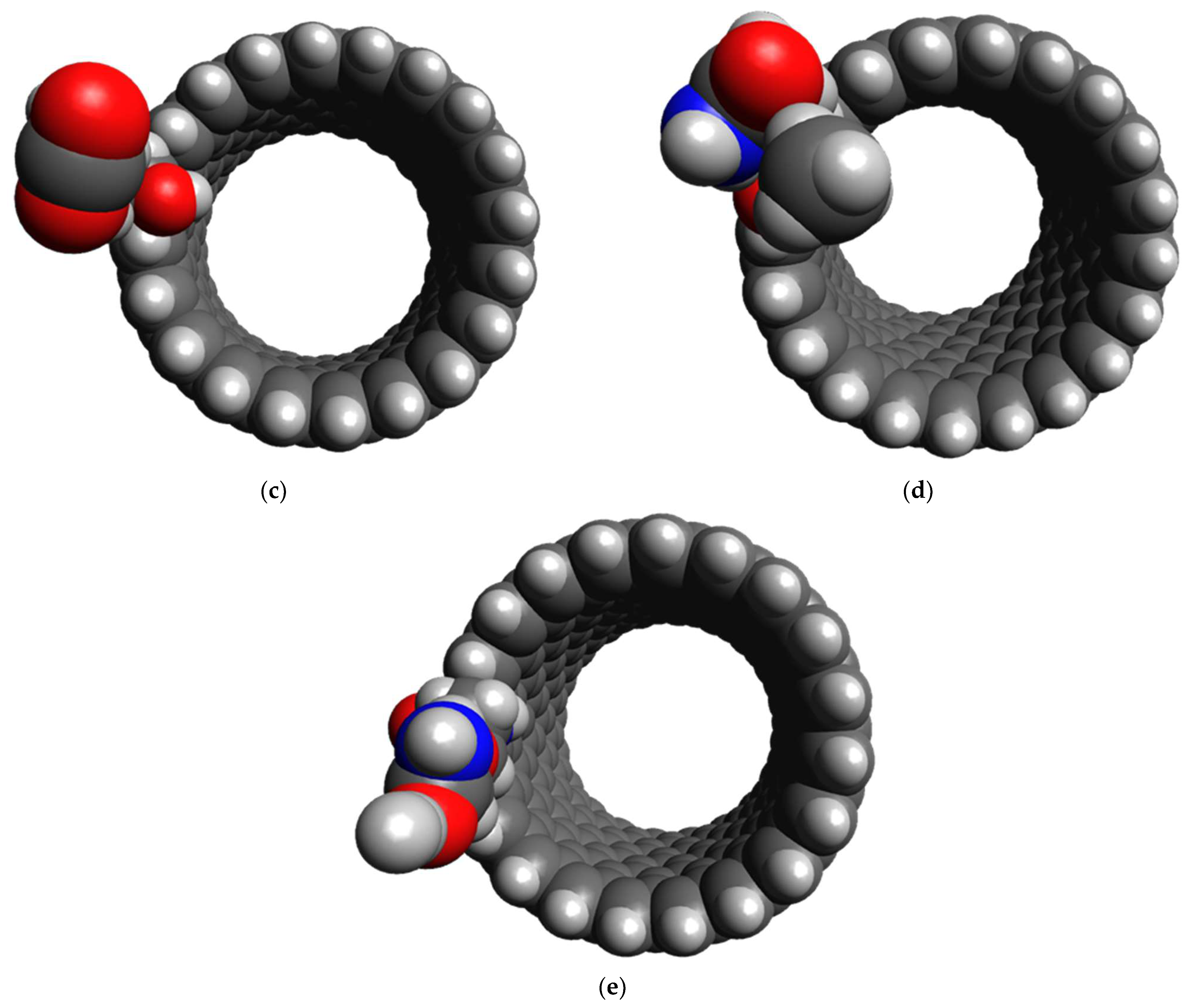
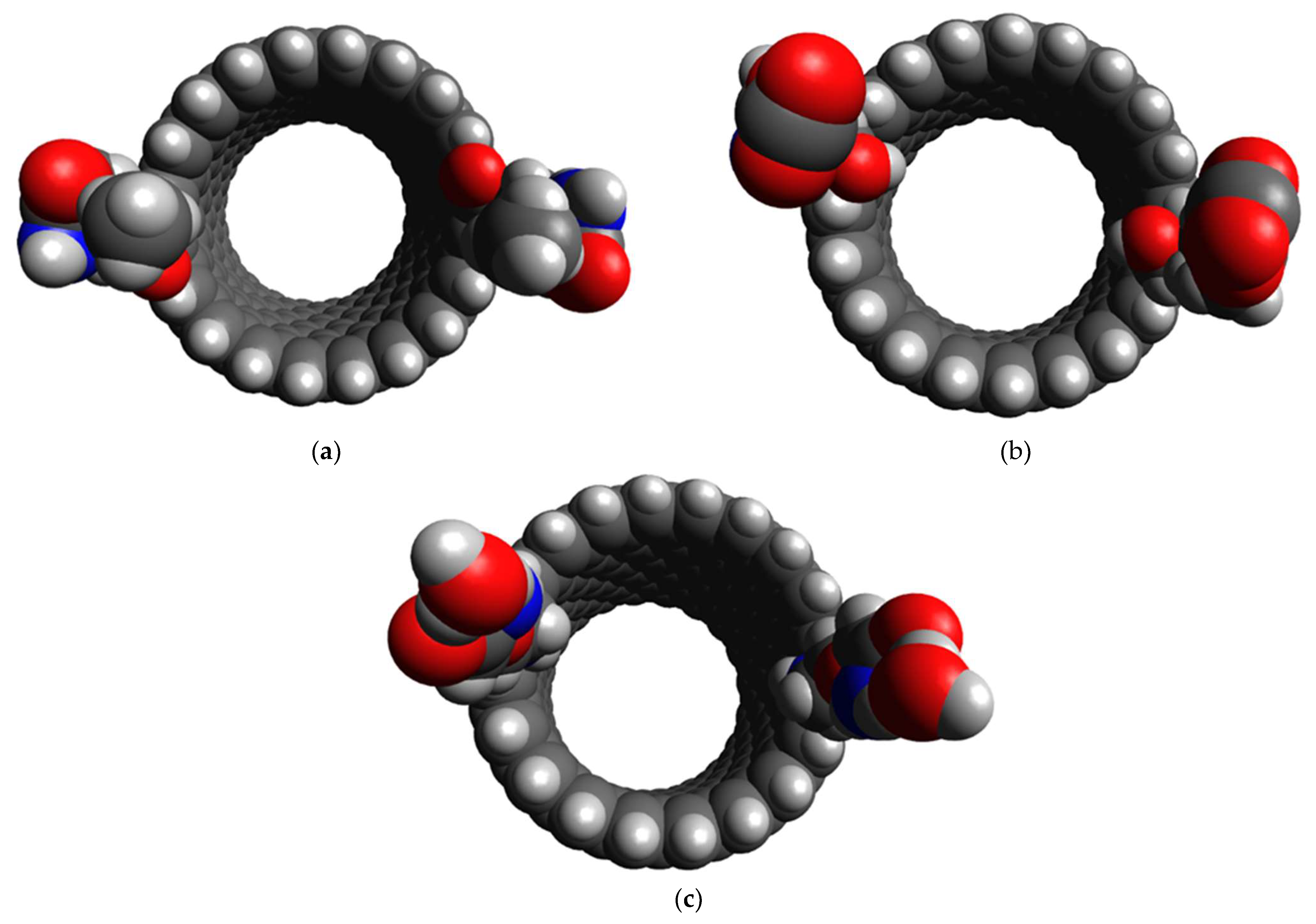
3.2. Affinity of the Functionalized CNT Edge toward the Target Gases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habib, R.; Yadollahi, B.; Saeed, A.; Doranehgard, M.H.; Karimi, N. On the Response of Ultralean Combustion of CH4/H2 Blends in a Porous Burner to Fluctuations in Fuel Flow—An Experimental Investigation. Energy Fuels 2021, 35, 8909–8921. [Google Scholar] [CrossRef]
- Lopez-Ruiz, G.; Alava, I.; Blanco, J.M. Impact of H2/CH4 blends on the flexibility of micromix burners applied to industrial combustion systems. Energy 2023, 270, 126882. [Google Scholar] [CrossRef]
- du Toit, M.; Engelbrecht, N.; Oelofse, S.P.; Bessarabov, D. Performance evaluation and emissions reduction of a micro gas turbine via the co-combustion of H2/CH4/CO2 fuel blends. Sustain. Energy Technol. Assess. 2020, 39, 100718. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Q. A review on mixing laws of laminar flame speed and their applications on H2/CH4/CO/air mixtures. Int. J. Hydrogen Energy 2020, 45, 20482–20490. [Google Scholar] [CrossRef]
- Yang, M.; Liu, W.; Sun, C.; Yu, W.; Lu, Y.; Liu, H.; An, Z.; Wang, J.; Lan, X.; Shi, T.; et al. Applicability Evaluation and Demonstration of Domestic Gas Appliance Using Natural Gas-Hydrogen Mixture. Mech. Eng. 2022, 44, 794–800. [Google Scholar]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Liu, X.; Elgowainy, A.; Wang, M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green Chem. 2020, 22, 5751–5761. [Google Scholar] [CrossRef]
- Su, B.; Luo, Z.; Wang, T.; Xie, C.; Cheng, F. Chemical kinetic behaviors at the chain initiation stage of CH4/H2/air mixture. J. Hazard. Mater. 2021, 403, 123680. [Google Scholar] [CrossRef]
- Kıymaz, T.B.; Böncü, E.; Güleryüz, D.; Karaca, M.; Yılmaz, B.; Allouis, C.; Gökalp, İ. Numerical investigations on flashback dynamics of premixed methane-hydrogen-air laminar flames. Int. J. Hydrogen Energy 2022, 47, 25022–25033. [Google Scholar] [CrossRef]
- Zhen, X.; Li, X.; Wang, Y.; Liu, D.; Tian, Z. Comparative study on combustion and emission characteristics of methanol/hydrogen, ethanol/hydrogen and methane/hydrogen blends in high compression ratio SI engine. Fuel 2020, 267, 117193. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.T.; Tsang, Y.F.; Lee, J. Integration of thermochemical conversion processes for waste-to-energy: A review. Korean J. Chem. Eng. 2023, 40, 1815–1821. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, C.; Zhang, D.; Wen, X.; Deng, H.; Chen, G. The effect of CH4 addition on high-H2 syngas combustion characteristics under N2/CO2 dilution. J. Energy Inst. 2024, 114, 101600. [Google Scholar] [CrossRef]
