Effects of Tungsten Addition on the Microstructure and Properties of FeCoCrNiAl High-Entropy Alloy Coatings Fabricated via Laser Cladding
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
3. Results and Analysis
3.1. Macroscopic Morphology Analysis of Cladding Coating
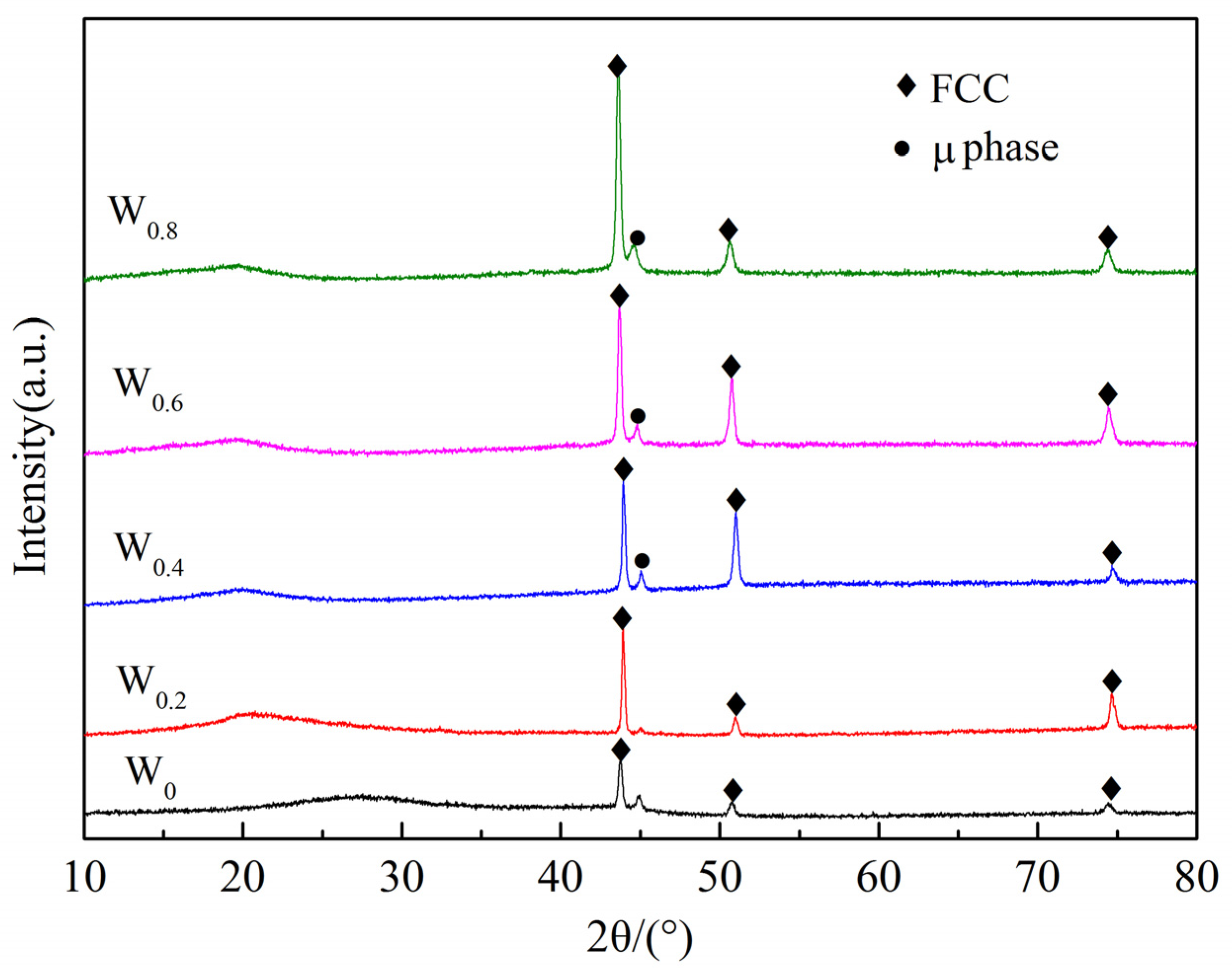
3.2. Phase Analysis
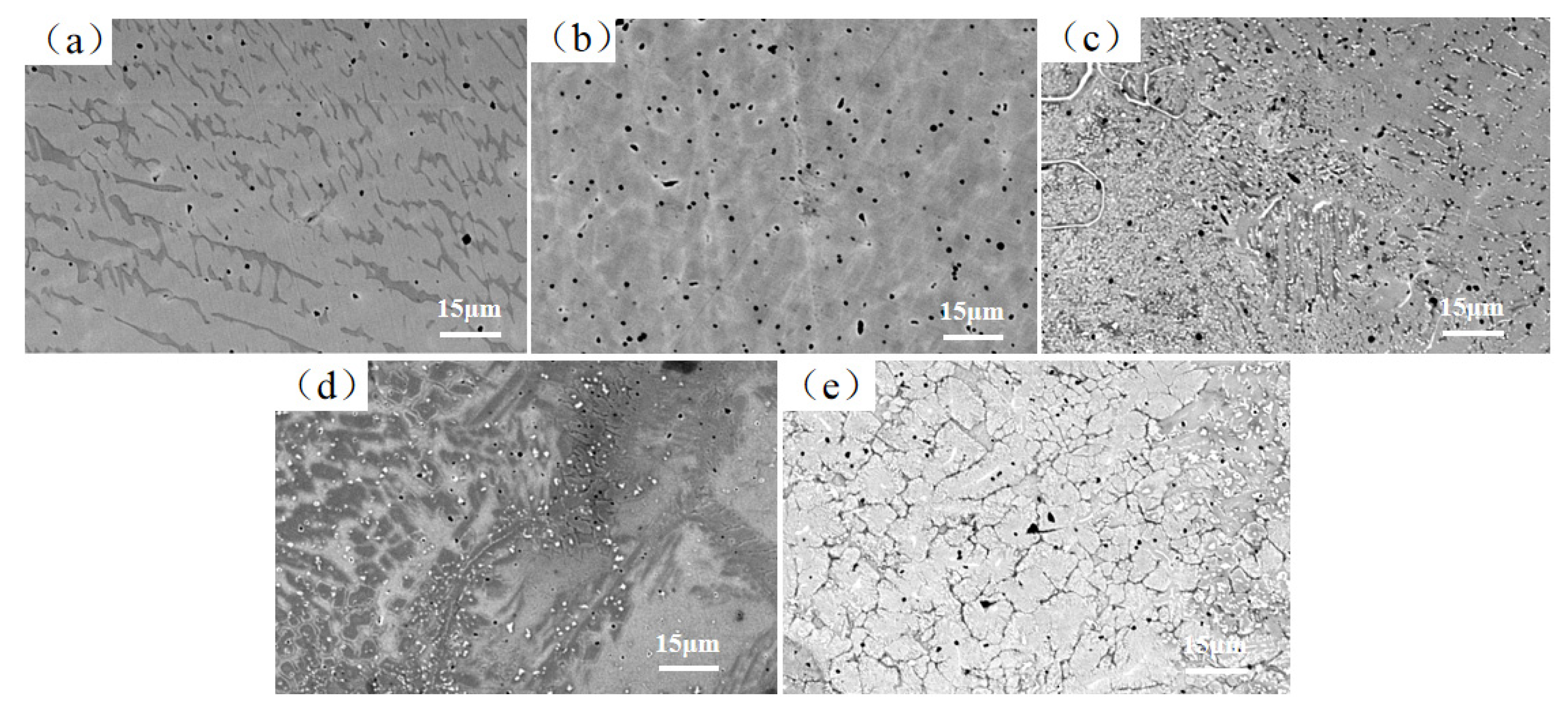
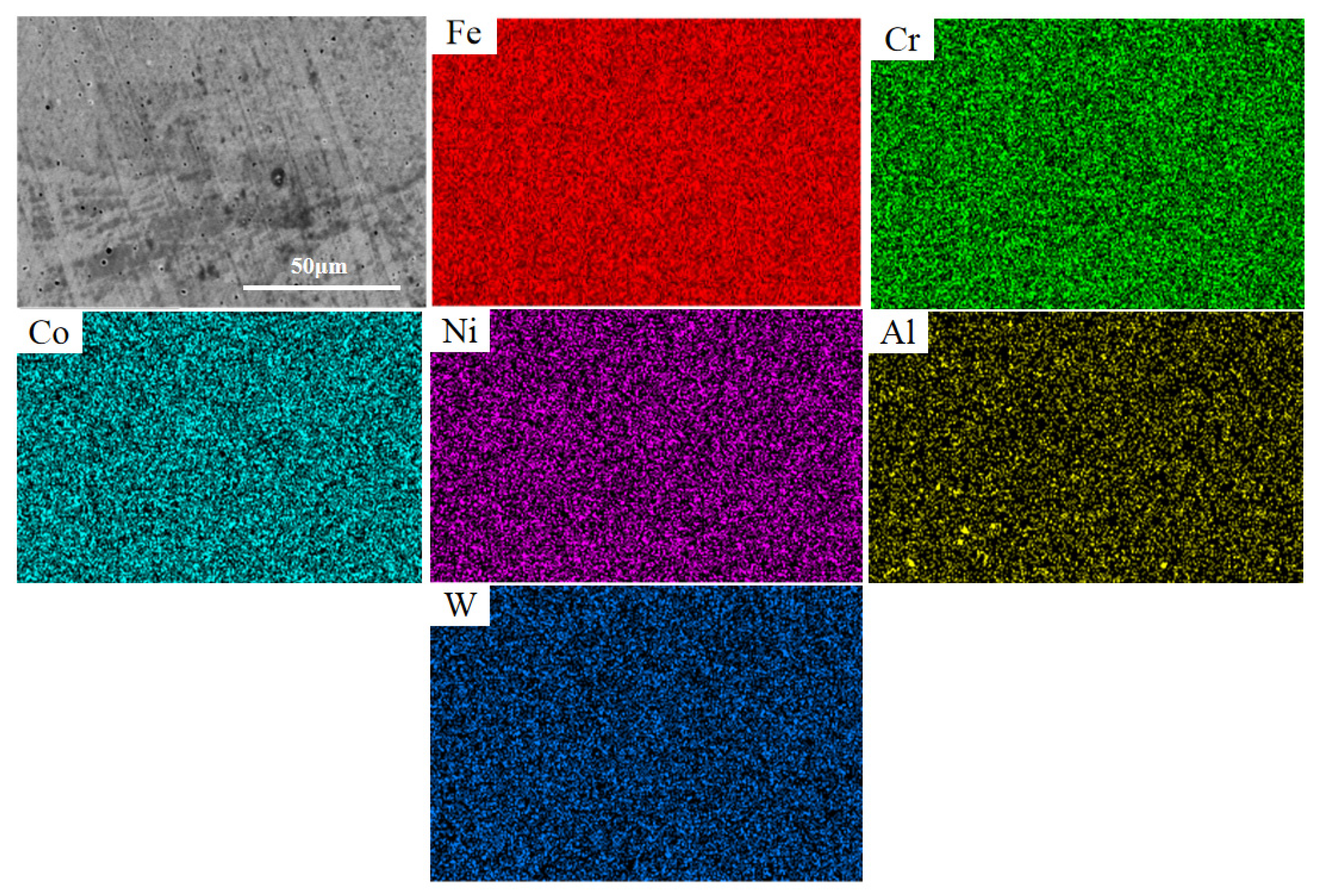
3.3. Microstructure Analysis
3.4. Microhardness Analysis
- (1)
- The atomic radii of these elements are 124.6 pm (Ni), 124.1 pm (Fe), 124.9 pm (Cr), 125.1 pm (Co), 143 pm (Al), and 137 pm (W). The atomic radius of W is relatively large. When it enters the lattice to form substitutional solid solutions, it induces lattice distortion. As the W content increases, the lattice distortion effect gradually intensifies, leading to significant solid-solution strengthening and thereby increasing the hardness of the cladding layer.
- (2)
- The laser cladding process, with its high energy density and rapid cooling rate, leads to a pronounced grain refinement effect during the alloy solidification process.
- (3)
- The inclusion of W encourages the development of µ phase, increases dislocation slip resistance, and to some extent inhibits grain growth [40]. This leads to further grain refinement, resulting in an increase in the microhardness of the cladding coating.
