Effects of Fineness and Morphology of Quartz in Siliceous Limestone on the Calcination Process and Quality of Cement Clinker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.2.1. Product Fineness
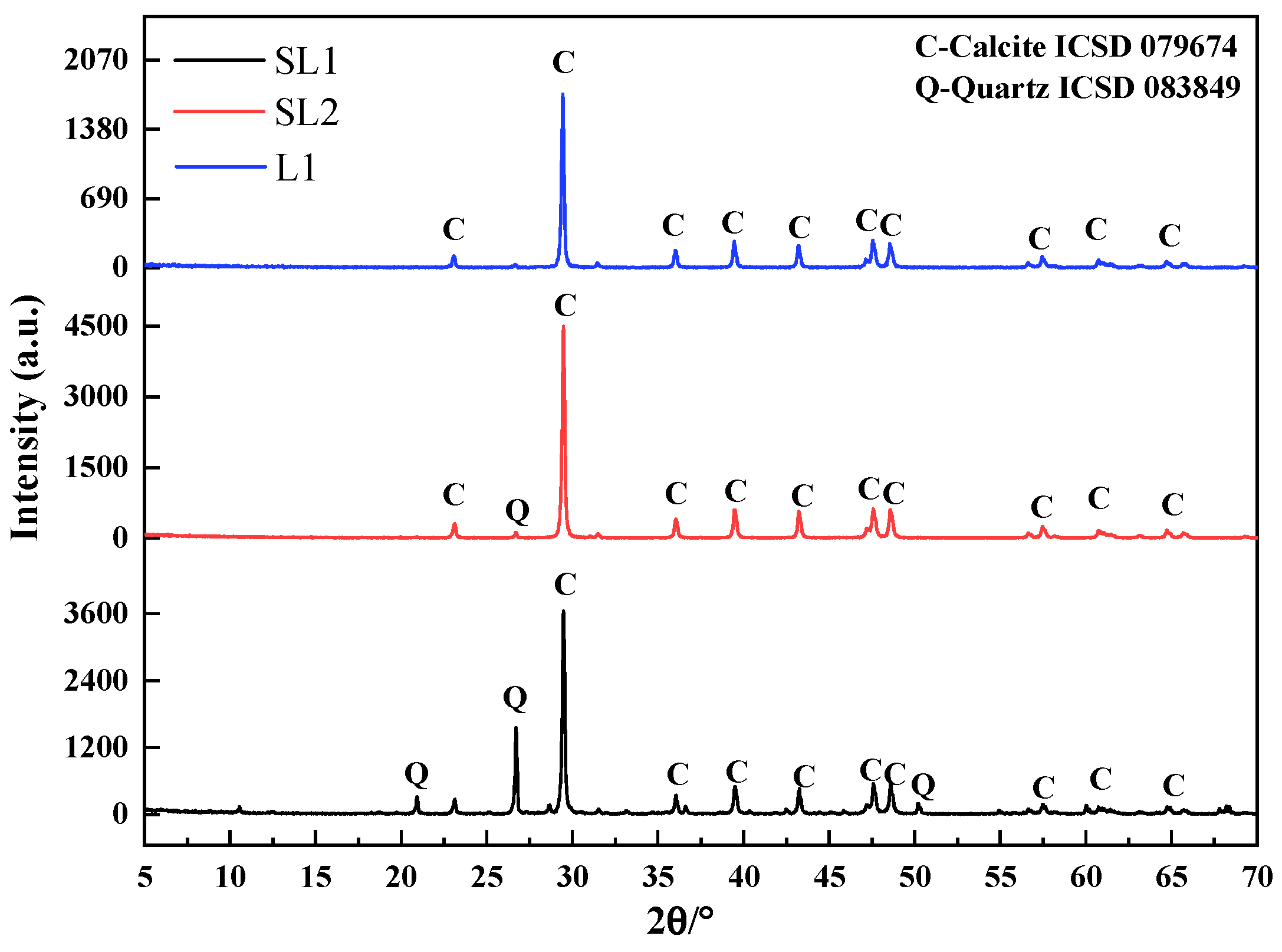
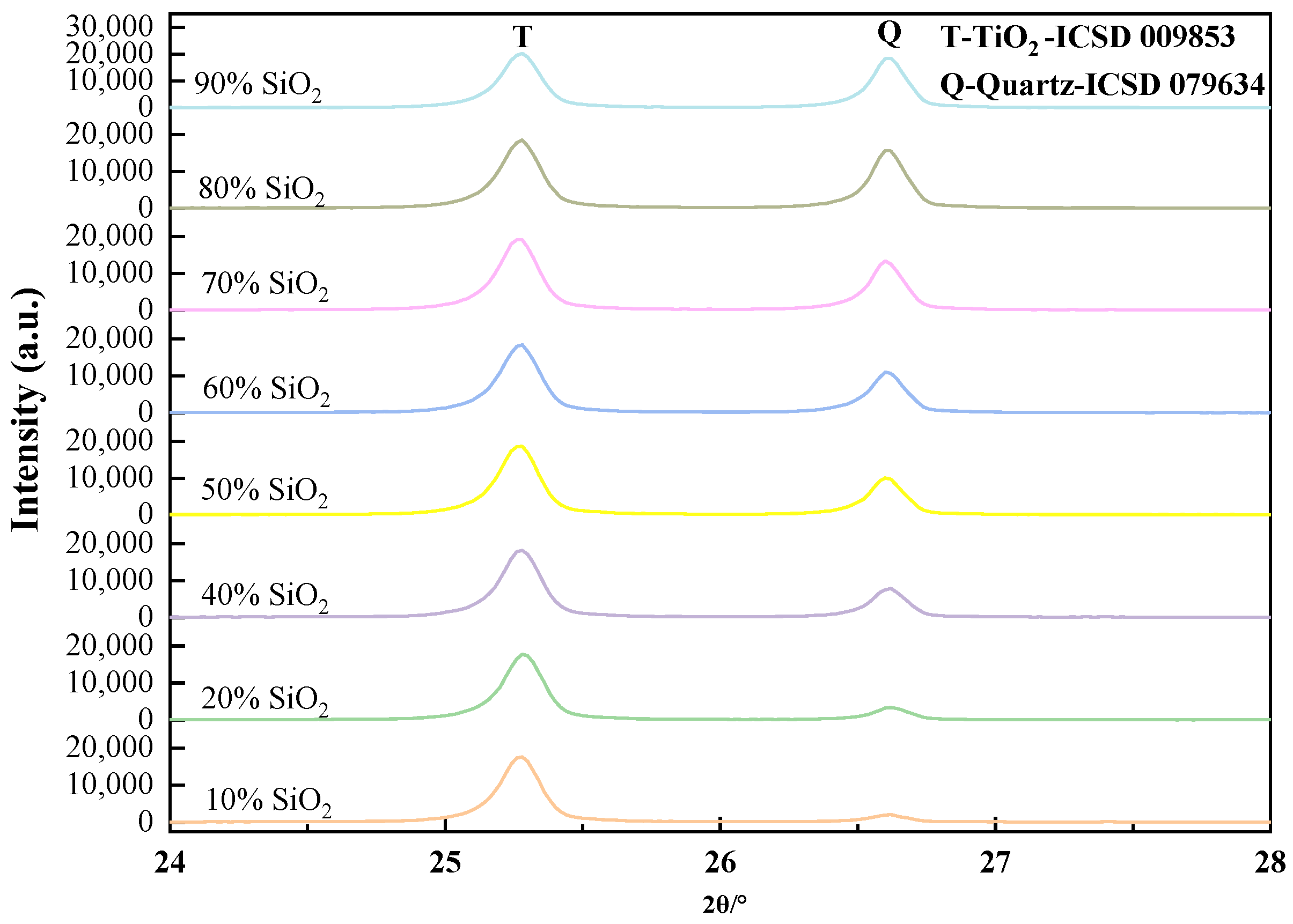
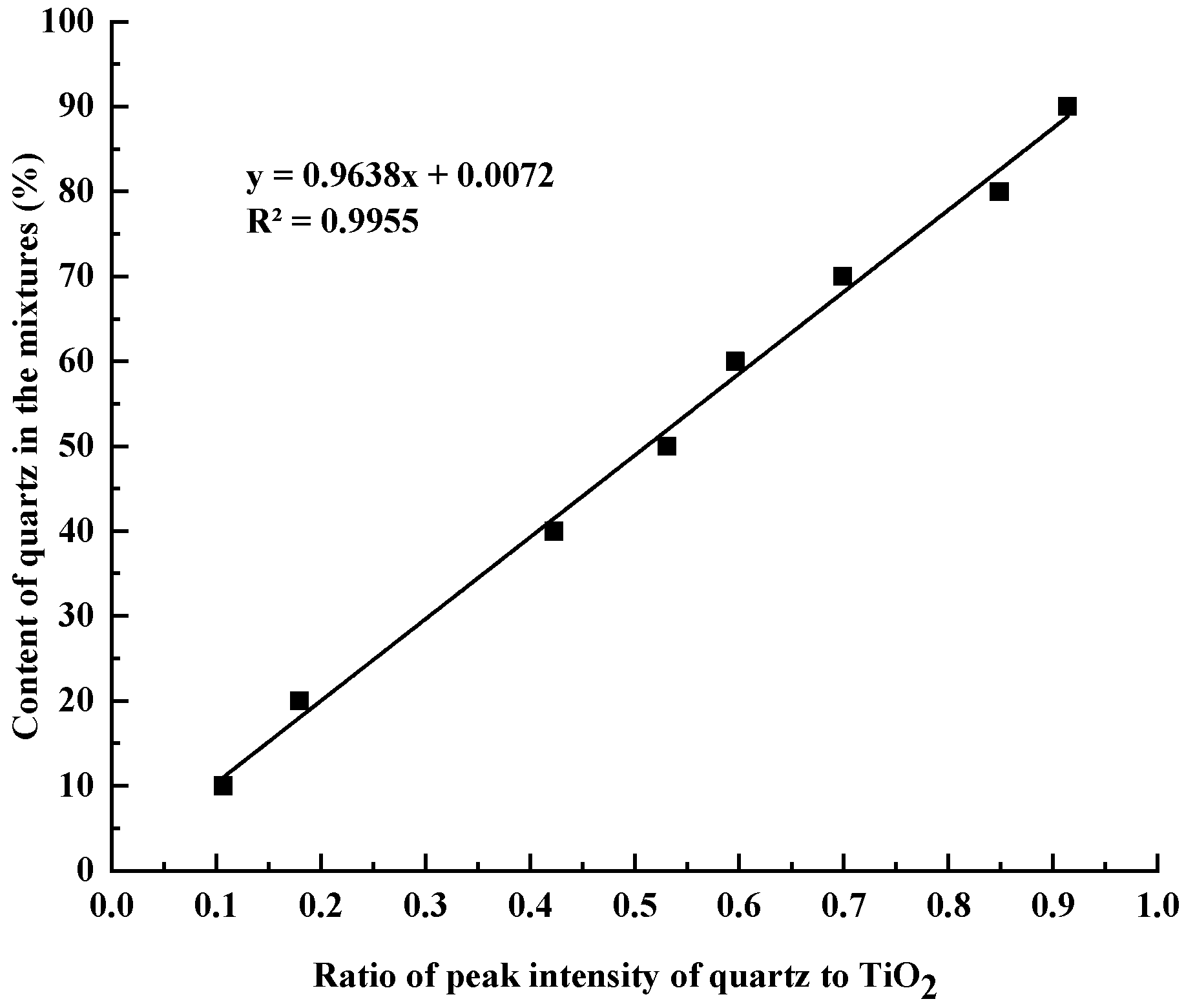
2.2.2. Measurement of Quartz Content in Siliceous Limestones
2.2.3. Quantification of Clinker Minerals
