Co-Extraction of Aluminum and Silicon and Kinetics Analysis in Carbochlorination Process of Low-Grade Bauxite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
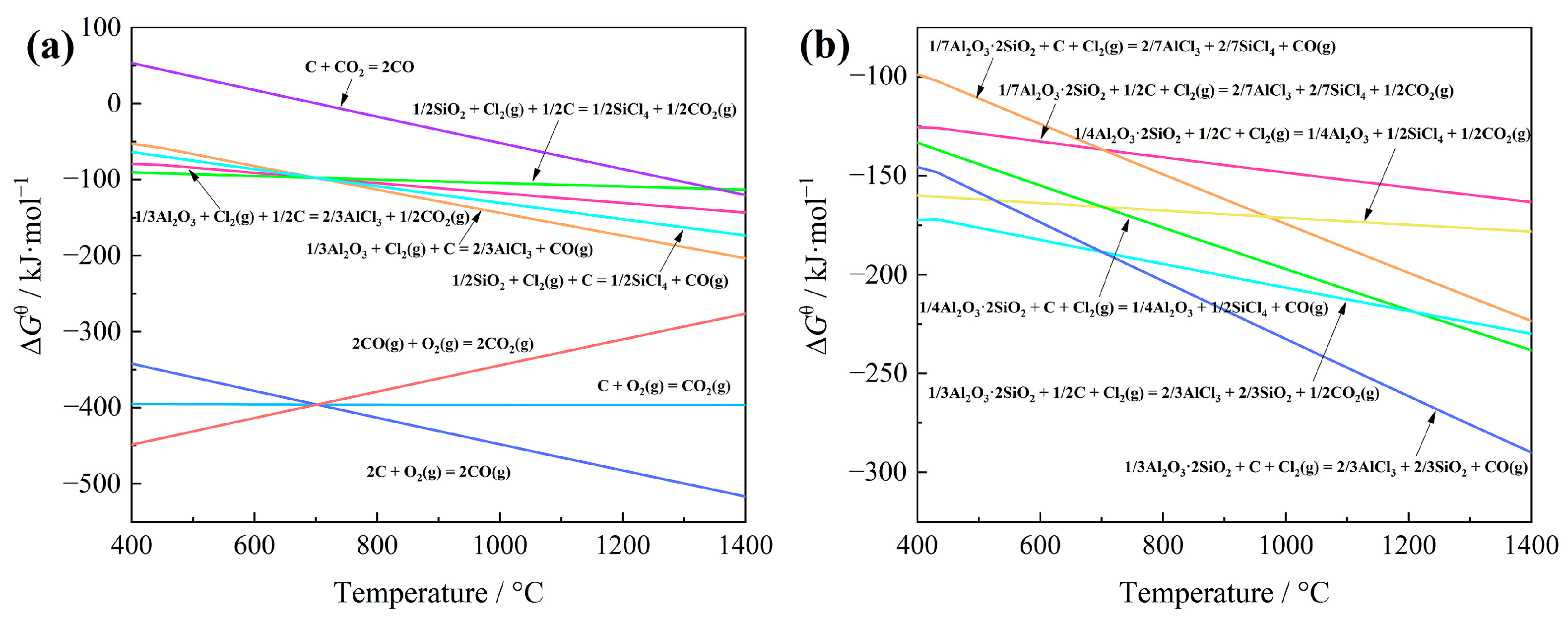