| HEAs | Average Microhardness | Ref. |
|---|---|---|
| FeCoCrNiAlW0.0 | 523.85/HV0.2 | This work |
| FeCoCrNiAlW0.2 | 601.99/HV0.2 | This work |
| FeCoCrNiAlW0.4 | 647.90/HV0.2 | This work |
| FeCoCrNiAlW0.6 | 698.82/HV0.2 | This work |
| FeCoCrNiAlW0.8 | 756.83/HV0.2 | This work |
| CoCrFeNiW0.8 | 432.02/HV0.3 | [35] |
| CoCrFeNiW0.75 | 303.6/HV0.2 | [41] |
| Al1.0FeMnNiCrCu0.5 | 541/HV0.2 | [42] |
| FeMnNiCrCu0.5 | 193/HV0.2 | [42] |
| CoCrFeNiTi0.8 | 502.39/HV0.3 | [34] |
| CoCrFeNiSi2.0 | 566.5/HV0.5 | [43] |
| FeCoNiCrSiAl1.0 | 439/HV0.2 | [44] |
| AlCrFeCoNi | 421/HV0.2 | [45] |
| CoCrFeNiW | 378/HV0.2 | [46] |
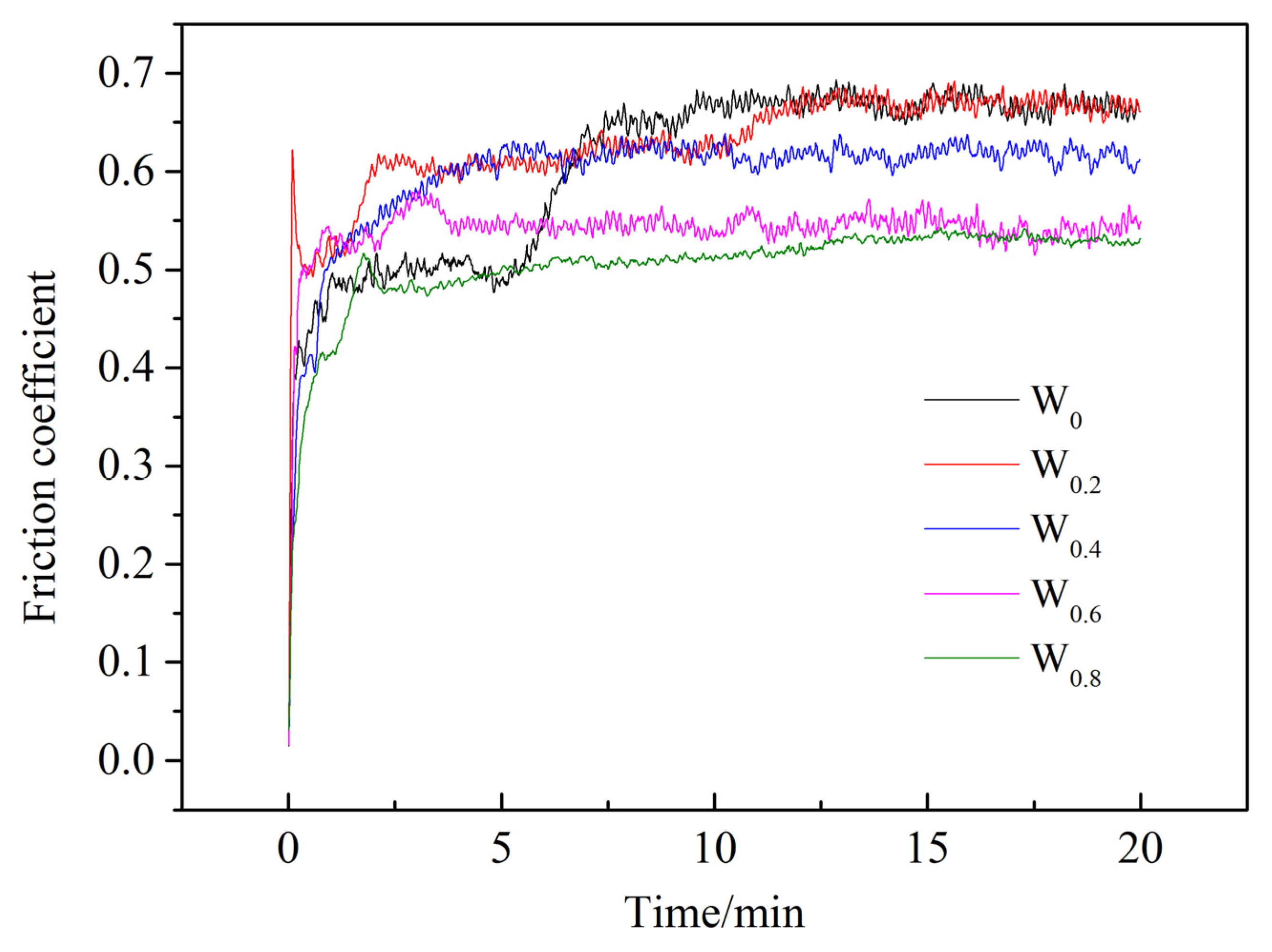
3.5. Friction and Wear Properties
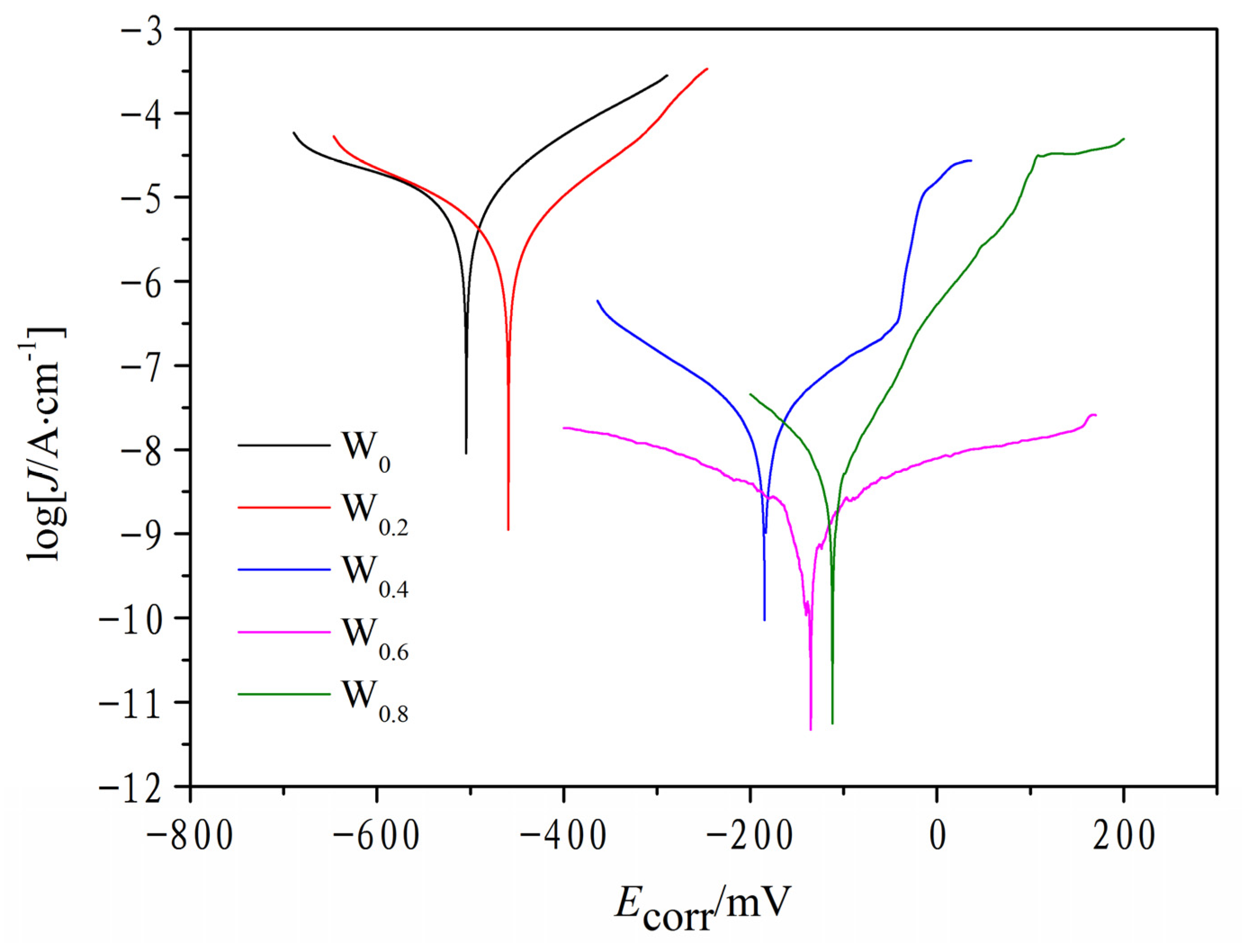
3.6. Corrosion Resistance Analysis
| HEAs | Ecorr/mV | Jcorr/A·cm−2 | Ref. |
|---|---|---|---|
| FeCoCrNiAlW0.0 | −515 | 5.43 × 10−6 | This work |
| FeCoCrNiAlW0.2 | −456 | 3.46 × 10−6 | This work |
| FeCoCrNiAlW0.4 | −186 | 2.73 × 10−8 | This work |
| FeCoCrNiAlW0.6 | −134 | 1.62 × 10−9 | This work |
| FeCoCrNiAlW0.8 | −112 | 5.26 × 10−9 | This work |
| CoCrFeNiTi0.6 | −509 | 4.22 × 10−5 | [34] |
| CoCrFeNiSi2.0 | −598.6 | 6.06 × 10−7 | [43] |
| FeCoNiCrMnW1.0 | −282 | 2.74 × 10−5 | [48] |
| CoCrFeNiAl0.3 | −451 | 1.03 × 10−5 | [50] |
4. Conclusions
- (1)
- The FeCoCrNiAlWx HEA cladding coatings mainly consist of FCC solid solution and µ phase. The microstructure is primarily composed of dendritic and cellular crystals, with the transition from dendritic to cellular crystals occurring gradually as the W content increases, and its doped W is evenly distributed throughout the cladding coating.
- (2)
- The microhardness of the FeCoCrNiAlWx HEA cladding coating increases with the increase in W content. When x = 0.8, the hardness reaches 756.83 HV0.2. The wear resistance of the cladding coating also improves accordingly, with the friction coefficient gradually decreasing. When x = 0.6 and x = 0.8, the friction coefficients are relatively close, with the average friction coefficient reaching a minimum of 0.527. The wear of the cladding coating mainly manifests as adhesive wear and abrasive wear.
- (3)
- The increase in the W element content made a significant impact on the corrosion resistance of the FeCoCrNiAlWx HEA cladding coating in a 3.5 wt.% NaCl solution, showing an upward trend in corrosion potential while the current density decreased accordingly. This change directly indicates that the corrosion resistance of the HEA coating is significantly improved with the increase in W content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.-S.; Liu, X.-B.; Wang, G.; Liu, Y.-F.; Meng, Y.; Zhang, S.-H. Effect of Cu content on microstructure evolution and tribological behaviors of Ni60 composite coatings on 45# steel by laser cladding. Opt. Laser Technol. 2022, 156, 108549. [Google Scholar]