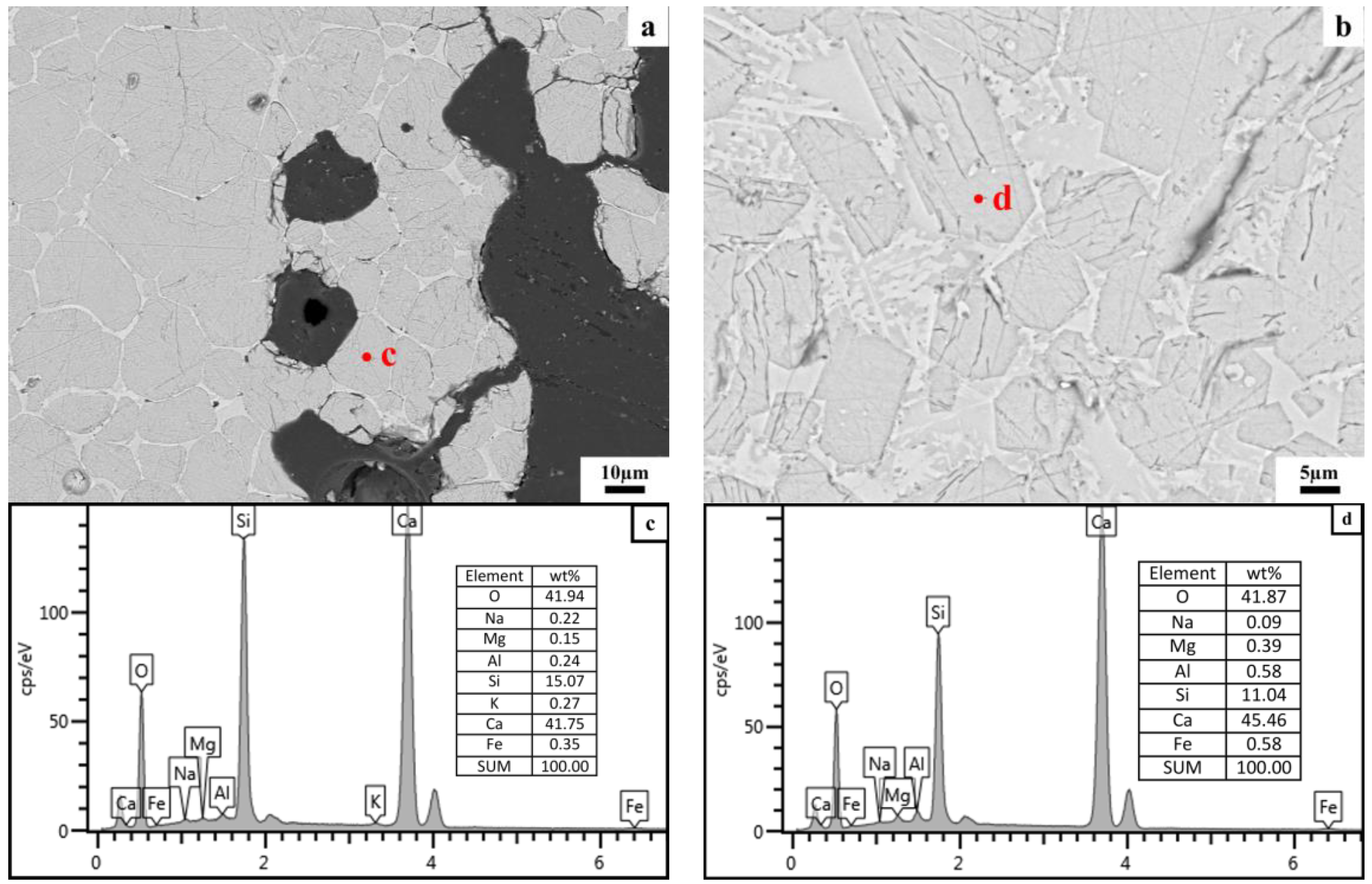
2.2.4. Microstructure Analysis
2.2.5. Mechanical Property
3. Results
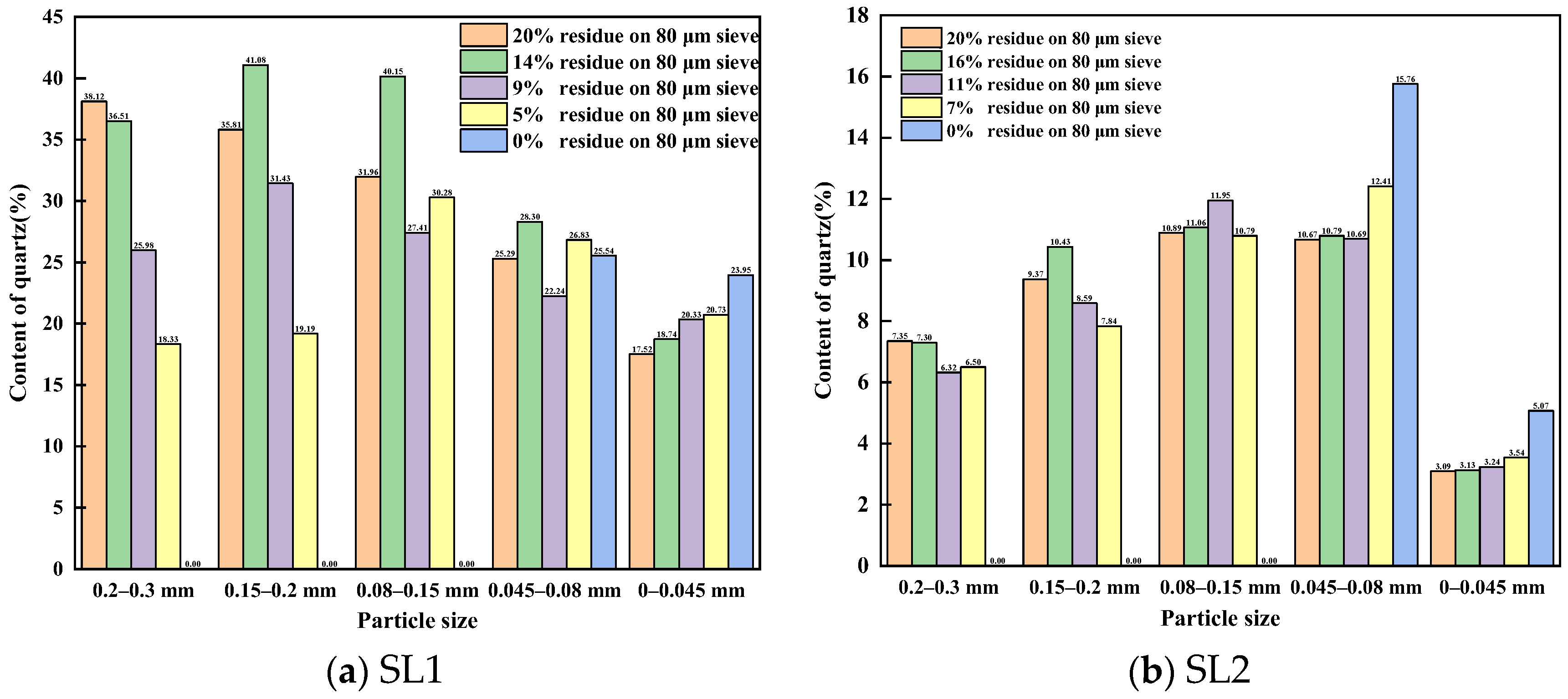
3.1. Quartz Distribution in Different Particle Size of Siliceous Limestones
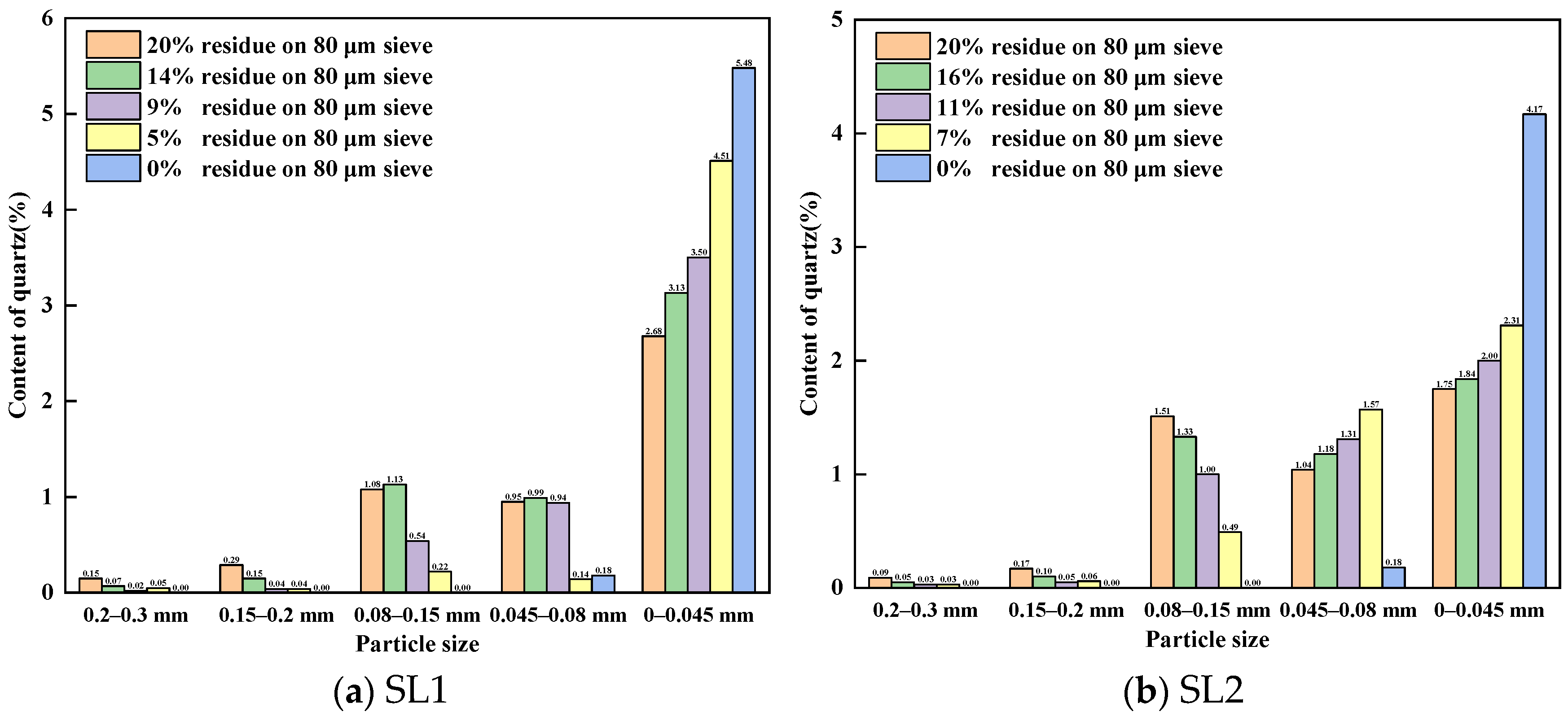
3.1.1. Content of Quartz in Different Particle Size of Siliceous Limestones
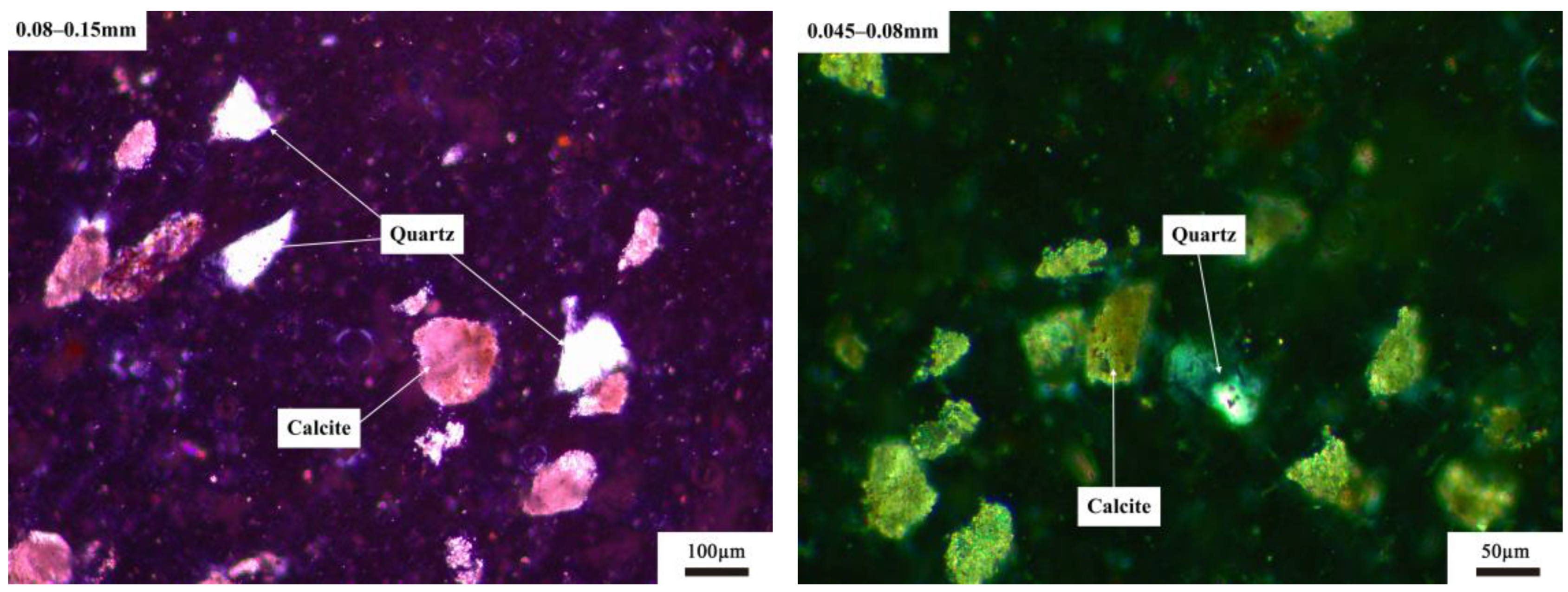
3.1.2. Microstructure of Quartz with Different Particle Size
