Properties of Cemented Filling Materials Prepared from Phosphogypsum-Steel Slag–Blast-Furnace Slag and Its Environmental Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Proportions and Preparation of PBM
2.2.1. Mix Proportions
2.2.2. Preparation of PBM
2.3. Test Methods
2.3.1. Compressive Strength Test
2.3.2. Toxicity Leaching Test
2.3.3. SEM Test
2.3.4. XRD Test
3. Results and Discussion
3.1. Unconfined Compressive Strength
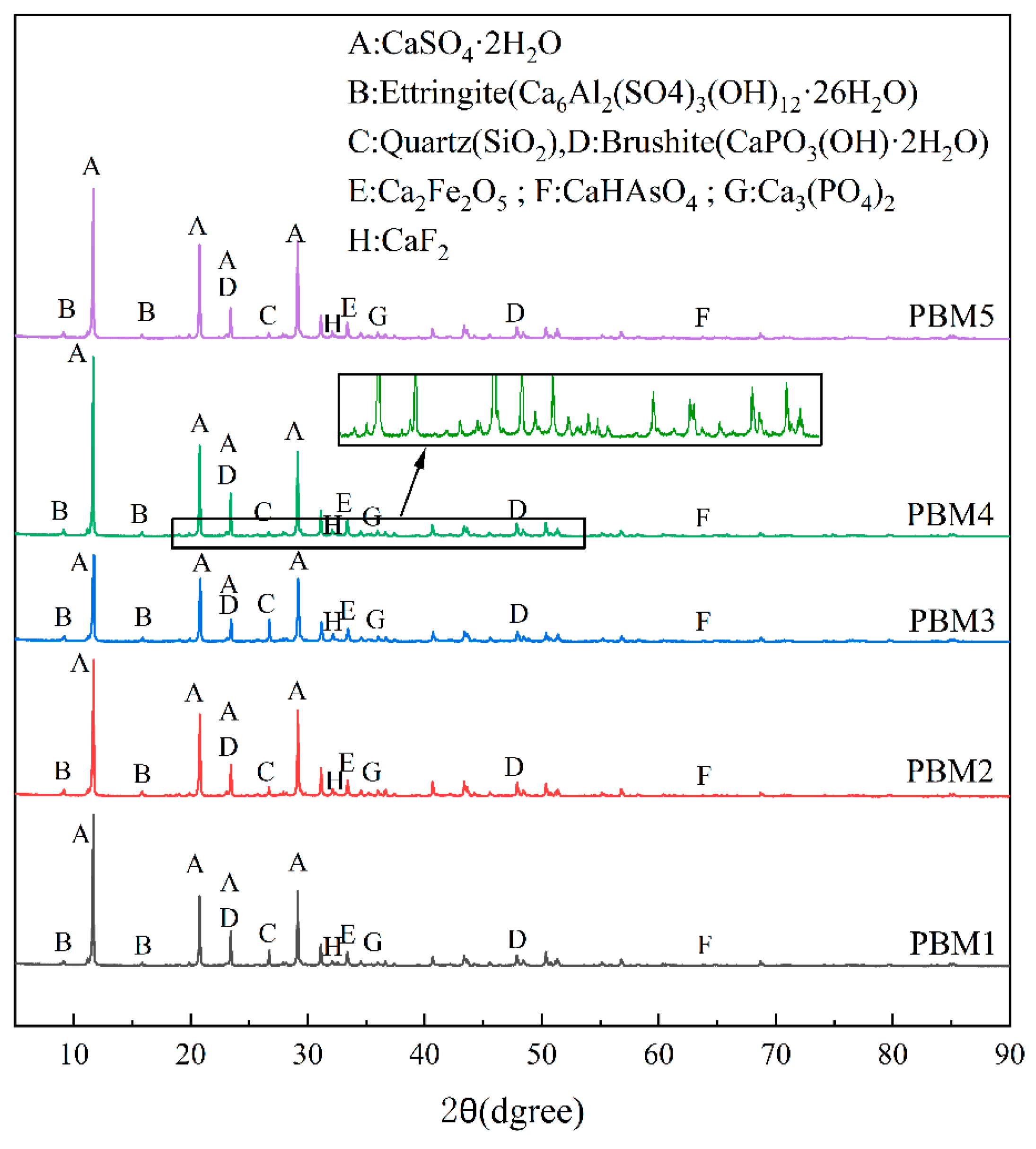
3.2. XRD Analysis
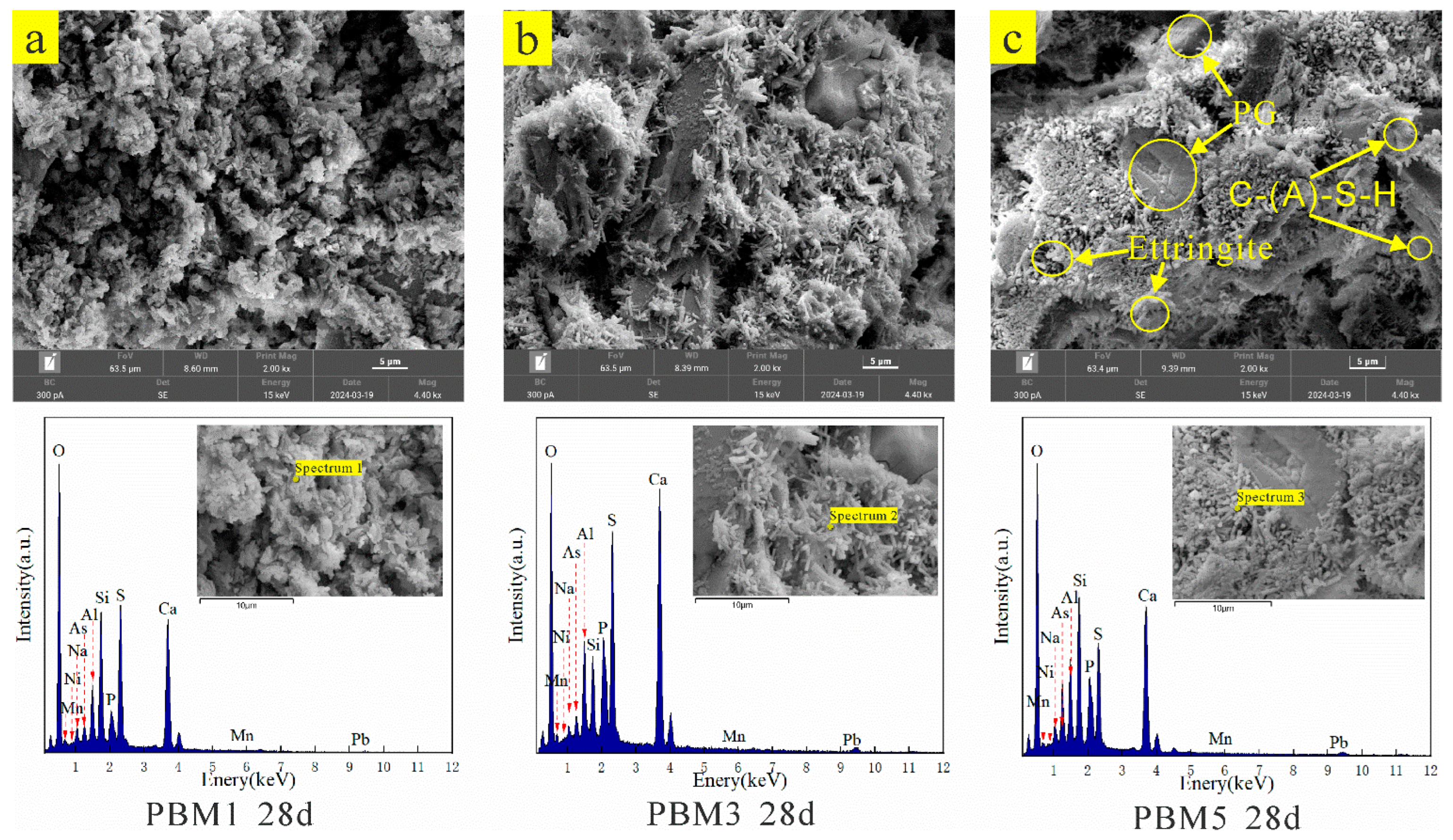
3.3. SEM-EDS Analysis