- Jiang, W.; Zhang, C.Y.; Gu, Q.M.; Xu, K.; Zhu, H.; Cao, Z.H. Tribological properties of micro-pit texture generated by composite processing. Lubr. Eng. 2019, 44, 85–89. [Google Scholar]
- Malatji, N.; Popoola, A.P.; Lenggopeng, T.; Pityana, S.L. Tribological and corrosion properties of laser additive manufactured AlCrFeNiCu high entropy alloy. Mater. Today Proc. 2020, 28, 944–948. [Google Scholar] [CrossRef]
- Wu, T.; Chen, Y.; Shi, S.; Wu, M.; Gui, W.; Tan, Y.; Li, J.; Wu, Y. Effects of W alloying on the lattice distortion and wear behavior of laser cladding AlCoCrFeNiWxhigh-entropy alloy coatings. Materials 2021, 14, 5450. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhang, C.; Li, L.; Yang, Y. Microstructure and properties of CoCrFeNiMnTix high-entropy alloy coated by laser cladding. Coatings 2024, 14, 620. [Google Scholar] [CrossRef]
- Guo, J.; Liu, C.H.; Wang, D.X.; Xu, L.F.; Song, K.K.; Gao, M. Structure and wear resistance of TiC-reinforced Al1.8CrCuFeNi2 high-entropy alloy coating using laser cladding. Materials 2023, 16, 3422. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Li, G.; Wu, B.; Xu, S.; Wang, L.Y.; Guo, Y.P. Effect of TiC content on microstructure and properties of TiC / Ni60 coatings on Ti6Al4V alloy deposited by laser cladding. Opt. Laser Technol. 2024, 168, 109854. [Google Scholar] [CrossRef]
- Li, Q.; Song, G.M.; Zhang, Y.Z.; Lei, T.C.; Chen, W.Z. Microstructure and dry sliding wear behavior of laser clad Ni-based alloy coating with the addition of SiC. Wear 2024, 254, 222–229. [Google Scholar] [CrossRef]
- Zhang, C.L.; Luo, X.; Ma, L.F.; Hou, L.; Huang, B.; Hu, R. Study on microstructure and properties of WC particle-reinforced FeCoCrNi-matrix high entropy alloy composites. Materials 2023, 16, 7380. [Google Scholar] [CrossRef]
- He, L.; Tan, Y.F.; Tan, H.; Zhou, C.H.; Gao, L. Tribological properties of nanostructured Al2O3−40%TiO2 multiphase ceramic particles reinforced Ni-based alloy composite coatings. Trans. Nonferrous Met. Soc. China 2013, 23, 2618–2627. [Google Scholar] [CrossRef]
- Ananta, B.K.; Ranga, J.G. Influence of Al2O3 and ZrO2 alloying on microstructure, hardness and flexural strength of Ni based functionally graded composites by vacuum sintering. Compos. Commun. 2020, 22, 100458. [Google Scholar]
- Khan, A.R.; Patra, A.; Chaira, D.; Arvindha Babu, D.; Majumdar, B. Study of microstructure, thermodynamic and powder properties of nano Y2O3, TiO2, ZrO2 dispersed W–Ni–Nb–Mo–Zr alloys. Mater. Chem. Phys. 2024, 311, 128567. [Google Scholar] [CrossRef]