3.2. Effects of Siliceous Limestone Fineness on Clinker Minerals
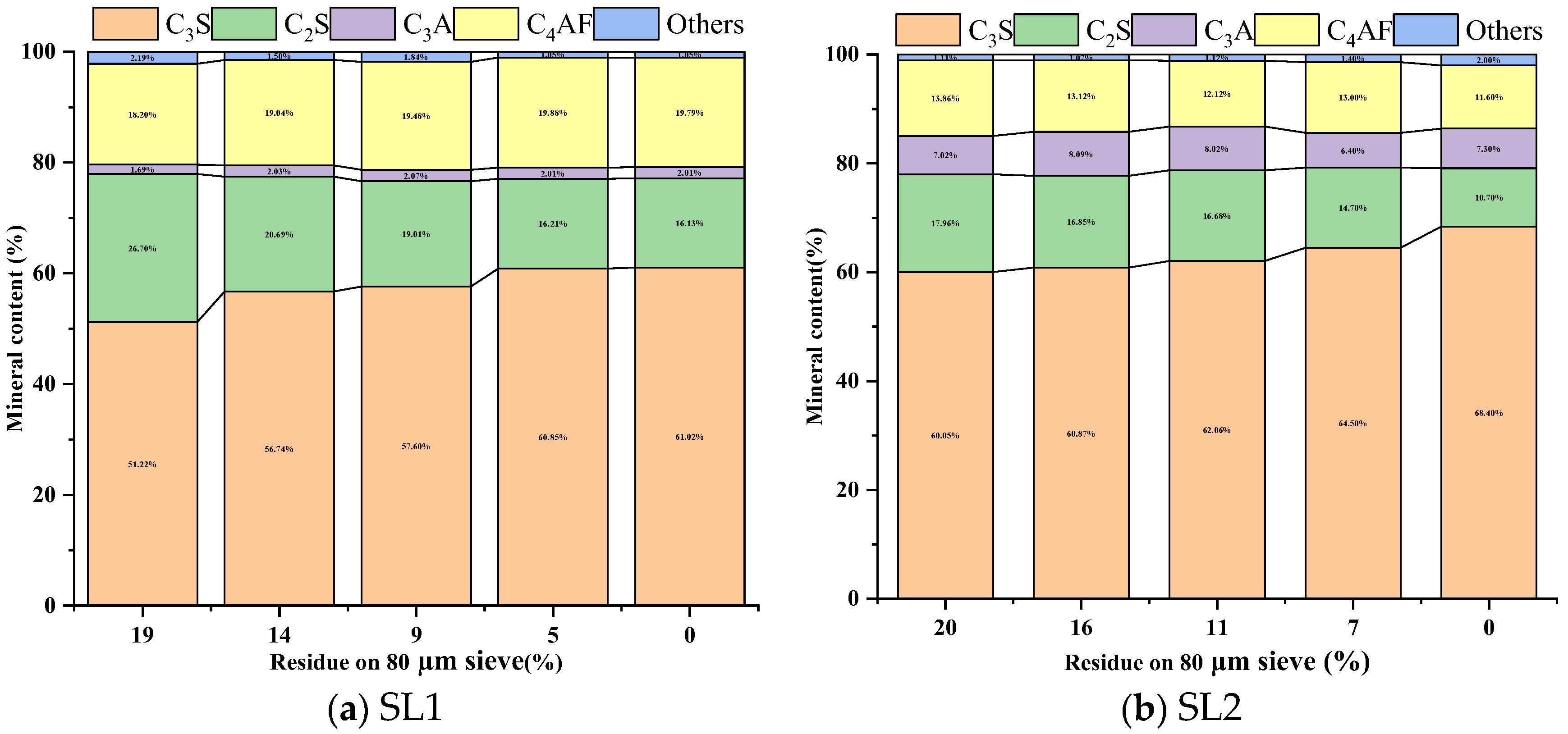
3.2.1. Burnability and Mineral Composition
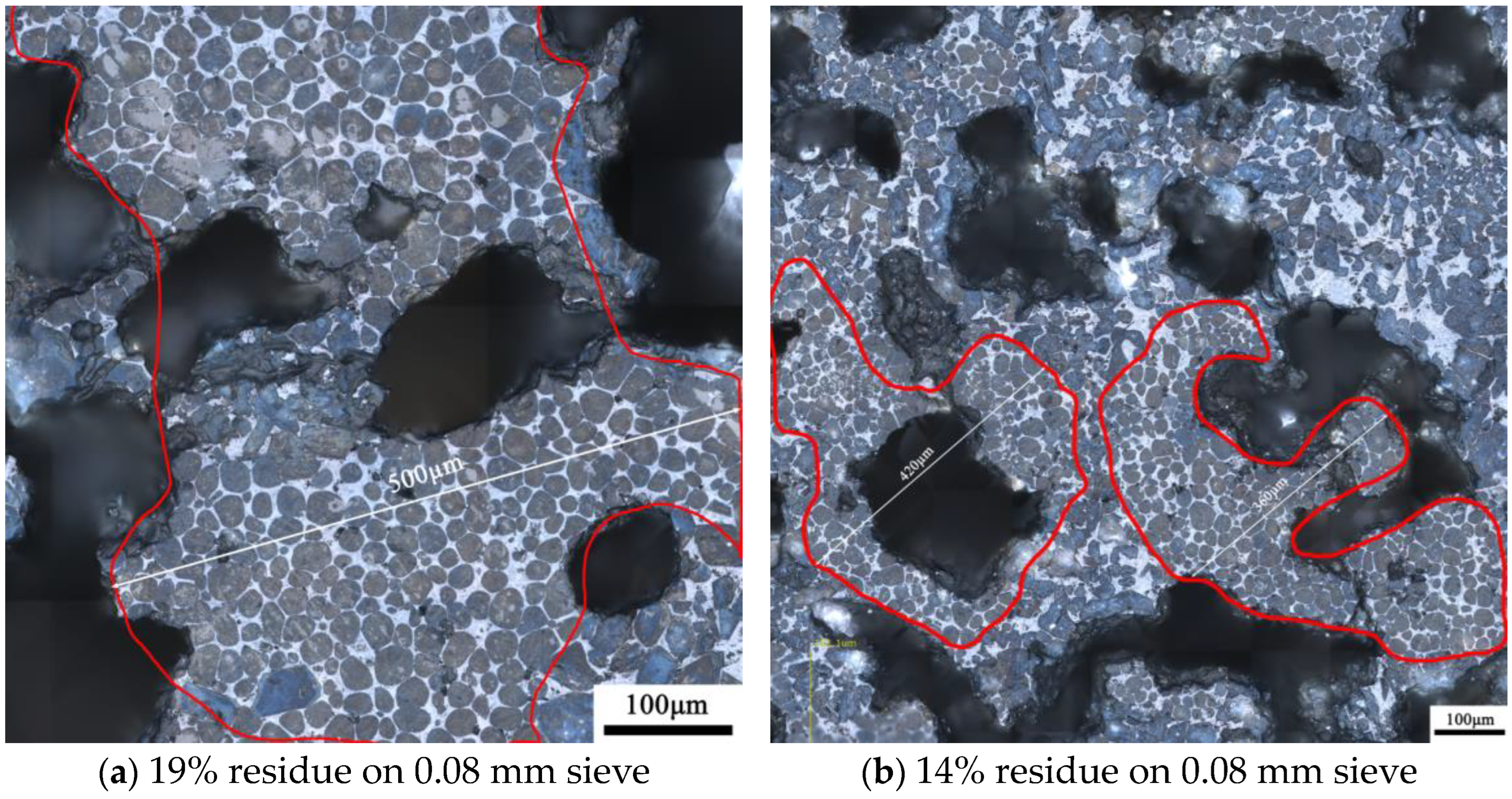
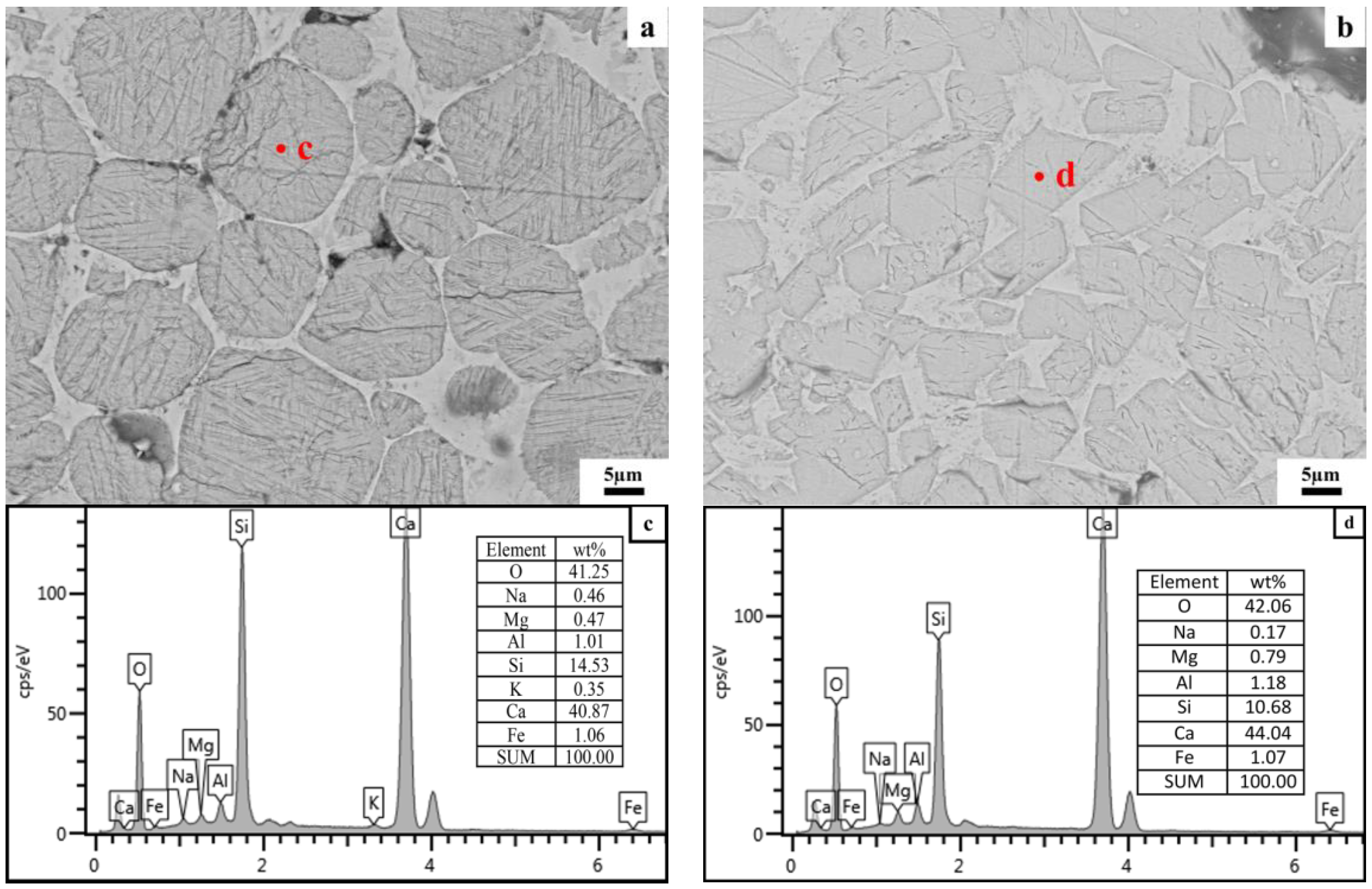
3.2.2. Microstructure of Clinker Minerals
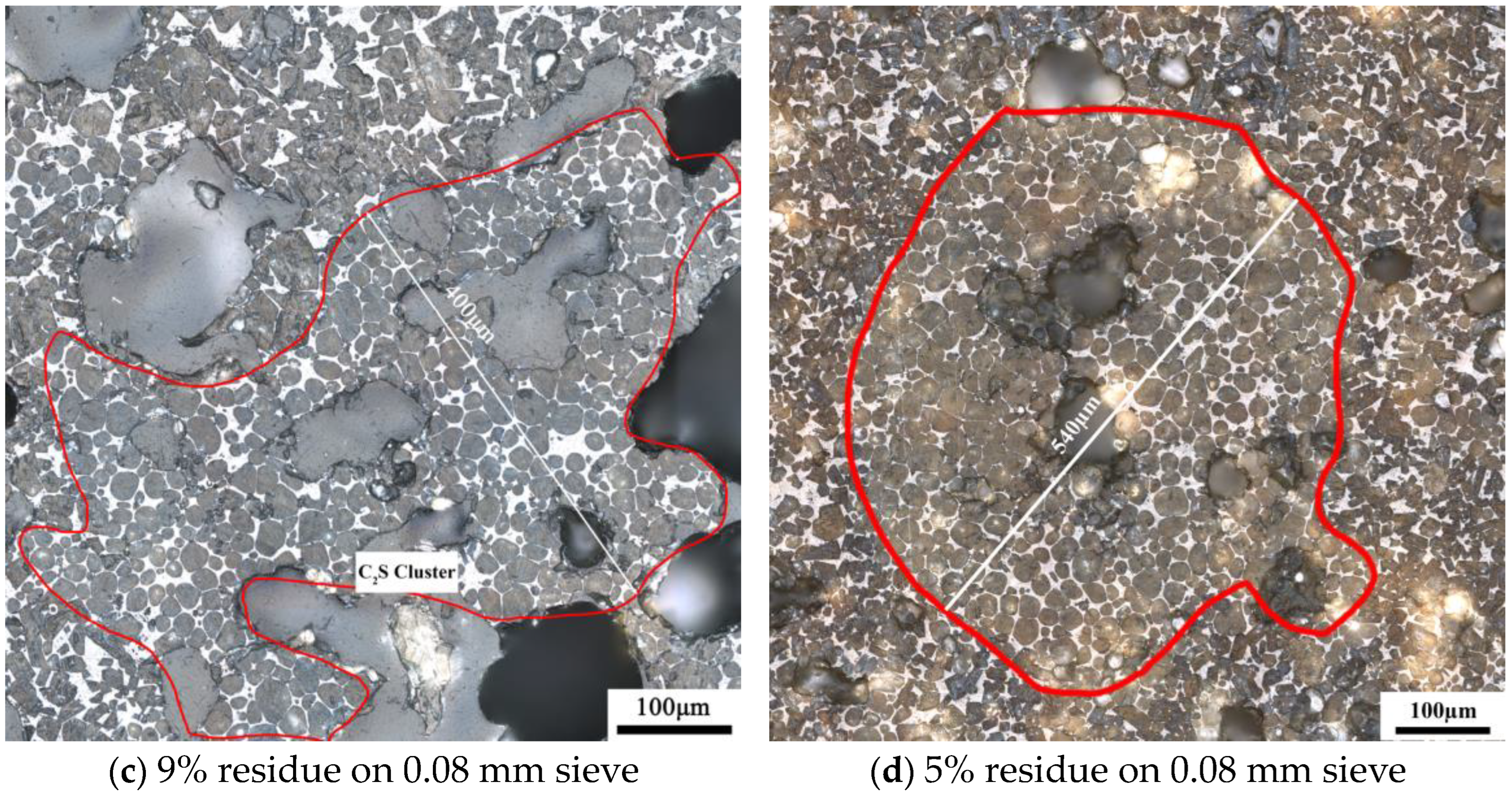
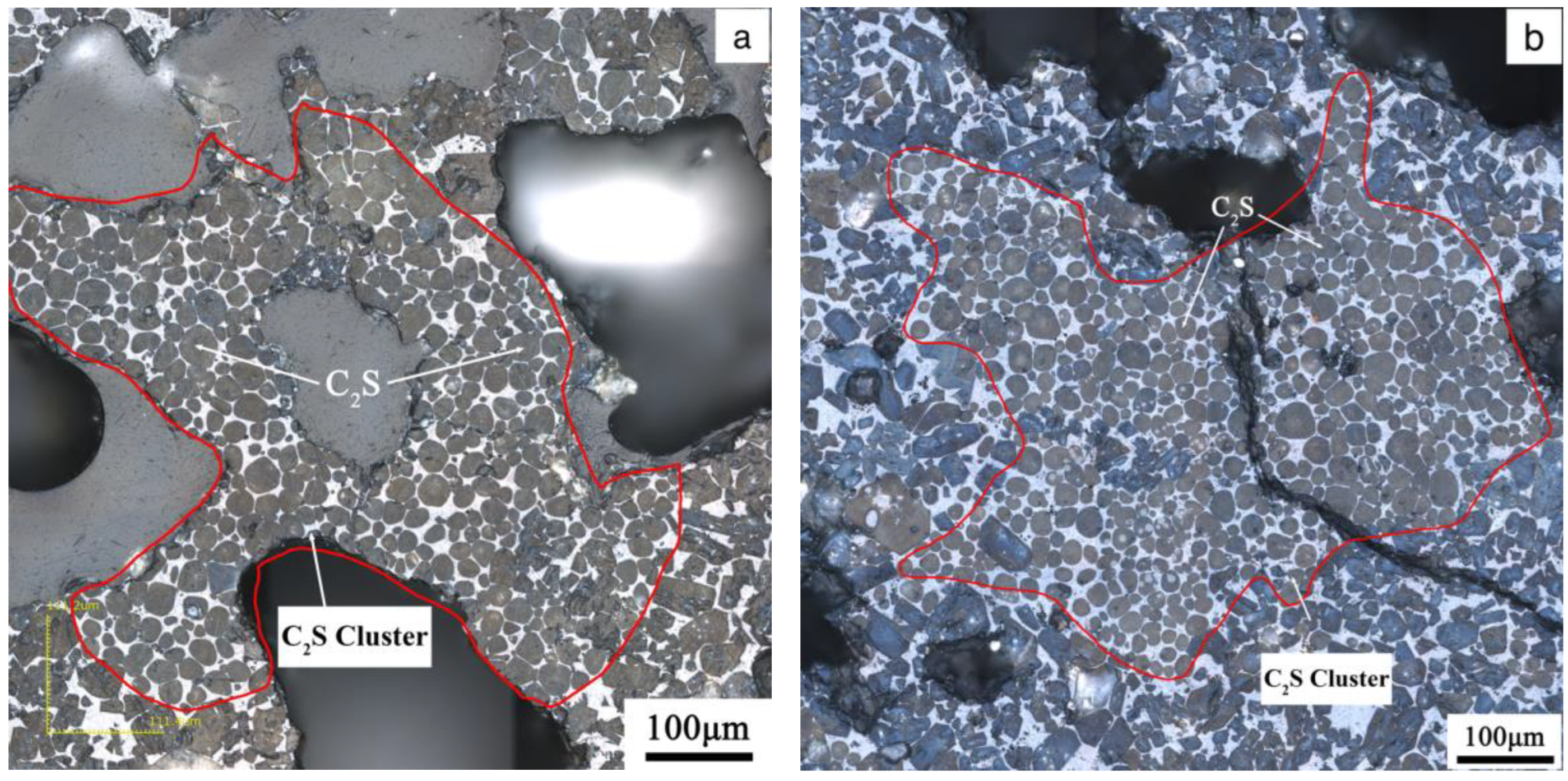
3.3. Effects of the Morphology of Quartz Crystal on the Microstructure of C2S Clusters