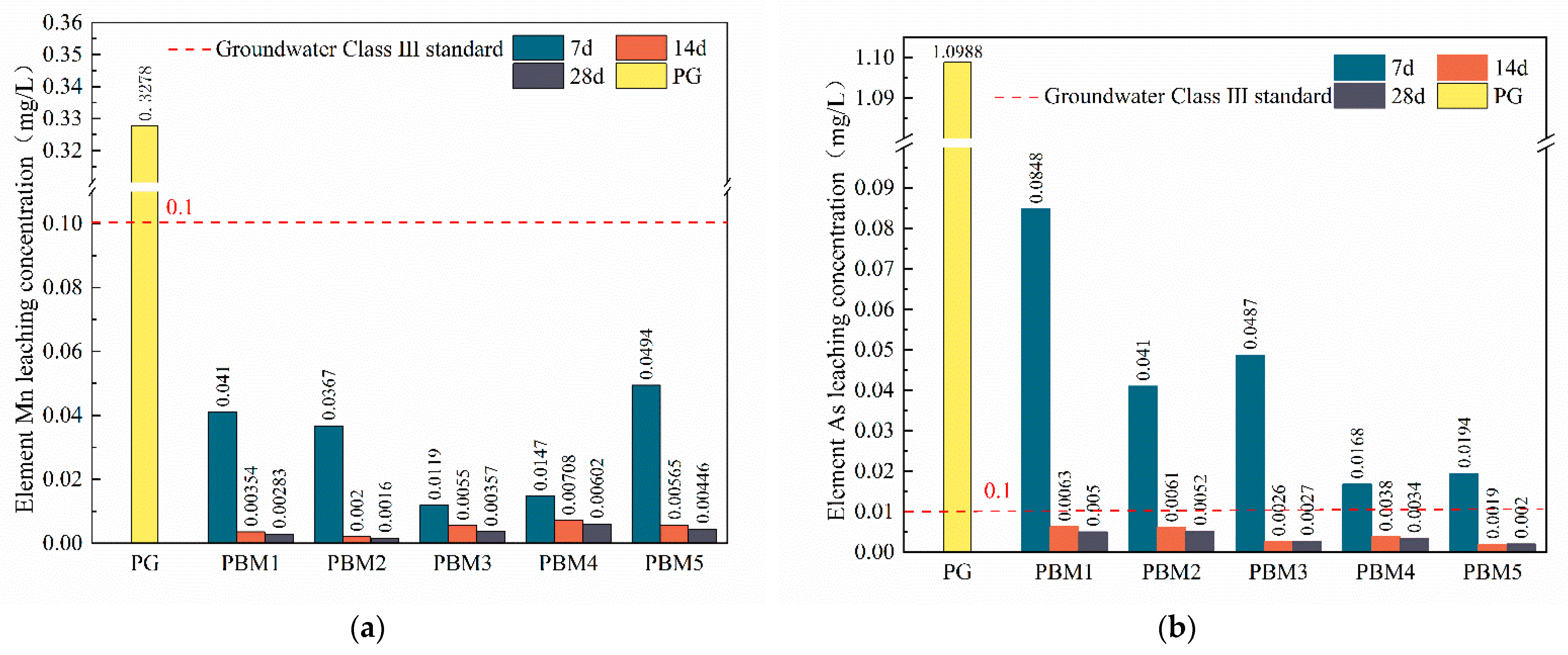
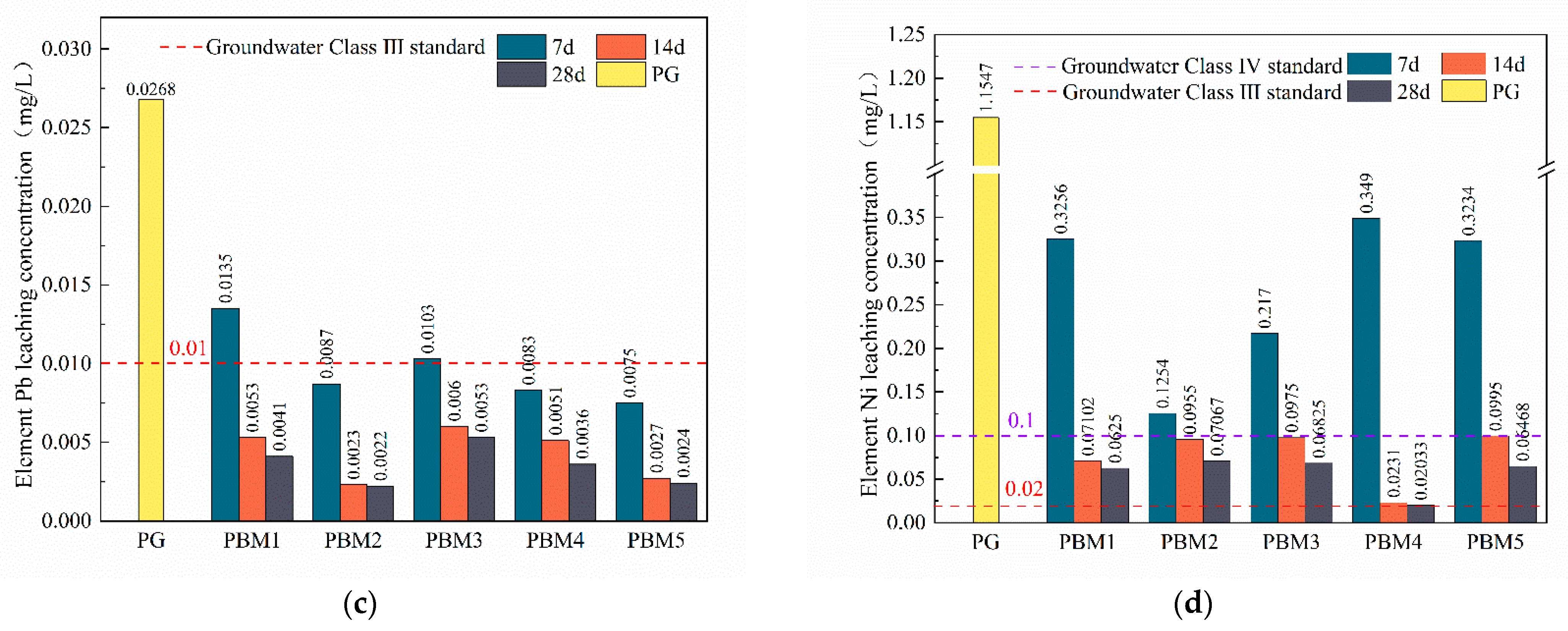
3.4. Leaching of Toxic Impurities in PBM
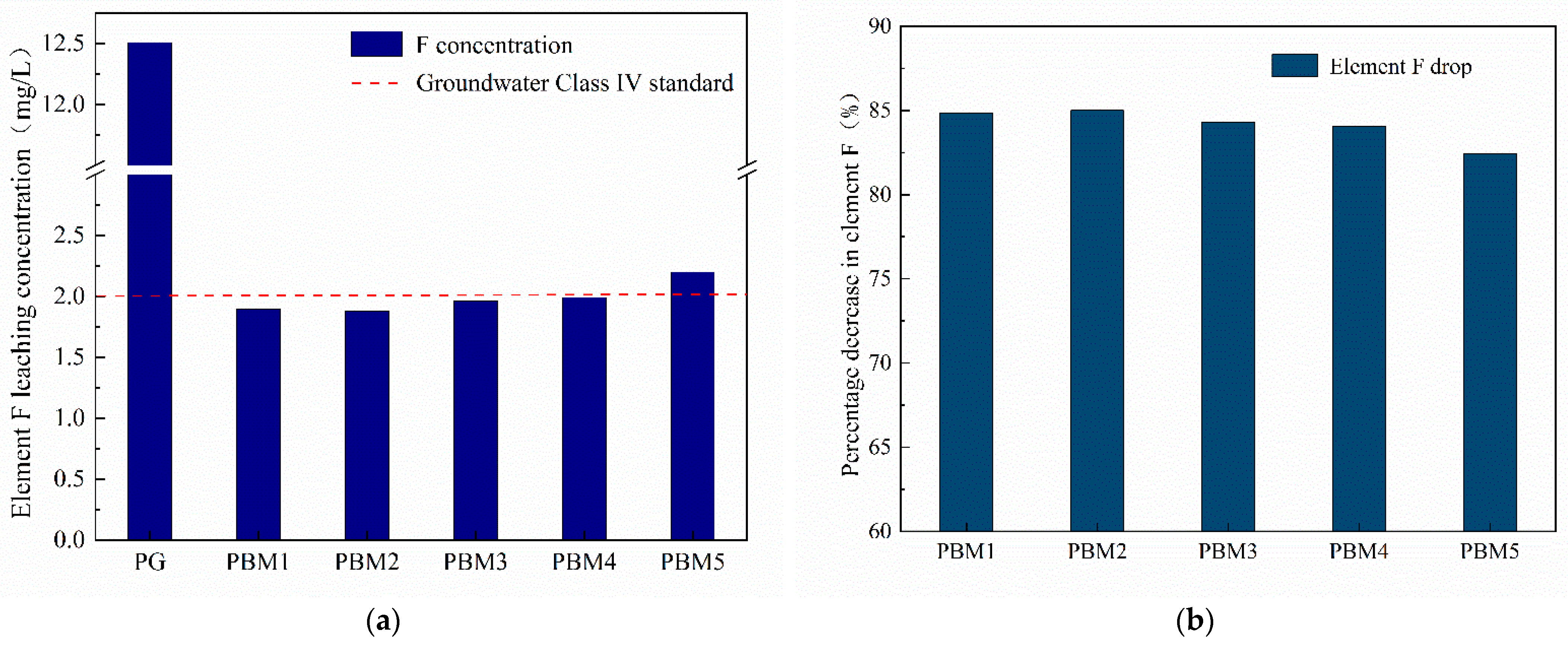
3.4.1. Leaching of Element Fluorine in PBM
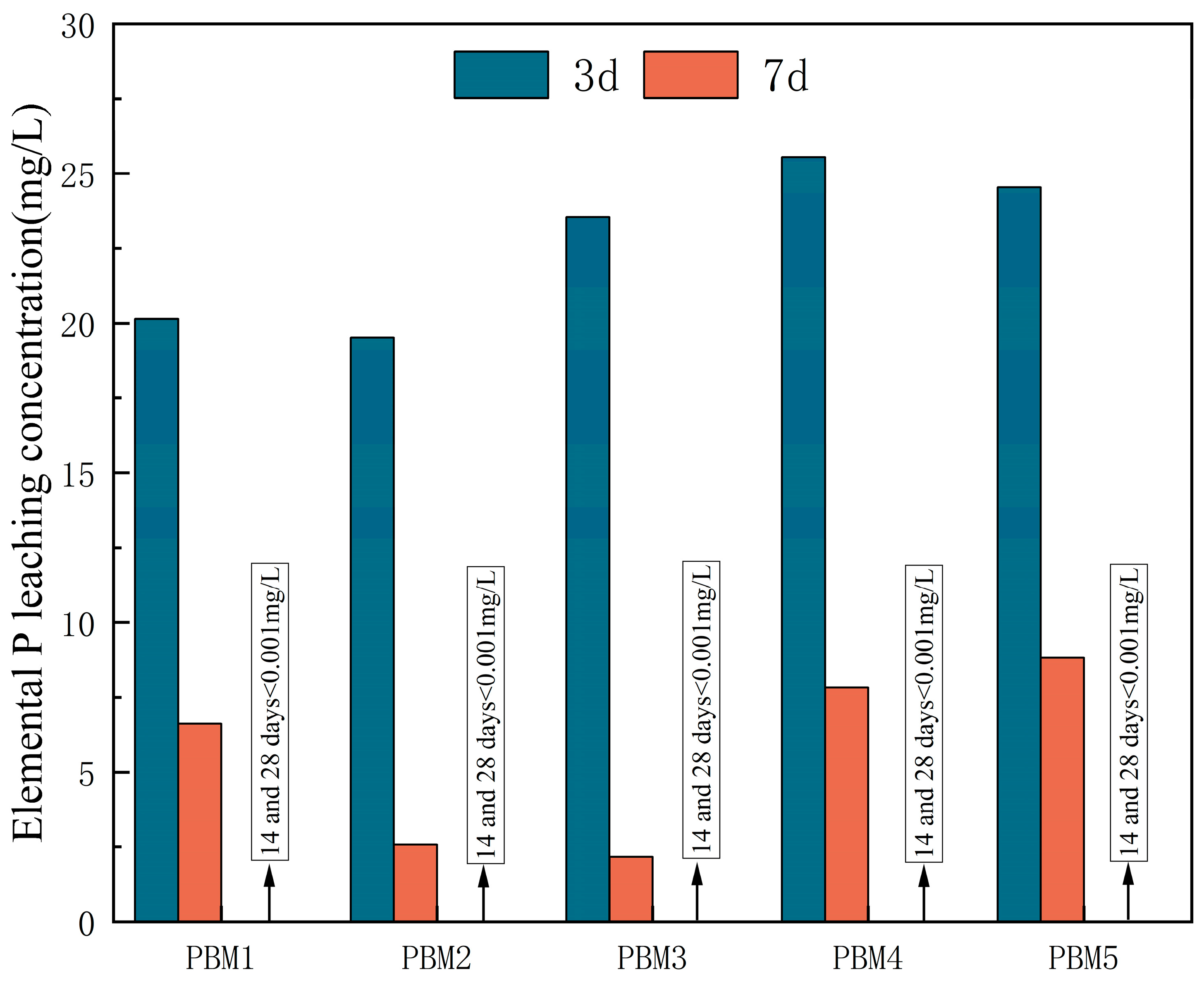
3.4.2. Leaching of Element Phosphorus in PBM
3.4.3. Heavy Metal Leaching
4. Conclusions
- (1)
- The compressive strength of PBM increased with the increase in GGBS, which increased from 4% to 16%, and the 28 d strength increased from 0.89 MPa to 3.78 MPa, all of which could meet the requirements of backfill technical specification.