- Yeh, J.W. High-Entropy Alloys: Overview. Encycl. Mater. Met. Alloys 2022, 2, 294–307. [Google Scholar]
- Kumar, S. Comprehensive review on high entropy alloy-based coating. Surf. Coat. Technol. 2024, 477, 130327. [Google Scholar] [CrossRef]
- Zirari, T.; Trabadelo, V. A review on wear, corrosion, and wear-corrosion synergy of high entropy alloys. Heliyon 2021, 10, e25867. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. High Entropy Alloy Coatings and Technology. Coatings 2021, 11, 372. [Google Scholar] [CrossRef]
- Liu, T.; Chang, M.; Cheng, X.D.; Zeng, X.; Shao, H.; Liu, F. Characteristics of WC reinforced Ni-based alloy coatings prepared by PTA + PMI method. Surf. Coat. Technol. 2020, 383, 125232. [Google Scholar] [CrossRef]
- Vallimanalan, A.; Kumaresh, B.S.P.; Muthukumaran, S.; Murali, M.; Vivek, G.; Mahendran, R. Corrosion behaviour of thermally sprayed Mo added AlCoCrNi high entropy alloy coating. Mater. Today Proc. 2020, 27, 2398–2400. [Google Scholar] [CrossRef]
- Mengiste, B.T.; Arab, A.; Guo, Y.; Lei, Y.; Li, X.; Chen, P.; Xie, J. Tuning the mechanical properties of CrCoFeMnNi high entropy alloy via cold spray additive manufacturing associated with heat treatment. Mater. Sci. Eng. A 2024, 894, 146214. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Luzin, V.; Schulz, C.; Hall, C.; Murty, B.S.; Kottada, R.S.; Berndt, C.C.; Ang, A.S. Multiscale mechanical performance and corrosion behaviour of plasma sprayed AlCoCrFeNi high-entropy alloy coatings. J. Alloys Compd. 2021, 854, 157140. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Zhang, X.M.; Quan, H.; Chen, Y.J.; Wang, S.; Zhang, S. Effect of Mo addition on structures and properties of FeCoNiCrMn high entropy alloy film by direct current magnetron sputtering. J. Alloys Compd. 2021, 895, 162709. [Google Scholar] [CrossRef]
- Ma, S.B.; Su, B.B.; Wang, X.; Xia, Z.W.; Liu, J.; Xu, Y. Wear resistance of SiC/Ni composite coating based on laser cladding. J. Mater. Eng. 2016, 44, 77–82. [Google Scholar]
- Xiang, D.D.; Liu, Y.S.; Yu, T.B.; Wang, D.; Leng, X.X.; Wang, K.M.; Liu, L.; Pan, J.; Yao, S.; Chen, Z.B. Review on wear resistance of laser cladding high-entropy alloy coatings. J. Mater. Res. Technol. 2024, 28, 911–934. [Google Scholar] [CrossRef]
- Huang, R.R.; Zhou, F.; Kuang, S.H.; Liu, Q.B. Microstructure evolution and properties of laser cladding AlFeCrMoNbx refractory high entropy alloy coatings. J. Mater. Res. Technol. 2023, 27, 5709–5719. [Google Scholar] [CrossRef]