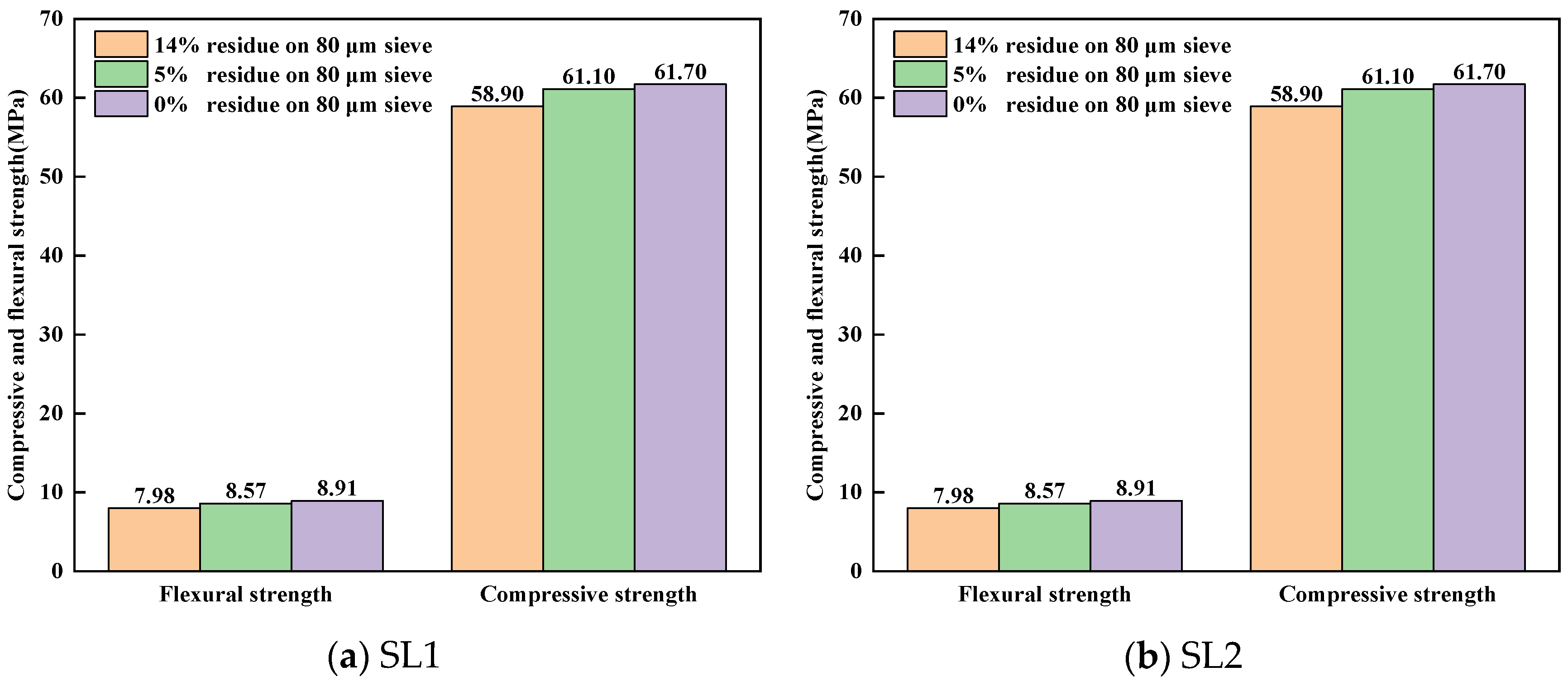
3.4. Effect of Fineness of Siliceous Limestones on the Mechanical Properties of Cement
4. Discussion
4.1. Effect of Particle Size of Quartz on the Formation of Clinker Minerals
4.2. Effects of the Morphology of Quartz Crystal on the Microstructure of C2S Clusters
4.3. The Reason for Low C3A Content in Clinker Calcined from Siliceous Limestone SL1
5. Conclusions
- The research reveals that larger quartz crystals and chert nodules in siliceous limestone are more difficult to grind than calcite. Decreasing the fineness of siliceous limestone reduces the quantity of large quartz particles, which is beneficial for the burnability of raw meal.
- A direct correlation between the fineness of siliceous limestone and the quality of the clinker minerals produced is noted. The fineness affects the mineral content of the clinker, which in turn affects the mechanical properties. As the fineness of siliceous limestones SL1 and SL2 decreases from 15% to 0% of residue on a 0.08 mm sieve, the content of C3S increases from 56.74% to 61.02% and 60.87% to 68.40%, respectively, while the content of C2S decreases from 20.69% to 16.13% and 16.85% to 10.70%, respectively. The compressive strength increases from 58.90 MPa to 61.70 MPa and 57.90 MPa to 62.30 MPa, respectively.
- The structure of quartz, whether as chert nodules or single crystals, can produce C2S clusters during the calcination, affecting the microstructure and mineral content of the clinker. Chert nodules tend to produce C2S clusters with more intermediate phases, whereas large-grained single quartz crystals lead to denser C2S clusters with minimal intermediate phases.