- Alavandi, S.K.; Agrawal, A.K. Experimental study of combustion of hydrogen–syngas/methane fuel mixtures in a porous burner. Int. J. Hydrogen Energy 2008, 33, 1407–1415. [Google Scholar] [CrossRef]
- Peters, R.; Baltruweit, M.; Grube, T.; Samsun, R.C.; Stolten, D. A techno economic analysis of the power to gas route. J. CO2 Util. 2019, 34, 616–634. [Google Scholar] [CrossRef]
- Ozturk, M.; Dincer, I. A comprehensive review on power-to-gas with hydrogen options for cleaner applications. Int. J. Hydrogen Energy 2021, 46, 31511–31522. [Google Scholar] [CrossRef]
- Reiter, G.; Lindorfer, J. Global warming potential of hydrogen and methane production from renewable electricity via power-to-gas technology. Int. J. LCA 2015, 20, 477–489. [Google Scholar] [CrossRef]
- Mehrjerdi, H. Optimal correlation of non-renewable and renewable generating systems for producing hydrogen and methane by power to gas process. Int. J. Hydrogen Energy 2019, 44, 9210–9219. [Google Scholar] [CrossRef]
- Belacel, M.; Hadef, A.; Mameri, A.; Tou, I.; Skender, A.; Salhi, N. Statistical approach and numerical analysis of turbulent diffusion flame of CH4/H2 mixture. Energy Source Part A 2024, 46, 636–658. [Google Scholar] [CrossRef]
- Mendoza Morales, M.J.; Blondeau, J.; De Paepe, W. Assessing the impact of CH4/H2 blends on the thermodynamic performance of aero-derivative gas turbine CHP configurations. Int. J. Hydrogen Energy 2024, 67, 159–171. [Google Scholar] [CrossRef]
- Ziani, L.; Chaker, A. Ambient pressure effect on non-premixed turbulent combustion of CH4–H2 mixture. Int. J. Hydrogen Energy 2016, 41, 11842–11847. [Google Scholar] [CrossRef]
- Fan, G.; Hou, Y.; Huang, D.; Dang, J.; Zhang, R.; Xiang, J.; Lv, X.; Ding, X. Synthesis of Ti(C, N, O) ceramic from rutile at low temperature by CH4-H2-N2 gas mixture. Int. J. Refract. Met. Hard Mater. 2021, 101, 105659. [Google Scholar] [CrossRef]