- Jin, J.J.; Chen, B.; Zhang, Z.Y.; Wu, Y.B.; Luo, Z.Y.; Gou, G.Q.; Chen, W.J. Effects of Mo content on the microstructure and mechanical properties of laser cladded FeCoCrNiMox (x = 0.2, 0.5) high-entropy alloy coatings. Surf. Coat. Technol. 2024, 482, 130697. [Google Scholar] [CrossRef]
- Menghani, J.V.; Vyas, A.V.; Patel, P.; Natu, H.; More, S.R. Wear, erosion and corrosion behavior of laser cladded high entropy alloy coatings—A review. Mater. Today Proc. 2020, 38, 2824–2829. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Sun, Y.N.; Cheng, W.J.; Han, C.Y.; Huang, L.F.; Su, C.J.; Jiang, L.H. Enhanced Wear and Corrosion Resistances of Alcocrfeni High-Entropy Alloy Coatings Via High-Speed Laser Cladding. Mater. Today Commun. 2020, 33, 104417. [Google Scholar] [CrossRef]
- Hao, X.H.; Liu, H.X.; Zhang, X.W.; Chen, L.; Wang, Y.Y.; Yang, C.; Liu, Y.X. Friction–wear behaviors and microstructure of AlTiVCrNb lightweight refractory high-entropy alloy coating prepared by laser cladding on Ti–6Al–4V substrate. J. Mater. Res. Technol. 2024, 29, 1–11. [Google Scholar] [CrossRef]
- Chen, Z.B.; Zhang, G.G.; Chen, J.J.; Guo, C.H.; Sun, W.Y.; Yang, Z.L.; Li, H.X.; Jiang, F.C.; Han, B. Microstructure and wear property of WMoTaNb refractory high entropy alloy coating by laser cladding. J. Mater. Res. Technol. 2024, 28, 1557–1569. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Jiang, F.L.; Song, P.F.; Yang, F.Z.; Wang, Y.L.; Yang, Y.; Liang, P. Advances in corrosion resistance of high entropy alloy coating prepared by laser cladding. Surf. Technol. 2021, 50, 257–270. [Google Scholar]
- Liu, Y.F.; Chang, T.; Liu, X.B.; Zhu, Y.; Wang, G.; Meng, Y.; Liang, J.; Xie, J.C. Research progress on tribological properties of high-entropy alloy coating. Surf. Technol. 2021, 50, 156–169. [Google Scholar]
- Liao, T.H.; Wang, Z.L.; Wu, X.H.; Liu, Q.B.; Guo, Y.X.; Ding, K.L.; Shang, X.J. Effect of V on microstructure, wear and corrosion properties in AlCoCrMoV high entropy alloy coatings by laser cladding. J. Mater. Res. Technol. 2023, 23, 4420–4431. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Z.X.; Xu, G.J.; Chen, H.Y. Influence of Al Addition on the Microstructure and Wear Behavior of Laser Cladding FeCoCrNiAlx High-Entropy Alloy Coatings. Coatings 2023, 13, 426. [Google Scholar] [CrossRef]
- Zuo, R.; Sun, R.; Niu, W.; Hao, W.J.; Gu, M.; Li, X.L. Microstructure and properties of CoCrFeNiTix high entropy alloy coated by laser cladding. Surf. Technol. 2022, 51, 363–370. [Google Scholar]
- Cai, Y.C.; Zhu, L.S.; Cui, Y.; Han, J. Manufacturing of FeCoCrNi + FeCoCrNiAl laminated high-entropy alloy by laser melting deposition (LMD). Mater. Lett. 2021, 289, 129445. [Google Scholar] [CrossRef]