- It is important to optimize the fineness of siliceous limestone to mitigate the adverse effects of large quartz particles on clinker quality. The sufficient fineness values of siliceous limestones SL1 and SL2 are 5% and 7% of residue on a 0.08 mm sieve, respectively, which can produce a clinker with a 28d compressive strength greater than 60 MPa. Different grinding requirements are needed for the different morphologies of quartz in the raw meal, to strike a balance between clinker quality and energy consumption without having to grind siliceous limestone at very fine grades.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogli, J.Z.A. Chemical composition of Portland cement clinker. Am. J. Pedagog. Educ. Res. 2023, 13, 111–114. [Google Scholar]
- Chatterjee, A.K. Cement Production Technology: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Chatterjee, T. Burnability and clinkerization of cement raw mixes. In Advances in Cement Technology; Elsevier: Amsterdam, The Netherlands, 1983; pp. 69–113. [Google Scholar]
- Zhang, W.; Liu, T.; Ren, X. Effects of siliceous raw materials on clinker formation. China Build. Mater. Acad. 2020, 23, 618–624. [Google Scholar]
- Makashev, S. Effect of Raw Materials Physico-Chemical Properties on Reactivity of Raw Mix and Clinker Minerogenesis Processes. In Proceedings of the 6th ICCC, Moscow, Russia, 6–10 September 1976; pp. 1–17. [Google Scholar]
- Mackenzie, K.J.D.; Hadipour, N. Formation kinetics of portland-cement clinker phases.3. beta-dicalcium silicate and tricalcium silicate. Trans. J. Br. Ceram. Soc. 1978, 77, 168–172. [Google Scholar]
- Christensen, N.; Smidth, F. Burnability of cement raw mixes at 1400 °C II the effect of the fineness. Cem. Concr. Res. 1979, 9, 285–294. [Google Scholar] [CrossRef]
- Dakang, Z.; Yuanbo, L. Influence and measures of large size SiO2 in raw material on quality of clinker. Cem. Eng. 2016, 5, 27–29. [Google Scholar]
- Jin, Z. Research on the Impact of Minerals and Particle Size on the Content of Free Calcium Oxide and Petrographic Structure; Guangxi University: Nanning, China, 2014. [Google Scholar]
- Maki, I.; Ito, S.; Tanioka, T.; Ohno, Y.; Fukuda, K. Clinker grindability and textures of alite and belite. Cem. Concr. Res. 1993, 23, 1078–1084. [Google Scholar] [CrossRef]
- Maki, I. Processing conditions of Portland cement clinker as viewed from the fine textures of the constituent minerals, Ceramic Transactions. Am. Ceram. Soc. 1994, 40, 3–17. [Google Scholar]
- Tan, L.; Xuehong, R.; Wensheng, Z. Research Progress on the Influence of SiO2 Crystalline Characteristics in Raw Materials on Clinker Sintering. Bull. Chin. Ceram. Soc. 2019, 38, 1777–1781. [Google Scholar]
- Huagang, Z. Effect of the properties of siliceous material on the burnability of raw meal. Cement 1998, 6, 12–16. [Google Scholar]
- He, S.; Ling, H.; Zhu, X. Influence of raw meal finess on burning of Portland cement clinker. Cem. Eng. 2012, 5, 23–26. [Google Scholar]
- Ishak, K.; Hashim, S.F.S.; Azizli, K.A.M.; Palaniandy, S.; Hussin, H. Effect of Quartz in Clay on Grindability of Raw Mixes for Cement Production. J. Phys. Sci. 2022, 33, 45–59. [Google Scholar] [CrossRef]
- Kakali, G.; Tsivilis, S. The effect of intergrinding and separate grinding of cement raw mix on the burning process. Cem. Concr. Res. 1993, 23, 651–662. [Google Scholar] [CrossRef]
- Fundal, E. The burnability of cement raw mixes. World Cem. Technol. 1979, 42, 195–204. [Google Scholar]
- Christensen, N. Burnability of cement raw mixes at 1400 °C I The effect of the chemical composition. Cem. Concr. Res. 1979, 9, 219–228. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, X.; Hu, J.; Xu, P.; Ni, X. The Influence of Grain Size and Crystal Structure of Quartz on Raw Meal Burnability. Cem. Technol. 2000, 3, 9–11. [Google Scholar]
- Hou, G.; Shen, X.; Xu, Z. Composition design for high C3S cement clinker and its mineral formation. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2007, 22, 56–60. [Google Scholar] [CrossRef]
- Mathew, B.; Erikandath, S.; Lele, G.; Khadilkar, S. Process Control & Clinker Quality Monitoring through Mineralogical and Micro-Structural Indices, Proceedings of the 14th NCB International Seminar on Cement and Building Materials, New Delhi, India, 1–4 December 2015; National Council for Cement and Building Materials: New Delhi, India, 2016. [Google Scholar]
- Maki, I.; Fukuda, K.; Imura, T.; Yoshida, H.; Ito, S. Formation of belite clusters from quartz grains in portland cement clinker. Cem. Concr. Res. 1995, 25, 835–840. [Google Scholar] [CrossRef]
- Korkmaz, A.V. Evaluation of chemical, mineralogical and clinker burnability properties of mudstones as cement raw materials. Case Stud. Constr. Mater. 2019, 11, e00254. [Google Scholar] [CrossRef]
- Castillo, J.A.; Broström, M.; Eriksson, M. Phase evolution and burnability of cement raw meal. Adv. Cem. Res. 2023, 35, 577–587. [Google Scholar] [CrossRef]
- Shirahama, N.; Yamashita, M.; Tanaka, H. The influence of crystal properties of quartz in raw materials on the formation of clinker minerals. In Proceedings of the 14th International Congress on the Chemistry of Cement, Beijing, China, 13–16 October 2015; Volume 42. [Google Scholar]
- De Matos, P.; Neto, J.A.; Sakata, R.; Kirchheim, A.; Rodríguez, E.; Campos, C. Strategies for XRD quantitative phase analysis of ordinary and blended Portland cements. Cem. Concr. Compos. 2022, 131, 104571. [Google Scholar] [CrossRef]
- GB/T 176-2017; Methods for chemical analysis of cement, Standardization Administration of PRC. China National Standardization Administration: Beijing, China, 2017.
- JY/T 0587-2020; General rules of polycrystalline X-ray diffraction method, Ministry of Education of PRC. Ministry of Education: Beijing, China, 2020.
- GB/T 17671-2021; Test method of cement mortar strength, Standardization Administration of PRC. China National Standardization Administration: Beijing, China, 2021.










| Samples | Chemical Composition/wt.% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOI | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | K2O | Na2O | SO3 | Total | |
| Siliceous limestone SL1 | 28.33 | 27.02 | 2.16 | 0.48 | 37.00 | 3.80 | 0.17 | 0.07 | 0.07 | 99.10 |
| Siliceous limestone SL2 | 40.08 | 6.48 | 1.08 | 0.24 | 50.91 | 0.24 | 0.16 | 0.05 | 0.07 | 99.31 |
| Limestone L1 | 42.83 | 0.45 | 0.33 | 0.12 | 53.84 | 0.26 | 0.03 | 0.03 | 0.05 | 97.94 |
| Sandstone S1 | 7.25 | 73.08 | 3.84 | 7.21 | 5.12 | 0.49 | 0.71 | 0.06 | 0.09 | 97.85 |
| Fly ash FA | 6.29 | 57.42 | 5.73 | 24.94 | 1.83 | 1.45 | 0.67 | 0.51 | 0.07 | 98.91 |
| Iron slag IS | 19.34 | 6.48 | 45.57 | 2.24 | 15.24 | 3.99 | 0.51 | 0.55 | 0.11 | 94.03 |
| Coal ash CA | 0.45 | 50.39 | 5.48 | 36.30 | 4.22 | 0.24 | - | - | 0.08 | 97.16 |
| No. | Raw Materials/% | ||||||
|---|---|---|---|---|---|---|---|
| Siliceous Limestone SL1 | Siliceous Limestone SL2 | Limestone L1 | Sandstone S1 | Fly Ash FA | Iron Slag IS | Coal Ash CA | |
| 1 | - | 83.5 | - | 2.6 | 7.2 | 5.4 | 1.5 |
| 2 | 23.6 | - | 61.5 | - | 8.3 | 5.1 | 1.5 |
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| CaCO3 | 10% | 20% | 40% | 50% | 60% | 70% | 80% | 90% |
| SiO2 | 90% | 80% | 60% | 50% | 40% | 30% | 20% | 10% |
| Total | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, D.; Li, W.; Xie, L.; Deng, M.; Ding, H.; Liu, K. Effects of Fineness and Morphology of Quartz in Siliceous Limestone on the Calcination Process and Quality of Cement Clinker. Materials 2024, 17, 3601. https://doi.org/10.3390/ma17143601
Nie D, Li W, Xie L, Deng M, Ding H, Liu K. Effects of Fineness and Morphology of Quartz in Siliceous Limestone on the Calcination Process and Quality of Cement Clinker. Materials. 2024; 17(14):3601. https://doi.org/10.3390/ma17143601
Chicago/Turabian StyleNie, Donggen, Wei Li, Lilan Xie, Min Deng, Hao Ding, and Kaiwei Liu. 2024. "Effects of Fineness and Morphology of Quartz in Siliceous Limestone on the Calcination Process and Quality of Cement Clinker" Materials 17, no. 14: 3601. https://doi.org/10.3390/ma17143601