- Berwal, P.; Khandelwal, B.; Kumar, S. Effect of ammonia addition on laminar burning velocity of CH4/H2 premixed flames at high pressure and temperature conditions. Int. J. Hydrogen Energy 2024, 49, 112–125. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Fatah, A. Impact of H2-CH4 mixture on pore structure of sandstone and limestone formations relevant to subsurface hydrogen storage. Fuel 2024, 358, 130192. [Google Scholar] [CrossRef]
- Ånestad, A.; Sampath, R.; Moeck, J.; Gruber, A.; Worth, N.A. The Structure and Stability of Premixed CH4, H2, and NH3/H2 Flames in an Axially Staged Can Combustor. J. Eng. Gas Turbine Power 2023, 146, V03BT04A022. [Google Scholar]
- Zhen, H.S.; Cheung, C.S.; Leung, C.W.; Choy, Y.S. A comparison of the emission and impingement heat transfer of LPG–H2 and CH4–H2 premixed flames. Int. J. Hydrogen Energy 2012, 37, 10947–10955. [Google Scholar] [CrossRef]
- Hou, S.-S.; Chiang, C.-Y.; Lin, T.-H. Oxy-Fuel Combustion Characteristics of Pulverized Coal under O2/Recirculated Flue Gas Atmospheres. Appl. Sci. 2020, 10, 1362. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S.; He, Y.; Yang, Y.; Lu, Q. Radiation effects of CO2 and H2O dilution on laminar flame speeds of syngas flames under elevated pressures. Int. J. Hydrogen Energy 2023, 48, 38870–38877. [Google Scholar] [CrossRef]
- Barker, R.; Hua, Y.; Neville, A. Internal corrosion of carbon steel pipelines for dense-phase CO2 transport in carbon capture and storage (CCS)—A review. Int. Mater. Rev. 2017, 62, 1–31. [Google Scholar] [CrossRef]
- Siažik, J.; Malcho, M. Accumulation of Primary Energy Into Natural Gas Hydrates. Procedia Eng. 2017, 192, 782–787. [Google Scholar] [CrossRef]
- Katsoulakos, N.; Doulos, I.; Kaliampakos, D. Sustainable organic waste treatment in mountainous areas through small biogas plants: Insights from Metsovo, Greece. In Proceedings of the 5th International Conference on Sustainable Solid Waste Management, Athens, Greece, 21–24 June 2017. [Google Scholar]
- Dai, Z.; Deng, L. Membranes for CO2 capture and separation: Progress in research and development for industrial applications. Sep. Purif. Technol. 2024, 335, 126022. [Google Scholar] [CrossRef]
- Roozitalab, A.; Hamidavi, F.; Kargari, A. A review of membrane material for biogas and natural gas upgrading. Gas Sci. Eng. 2023, 114, 204969. [Google Scholar] [CrossRef]