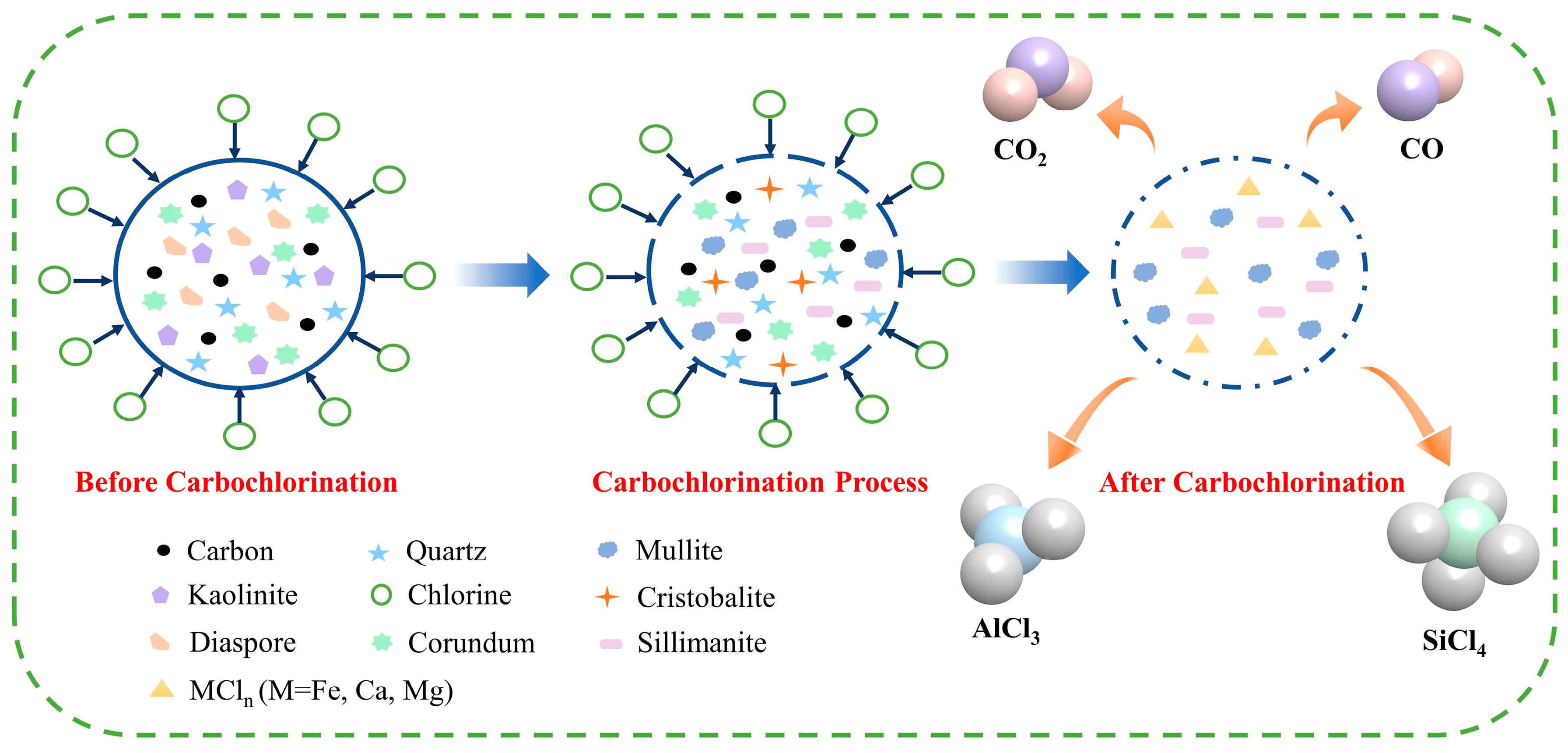
3.1. Thermodynamics of Carbochlorination Reactions
3.2. Carbochlorination of Bauxite
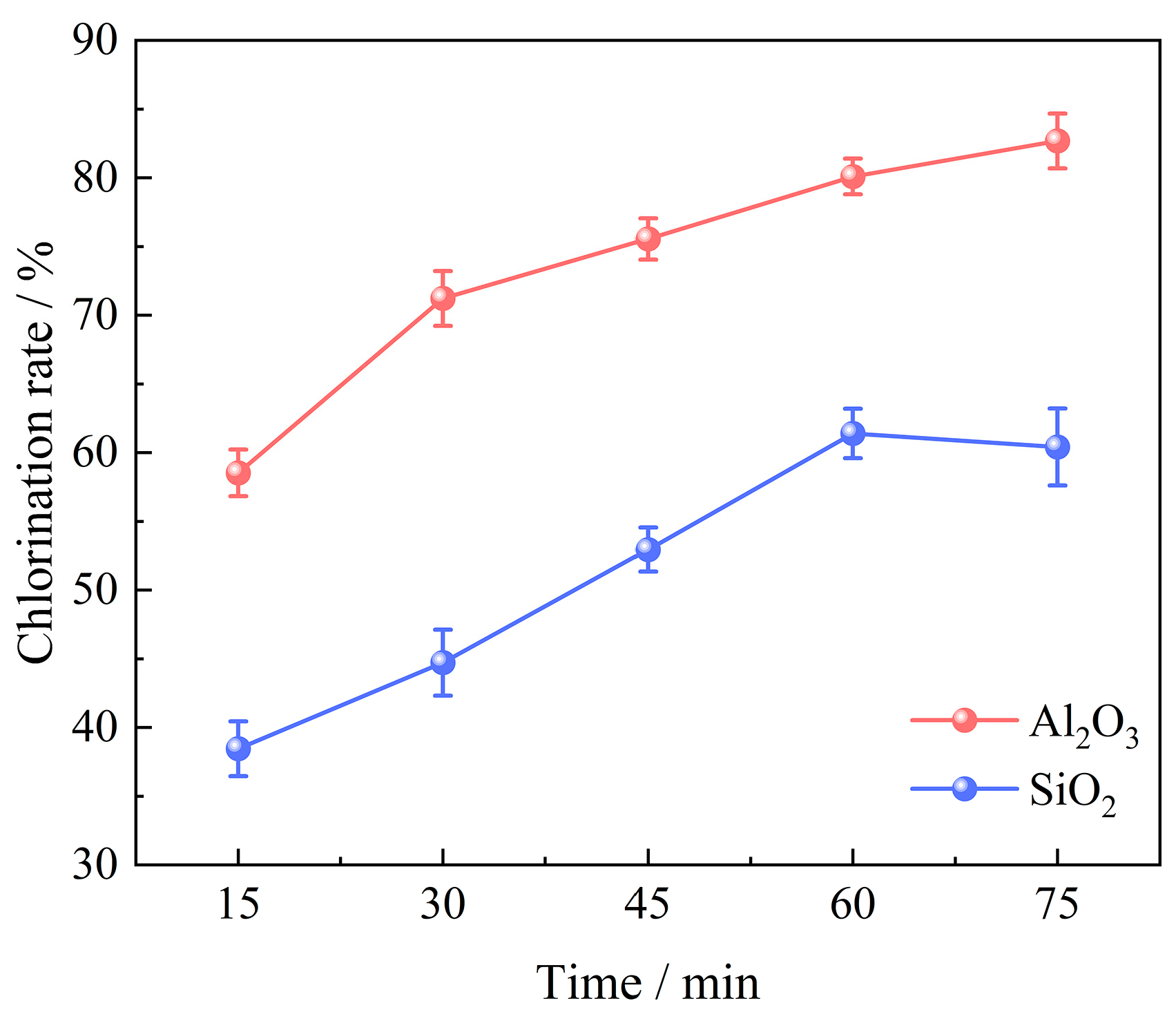
3.2.1. Effect of Chlorination Time