- (2)
- The hydration products of PBM are mainly ettringite and C-(A)-S-H gel, and the production of ettringite is conducive to the formation of a network skeleton to enhance the microstructure of PBM, while the C-(A)-S-H gel effectively binds the unreacted PG, and at the same time, fills up the structural pores to form a dense microstructure, which improves the strength.
- (3)
- Toxic leaching showed that fluorine, phosphorus, and heavy metal elements were effectively stabilized and applied in the backfill technology without toxic leaching contamination. The heavy metal curing mechanism is manifested as follows: F− reacts with Ca2+ to generate the infusibility compound CaF2, phosphorus generates infusibility substances Ca2(PO4)3 and CaHPO4 by reacting with Ca2+, and the addition of SS can provide the system with more CaO, which is conducive to the stabilization of fluorine and phosphorus. Heavy metals can be chemically adsorbed or ionically displaced by ettringite and gel products to achieve the curing purpose.
- (4)
- The use of alkali-activated all-solid waste as a curing agent for PG backfill material was shown to provide a solution for recycling large quantities of industrial solid waste into utilization to alleviate the pressure of environmental pollution based on the mechanical properties and environmental suitability of PBM.
- (5)
- However, 20% of the binder was used in this study compared to about 10% used for common backfill materials, which may be attributed to the high acid and sulfate content of PG. The leaching concentrations of F ions and some pollutant elements were higher than the groundwater class III standard. Therefore, further studies are needed to develop effective curing contaminants and low-cost binders for PG-based CPB.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, R.; Wang, Q.; Luo, T. Reuse of phosphogypsum as hemihydrate gypsum: The negative effect and content control of H3PO4. Resour. Conserv. Recycl. 2021, 174, 105830. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Al-Masri, M.S.; Amin, Y.; Ibrahim, S.; Al-Bich, F. Distribution of some trace metals in Syrian phosphogypsum. Appl. Geochem. 2004, 19, 747–753. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z.; Wang, X.; Yang, L.; Zhong, B.; Liu, J. Thermodynamic study of phosphogypsum decomposition by sulfur. J. Chem. Thermodyn. 2013, 57, 39–45. [Google Scholar] [CrossRef]
- López, F.A.; Gázquez, M.; Alguacil, F.J.; Bolívar, J.P.; García-Díaz, I.; López-Coto, I. Microencapsulation of phosphogypsum into a sulfur polymer matrix: Physico-chemical and radiological characterization. J. Hazard. Mater. 2011, 192, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Chen, Q.; Sun, H.; Peng, T. Modified mineral carbonation of phosphogypsum for CO2 sequestration. J. CO2 Util. 2019, 34, 507–515. [Google Scholar] [CrossRef]
- Altun, İ.A.; Sert, Y. Utilization of weathered phosphogypsum as set retarder in Portland cement. Cem. Concr. Res. 2004, 34, 677–680. [Google Scholar] [CrossRef]
- Hentati, O.; Abrantes, N.; Caetano, A.L.; Bouguerra, S.; Goncalves, F.; Römbke, J.; Pereira, R. Phosphogypsum as a soil fertilizer: Ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. J. Hazard. Mater. 2015, 294, 80–89. [Google Scholar] [CrossRef]
- Shen, W.; Zhou, M.; Ma, W.; Hu, J.; Cai, Z. Investigation on the application of steel slag-fly ash-phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [CrossRef]
- Campos, M.P.; Costa, L.; Nisti, M.B.; Mazzilli, B.P. Phosphogypsum recycling in the building materials industry: Assessment of the radon exhalation rate. J. Environ. Radioact. 2017, 172, 232–236. [Google Scholar] [CrossRef]
- Kumar, S. Fly ash-lime-phosphogypsum hollow blocks for walls and partitions. Build. Environ. 2003, 38, 291–295. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Li, H.; Jiang, Z.; Gong, Z.; Yang, X.; Chen, Q. Utilization of the black tea powder as multifunctional admixture for the hemihydrate gypsum. J. Clean. Prod. 2019, 210, 231–237. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, H.; Shu, Z.; Wang, Y.; Yan, C. Utilization of waste phosphogypsum to prepare non-fired bricks by a novel Hydration-Recrystallization process. Constr. Build. Mater. 2012, 34, 114–119. [Google Scholar] [CrossRef]
- Garg, M.; Singh, M.; Kumar, R. Some aspects of the durability of a phosphogypsum-lime-fly ash binder. Constr. Build. Mater. 1996, 10, 273–279. [Google Scholar] [CrossRef]