- Singla, S.; Shetti, N.P.; Basu, S.; Mondal, K.; Aminabhavi, T.M. Hydrogen production technologies—Membrane based separation, storage and challenges. J. Environ. Manag. 2022, 302, 113963. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Seong, J.G.; Hu, X.; Lee, Y.M. Recent progress in microporous polymers from thermally rearranged polymers and polymers of intrinsic microporosity for membrane gas separation: Pushing performance limits and revisiting trade-off lines. J. Polym. Sci. 2020, 58, 2450–2466. [Google Scholar] [CrossRef]
- León, N.E.; Liu, Z.; Irani, M.; Koros, W.J. How to Get the Best Gas Separation Membranes from State-of-the-Art Glassy Polymers. Macromolecules 2022, 55, 1457–1473. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Wong, D.A.; Yang, J. Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation. Polymers 2021, 13, 2539. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Z.; Dai, Y.; Mu, P.; Li, J. Two-dimensional nanomaterial MXenes for efficient gas separation: A review. Nanoscale 2023, 15, 4170–4194. [Google Scholar] [CrossRef] [PubMed]
- Bilchak, C.R.; Jhalaria, M.; Huang, Y.; Abbas, Z.; Midya, J.; Benedetti, F.M.; Parisi, D.; Egger, W.; Dickmann, M.; Minelli, M.; et al. Tuning Selectivities in Gas Separation Membranes Based on Polymer-Grafted Nanoparticles. ACS Nano 2020, 14, 17174–17183. [Google Scholar] [CrossRef]
- Goh, P.S.; Wong, K.C.; Ismail, A.F. Nanocomposite Membranes for Liquid and Gas Separations from the Perspective of Nanostructure Dimensions. Membranes 2020, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Buonomenna, M.G.; Yave, W.; Golemme, G. Some approaches for high performance polymer based membranes for gas separation: Block copolymers, carbon molecular sieves and mixed matrix membranes. RSC Adv. 2012, 2, 10745–10773. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Sears, K.; Dumée, L.; Schütz, J.; She, M.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Recent Developments in Carbon Nanotube Membranes for Water Purification and Gas Separation. Materials 2010, 3, 127–149. [Google Scholar] [CrossRef]
- Amirkhani, F.; Mosadegh, M.; Asghari, M.; Parnian, M.J. The beneficial impacts of functional groups of CNT on structure and gas separation properties of PEBA mixed matrix membranes. Polym. Test. 2020, 82, 106285. [Google Scholar] [CrossRef]
- Ashirov, T.; Yazaydin, A.O.; Coskun, A. Tuning the Transport Properties of Gases in Porous Graphene Membranes with Controlled Pore Size and Thickness. Adv. Mater. 2022, 34, 2106785. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.M.; Baniasadi, A.; Moradi, M. Recent progress in molecular simulation of nanoporous graphene membranes for gas separation. J. Korean Phys. Soc. 2017, 71, 54–62. [Google Scholar] [CrossRef]