3.2.2. Effect of Coking Coal Addition
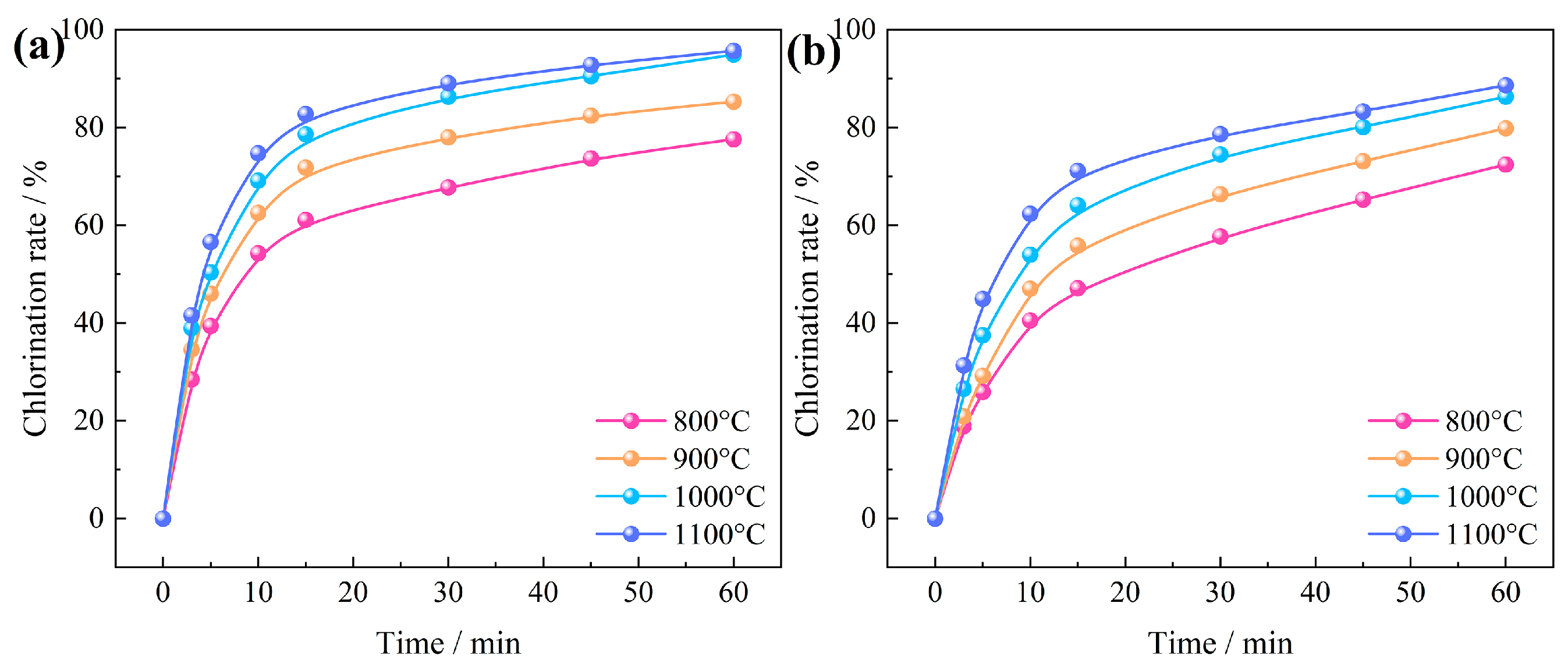
3.2.3. Effect of Chlorination Temperature
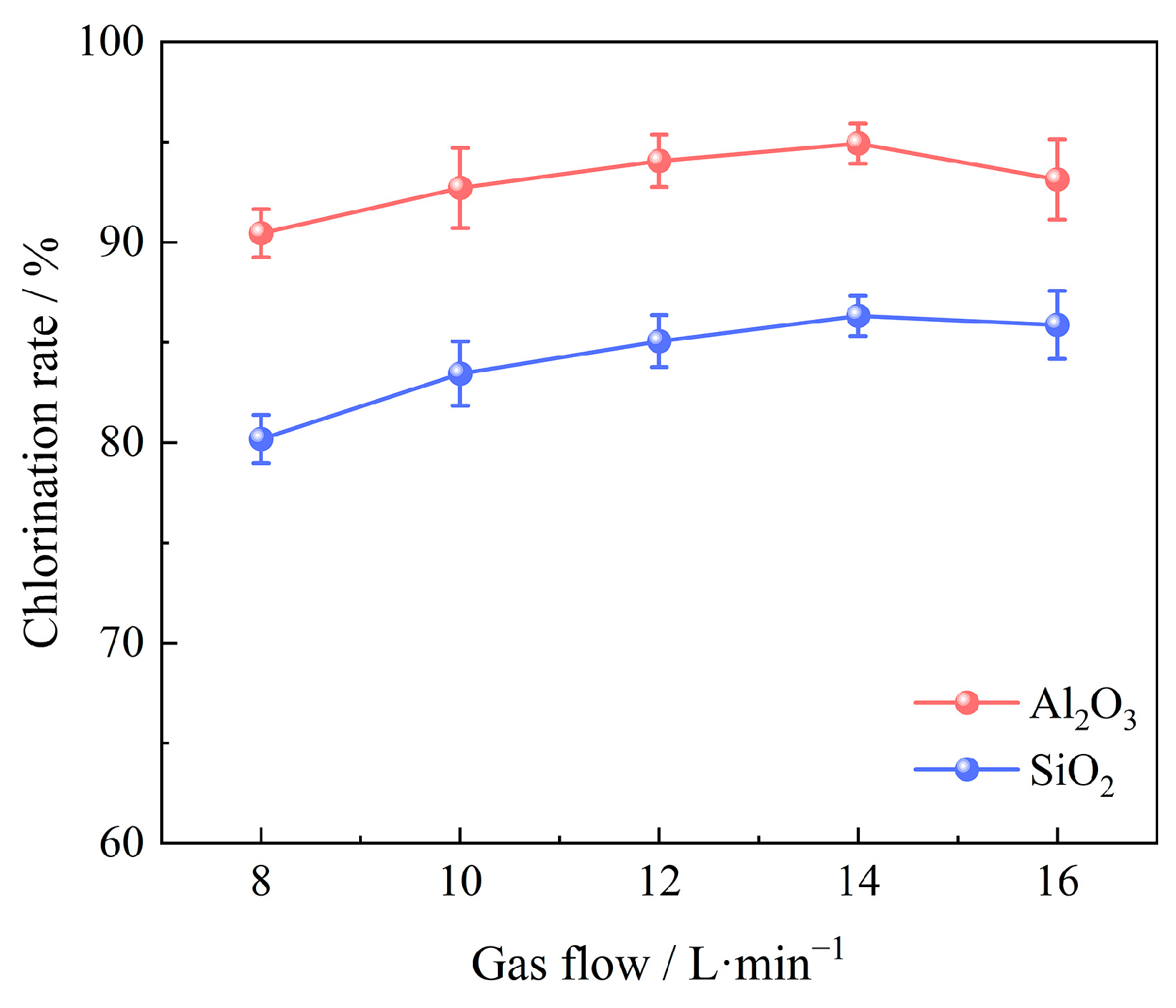
3.2.4. Effect of Gas Flow