- Kumar, S. A perspective study on fly ash-lime-gypsum bricks and hollow blocks for low cost housing development. Constr. Build. Mater. 2002, 16, 519–525. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, H.; Zheng, L.; Wang, K.; Li, H.; Wang, Y.; Feng, H. Utilization of waste phosphogypsum to prepare hydroxyapatite nanoparticles and its application towards removal of fluoride from aqueous solution. J. Hazard. Mater. 2012, 241, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Pérez-López, R.; Gázquez, M.J.; Morales-Flórez, V.; Santos, A.; Esquivias, L.; Bolívar, J.P. Fractionation and fluxes of metals and radionuclides during the recycling process of phosphogypsum wastes applied to mineral CO2 sequestration. Waste Manag. 2015, 45, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.M. Potential use of phosphogypsum in alkali-activated fly ash under the effects of elevated temperatures and thermal shock cycles. J. Clean. Prod. 2015, 87, 717–725. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Zhou, Y.; Min, C.; Shi, Y. Effect of phosphorus on the properties of phosphogypsum-based cemented backfill. J. Hazard. Mater. 2020, 399, 122993. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Qi, C.; Fourie, A.; Xiao, C. Recycling phosphogypsum and construction demolition waste for cemented paste backfill and its environmental impact. J. Clean. Prod. 2018, 186, 418–429. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Yin, X.; Ma, L.; Li, K.; Du, W.; Hou, P.; Dai, Q.; Xiong, X.; Xie, L. Preparation of phosphogypsum-based cemented paste backfill and its environmental impact based on multi-source industrial solid waste. Constr. Build. Mater. 2023, 404, 133314. [Google Scholar] [CrossRef]
- Zheng, P.; Li, W.; Ma, Q.; Xi, L. Mechanical properties of phosphogypsum-soil stabilized by lime activated ground granulated blast-furnace slag. Constr. Build. Mater. 2023, 402, 132994. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Fall, M.; Belem, T. A contribution to understanding the hardening process of cemented pastefill. Miner. Eng. 2004, 17, 141–152. [Google Scholar] [CrossRef]
- Tariq, A.; Yanful, E.K. A review of binders used in cemented paste tailings for underground and surface disposal practices. J. Environ. Manag. 2013, 131, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Singh, M. Effect of phosphatic and fluoride impurities of phosphogypsum on the properties of selenite plaster. Cem. Concr. Res. 2003, 33, 1363–1369. [Google Scholar] [CrossRef]
- Tabikh, A.A.; Miller, F.M. The nature of phosphogypsum impurities and their influence on cement hydration. Cem. Concr. Res. 1971, 1, 663–678. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Wang, D.; Yan, P.; Luo, L.; Zhang, H.; Zhang, H.; Gu, X. Hydration superposition effect and mechanism of steel slag powder and granulated blast furnace slag powder. Constr. Build. Mater. 2023, 366, 130101. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Hu, L. Efficient use of steel slag in alkali-activated fly ash-steel slag-ground granulated blast furnace slag ternary blends. Constr. Build. Mater. 2020, 259, 119814. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, Z.; Yan, X.; Zhang, Y.; Dong, S.; Li, K.; Briseghella, B.; Marano, G.C. Mechanical properties and microstructure of engineered cementitious composites with high volume steel slag and GGBFS. Constr. Build. Mater. 2023, 398, 132512. [Google Scholar] [CrossRef]
- Huo, Y.; Huang, J.; Han, X.; Sun, H.; Liu, T.; Zhou, J.; Yang, Y. Mass GGBFS concrete mixed with recycled aggregates as alkali-active substances: Workability, temperature history and strength. Materials 2023, 16, 5632. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Shi, Y.; Liu, Z. Properties of cemented phosphogypsum (PG) backfill in case of partially substitution of composite Portland cement by ground granulated blast furnace slag. Constr. Build. Mater. 2021, 305, 124786. [Google Scholar] [CrossRef]
- Aperador, W.; Bautista-Ruiz, J.; Sánchez-Molina, J. Geopolymers Based on a Mixture of Steel Slag and Fly Ash, Activated with Rice Husks and Reinforced with Guadua angustifolia Fibers. Sustainability 2023, 15, 12404. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Cheng, Y.; Huseien, G.F.; Shah, K.W. Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Santos, W.F.; Botterweg, J.; Figueiredo, S.C.; Schollbach, K.; van der Laan, S.; Brouwers, H. Sodium oxalate activation of basic oxygen furnace slag for building materials. Resour. Conserv. Recycl. 2023, 198, 107174. [Google Scholar] [CrossRef]
- GB/T 14848-2017; Standard for Groundwater Quality [S]. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China; Standardization Administration: Beijing, China, 2017.
- Sas, Z.; Sha, W.; Soutsos, M.; Doherty, R.; Bondar, D.; Gijbels, K.; Schroeyers, W. Radiological characterisation of alkali-activated construction materials containing red mud, fly ash and ground granulated blast-furnace slag. Sci. Total Environ. 2019, 659, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- GB/T 39489-2020; Technical Specification for the Total Tailings Paste Backfill [S]. State Administration for Market Regulation: Beijing, China; Standardization Administration: Beijing, China, 2020.
- JGJ/T 70-2009; Standard for Test Method of Performance on Building Mortar [S]. Industry Standards-Construction Industry. Chinese Standard: Beijing, China, 2009.
- HJ 557-2010; Solid Waste-Extraction Procedure for Leaching Toxicity-Horizontal Vibration Method [S]. Industry Standard-Environmental Protection: Beijing, China, 2010.