- Gu, Z.; Shi, Z.; Lin, G.; Zeng, S.; Elmegreen, B.; Luan, B. Highly efficient and selective H2/CH4 separation by graphene membranes with embedded crown ethers. Int. J. Hydrogen Energy 2022, 47, 24835–24842. [Google Scholar] [CrossRef]
- Favvas, E.; Nitodas, S.; Stefopoulos, A.; Papageorgiou, S.; Stefanopoulos, K.; Mitropoulos, A. High purity multi-walled carbon nanotubes: Preparation, characterization and performance as filler materials in co-polyimide hollow fiber membranes. Sep. Purif. Technol. 2014, 122, 262–269. [Google Scholar] [CrossRef]
- Favvas, E.; Stefanopoulos, K.; Nolan, J.; Papageorgiou, S.; Mitropoulos, A.; Lairez, D. Mixed Matrix Hollow Fiber Membranes with enhanced gas permeation properties. Sep. Purif. Technol. 2014, 132, 336–345. [Google Scholar] [CrossRef]
- Sharma, A.; Tripathi, B.; Vijay, Y.K. Dramatic Improvement in properties of magnetically aligned CNT/polymer nanocomposites. J. Memb. Sci. 2010, 361, 89–95. [Google Scholar] [CrossRef]
- Labropoulos, A.; Veziri, C.; Kapsi, M.; Pilatos, G.; Likodimos, V.; Tsapatsis, M.; Kanellopoulos, N.K.; Romanos, G.E.; Karanikolos, G.N. Carbon Nanotube Selective Membranes with Subnanometer, Vertically Aligned Pores, and Enhanced Gas Transport Properties. Chem. Mater. 2015, 27, 8198–8210. [Google Scholar] [CrossRef]
- Thornton, A.; Ahmed, A.; Stelzl, K.; Schaefer, A.; Majumder, M.; Park, H.B.; Hill, A.; Todd, B. Chapter 11 Ultrafast Transport in Nanotubes and Nanosheets. In Nanotubes and Nanosheets Functionalization and Applications of Boron Nitride and Other Nanomaterials; Chen, Y.I., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 271–304. [Google Scholar]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.M.; Lillepaerg, J.; Abetz, V. Enhanced gas permeability by fabricating mixed matrix membranes of functionalized multiwalled carbon nanotubes and polymers of intrinsic microporosity (PIM). J. Memb. Sci. 2013, 436, 109–120. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Wang, C.; Guo, R. Carbon nanotube-based mixed-matrix membranes with supramolecularly engineered interface for enhanced gas separation performance. J. Memb. Sci. 2019, 598, 117794. [Google Scholar] [CrossRef]