3.2.5. Effect of Oxygen Content
3.3. Carbochlorination Kinetics of Low-Grade Bauxite
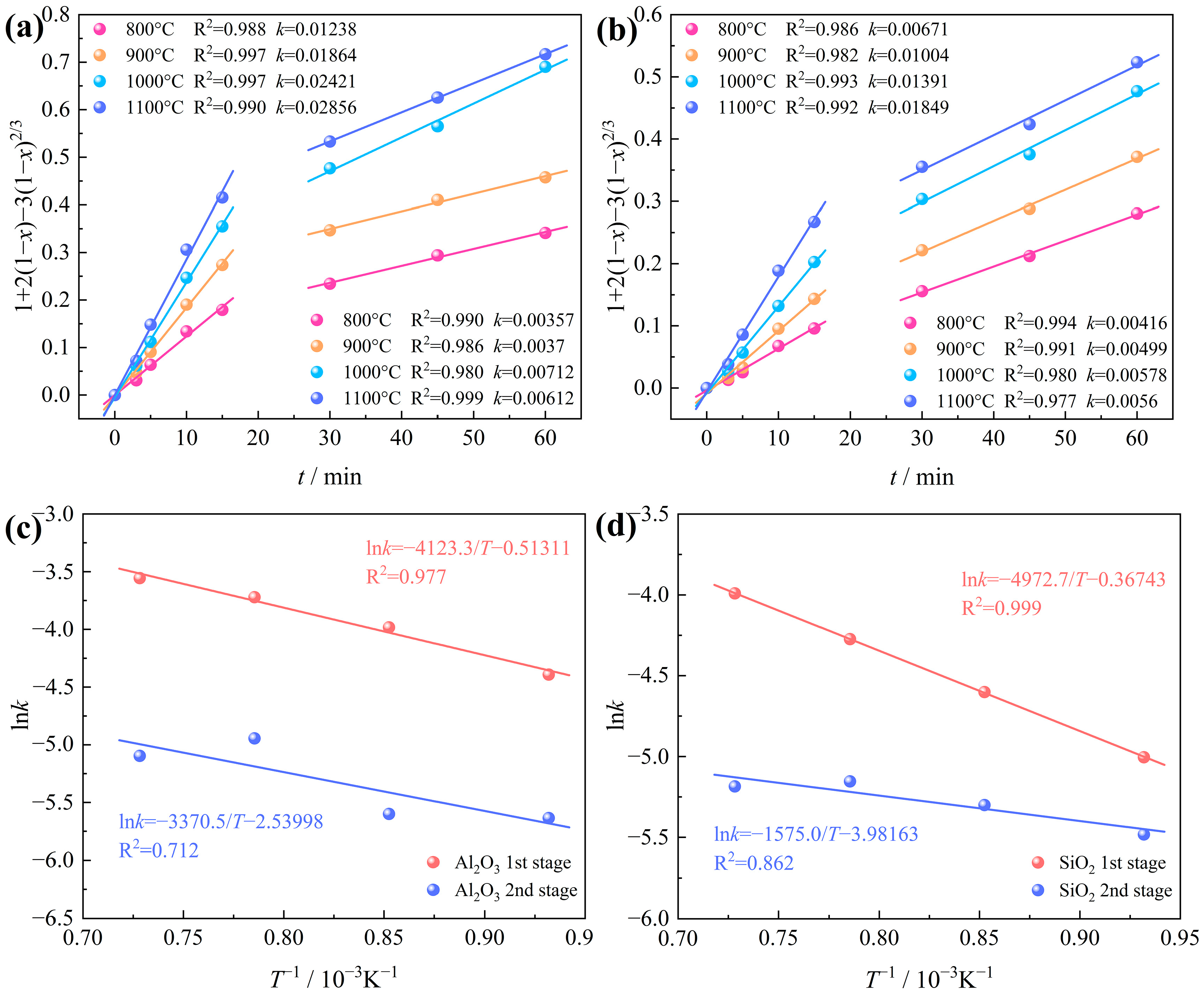
3.3.1. Effects of Chlorination Temperature and Time
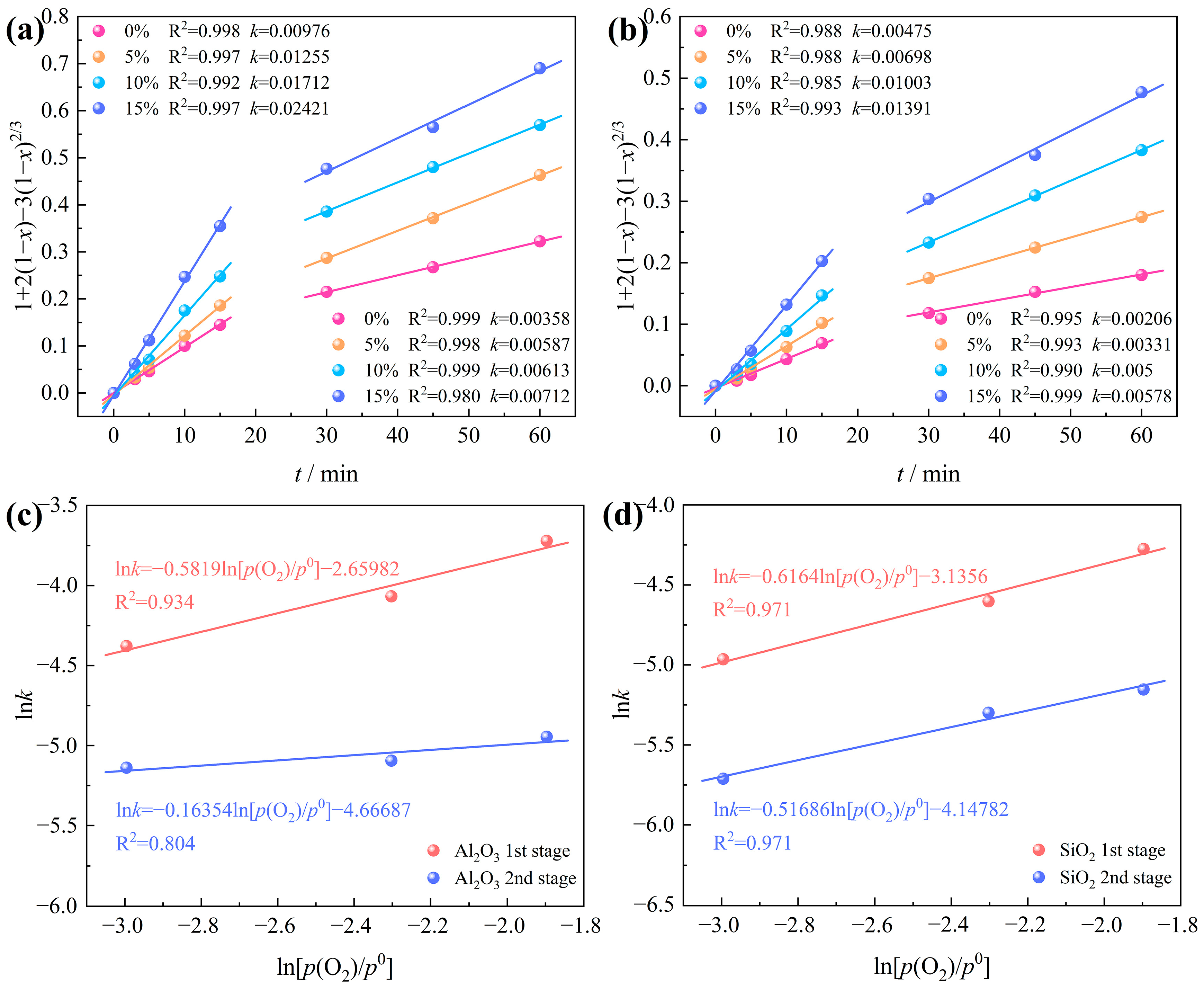
3.3.2. Effects of Oxygen Content and Chlorination Time
3.3.3. Determination of Chlorination Kinetic Model
- (1)
- Cl2 permeated the surface of bauxite pellets via the gas phase diffusion boundary layer.