- Shi, Y.; Cheng, L.; Tao, M.; Tong, S.; Yao, X.; Liu, Y. Using modified quartz sand for phosphate pollution control in cemented phosphogypsum (PG) backfill. J. Clean. Prod. 2021, 283, 124652. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Wang, Y.; Zhang, Q.; Liu, Y. Highly-efficient fluoride retention in on-site solidification/stabilization of phosphogypsum: Cemented paste backfill synergizes with poly-aluminum chloride activation. Chemosphere 2022, 309, 136652. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Yang, S.; Chu, J.; Liu, Y. Study on the early effect of excitation method on the alkaline steel slag. Sustainability 2023, 15, 4714. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-activated binders based on ground granulated blast furnace slag and phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Fu, Q.; Bu, M.; Zhang, Z.; Xu, W.; Yuan, Q.; Niu, D. Hydration characteristics and microstructure of alkali-activated slag concrete: A review. Engineering 2023, 20, 162–179. [Google Scholar] [CrossRef]
- Ding, X.; Ao, Z.; Zhou, W.; Qin, H.; Yang, Z.; An, W.; Li, X.; Liu, H. Geopolymer-based modification of blasting sealing materials and optimization of blasting block size in coal seams of open pit mines. Int. J. Min. Sci. Technol. 2023, 33, 1551–1562. [Google Scholar] [CrossRef]
- Zhao, Y.; Taheri, A.; Karakus, M.; Chen, Z.; Deng, A. Effects of water content, water type and temperature on the rheological behaviour of slag-cement and fly ash-cement paste backfill. Int. J. Min. Sci. Technol. 2020, 30, 271–278. [Google Scholar] [CrossRef]
- Li, L.; Xie, J.; Zhang, B.; Feng, Y.; Yang, J. A state-of-the-art review on the setting behaviours of ground granulated blast furnace slag-and metakaolin-based alkali-activated materials. Constr. Build. Mater. 2023, 368, 130389. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Qiu, J.; Xing, J. Review: Alkali-activated blast furnace slag for eco-friendly binders. J. Mater. Sci. 2022, 57, 1599–1622. [Google Scholar] [CrossRef]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum recycling: A review of environmental issues, current trends, and prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P. Hydration properties of basic oxygen furnace steel slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Cheng, F.; Song, H.; Yang, H. Investigation into the synergistic effects in hydrated gelling systems containing fly ash, desulfurization gypsum and steel slag. Constr. Build. Mater. 2018, 187, 1113–1120. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Mi, G. Effect of blended steel slag–GBFS mineral admixture on hydration and strength of cement. Constr. Build. Mater. 2012, 35, 8–14. [Google Scholar] [CrossRef]
- Hu, S.; Wang, H.; Zhang, G.; Ding, Q. Bonding and abrasion resistance of geopolymeric repair material made with steel slag. Cem. Concr. Compos. 2008, 30, 239–244. [Google Scholar] [CrossRef]
- Ma, W.; Brown, P.W. Hydrothermal reactions of fly ash with Ca(OH)2 and CaSO4·2H2O. Cem. Concr. Res. 1997, 27, 1237–1248. [Google Scholar] [CrossRef]
- Chen, R.; Lai, H.; Cui, D.; Zhu, Y. Alkali-activated mortar for tunnel-lining structure repair. J. Mater. Civ. Eng. 2019, 31, 4019217. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Li, L.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; Van Deventer, J.S. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Liu, Q.; He, Q.; Li, R.; Feng, Y.; Lyu, X.; Wang, J.; Li, L. Influence of colloidal nanosilica on hydration kinetics and properties of CaO/CaSO4-activated slag binder. Int. J. Min. Sci. Technol. 2022, 32, 1407–1418. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Li, Y.; Shen, L.; Mirmoghtadaei, R.; Ai, L. A design of experiment approach to study the effects of raw material on the performance of geopolymer concrete. Adv. Civ. Eng. Mater. 2017, 6, 526–549. [Google Scholar] [CrossRef]
- Pu, S.; Zhu, Z.; Huo, W. Evaluation of engineering properties and environmental effect of recycled gypsum stabilized soil in geotechnical engineering: A comprehensive review. Resour. Conserv. Recycl. 2021, 174, 105780. [Google Scholar] [CrossRef]
- Singh, B.; Rahman, M.R.; Paswan, R.; Bhattacharyya, S.K. Effect of activator concentration on the strength, ITZ and drying shrinkage of fly ash/slag geopolymer concrete. Constr. Build. Mater. 2016, 118, 171–179. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y.; Ge, W.; Zhang, A. Mechanical, microstructure and reaction process of calcium carbide slag-waste red brick powder based alkali-activated materials (CWAAMs). J. Clean. Prod. 2022, 331, 129845. [Google Scholar] [CrossRef]