- Ma, S.Z.; Sun, R.L.; Niu, W.; Gu, M.; Zuo, R.Y.; Zhang, L.W. Microstructure and properties of CoCrFeNiWx High entropy alloy coated by laser cladding. Surf. Technol. 2022, 52, 429–437. [Google Scholar]
- Annie, A.; Thierry, C.; Jean, J. Partial redetermination of the Fe-W phase diagram. Metall. Mater. Trans. A 2013, 44A, 2996. [Google Scholar]
- Jonathan, W.P.; Michael, A.M.; Mark, A.R.; Tomas, F.B.; Benjamin, G.; Nicolas, A.; Aaron, G.; Andrew, B.K. In situ synchrotron X-ray imaging and mechanical properties characterization of additively manufactured high-entropy alloy composites. J. Alloys Compd. 2021, 876, 159505. [Google Scholar]
- Xiao, N.; Guan, X.; Wang, D.; Yan, H.L.; Cai, M.H.; Jia, N.; Zhang, Y.D.; Claude, E.; Zhao, X.; Zuo, L. Impact of W alloying on microstructure, mechanical property and corrosion resistance of face-centered cubic high entropy alloys: A review. Int. J. Miner. Metall. Mater. 2023, 30, 1667–1679. [Google Scholar] [CrossRef]
- Liu, J.Z.; Liu, H.X.; Di, Y.N.; Lin, J.Q.; Hao, X.H.; Wang, Y.Y.; Chen, L.; Zhang, X.W. Effects of carbon content on friction and wear behavior and corrosion resistance of laser cladding CoCrFeMnNiCx high entropy alloy coating. China Surf. Eng. 2020, 33, 118–127. [Google Scholar]
- Liu, H.; Gao, Q.; Dai, J.B.; Chen, P.J.; Gao, W.P.; Hao, H.B.; Yang, H.F. Microstructure and high-temperature wear behavior of CoCrFeNiWx high-entropy alloy coatings fabricated by laser cladding. Tribol. Int. 2022, 172, 107574. [Google Scholar] [CrossRef]
- Thanhhung, N.; Xuannam, L.; Ming, H.; Qin, Y.; Yang, S. Fabriaction and characterization of AlFeMnNiCrCu0.5 (x = 0.0; 0.5; 1.0) high-entropy alloy coating by laser cladding. J. Therm Spray Tech. 2020, 31, 980–990. [Google Scholar]
- Hao, W.J.; Sun, R.L.; Niu, W.; Tan, J.H.; Li, X.L. Microstructure and Properties of Laser Cladding CoCrFeNiSix High-entropy Alloy Coating. Surf. Technol. 2021, 50, 87–94. [Google Scholar]
- Xiao, J.K.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and tribological properties of plasma sprayed FeCoNiCrSiAlx high entropy alloy coatings. Wear 2020, 448–449, 203209. [Google Scholar] [CrossRef]
- Ashok, M.; Ameey, A.; Murty, B.S.; Christopher, C.B.; Ravi, S.K.; Andrew, S.M.A. Thermal spray high-entropy alloy coatings: A review. J. Therm Spray Tech. 2020, 29, 857–893. [Google Scholar]
- Liu, Y.; Tan, N.; Li, Y.; Zhang, G.L.; Yin, W.; Li, G.H.; Cai, Y.J.; Zhou, Y.J.; Han, S.X.; Liu, C.Y. Microstructure, hardness, and tribological properties of CoCrFeNiX (X = Mo, Ti, W) high entropy alloy coating by red-blue composite laser cladding on copper alloy. Surf. Coat. Technol. 2024, 483, 130761. [Google Scholar] [CrossRef]