- (2)
- Cl2 was diffused through porous solid media and subsequently adsorbed by the aluminum–silicon phase in the pellets.
- (3)
- The carbochlorination reaction between Cl2 and the aluminum–silicon phase resulted in the production of gaseous AlCl3 and SiCl4.
- (4)
- AlCl3 and SiCl4 diffused into the gas phase through the porous medium layer.
- (5)
- AlCl3 and SiCl4 underwent diffusion from the gas boundary layer into the surrounding air.
- (1)
- Kinetics equation of chemical reaction control:
- (2)
- Kinetics equation of external diffusion control:
- (3)
- Kinetics equation of internal diffusion control:
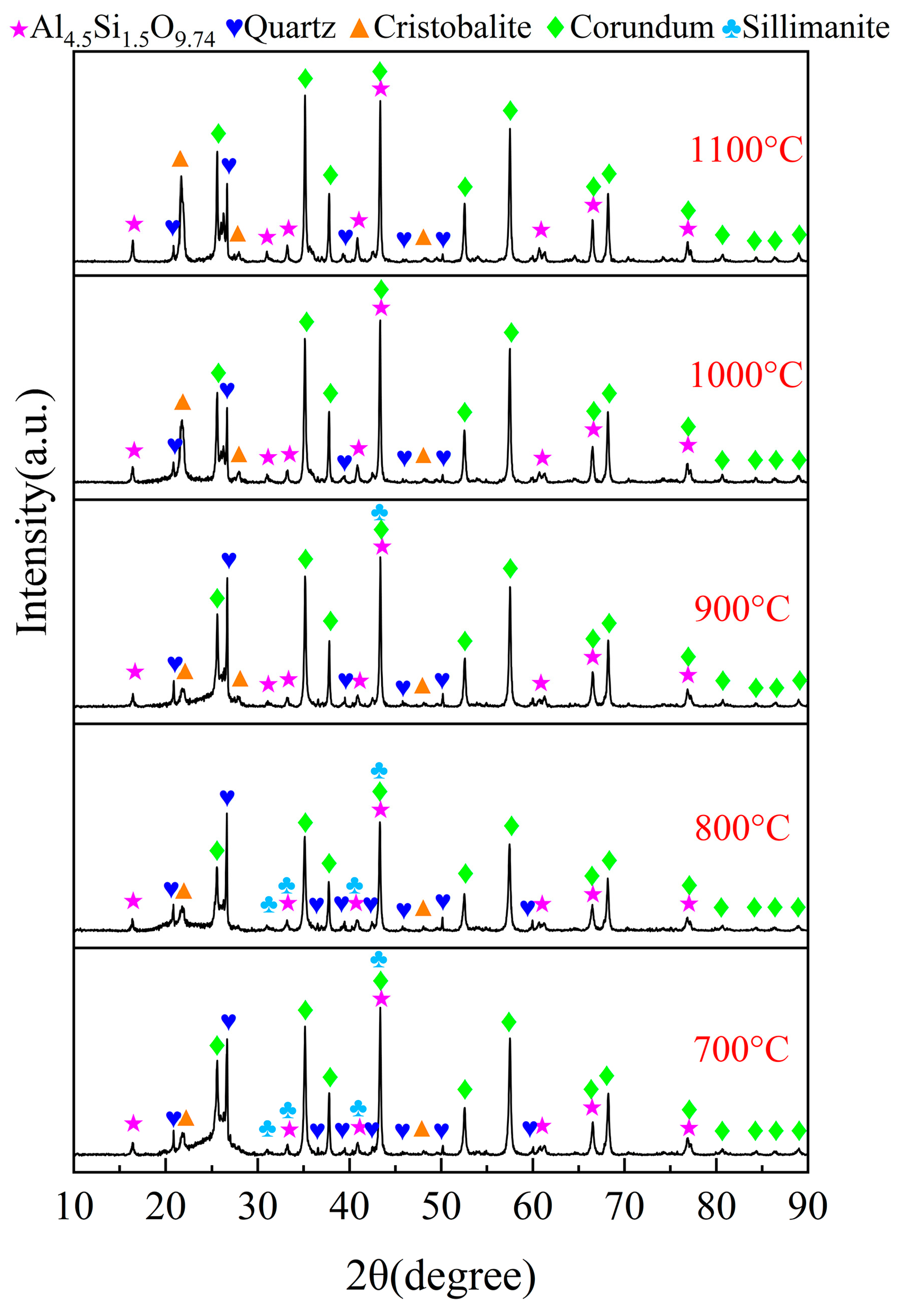
3.4. Carbochlorination Mechanism of Low-Grade Bauxite
4. Conclusions
- (1)
- The feasibility of recovering aluminum and silicon from low-grade bauxite via carbochlorination was verified through thermodynamic analysis. The carbochlorination of aluminum has precedence over that of silicon.