- Du, F.; Qu, L.; Xia, Z.; Feng, L.; Dai, L. Membranes of Vertically Aligned Superlong Carbon Nanotubes. Langmuir 2011, 27, 8437–8443. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Chopra, N.; Andrews, R.; Hinds, B.J. Enhanced flow in carbon nanotubes. Nature 2005, 438, 44. [Google Scholar] [CrossRef]
- Kim, S.; Fornasiero, F.; Park, H.G.; In, J.B.; Meshot, E.; Giraldo, G.; Stadermann, M.; Fireman, M.; Shan, J.; Grigoropoulos, C.P.; et al. Fabrication of flexible, aligned carbon nanotube/polymer composite membranes by in-situ polymerization. J. Memb. Sci. 2014, 460, 91–98. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Memb. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Majumder, M.; Chopra, N.; Hinds, B.J. Mass Transport through Carbon Nanotube Membranes in Three Different Regimes: Ionic Diffusion and Gas and Liquid Flow. ACS Nano 2011, 5, 3867–3877. [Google Scholar] [CrossRef]
- Lee, K.-J.; Park, H.-D. The most densified vertically-aligned carbon nanotube membranes and their normalized water permeability and high pressure durability. J. Memb. Sci. 2016, 501, 144–151. [Google Scholar] [CrossRef]
- Favvas, E.; Heliopoulos, N.; Papageorgiou, S.; Ch, A.; Mitropoulos; Kapantaidakis, G.; Kanrllopoulos, N. Helium and hydrogen selective carbon hollow fiber membranes: The effect of pyrolysis isothermal time. Sep. Purif. Technol. 2015, 142, 176. [Google Scholar] [CrossRef]
- Gotzias, A.; Sapalidis, A.; Favvas, E. Hydrogen adsorption simulations in isomorphous borohydride and imidazolate frameworks: Evaluations using interpolation. Int. J. Hydrogen Energy 2021, 46, 19778–19787. [Google Scholar] [CrossRef]
- Karousos, D.S.; Qadir, D.; Sapalidis, A.A.; Ahmad, F.; Favvas, E.P. Polymeric, metallic and carbon membranes for hydrogen separation: A review. Gas Sci. Eng. 2023, 120, 205167. [Google Scholar] [CrossRef]
- Agbadaola, O.; Qadir, D.; Ahmad, F.; Suleman, H.; Karousos, D.; Favvas, E. Hydrogen technologies and policies for sustainable future: A review. Chem. Pap. 2024, 78, 4057–4073. [Google Scholar] [CrossRef]
- Mahboubian, L.; Pakdel, S.; Azamat, J.; Erfan-Niya, H. Separation of H2/CH4 gas mixture through graphenylene membrane with functionalized nanopore: A computational study. Int. J. Hydrogen Energy 2022, 47, 28025–28033. [Google Scholar] [CrossRef]