- Sun, P.; Yan, N.; Wei, S.; Wang, D.; Song, W.; Tang, C.; Yang, J.; Xu, Z.; Hu, Q.; Zeng, X. Microstructural evolution and strengthening mechanisms of Inconel 718 alloy with different W addition fabricated by laser cladding. Mater. Sci. Eng. A 2023, 868, 144535. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Wang, L.; Zhao, X.H.; Wu, J.L.; Zhang, F.; Pi, J.H. Effects of W addition on the corrosion behaviors of FeCoNiCrMn high entropy alloy composites in the 3.5 wt.% NaCl solution. Surf. Interfaces 2021, 23, 100956. [Google Scholar] [CrossRef]
- Niu, Z.Z.; Xu, J.; Wang, T.; Wang, N.R.; Han, Z.H.; Wang, Y. Microstructure, mechanical properties and corrosion resistance of CoCrFeNiWx (x = 0, 0.2, 0.5) high entropy alloys. Intermetallics 2019, 112, 106550. [Google Scholar] [CrossRef]
- Gao, L.; Liao, W.B.; Zhang, H.T.; James, U.S.; Sun, D.; Lu, Y. Microstructure, mechanical and corrosion behaviors of CoCrFeNiAl0.3 high entropy alloy (HEA) films. Coatings 2017, 7, 156. [Google Scholar] [CrossRef]
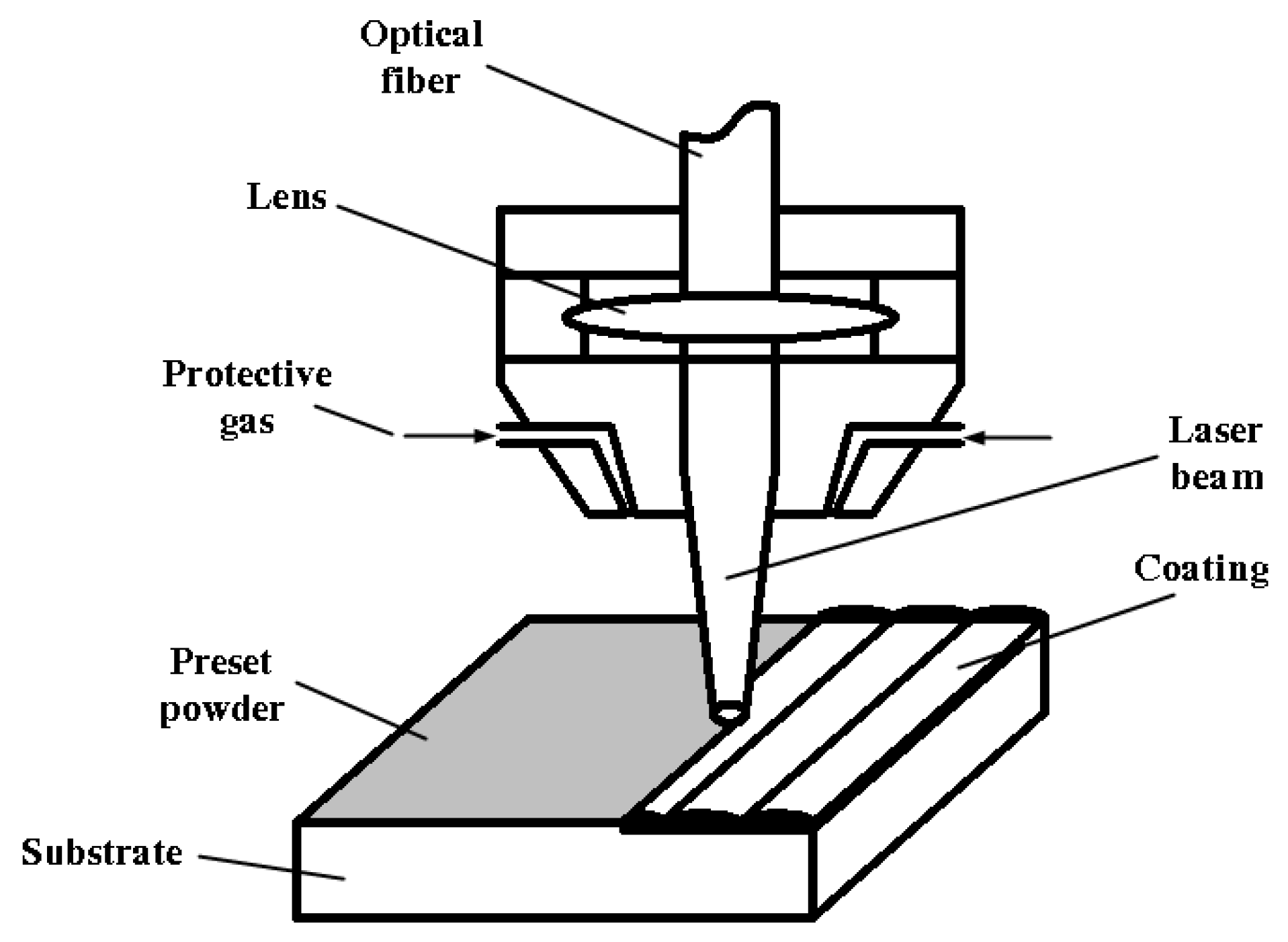
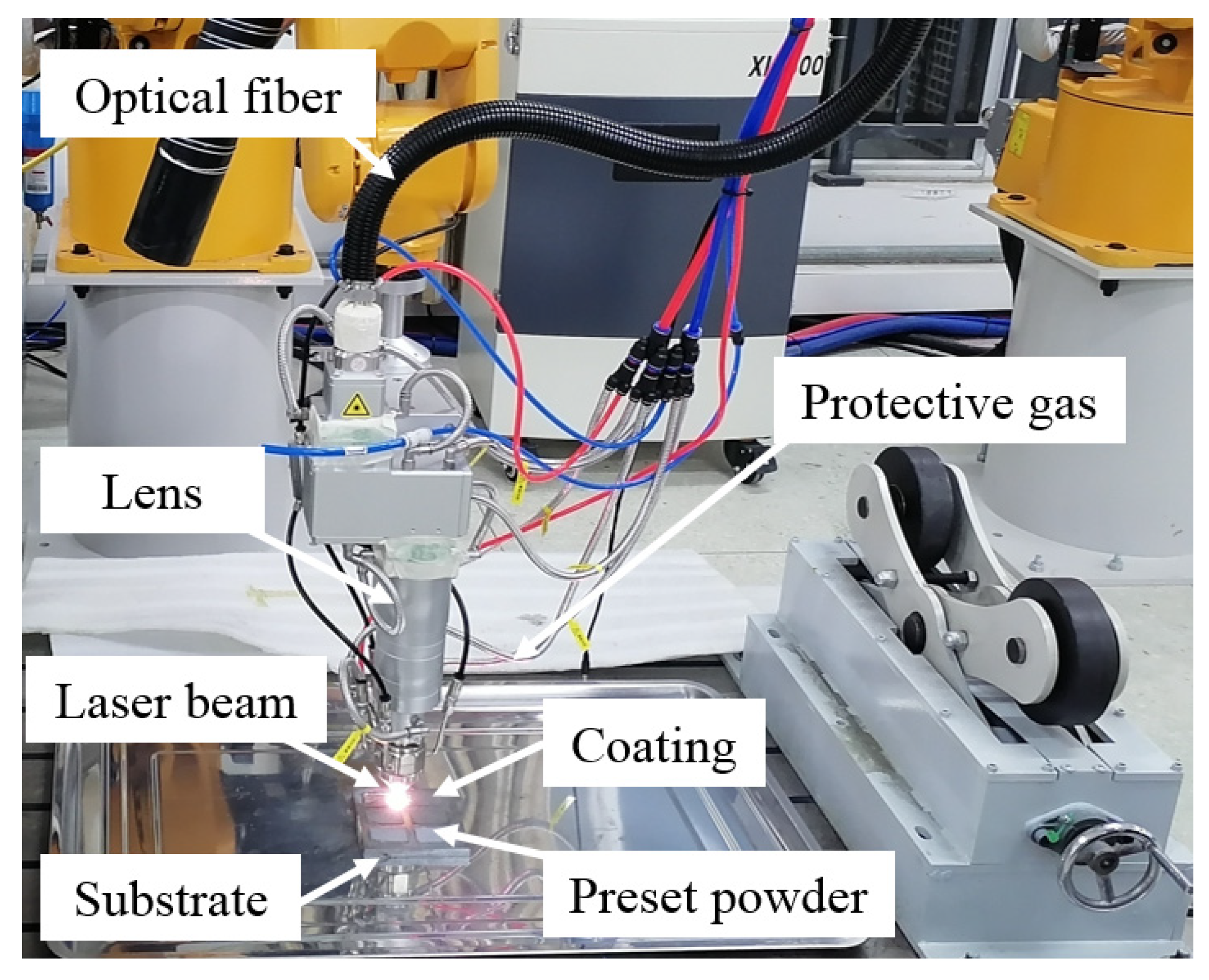
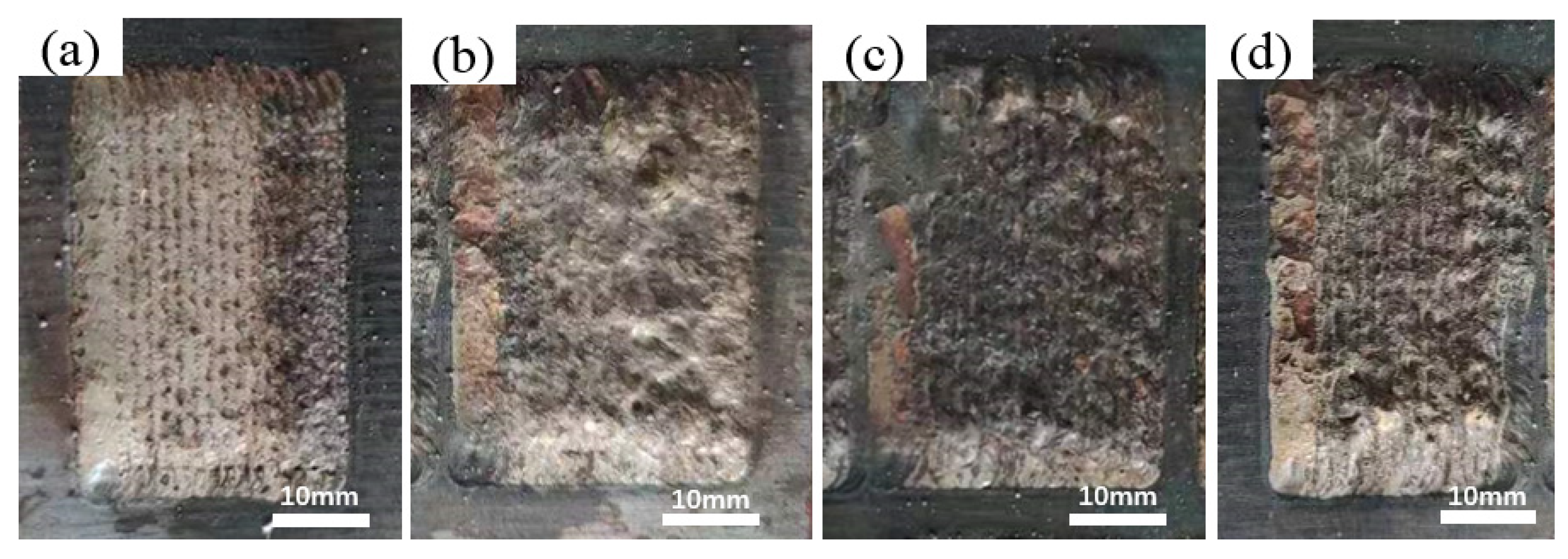
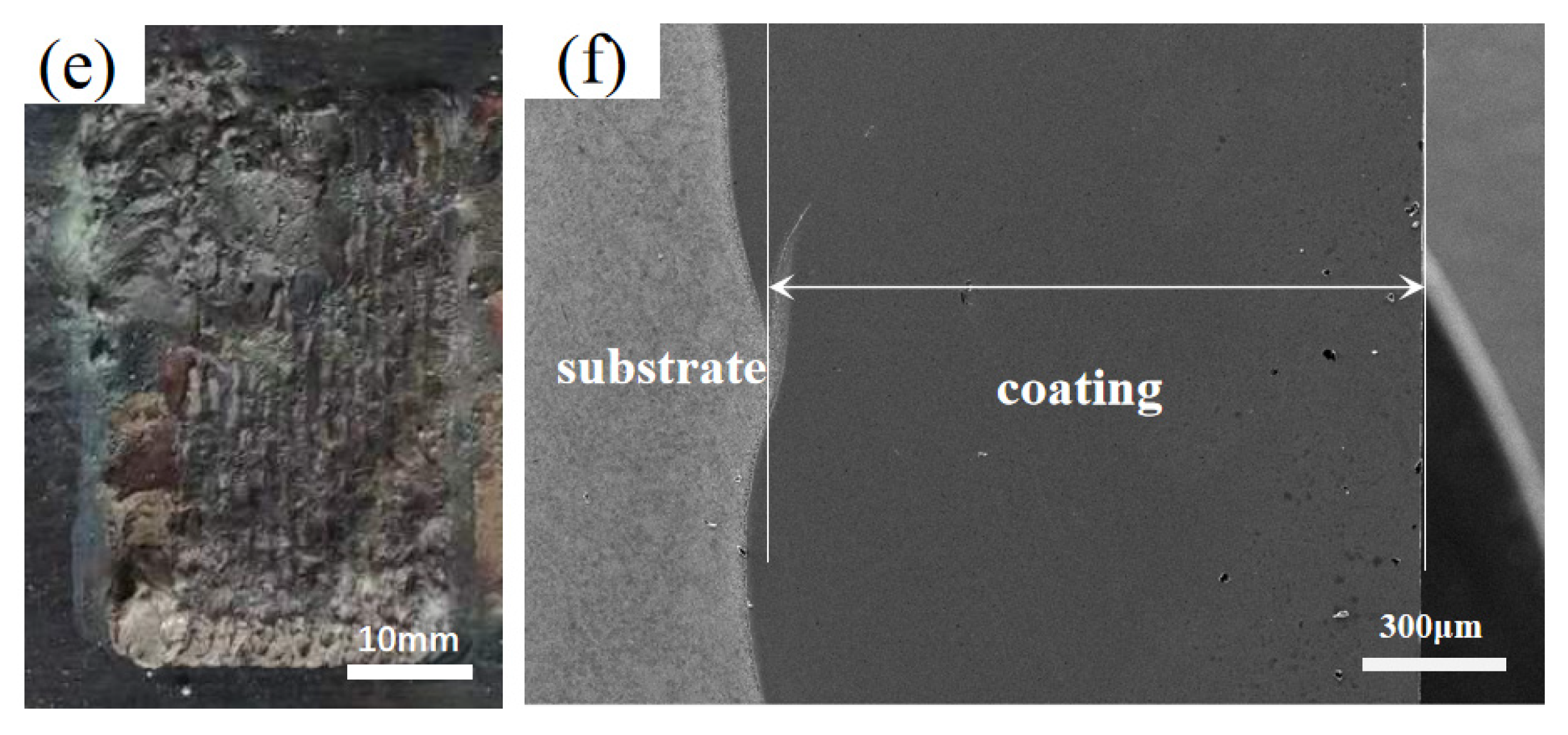


| HEAs | Fe | Co | Cr | Ni | Al | W |
|---|---|---|---|---|---|---|
| FeCoCrNiAlW0.0 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 0.00 |
| FeCoCrNiAlW0.2 | 19.23 | 19.23 | 19.23 | 19.23 | 19.23 | 3.85 |
| FeCoCrNiAlW0.4 | 18.52 | 18.52 | 18.52 | 18.52 | 18.52 | 7.4 |
| FeCoCrNiAlW0.6 | 17.86 | 17.86 | 17.86 | 17.86 | 17.86 | 10.7 |
| FeCoCrNiAlW0.8 | 17.24 | 17.24 | 17.24 | 17.24 | 17.24 | 13.8 |
| Parameter | Value |
|---|---|
| Laser power | 1200 W |
| Scanning speed | 5 mm/s |
| Overlap ratio | 50% |
| Laser spot diameter | 3 mm |
| Argon flow rate | 12 L/min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Zhang, C.; Li, L.; Chen, H.; Yang, Y. Effects of Tungsten Addition on the Microstructure and Properties of FeCoCrNiAl High-Entropy Alloy Coatings Fabricated via Laser Cladding. Materials 2024, 17, 3592. https://doi.org/10.3390/ma17143592
Ma S, Zhang C, Li L, Chen H, Yang Y. Effects of Tungsten Addition on the Microstructure and Properties of FeCoCrNiAl High-Entropy Alloy Coatings Fabricated via Laser Cladding. Materials. 2024; 17(14):3592. https://doi.org/10.3390/ma17143592
Chicago/Turabian StyleMa, Shibang, Congzheng Zhang, Liang Li, Haodong Chen, and Yinhai Yang. 2024. "Effects of Tungsten Addition on the Microstructure and Properties of FeCoCrNiAl High-Entropy Alloy Coatings Fabricated via Laser Cladding" Materials 17, no. 14: 3592. https://doi.org/10.3390/ma17143592