- (2)
- The primary determinants influencing the carbochlorination process of low-grade bauxite include the chlorination time, amount of added coking coal, chlorination temperature, gas flow, and oxygen content. Optimal results are achieved when operating under conditions of a chlorination temperature of 1000 °C, a chlorination time of 60 min, a gas flow of 14 L/min, a C/O molar ratio of 2.206, and an oxygen content of 15%, with which the chlorination efficiency for Al2O3 and SiO2 can reach 94.93% and 86.32%, respectively.
- (3)
- The kinetic analysis of the carbochlorination process involving Al2O3 and SiO2 in bauxite reveals that the reaction adheres to a shrinking, unreacted core model. This model is primarily governed by gas diffusion within the product layer, encompassing two distinct stages. The apparent activation energies for the initial and subsequent stages of the Al2O3 chlorination reaction, occurring at temperatures ranging from 800 to 1100 °C, are 34.281 kJ/mol and 28.022 kJ/mol, respectively. Likewise, the apparent activation energies for the two stages of the SiO2 chlorination reaction stand at 41.343 kJ/mol and 13.095 kJ/mol, respectively.
- (4)
- The observed variation in oxygen content, ranging from 0% to 15%, yielded apparent reaction orders of 0.58 and 0.16 for the first and second stages of Al2O3 chlorination, respectively. Similarly, for SiO2 chlorination, the evident reaction orders were determined to be 0.62 and 0.52 for the two stages, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shah, S.S.; Palmieri, M.C.; Sponchiado, S.R.P.; Bevilaqua, D. A sustainable approach on biomining of low-grade bauxite by P. simplicissimum using molasses medium. Braz. J. Microbiol. 2022, 53, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Vakilchap, F.; Mousavi, S.M.; Shojaosadati, S.A. Role of Aspergillus niger in recovery enhancement of valuable metals from produced red mud in Bayer process. Bioresour. Technol. 2016, 218, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.C.; Zhang, F.; Deng, X.J.; Guo, H.C.; Zhang, C.; Shi, C.S.; Zeng, M. Extraction of alumina from alumina rich coal gangue by a hydro-chemical process. R. Soc. Open Sci. 2020, 7, 192132. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.P.; Chen, C.Y.; Lan, Y.P.; Wang, L.Z.; Li, J.Q. Desilication and recycling of alkali-silicate solution seeded with red mud for low-grade bauxite utilization. J. Mater. Res. Technol. 2020, 9, 7418–7426. [Google Scholar] [CrossRef]
- Birinci, M.; Gök, R. Characterization and flotation of low-grade boehmitic bauxite ore from Seydisehir (Konya, Turkey). Miner. Eng. 2021, 161, 106714. [Google Scholar] [CrossRef]