- Jiang, D.; Shi, C.; Zhang, Z. Recent progress in understanding setting and hardening of alkali-activated slag (AAS) materials. Cem. Concr. Compos. 2022, 134, 104795. [Google Scholar] [CrossRef]
- Battistoni, P.; Carniani, E.; Fratesi, V.; Balboni, P.; Tornabuoni, P. Chemical-physical pretreatment of phosphogypsum leachate. Ind. Eng. Chem. Res. 2006, 45, 3237–3242. [Google Scholar] [CrossRef]
- Tao, W.; Fattah, K.P.; Huchzermeier, M.P. Struvite recovery from anaerobically digested dairy manure: A review of application potential and hindrances. J. Environ. Manag. 2016, 169, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [PubMed]
- Lan, J.; Dong, Y.; Sun, Y.; Fen, L.; Zhou, M.; Hou, H.; Du, D. A novel method for solidification/stabilization of Cd (II), Hg (II), Cu (II), and Zn (II) by activated electrolytic manganese slag. J. Hazard. Mater. 2021, 409, 124933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Xu, Y.; Tang, B.; Wang, Y. Preparation of road base material by utilizing electrolytic manganese residue based on Si-Al structure: Mechanical properties and Mn2+ stabilization/solidification characterization. J. Hazard. Mater. 2020, 390, 122188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef]
- Choi, W.; Lee, S.; Park, J. Cement based solidification/stabilization of arsenic-contaminated mine tailings. Waste Manag. 2009, 29, 1766–1771. [Google Scholar] [CrossRef]
- He, S.; Jiang, D.; Hong, M.; Liu, Z. Hazard-free treatment and resource utilisation of electrolytic manganese residue: A review. J. Clean. Prod. 2021, 306, 127224. [Google Scholar] [CrossRef]
- Gougar, M.; Scheetz, B.E.; Roy, D.M. Ettringite and C-S-H Portland cement phases for waste ion immobilization: A review. Waste Manag. 1996, 16, 295–303. [Google Scholar] [CrossRef]
- Myneni, S.C.; Traina, S.J.; Logan, T.J.; Waychunas, G.A. Oxyanion behavior in alkaline environments: Sorption and desorption of arsenate in ettringite. Environ. Sci. Technol. 1997, 31, 1761–1768. [Google Scholar] [CrossRef]





| Materials | Chemical Composition | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | SO3 | Others | |
| PG | 2.45 | 36.59 | 0.34 | 0.34 | 0 | 59.78 | 0.5 |
| GGBS | 34.5 | 34 | 17.7 | 1.03 | 6.01 | 1.64 | 5.58 |
| SS | 16.22 | 42.19 | 6.33 | 18.93 | 8.37 | 0.97 | 6.99 |
| Hazardous Element Content (mg/L) | Mn | Pb | Se | As | Ni | Be | Cu | Zn | Cd | Ba |
|---|---|---|---|---|---|---|---|---|---|---|
| PG-1 | 0.5442 | 0.0281 | 0.0058 | 1.1537 | 0.8124 | 0.0004 | 0.0441 | 0.1365 | 0.0010 | 0.0105 |
| PG-2 | 0.1147 | 0.0257 | 0.0013 | 1.5548 | 1.1085 | 0.0002 | 0.0203 | 0.1748 | 0.0002 | 0.0076 |
| PG-3 | 0.3245 | 0.0265 | 0.0094 | 0.5878 | 1.5432 | 0.0006 | 0.0616 | 0.0787 | 0.0009 | 0.0119 |
| Average concentration | 0.3278 | 0.0268 | 0.0055 | 1.0988 | 1.1547 | 0.0004 | 0.0420 | 0.1300 | 0.0007 | 0.0100 |
| Class III Standard | 0.1000 | 0.0100 | 0.0100 | 0.0100 | 0.0200 | 0.0020 | 1.0000 | 1.0000 | 0.0050 | 0.7000 |
| Sample ID | Raw Material (%) | Molar Concentration of Alkali-Activated (mol/L) | Liquid–Solid Ratio | Age of Hardening (Days) | ||
|---|---|---|---|---|---|---|
| PG | SS | GGBS | ||||
| PBM1 | 80 | 16 | 4 | 1 | 0.4 | 7, 14, 28 |
| PBM2 | 80 | 13 | 7 | 1 | 0.4 | |
| PBM3 | 80 | 10 | 10 | 1 | 0.4 | |
| PBM4 | 80 | 7 | 13 | 1 | 0.4 | |
| PBM5 | 80 | 4 | 16 | 1 | 0.4 | |
| CPBM | 80 | 10 | 10 | 0 | 0.4 | |
| Elemental | O | Na | Al | Si | P | S | Ca | Mn | Ni | As | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectrum 1 | Wt% | 54.18 | 1.27 | 3.27 | 8.08 | 1.12 | 11.49 | 18.46 | 0.14 | 0.03 | 0.87 | 100 |
| At% | 71.69 | 1.17 | 2.56 | 6.08 | 0.77 | 7.57 | 9.73 | 0.06 | 0.01 | 0.24 | 100 | |
| Spectrum 2 | Wt% | 47.75 | 0.79 | 4.24 | 3.54 | 1.21 | 12.09 | 26.19 | 0.19 | 0.22 | 0.87 | 100 |
| At% | 67.82 | 0.78 | 3.56 | 2.86 | 0.88 | 8.54 | 14.8 | 0.08 | 0.1 | 0.26 | 100 | |
| Spectrum 3 | Wt% | 50.89 | 1.72 | 4.64 | 8.97 | 1.06 | 8.17 | 19.02 | 0.17 | 0.18 | 2.36 | 100 |
| At% | 69.78 | 1.63 | 3.76 | 6.99 | 0.75 | 5.58 | 10.38 | 0.07 | 0.07 | 0.69 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhu, L.; Wu, Z.; Wang, X. Properties of Cemented Filling Materials Prepared from Phosphogypsum-Steel Slag–Blast-Furnace Slag and Its Environmental Effect. Materials 2024, 17, 3618. https://doi.org/10.3390/ma17143618
Li K, Zhu L, Wu Z, Wang X. Properties of Cemented Filling Materials Prepared from Phosphogypsum-Steel Slag–Blast-Furnace Slag and Its Environmental Effect. Materials. 2024; 17(14):3618. https://doi.org/10.3390/ma17143618
Chicago/Turabian StyleLi, Kai, Lishun Zhu, Zhonghu Wu, and Xiaomin Wang. 2024. "Properties of Cemented Filling Materials Prepared from Phosphogypsum-Steel Slag–Blast-Furnace Slag and Its Environmental Effect" Materials 17, no. 14: 3618. https://doi.org/10.3390/ma17143618