- Zhu, L.; Jin, Y.; Xue, Q.; Li, X.; Zheng, H.; Wu, T.; Ling, C. Theoretical study of a tunable and strain-controlled nanoporous graphenylene membrane for multifunctional gas separation. J. Mater. Chem. A 2016, 4, 15015–15021. [Google Scholar] [CrossRef]
- Rudkevich, D.M. Emerging Supramolecular Chemistry of Gases. Angew. Chem. Int. Ed. 2004, 43, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Wallen, S.L. Sugar Acetates as Novel, Renewable CO2-philes. J. Am. Chem. Soc. 2002, 124, 7274–7275. [Google Scholar] [CrossRef]
- Wu, L.-g.; Yang, C.-h.; Wang, T.; Zhang, X.-y. Enhanced the performance of graphene oxide/polyimide hybrid membrane for CO2 separation by surface modification of graphene oxide using polyethylene glycol. Appl. Surf. Sci. 2018, 440, 1063–1072. [Google Scholar] [CrossRef]
- Zhao, X.; Xuan, H.; He, C. Enhanced separation and antifouling properties of PVDF ultrafiltration membranes with surface covalent self-assembly of polyethylene glycol. RSC Adv. 2015, 5, 81115–81122. [Google Scholar] [CrossRef]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Hertwig, R.H.; Koch, W. On the parameterization of the local correlation functional. What is Becke-3-LYP? Chem. Phys. Lett. 1997, 268, 345–351. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Schwabe, T.; Mück-Lichtenfeld, C. Density functional theory with dispersion corrections for supramolecular structures, aggregates, and complexes of (bio)organic molecules. Org. Biomol. Chem. 2007, 5, 741–758. [Google Scholar] [CrossRef]
- Simon, S.; Duran, M.; Dannenberg, J.J. How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J. Chem. Phys. 1996, 105, 11024–11031. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Muller, R.P.; Goddard, W.A. An extended hybrid density functional (X3LYP) with improved descriptions of nonbond interactions and thermodynamic properties of molecular systems. J. Chem. Phys. 2005, 122, 14105. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Lin, T.-W.; Salzmann, C.; Shao, L.-D.; Yu, C.-H.; Green, M.; Tsang, S.C.E. Polyethylene glycol grafting and attachment of encapsulated magnetic iron oxide silica nanoparticles onto chlorosilanized single-wall carbon nanotubes. Carbon 2009, 47, 1415–1420. [Google Scholar] [CrossRef]
- Gong, C.; Xue, Z.; Wang, X.; Zhou, X.-P.; Xie, X.-L.; Mai, Y.-W. Poly(ethylene glycol) grafted multi-walled carbon nanotubes/LiFePO4 composite cathodes for lithium ion batteries. J. Power Sources 2014, 246, 260–268. [Google Scholar] [CrossRef]