- Lakshmanan, V.I.; Sridhar, R.; Chen, J.; Halim, M.A. Development of Mixed-Chloride Hydrometallurgical Processes for the Recovery of Value Metals from Various Resources. Trans. Indian Inst. Met. 2016, 69, 39–50. [Google Scholar] [CrossRef]
- Grjotheim, K. Aluminium Electrolysis: The Chemistry of the Hall-Hult Process; Aluminium-Verlag GmbH: Düsseldorf, Germany, 1977. [Google Scholar]
- Liang, X.M. Development of Alcoa’s aluminum electrolysis technology. Light Met. 2020, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Wyndham, R. Controlled Carbo-Chlorination of Kaolinitic Ores. U.S. Patent 4,083,927A, 11 April 1978. [Google Scholar]
- Reynolds, J.E.; Williams, A.R. Process for Chlorinating Clay and Bauxite. U.S. Patent 4,288,414A, 1981. [Google Scholar]
- Wang, L.; Zhao, X.X.; Zhang, Z.M.; Zhang, T.A.; Lv, G.Z.; Dou, Z.H.; Zhao, A.C.; Zhang, X.Y. Synergistic separation and extraction of valuable elements from high-alumina fly ash with carbochlorination method. Trans. Nonferr. Met. Soc. China 2023, 1–20. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the greenhouse gas CO2 to the fuel gas CO via the Boudouard reaction: A review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Deng, P.; Li, L.; Jia, Y.Q.; Liu, D.C.; Jiang, W.L.; Kong, L.X. Chlorination behavior of low-grade titanium slag in AlCl3-NaCl molten salt. JOM 2022, 74, 213–221. [Google Scholar] [CrossRef]
- Zhao, X.X.; Wang, L.; Cheng, T.H.; Liu, Y.; Zhang, T.G.; Zhao, Q.Y. Synergistic extraction of valuable elements from high-alumina fly ash via carbochlorination. J. Sustain. Metall. 2024. [Google Scholar] [CrossRef]
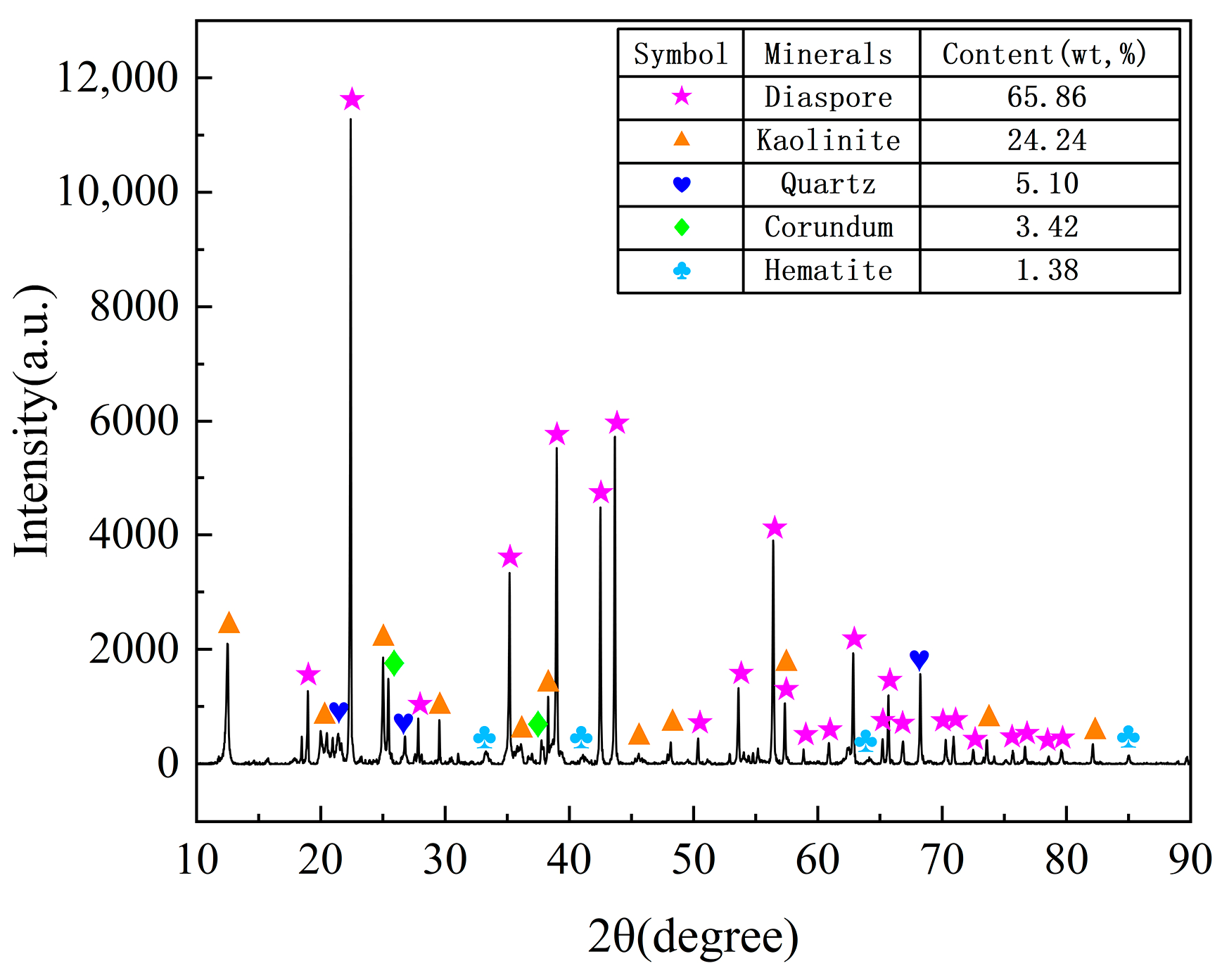






| Component | Al2O3 | SiO2 | CaO | Fe2O3 | TiO2 | MgO | K2O | A/S |
|---|---|---|---|---|---|---|---|---|
| Content, wt.% | 61.11 | 22.49 | 1.25 | 8.42 | 3.73 | 0.37 | 1.08 | 2.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, Y.; Wang, L.; Hua, Y.; Cheng, T.; Zhang, T.; Zhao, Q. Co-Extraction of Aluminum and Silicon and Kinetics Analysis in Carbochlorination Process of Low-Grade Bauxite. Materials 2024, 17, 3613. https://doi.org/10.3390/ma17143613
Zhao X, Liu Y, Wang L, Hua Y, Cheng T, Zhang T, Zhao Q. Co-Extraction of Aluminum and Silicon and Kinetics Analysis in Carbochlorination Process of Low-Grade Bauxite. Materials. 2024; 17(14):3613. https://doi.org/10.3390/ma17143613
Chicago/Turabian StyleZhao, Xinxin, Yan Liu, Long Wang, Yutong Hua, Tianhao Cheng, Tingan Zhang, and Qiuyue Zhao. 2024. "Co-Extraction of Aluminum and Silicon and Kinetics Analysis in Carbochlorination Process of Low-Grade Bauxite" Materials 17, no. 14: 3613. https://doi.org/10.3390/ma17143613