- Lay, L.; Liu, H.; Tan, H.R.; Liu, Y. Delivery of paclitaxel by physically loading onto poly(ethylene glycol) (PEG)-graft-carbon nanotubes for potent cancer therapeutic. Nanotechnology 2010, 21, 065101. [Google Scholar] [CrossRef]
- Singh, S.; Varghese, A.M.; Reddy, K.S.K.; Romanos, G.E.; Karanikolos, G.N. Polysulfone Mixed-Matrix Membranes Comprising Poly(ethylene glycol)-Grafted Carbon Nanotubes: Mechanical Properties and CO2 Separation Performance. Ind. Eng. Chem. Res. 2021, 60, 11289–11308. [Google Scholar] [CrossRef]

| Head Group | Binding Energy (kcal/mol) |
|---|---|
| CH3CSCH3 | 2.44 |
| CH3COCH3 | 3.14 |
| CH3COH | 3.30 |
| CH3OCOCH3 | 3.47 |
| CH3OCOSH | 3.75 |
| HOCH2CH2NH2 | 4.29 |
| CH3CSOH | 4.63 |
| CH3CSNH2 | 4.65 |
| CH3SOONH2 | 5.05 |
| HCONH2 | 5.06 |
| CH3COOH | 5.10 |
| CH3OCONH2 | 5.29 |
| CH3CONH2 | 5.34 |
| Functional Group | Binding Energy (kcal/mol) |
|---|---|
| 1 ethylene glycol-CH2OCOCH3 | −3.54 |
| 2 ethylene glycol-CH2OCOCH3 | −3.57 |
| 3 ethylene glycol-CH2OCOCH3 | −3.57 |
| 1 ethylene glycol-CH2CONH2 | −5.65 |
| 2 ethylene glycol-CH2CONH2 | −5.64 |
| 3 ethylene glycol-CH2CONH2 | −5.65 |
| Molecule | Binding Energy (kcal/mol) |
|---|---|
| H2O | 15.17 |
| H2S | 8.00 |
| CO2 | 6.94 |
| CH4 | 1.73 |
| H2 | 1.10 |
| Molecule | Binding Energy (kcal/mol) |
|---|---|
| H2O | 14.84 |
| H2S | 7.85 |
| CO2 | 6.84 |
| CH4 | 1.46 |
| H2 | 1.08 |
| Molecule | Binding Energy (kcal/mol) |
|---|---|
| H2O | 17.67 |
| H2S | 11.84 |
| CO2 | 8.11 |
| CH4 | 2.90 |
| H2 | 2.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmanzadeh, A.; AL-Hamdani, N.; Favvas, E.P.; De Luca, G. Insights into Cis-Amide-Modified Carbon Nanotubes for Selective Purification of CH4 and H2 from Gas Mixtures: A Comparative DFT Study. Materials 2024, 17, 3588. https://doi.org/10.3390/ma17143588
Rahmanzadeh A, AL-Hamdani N, Favvas EP, De Luca G. Insights into Cis-Amide-Modified Carbon Nanotubes for Selective Purification of CH4 and H2 from Gas Mixtures: A Comparative DFT Study. Materials. 2024; 17(14):3588. https://doi.org/10.3390/ma17143588
Chicago/Turabian StyleRahmanzadeh, Atyeh, Nasser AL-Hamdani, Evangelos P. Favvas, and Giorgio De Luca. 2024. "Insights into Cis-Amide-Modified Carbon Nanotubes for Selective Purification of CH4 and H2 from Gas Mixtures: A Comparative DFT Study" Materials 17, no. 14: 3588. https://doi.org/10.3390/ma17143588





